Minimization of Chemical Kinetic Reaction Set for System-Level Study of Non-Thermal Plasma NOx Abatement Process
Abstract
1. Introduction
2. Materials and Methods
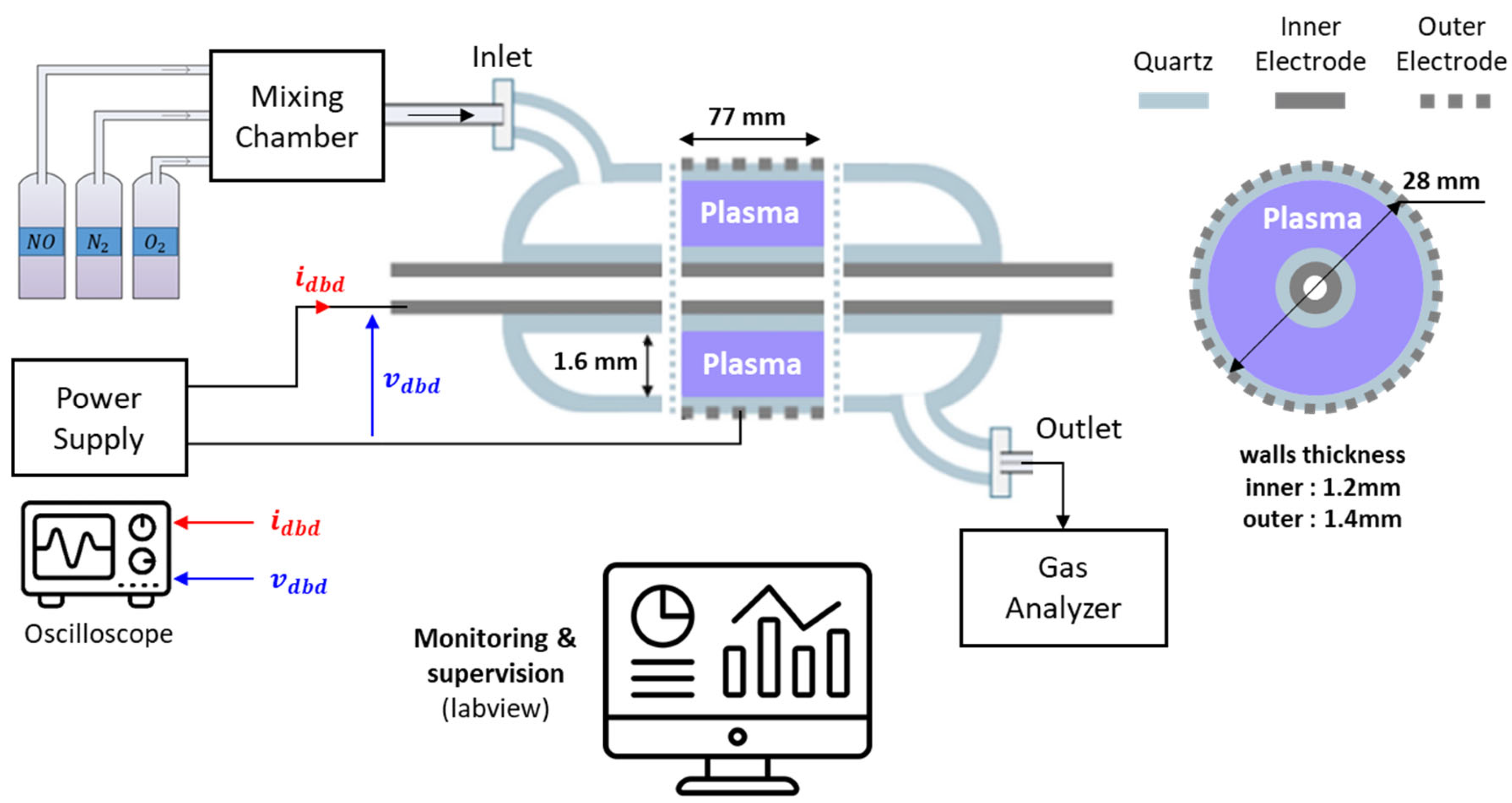
2.1. Experimental Setup
- The reactor has a coaxial cylindrical geometry made of quartz. The inner electrode is stainless-steel foil, and the outer electrode is a metallic mesh (knitted thin wires made of tinned copper) wrapped around the quartz tube. The length of the mesh is 77 mm. Detailed dimensions are shown in Figure 1.
- The treated gas mixture is composed of , and and flows through the DBD reactor. The gas composition and total flow rate are adjustable: mass flow controllers (Bronkhorst EL-FLOW Prestige) are used to measure and regulate them. This mixture is intended to support research into the use of hydrogen in burners or combustion engines. It also allows for a comparison of the solutions and performance obtained in numerous studies available in the literature [1,2,3,4,5,6,7,9].Figure 1. Experimental setup and reactor dimensions.
- The DBD voltage is measured with a 200 MHz digital oscilloscope (LeCroy HDO4024) connected through a 1000:1 voltage probe (Testec TT-SI 9010), and the current is measured using a current probe (LeCroy AP015). Gas analyzers (Testo 350 and Serinus 40, specifically for low NOx concentrations) measure the and concentrations.
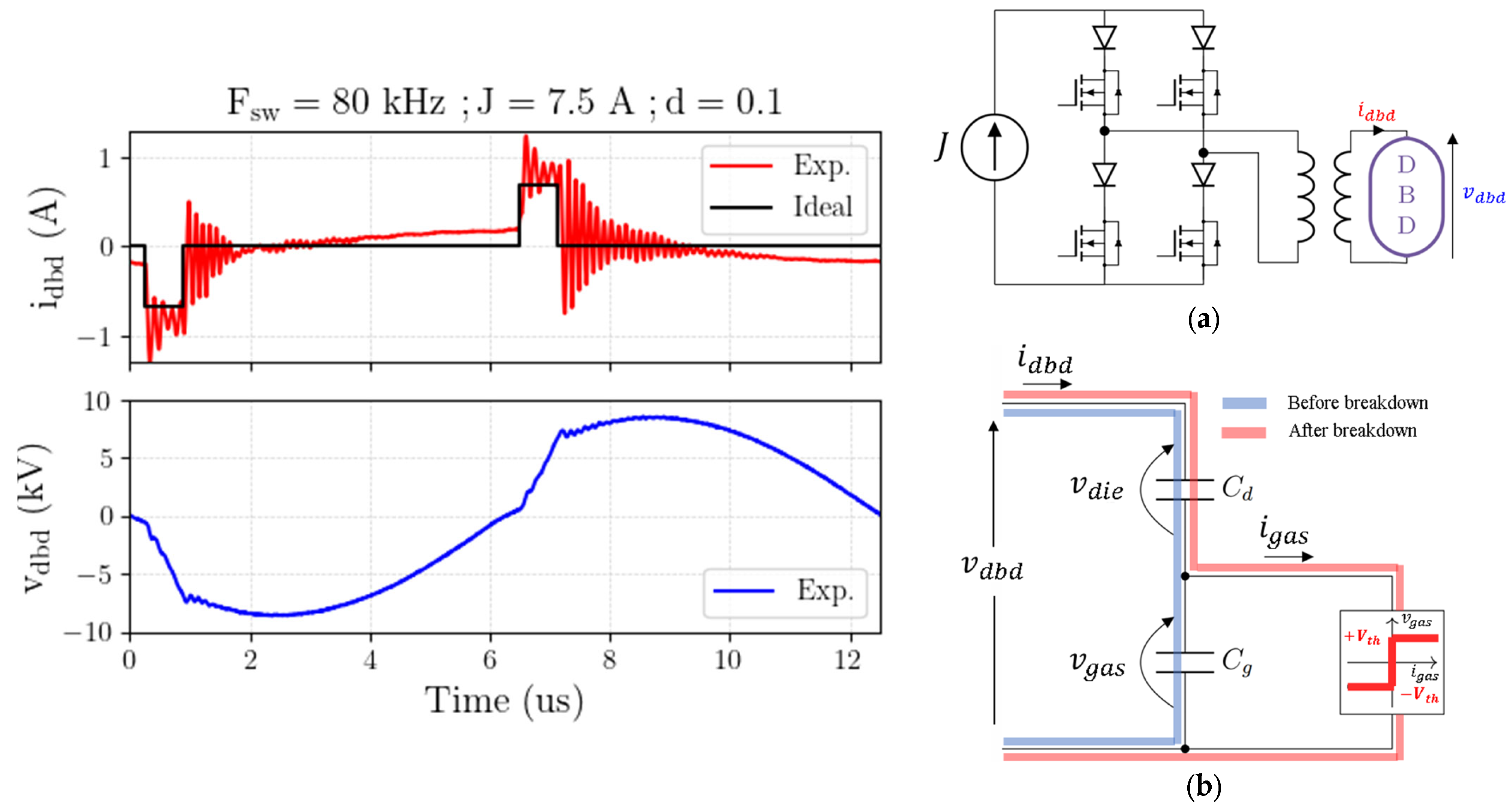
- A specific power supply [20,21] was developed to ignite the plasma and control the energy injected into the NTP. It delivers square current waveforms, as shown in Figure 2. This circuit integrates a DC current source connected in cascade with a full-bridge current inverter and a step-up transformer. The converter delivers current pulses, whose rectangular shape is controlled by three degrees of freedom, namely frequency , current amplitude , and pulse duration (, being the duty cycle in the range of 5–95%). The maximum current delivered by the current source (primary side of the transformer) is 12 A. The high-voltage transformer is designed to withstand a maximum voltage of 11 kV; its turn ratio is 11. The frequency can be varied from 80 kHz to 200 kHz.
- Defines the configuration of each device;
- Defines the set point of controlled devices (mass flow controllers, power supply);
- Sets up and monitors batch experiments (gas mixtures, power supply control);
- Monitors data acquisition and measurement storage (database);
- Prepares measurements for post-treatment analyses.
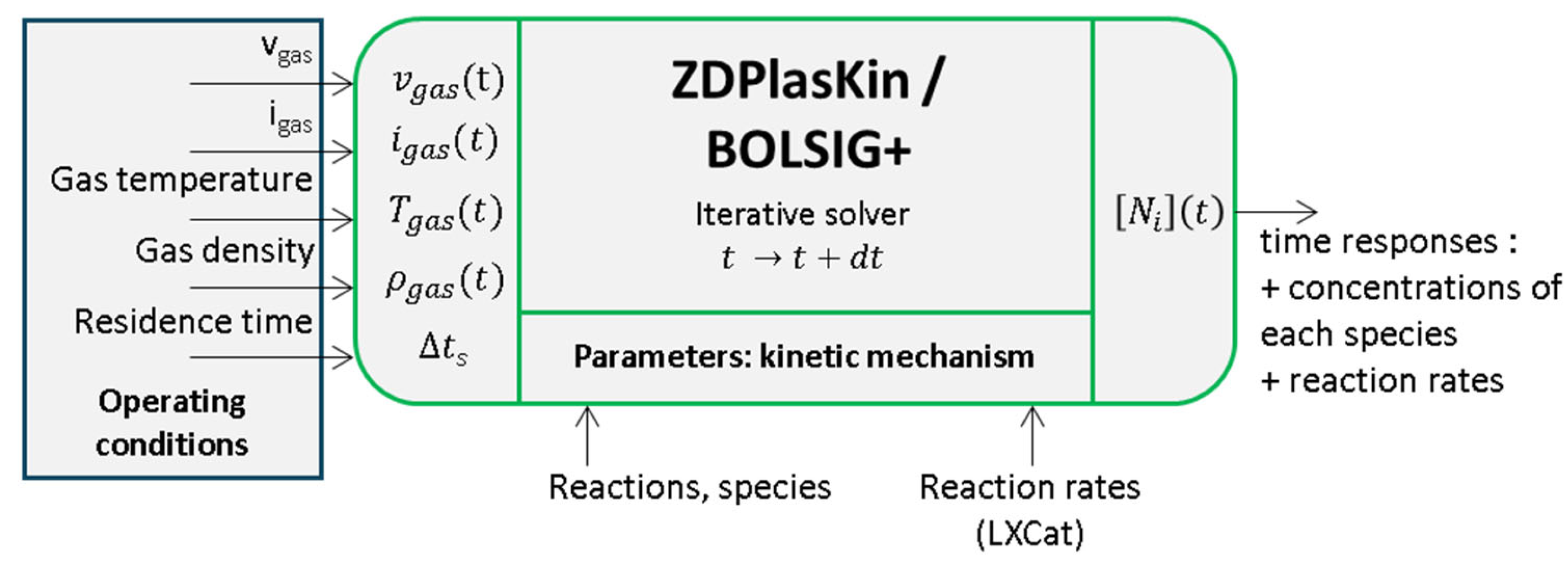
2.2. System-Level Analysis and Kinetics of Plasma Gas Treatment
- For gas-phase reactions, rate coefficients can be constant or temperature-dependent, often following an Arrhenius-type law.
- For electron impact reactions, rate coefficients depend on the electron collision cross-sections (cross-sections are a measure of the probability that a specific process will take place in a collision of two particles). Since the cross-sections also vary with electron energy, ZdPlaskin first calls BOLSIG+ to solve the electron energy distribution function (EEDF) and then computes the rate coefficients accordingly. The required cross-section data are sourced from LXCat, an open access, community-driven database for electron scattering cross-sections [13,14,15]. It should be noted that these databases are also used by commercial simulation products (for example, Comsol).
- Favorable reduction, which transforms into ;
- Unfavorable oxidation, which increases the amount.
- The electric field is obtained from the voltage divided by the gap length. However, is not a directly measurable quantity (due to dielectric barriers in the reactor being present). With the records of and being measurements or simulation results, the evaluation of is straightforward [20] using the classical Equation (1) for the DBD:where is the equivalent capacitance of the dielectric barriers in the reactor, as shown in Figure 2b. It is worth mentioning in this context that the DBD device is represented by an equivalent lumped parameter circuit [21]. Experimental records are used to identify the parameters of this circuit on the basis of the Manley diagram [22,23]. This equivalent circuit is also useful to build the gas current waveform from the DBD voltage and current, and . Uniform behavior of the plasma is assumed, and no local phenomena (micro discharges, for instance, streamers) can be individually considered; this is in good agreement with the “zero dimensional” characteristic of the ZdPlaskin solver.
- The temperature is assumed to be uniform within the reactor. The gas number density is also uniform, being closely related to —according to the ideal gases law, one can state thatwhere is the Avogadro constant, is the ideal gas constant, and is the gas pressure (1 atm, constant in our case).
- The gas current is used together with the electric field to calculate the electron density necessary for electron impact reactions.
2.2.1. Modeling Equations
2.2.2. Equation Solving
2.2.3. Simulation Results and Performance
2.3. Minimization of the Reaction Set for Kinetics Modeling
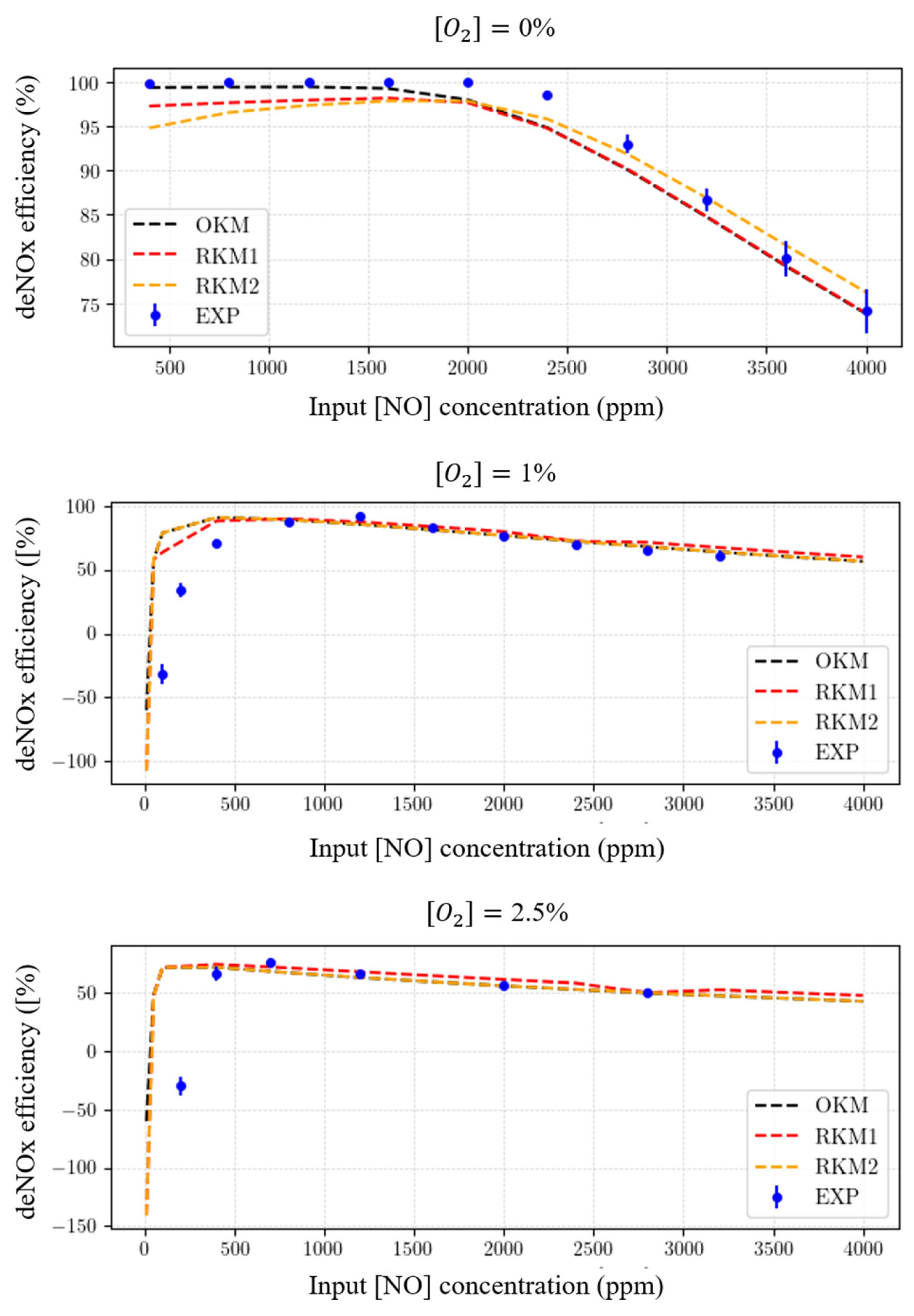
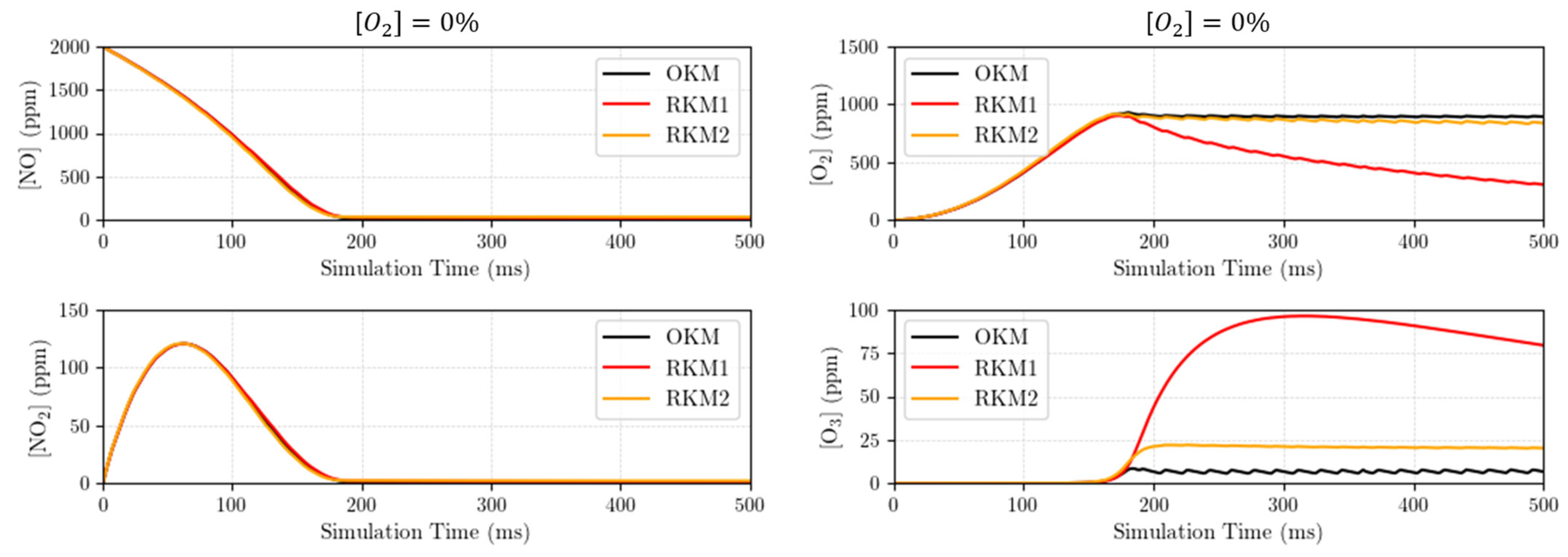
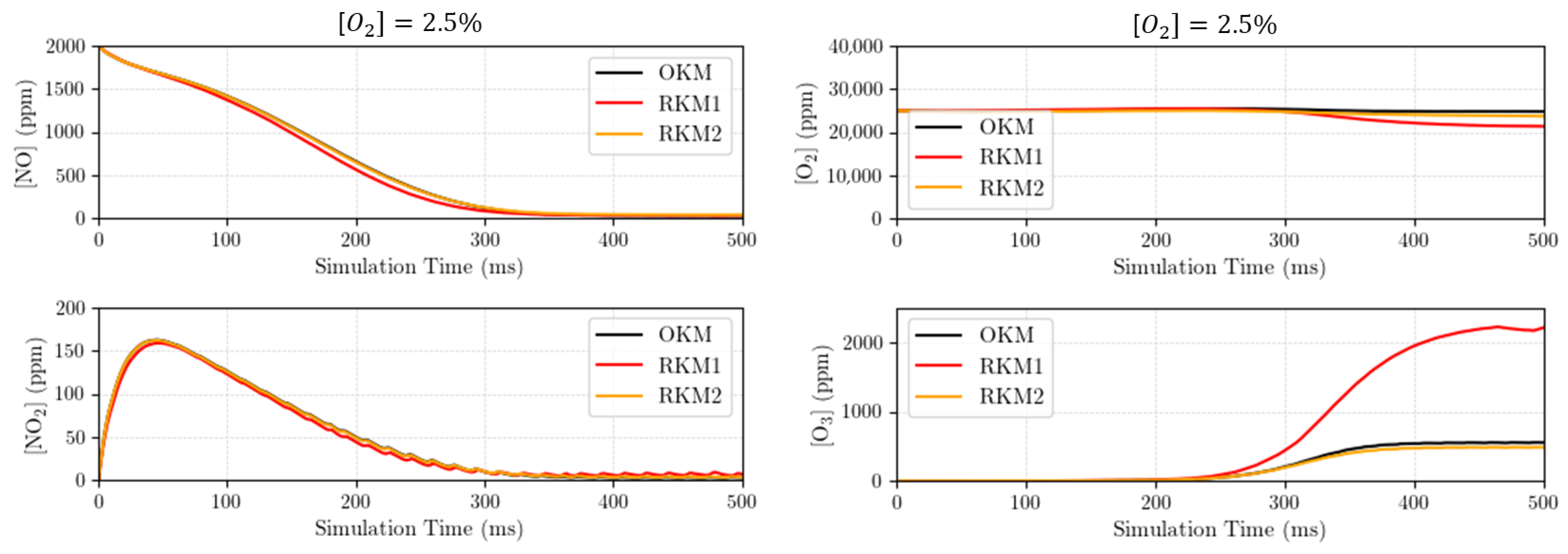
- Using the “original kinetic mechanism” (OKM) as the reference (in our case, it includes species and reactions). Indeed, this OKM has shown to very accurately reflect the experimental measurements (see Figure 6). Each tentative reduced kinetic mechanism will be compared to the later one (OKM);
- Proceeding with a two-step approach to build a “reduced kinetic mechanism” (RKM), as described below. The construction of the RKM, as well as the scans presented in Section 3 for robustness analysis, were performed using a toolkit called “Zukini,” developed by A. Cuellar.
2.3.1. First Step: Chemical Analysis-Based Selection of an “Initial Kinetic Mechanism”
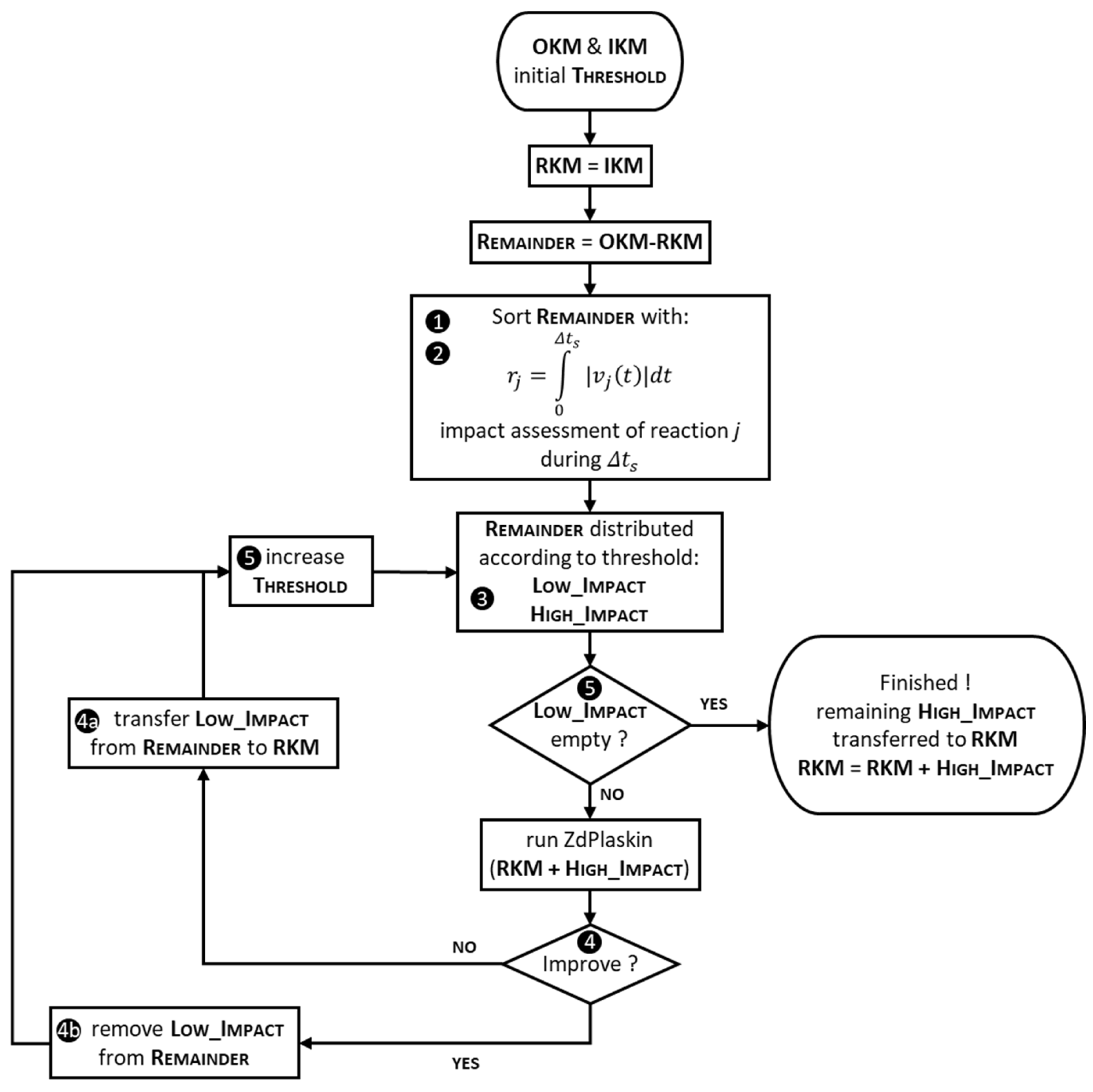
2.3.2. Second Step: Numerical Stability and Completeness Approach
- Each reaction j in the “remainder” set is given an individual score, which is calculated as the integral of its reaction rate (as defined in Equation (4); the absolute value is considered) over the residence time . The reaction rate generally varies during the simulation; it is computed by ZdPlaskin running the OKM (each one of the reactions rates can be plotted among the curves built by ZdPlaskin). These scores translate the assessment of the impact of each reaction into a single figure based on the variation in the concentrations of products and reactants resulting from that reaction.
- All the reactions are sorted according to their score.
- A threshold level is introduced and reactions with higher scores than the threshold (reactions with “high impact”), together with their reactants and products, are used together with the “initial sets” (IKM) to build a new “kinet file”, which is then run with ZdPlaskin; other reactions (with scores below the threshold, considered as a “low impact” subset) are temporarily discarded.
- If the simulation time is longer than the simulation time of the previous iteration (or the one obtained with the OKM for the first iteration) or if the obtained time profiles of the species of interest differ too much from the OKM’s ones (more than 10% has appeared to be at a good level), then we consider that the attempt associated with the current threshold has failed:
- The temporarily eliminated reactions (“low impact” subset described at step 3) are thus considered necessary for a coherent description of the kinetic mechanism and are added, together with their associated species, to the “initial kinetic mechanism” (4a).
- If the attempt succeeds (both previous conditions are fulfilled), the temporarily discarded reactions and species are definitively eliminated (4b). It is worth mentioning at this step that the elimination process, which uses simulation results to check the relevancy of a kinetic reduction attempt, is in fact tightly bounded to the operating condition selected for the test (in some manner, it is similar to the “local linearization” of a nonlinear system, which is achieved in the vicinity of a given operating point)—see Table 4 below in the Section 3.
- The process is repeated with a higher threshold level until no reaction is left for potential elimination (“low impact” subset is empty).
- The result of these iterations is a reduced kinetic mechanism (RKM) focusing on the species that are initially selected among the species of interest (SOI).
3. Results
3.1. Performance of the Reduced Kinetic Mechanisms (RKM1 and RKM2)
- For {, } SOI, IKM incorporates 189 reactions and 11 species to be compared with RKM1;
- For {, , , } SOI, IKM incorporates 374 reactions and 24 species to be compared with RKM2;
3.2. Compared Time Responses of the Kinetic Mechanisms
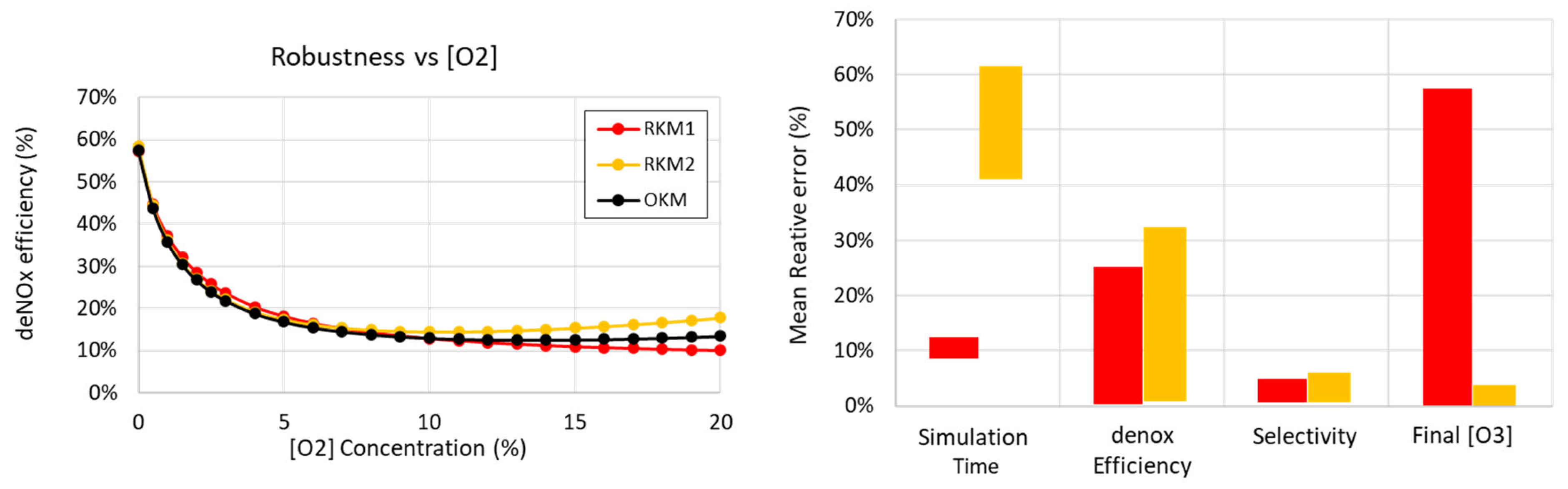
3.3. Robustness of Reduced Kinetic Mechanisms (RKM1 and RKM2) Concerning deNOX Process Performance
- The temperature is kept at 450 K (uniform temperature distribution assumed in the reactor);
- The electrical power injection is the same for all simulations at 20 W, with the pattern described in Table 4;
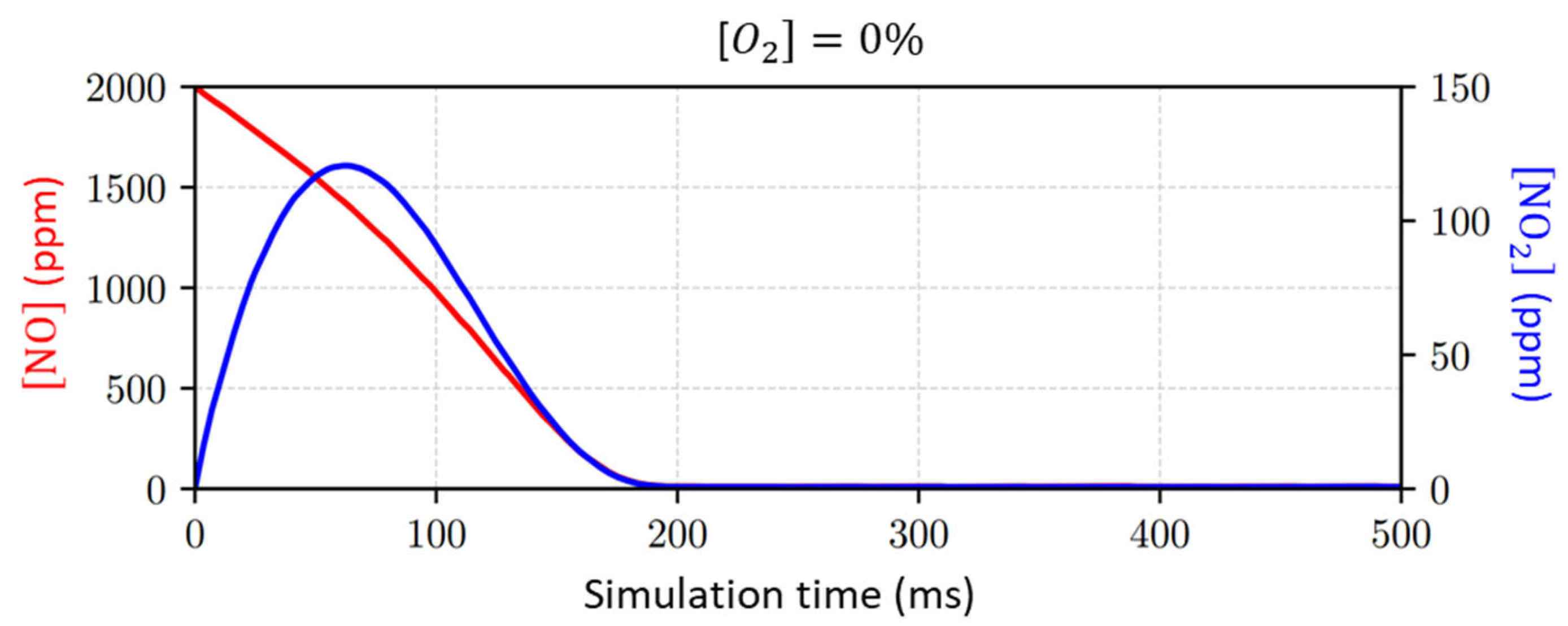
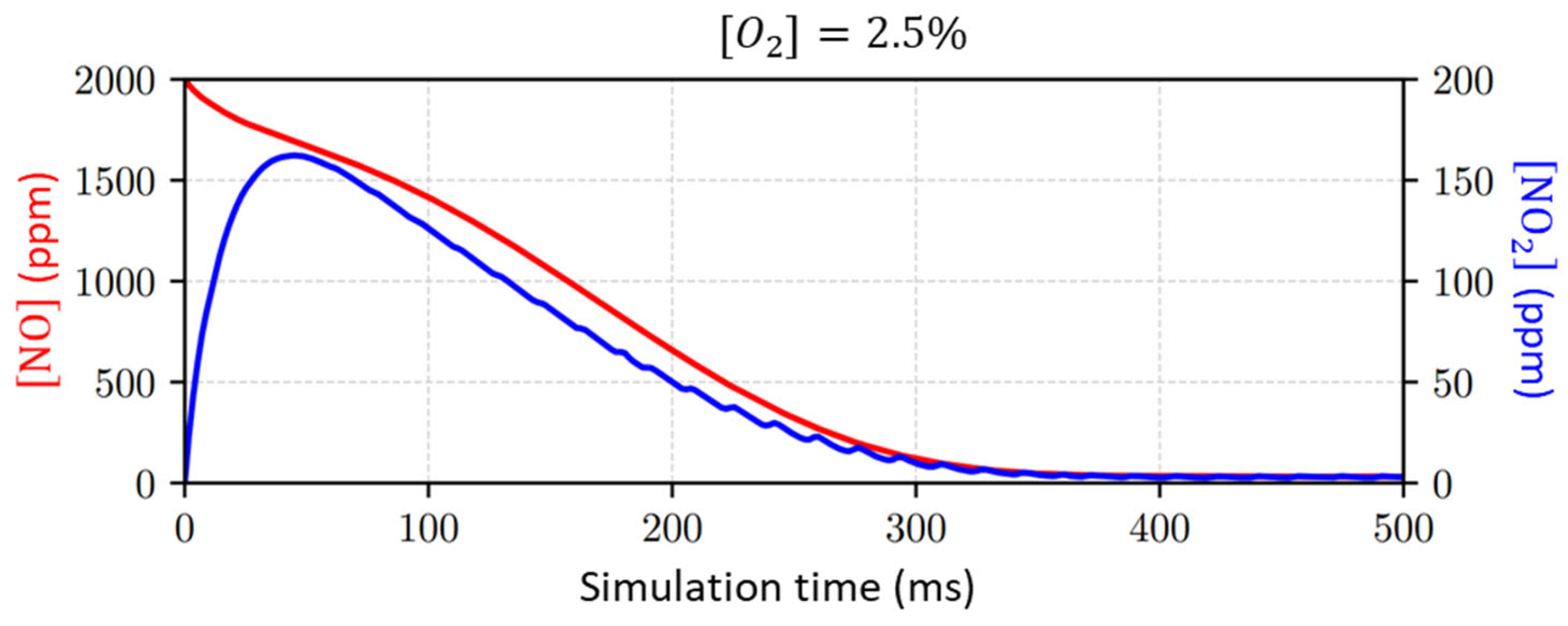
- Different concentrations of , which is known to be an effective quencher and significantly disturbs NTP processes, are considered.
3.4. Robustness of Reduced Kinetic Mechanisms (RKM1 and RKM2) with Respect to Experimental Conditions
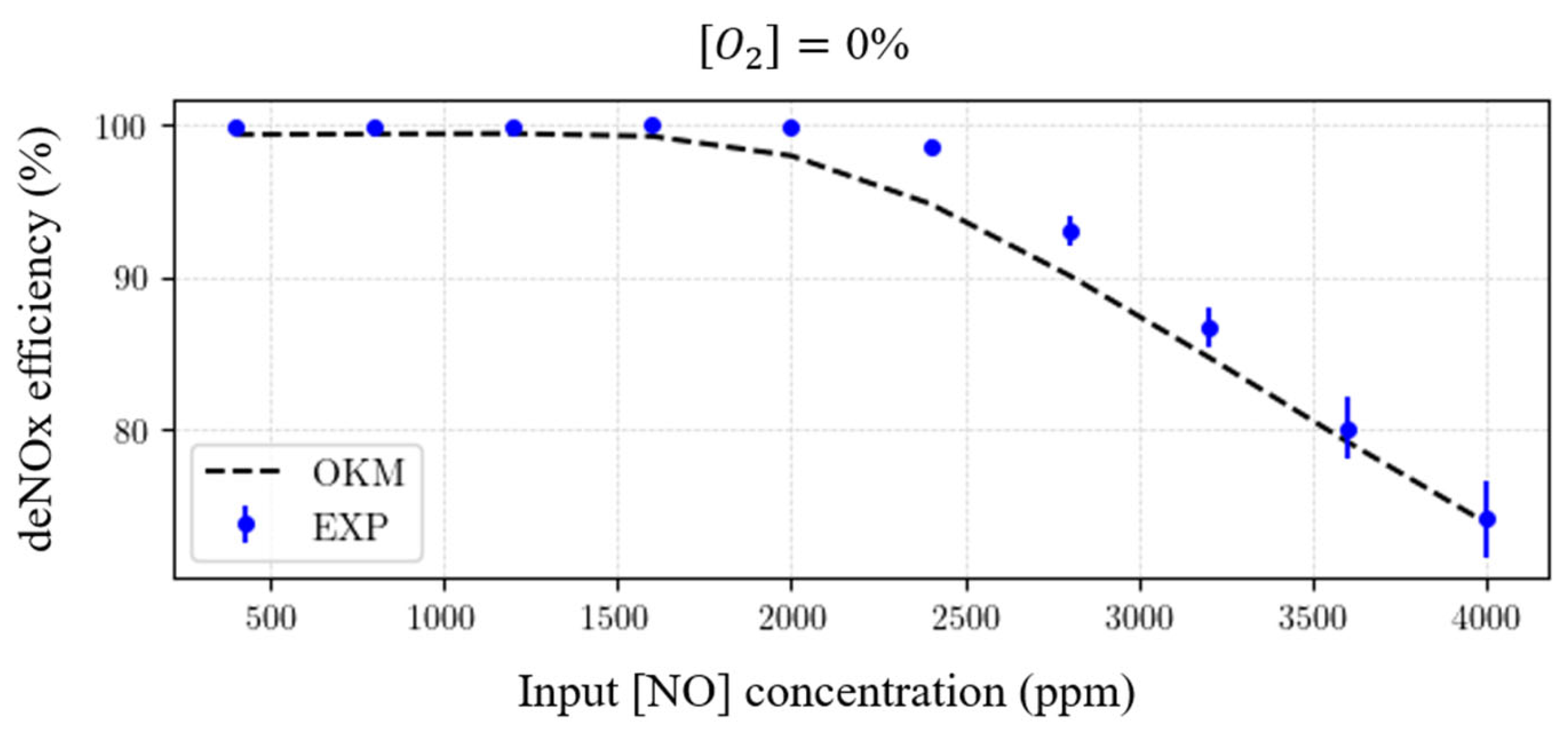
3.4.1. Robustness with Respect to
- The temperature is kept at 450 K (uniform temperature distribution assumed in the reactor);
- The electrical power injection is the same for all simulations at 20 W, with the pattern described in Table 4;
- concentration is considered.
- Simulation time;
- deNOx efficiency (already displayed differently in the left graph);
- selectivity = , giving the percentage of injected transformed into ;
- concentration in the outlet gas mixture (after the residence time).
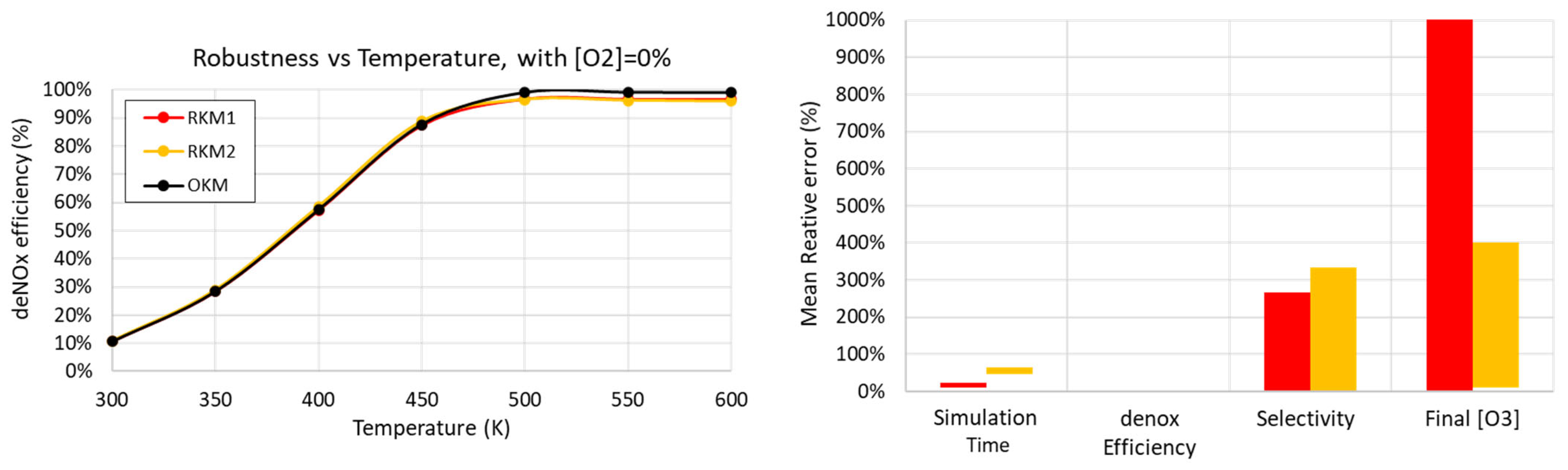
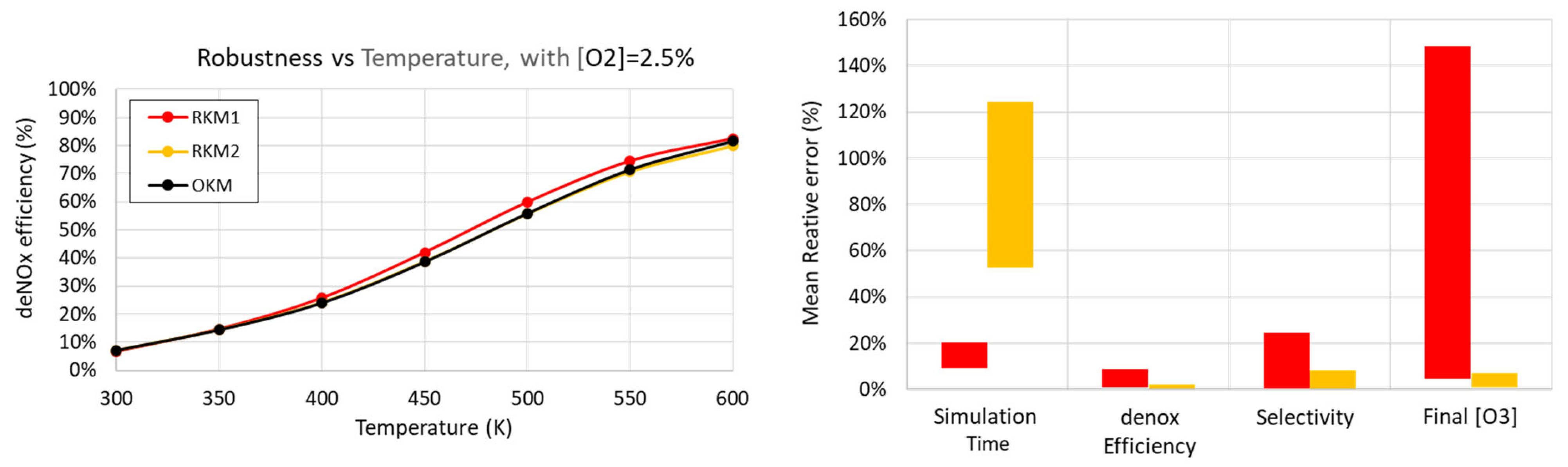
3.4.2. Robustness with Respect to Gas Temperature
- The electrical power injection is the same for all simulations at 20 W, with the pattern described in Table 4;
- concentration is considered;
- Simulation time;
- deNOx efficiency (already displayed differently in the left graph);
- selectivity;
- concentration in the outlet gas mixture (after the residence time).
4. Discussion
4.1. Accelerated Simulations to Facilitate the Design of Experimental and Industrial Configurations
4.2. Accuracy of Reduced Kinetic Mechanisms (RKM1, RKM2)
4.3. Robustness of Reduced Kinetic Mechanisms
- In the concentration in the input gas mixture; see Figure 11;
- In the concentration in the input gas mixture; see Figure 12;
- A time simulation (with the same 180 ms residence time);
- deNOx treatment efficiency;
- deNOx treatment sensitivity;
- concentration in the outlet gas mixture.
5. Conclusions
- NTP chemical simulation, using dedicated tools such as ZdPlaskin, can make a valuable contribution to the design of experimental setups;
- Reduced kinetic mechanisms focused on a specific set of species of interest (SOI) are able to drastically reduce the simulation time and allow for an intensive use of simulations;
- The proposed method for selecting a subset of chemical species and reactions focused on SOI based on the original kinetic mechanism has proven to be effective and capable of establishing robust kinetic mechanisms, particularly with regard to changes in the proportions of the input gas mixture; when temperature changes are taken into account, the robustness of the reduced mechanisms decreases when the selected operating conditions deviate too far from the reference temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DBD | Dielectric Barrier Discharge |
| NTP | Non-Thermal Plasma |
| SOI | Species of Interest |
| OKM | Original Kinetic Mechanism |
| IKM | Initial Kinetic Mechanism |
| RKM | Reduced Kinetic Mechanism |
References
- Talebizadeh, P.; Babaie, M.; Brown, R.; Rahimzadeh, H.; Ristovski, Z.; Arai, M. The role of non-thermal plasma technique in NOx treatment: A review. Renew. Sustain. Energy Rev. 2014, 40, 886–901. [Google Scholar] [CrossRef]
- Rueda, V. Reduction of Nitrogen Oxides in Diesel Exhaust Using Dielectric Barrier Discharges Driven by Current-Mode Power Supplies. Ph.D. Thesis, Institut National Polytechnique de Toulouse—INPT, Toulouse, France, Pontificia Universidad Javeriana, Bogotá, Colombia, 2022. Available online: https://theses.hal.science/tel-04248330v1 (accessed on 27 July 2025).
- Alves, L.; Holz, L.I.V.; Fernandes, C.; Ribeirinha, P.; Mendes, D.; Fagg, D.P.; Mendes, A. A comprehensive review of NOx and N2O mitigation from industrial streams. Renew. Sustain. Energy Rev. 2022, 155, 111916. [Google Scholar] [CrossRef]
- Wang, Z.; Kuang, H.; Zhang, J.; Chu, L.; Ji, Y. Nitrogen oxide removal by non-thermal plasma for marine diesel engines. RSC Adv. 2019, 9, 5402–5416. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, C.; Penetrante, B. Effect of Gas Composition on the NOx Conversion Chemistry in a Plasma. SAE Tech. Pap. 1998, 107, 982433. [Google Scholar] [CrossRef]
- Vinh, T.Q.; Watanabe, S.; Furuhata, T.; Arai, M. Fundamental study of NOx removal from diesel exhaust gas by dielectric barrier discharge reactor. J. Mech. Sci. Technol. 2012, 26, 1921–1928. [Google Scholar] [CrossRef]
- Anaghizi, S.J.; Talebizadeh, P.; Rahimzadeh, H.; Ghomi, H. The Configuration Effects of Electrode on the Performance of Dielectric Barrier Discharge Reactor for NOx Removal. IEEE Trans. Plasma Sci. 2015, 43, 1944–1953. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Wang, T.; Sun, B.M.; Xiao, H.P.; Zeng, J.Y.; Duan, E.P.; Xin, J.; Li, C. Effect of Reactor Structure in DBD for Nonthermal Plasma Processing of NO in N2 at Ambient Temperature. Plasma Chem. Plasma Process 2012, 32, 1189–1201. [Google Scholar] [CrossRef]
- Bente, N. Étude Système d’un Dispositif de Décharges à Barrières Diélectriques Dédié à la Réduction des Oxydes d’azote. Ph.D. Thesis, Institut National Polytechnique de Toulouse—INPT, Toulouse, France, 2025. Available online: https://theses.hal.science/tel-05185152v1 (accessed on 27 July 2025).
- Pancheshnyi, S.; Eismann, B.; Hagelaar, G.J.M.; Pitchford, L.C. Computer Code ZdPlaskin; University of Toulouse, LAPLACE, CNRS-UPS-INP: Toulouse, France, 2008; Available online: https://www.ZdPlaskin.laplace.univ-tlse.fr (accessed on 27 July 2025).
- Hagelaar, G.J.M.; Pitchford, L.C. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci. Technol. 2005, 14, 722–733. [Google Scholar] [CrossRef]
- Pitchford, L.C.; Alves, L.L.; Bartschat, K.; Biagi, S.F.; Bordage, M.-C.; Bray, I.; Brion, C.E.; Brunger, M.J.; Campbell, L.; Chachereau, A.; et al. LXCat: An Open-Access, Web-Based Platform for Data Needed for Modeling Low Temperature Plasmas. Plasma Process. Polym. 2017, 14, 1600098. [Google Scholar] [CrossRef]
- Pancheshnyi, S.; Biagi, S.; Bordage, M.; Hagelaar, G.; Morgan, L.; Phelps, A.; Pitchford, L. The LXCat project: Electron scattering cross sections and swarm parameters for low temperature plasma modeling. Chem. Phys. 2012, 398, 148–153. [Google Scholar] [CrossRef]
- Carbone, E.; Graef, W.; Hagelaar, G.; Boer, D.; Hopkins, M.M.; Stephens, J.C.; Yee, B.T.; Pancheshnyi, S.; van Dijk, J.; Pitchford, L. Data Needs for Modeling Low-Temperature Non-Equilibrium Plasmas: The LXCat Project, History, Perspectives and a Tutorial. Atoms 2021, 9, 16. [Google Scholar] [CrossRef]
- Koelman, P.; Heijkers, S.; Mousavi, S.T.; Graef, W.; Mihailova, D.; Kozak, T.; Bogaerts, A.; van Dijk, J. A Comprehensive Chemical Model for the Splitting of CO2 in Non-Equilibrium Plasmas. Plasma Process. Polym. 2017, 14, 1600155. [Google Scholar] [CrossRef]
- Shao, X.; Lacoste, D.A.; Im, H.G. ChemPlasKin: A general-purpose program for unified gas and plasma kinetics simulations. Appl. Energy Combust. Sci. 2024, 19, 100280. [Google Scholar] [CrossRef]
- Tatar, M.; Vashisth, V.; Iqbal, M.; Butterworth, T.; van Rooij, G.; Andersson, R. Analysis of a plasma reactor performance for direct nitrogen fixation by use of three-dimensional simulations and experiments. Chem. Eng. J. 2024, 497, 154756. [Google Scholar] [CrossRef]
- Malé, Q.; Barléon, N.; Shcherbanev, S.; Dharmaputra, B.; Noiray, N. Numerical study of nitrogen oxides chemistry during plasma assisted combustion in a sequential combustor. Combust. Flame 2024, 260, 113206. [Google Scholar] [CrossRef]
- Rueda, V.; Wiesner, A.; Diez, R.; Piquet, H. Power Estimation of a Current Supplied DBD Considering the Transformer Parasitic Elements. IEEE Trans. Ind. Appl. 2019, 55, 6567–6575. [Google Scholar] [CrossRef]
- Florez, D.; Schitz, D.; Piquet, H.; Diez, R. Efficiency of an Exciplex DBD Lamp Excited Under Different Methods. IEEE Trans. Plasma Sci. 2018, 46, 140–147. [Google Scholar] [CrossRef]
- Manley, T.C. The Electric Characteristics of the Ozonator Discharge. Trans. Electrochem. Soc. 1943, 84, 83. [Google Scholar] [CrossRef]
- Díez, R.; Salanne, J.-P.; Piquet, H.; Bhosle, S.; Zissis, G. Predictive model of a DBD lamp for power supply design and method for the automatic identification of its parameters. Eur. Phys. J. Appl. Phys. 2007, 37, 307–313. [Google Scholar] [CrossRef]
- Penetrante, B.M.; Hsiao, M.C.; Merritt, B.T.; Vogtlin, G.E.; Wallman, P.H.; Neiger, M.; Wolf, O.; Hammer, T.; Broer, S. Pulsed corona and dielectric-barrier discharge processing of NO in N2. Appl. Phys. Lett. 1996, 68, 3719–3721. [Google Scholar] [CrossRef]
- Clyne, M.A.A.; McDermid, I.S. Mass spectrometric determinations of the rates of elementary reactions of NO and of NO2 with ground state N4S atoms. J. Chem. Soc. Faraday Trans. 1975, 71, 2189–2202. [Google Scholar] [CrossRef]
- Zhu, A.-M.; Sun, Q.; Niu, J.-H.; Xu, Y.; Song, Z.-M. Conversion of NO in NO/N2, NO/O2/N2, NO/C2H4/N2 and NO/C2H4/O2/N2 Systems by Dielectric Barrier Discharge Plasmas. Plasma Chem. Plasma Process 2005, 25, 371–386. [Google Scholar] [CrossRef]
- Tsang, W.; Herron, J.T. Chemical Kinetic Data Base for Propellant Combustion I. Reactions Involving NO, NO2, HNO, HNO2, HCN and N2O. J. Phys. Chem. Ref. Data 1991, 20, 609–663. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F.; Kerr, J.A., Jr.; Troe, J. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement IV. UPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry. J. Phys. Chem. Ref. Data 1992, 21, 1125. [Google Scholar] [CrossRef]
- Bente, N.; Piquet, H.; Merbahi, N.; Bru, E. Thermal modelling of an atmospheric pressure cylindrical DBD reactor for NOx removal. In Proceedings of the Europhysics Conference on Atomic and Molecular Physics of Ionized Gases (ESCAMPIG XXVI), Brno, Czech Republic, 9–13 July 2024; Available online: https://hal.science/hal-04639116v1 (accessed on 27 July 2025).
- Talebizadeh, P.; Rahimzadeh, H.; Babaie, M.; Anaghizi, S.J.; Ghomi, H.; Ahmadi, G.; Brown, R. Evaluation of Residence Time on Nitrogen Oxides Removal in Non-Thermal Plasma Reactor. PLoS ONE 2015, 10, e0140897. [Google Scholar] [CrossRef] [PubMed]
- Capitelli, M.; Ferreira, C.M.; Gordiets, B.F.; Osipov, A.I. Plasma Kinetics in Atmospheric Gases; Springer: Berlin/Heidelberg, Germany, 2000; pp. 229–250. [Google Scholar]
- Flitti, A.; Pancheshnyi, S. Gas heating in fast pulsed discharges in N2–O2 mixtures. Eur. Phys. J. Appl. Phys. 2009, 45, 21001. [Google Scholar] [CrossRef]
- Phelps, A.V. Cross sections and swarm coefficients for nitrogen ions and neutrals in N2 and argon ions and neutrals in Ar for energies from 0.1 eV to 10 keV. J. Phys. Chem. Ref. Data 1991, 20, 557–573. [Google Scholar] [CrossRef]
- Türtscher, P.L.; Reiher, M. Pathfinder—Navigating and Analyzing Chemical Reaction Networks with an Efficient Graph-Based Approach. J. Chem. Inf. Model. 2022, 63, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Markosyan, A.H.; Luque, A.; Gordillo-Vázquez, F.J.; Ebert, U. PumpKin: A tool to find principal pathways in plasma chemical models. Comput. Phys. Commun. 2014, 185, 2697–2702. [Google Scholar] [CrossRef]
- Wang, T.; Sun, B. Effect of temperature and relative humidity on NOX removal by dielectric barrier discharge with acetylene. Fuel Process. Technol. 2016, 144, 109–114. [Google Scholar] [CrossRef]

| Reaction Rate | Bibl. Ref—Year | |
|---|---|---|
| +++ | [24]—1996 | |
| [25]—1975 | ||
| Reaction Rate | Bibl. Ref—year | |
| +++ | [26]—2005 | |
| [27]—1991 | ||
| [27]—1991 | ||
| [28]—1992 | ||
| [28]—1992 |
| Selected Species of Interest | Number of Reactions | Number of Species |
Simulation Time |
|---|---|---|---|
| original kinetic mechanism OKM | 656 | 68 | 500 s |
| }—RKM1 | 246 | 56 | 82 s |
| }—RKM2 | 416 | 66 | 213 s |
| OKM (68) | RKM1 (56) | RKM2 (66) | Status | |
|---|---|---|---|---|
| Placeholder electrons | ANY_NEUTRAL, e | ANY_NEUTRAL, e | ANY_NEUTRAL, e | Same for all |
| Atoms, incl. excited | N, N(2D), N(2P), O, O(1D), O(1S) | N, N(2D), N(2P), O, O(1D) | N, N(2D), N(2P), O, O(1D), O(1S) | OKM |
| Base neutrals (molecules) | N2, O2, O3, NO, NO2, NO3, N2O, N2O5 | N2, O2, O3, NO, NO2, NO3, N2O, N2O5 | N2, O2, O3, NO, NO2, NO3, N2O, N2O5 | Same for all |
| N2 vibrational | N2(v1), N2(v2), N2(v3), N2(v4), N2(v5), N2(v6), N2(v7), N2(v8), N2(v1RES) | N2(v1), N2(v2), N2(v3), N2(v4), N2(v5), N2(v6), N2(v7), N2(v8), N2(v1RES) | N2(v1), N2(v2), N2(v3), N2(v4), N2(v5), N2(v6), N2(v7), N2(v8), N2(v1RES) | Same for all |
| N2 electronic, excited | N2(A1), N2(A′1), N2(A″1), N2(A3), N2(A3,v5-9), N2(A3,v10-), N2(B3), N2(B′3), N2(C3), N2(E3), N2(W1), N2(W3) | N2(A1), N2(A′1), N2 (A3), N2(A3,v5-9), N2(A3,v10-), N2(B3), N2(B′3), N2(C3), N2(E3), N2(W1), N2(W3) | N2(A1), N2(A′1), N2(A3), N2(A3,v5-9), N2(A3,v10-), N2(B3), N2(B′3), N2(C3), N2(W1), N2(W3) | |
| O2 excited | O2(v1), O2(v2), O2(v3), O2(v4), O2(v1RES), O2(v2RES) O2(A1), O2(B1), O2(4.5eV), O2(6.0eV), O2(8.4eV), O2(9.97eV) | O2(A1), O2(B1) | O2(v1), O2(v2), O2(v3), O2(v4), O2(v1RES), O2(v2RES) O2(A1), O2(B1), O2(4.5eV), O2(6.0eV), O2(8.4eV), O2(9.97eV) | OKM |
| Negative ions | O−, O2−, O3−, O4, NO−, NO2−, NO3−, N2O− | O−, O2−, O3−, O4, NO−, NO2−, NO3−, N2O− | O−, O2−, O3−, O4, NO−, NO2−, NO3−, N2O− | Same for all |
| Positive ions | N+, N2+, N3+, N4+, O+, O2+, O4+, NO+, NO2+, N2O+ | N+, N2+, N3+, N4+, O+, O2+, O4+, NO+, NO2+, N2O+ | N+, N2+, N3+, N4+, O+, O2+, O4+, NO+, NO2+, N2O+ | Same for all |
| Ion, neutral cluster | O2+N2 | O2+N2 | O2+N2 | Same for all |
| Operating Condition | ZdPlaskin Input Variable | Value |
|---|---|---|
| Electrical power injection: 20 W | High-frequency current source generator | |
| Burst | ||
| Gas mixture—nominal values | Gas mixture | |
| Treated gas | Pressure | |
| Volume | ||
| Temperature | 450 K | |
| Residence time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bente, N.; Cuellar Valencia, A.; Piquet, H. Minimization of Chemical Kinetic Reaction Set for System-Level Study of Non-Thermal Plasma NOx Abatement Process. Plasma 2025, 8, 36. https://doi.org/10.3390/plasma8030036
Bente N, Cuellar Valencia A, Piquet H. Minimization of Chemical Kinetic Reaction Set for System-Level Study of Non-Thermal Plasma NOx Abatement Process. Plasma. 2025; 8(3):36. https://doi.org/10.3390/plasma8030036
Chicago/Turabian StyleBente, Nicolas, Alfredo Cuellar Valencia, and Hubert Piquet. 2025. "Minimization of Chemical Kinetic Reaction Set for System-Level Study of Non-Thermal Plasma NOx Abatement Process" Plasma 8, no. 3: 36. https://doi.org/10.3390/plasma8030036
APA StyleBente, N., Cuellar Valencia, A., & Piquet, H. (2025). Minimization of Chemical Kinetic Reaction Set for System-Level Study of Non-Thermal Plasma NOx Abatement Process. Plasma, 8(3), 36. https://doi.org/10.3390/plasma8030036