Trypan Blue Image-Guided Removal of Surface-Based Bacterial Biofilms from Chicken Tissue Using Cold Atmospheric Pressure Plasma
Abstract
1. Introduction
2. Experimental Procedures
2.1. Biofilm Sample Preparation
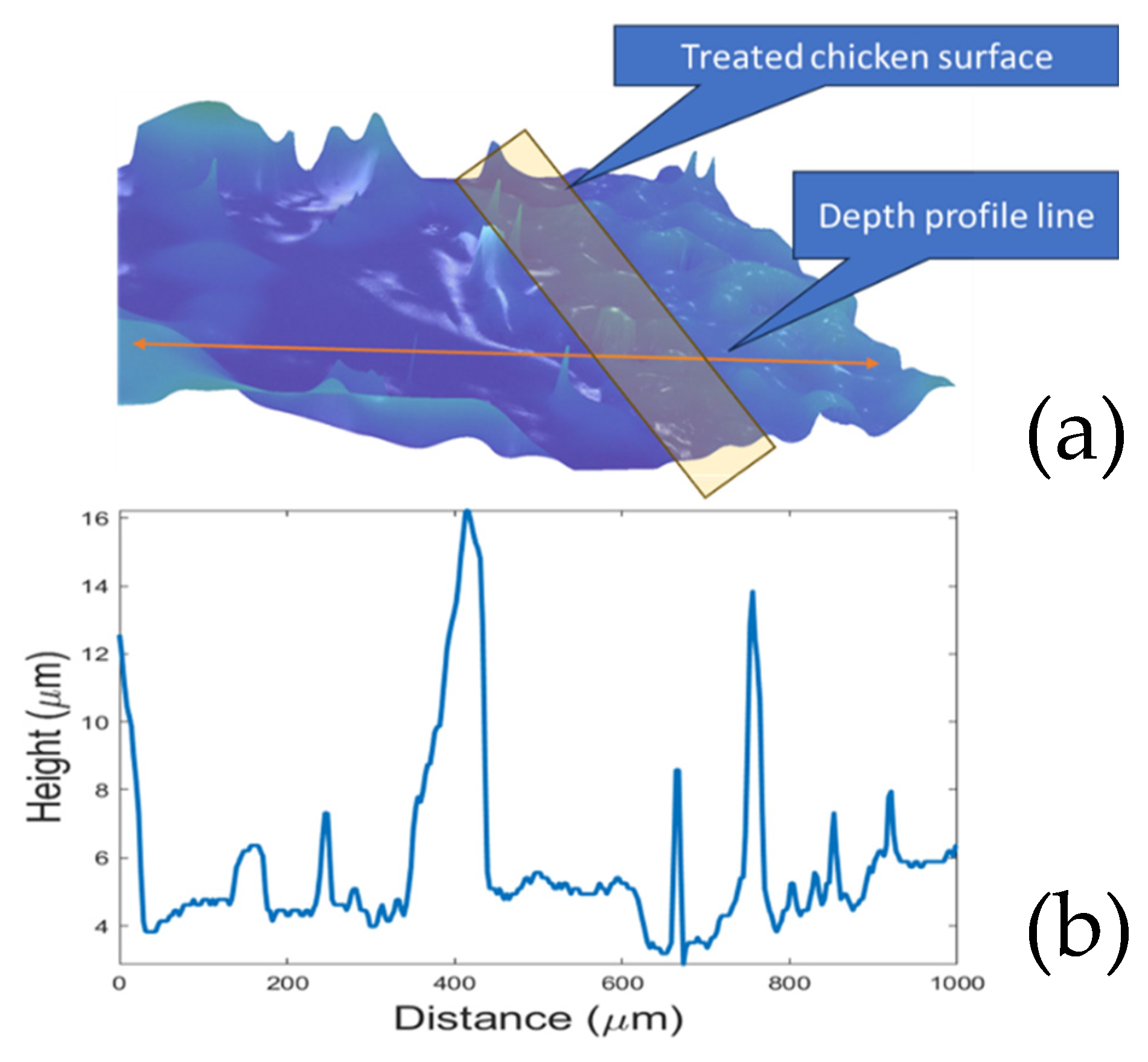
2.2. Biofilm Visualization and Imaging System
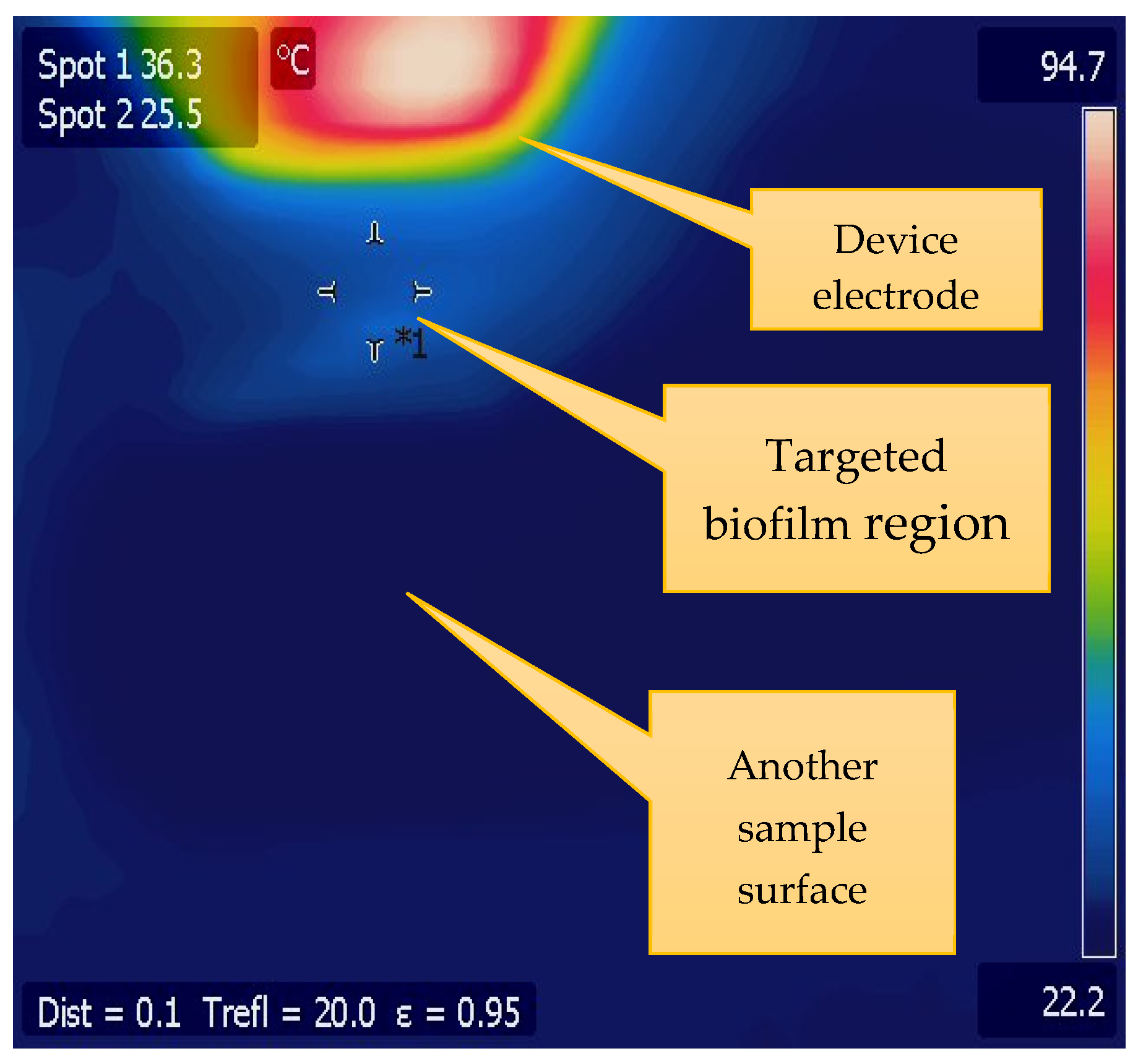
2.3. CAP Operation and Characterization
2.4. Microscopic Imaging and CFU Analysis
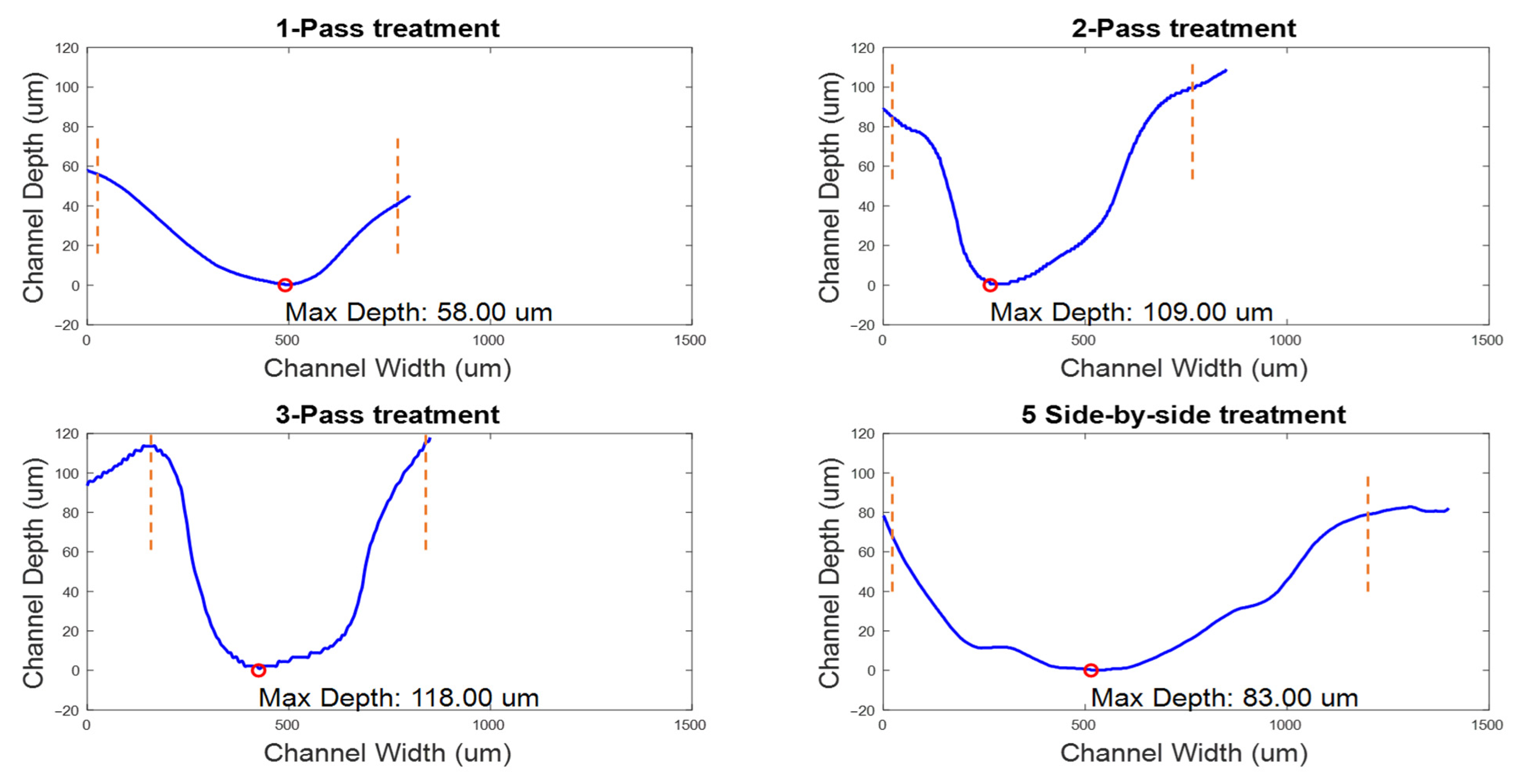
- A single pass over a channel at a speed of 0.1 mm/s.
- Two consecutive passes over the same channel, maintaining the speed at 0.1 mm/s.
- Three passes over the same channel, again at a speed of 0.1 mm/s.
- Creating five adjacent channels, each with a single pass at 0.1 mm/s.
3. Experimental Results
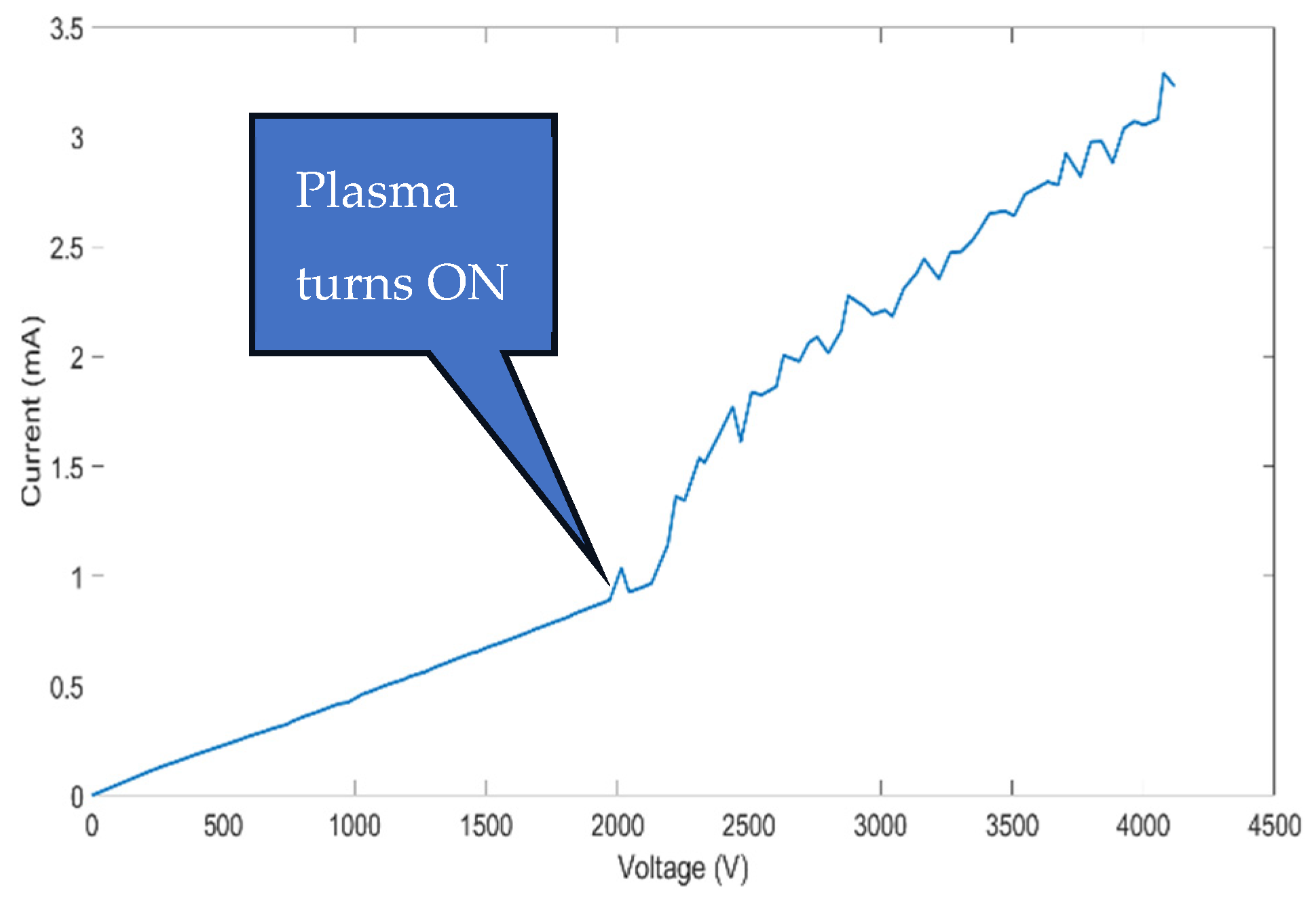
3.1. CAP Device Characterization
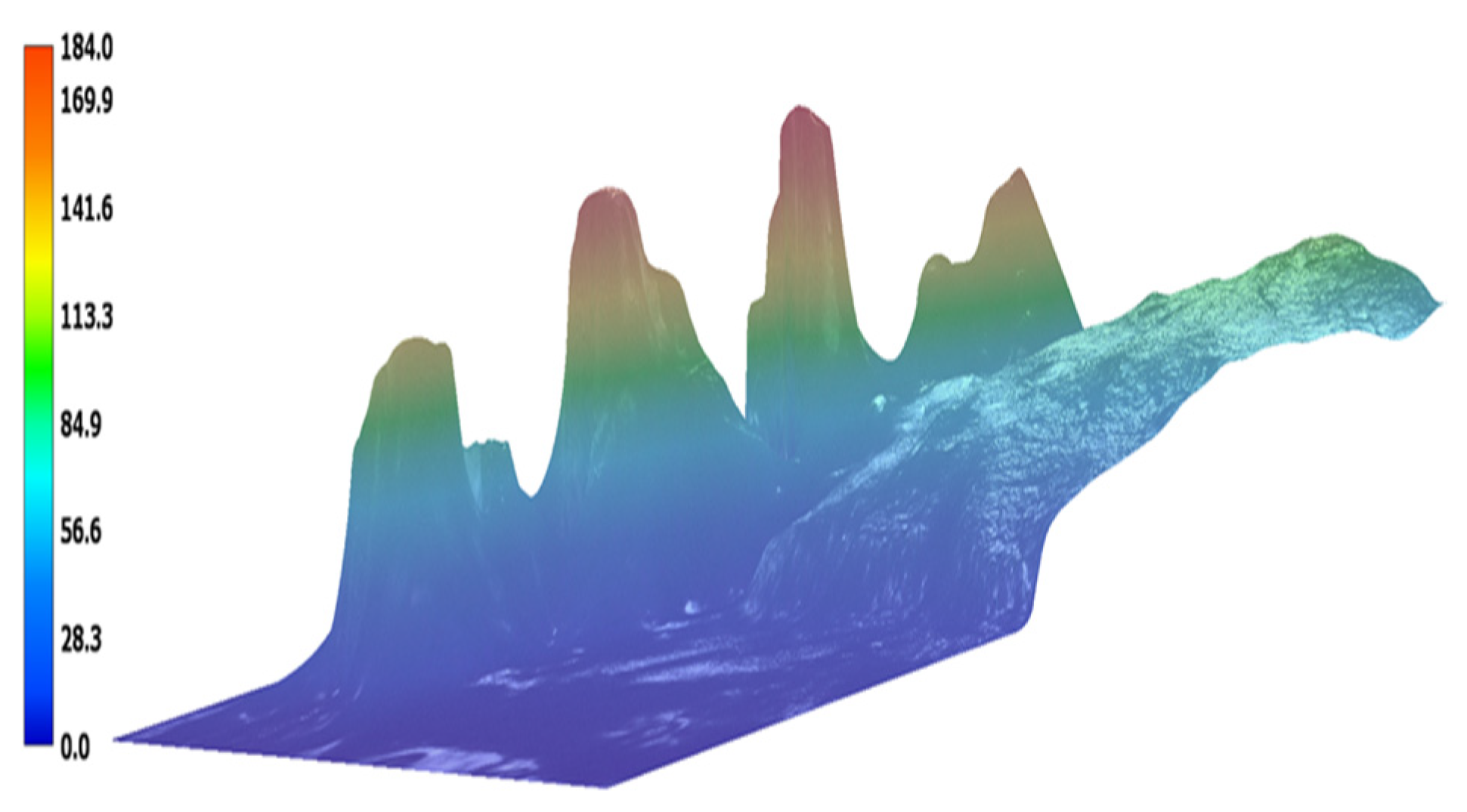
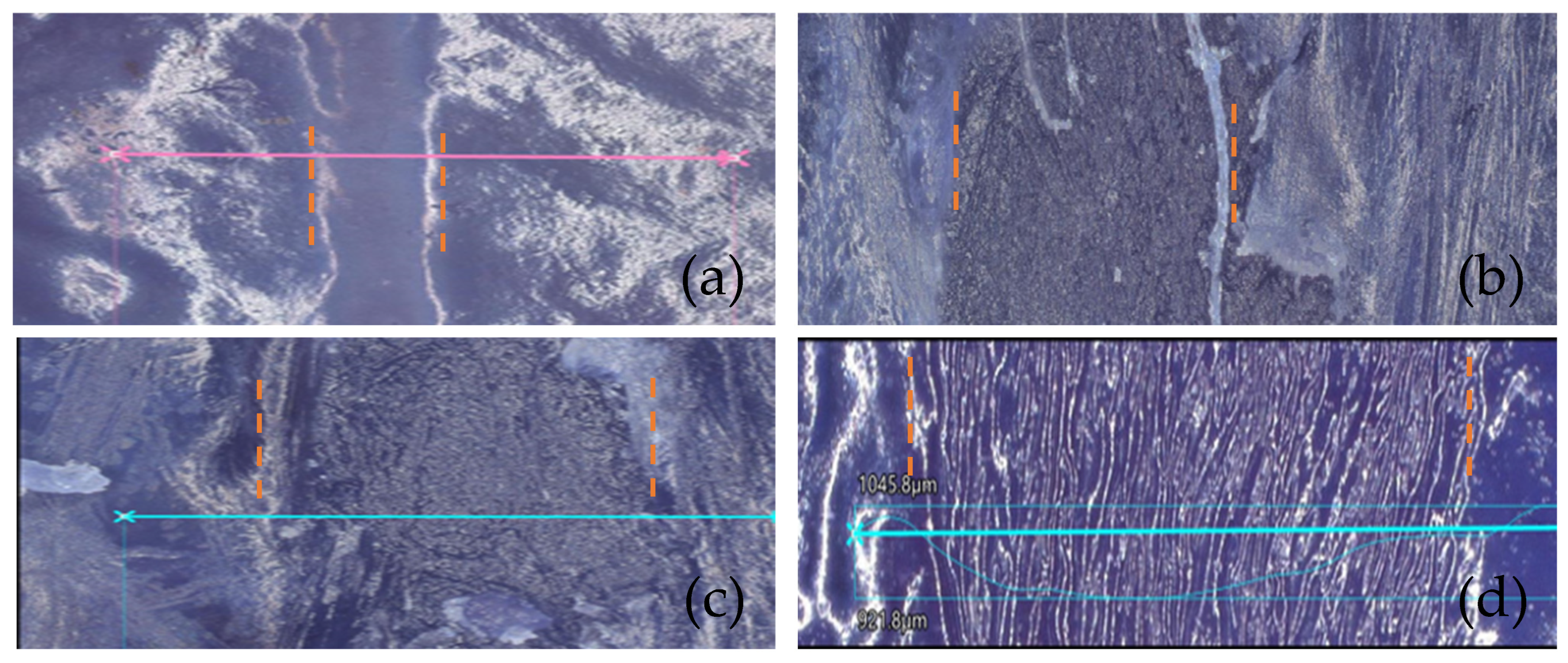
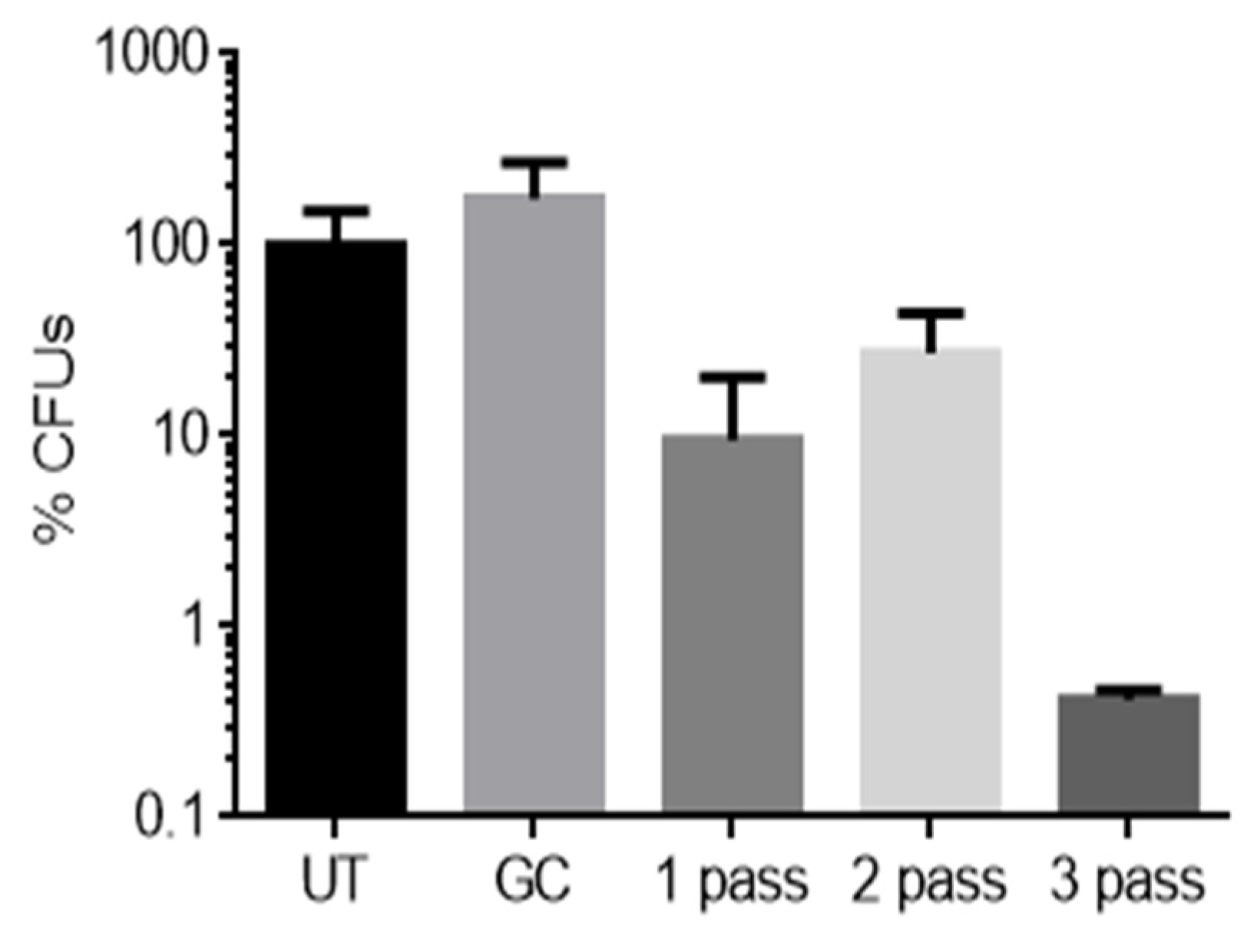
3.2. Biofilm Reduction Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weigelt, M.A.; McNamara, S.A.; Sanchez, D.; Hirt, P.A.; Kirsner, R.S. Evidence-Based Review of Antibiofilm Agents for Wound Care. Adv. Wound Care 2021, 10, 13–23. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Hurlow, J.; Blanz, E.; Gaddy, J.A. Clinical investigation of biofilm in non-healing wounds by high resolution microscopy techniques. J. Wound Care 2016, 25, 11–22. [Google Scholar] [CrossRef]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Farhan, N.; Jeffery, S. Utility of MolecuLight i:X for Managing Bacterial Burden in Pediatric Burns. J. Burn Care Res. 2019, 41, 328–338. [Google Scholar] [CrossRef]
- Xu, Y.; Dhaouadi, Y.; Stoodley, P.; Ren, D. Sensing the unreachable: Challenges and opportunities in biofilm detection. Curr. Opin. Biotechnol. 2020, 64, 79–84. [Google Scholar] [CrossRef]
- Rennie, M.; Dunham, D.; Lindvere-Teene, L.; Raizman, R.; Hill, R.; Linden, R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:XTM. Diagnostics 2019, 9, 22. [Google Scholar] [CrossRef]
- Pijpe, A.; Ozdemir, Y.; Sinnige, J.C.; Kwa, K.A.A.; Middelkoop, E.; De Vries, A.M. Detection of bacteria in burn wounds with a novel handheld autofluorescence wound imaging device: A pilot study. J. Wound Care 2019, 28, 548–554. [Google Scholar] [CrossRef]
- Okebiorun, M.; Oberbeck, C.; Waite, C.; Clark, S.; Miller, D.; Smith, E.H.B.; Cornell, K.A.; Browning, J. Selective Optical Imaging for Detection of Bacterial Biofilms in Tissues. J. Imaging 2023, 9, 160. [Google Scholar] [CrossRef]
- Dada, V.K.; Sharma, N.; Sudan, R.; Sethi, H.; Dada, T.; Pangtey, M.S. Anterior capsule staining for capsulorhexis in cases of white cataract: Comparative clinical study. J. Cataract. Refract. Surg. 2004, 30, 326–333. [Google Scholar] [CrossRef]
- Kuhn, F. Chromovitrectomy. In Vitreoretinal Surgery: Strategies and Tactics; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 325–330. [Google Scholar] [CrossRef]
- Jacob, S.; Agarwal, A.; Agarwal, A.; Agarwal, S.; Chowdhary, S.; Chowdhary, R.; Bagmar, A.A. Trypan blue as an adjunct for safe phacoemulsification in eyes with white cataract. J. Cataract. Refract. Surg. 2002, 28, 1819–1825. [Google Scholar] [CrossRef]
- Nanavaty, M.A.; Johar, K.; Sivasankaran, M.A.; Vasavada, A.R.; Praveen, M.R.; Zetterström, C. Effect of trypan blue staining on the density and viability of lens epithelial cells in white cataract. J. Cataract. Refract. Surg. 2006, 32, 1483–1488. [Google Scholar] [CrossRef]
- Farah, M.E.; Maia, M.; Furlani, B.; Bottós, J.; Meyer, C.H.; Lima, V.; Penha, F.M.; Costa, E.F.; Rodrigues, E.B. Current Concepts of Trypan Blue in Chromovitrectomy; Karger Medical and Scientific Publishers: Basel, Switzerland, 2008; Volume 42, pp. 91–100. [Google Scholar] [CrossRef]
- Chang, Y.S.; Tseng, S.Y.; Tseng, S.H. Comparison of dyes for cataract surgery. Part 2: Efficacy of capsule staining in a rabbit model. J. Cataract. Refract. Surg. 2005, 31, 799–804. [Google Scholar] [CrossRef]
- Lou, B.-S.; Lai, C.-H.; Chu, T.-P.; Hsieh, J.-H.; Chen, C.-M.; Su, Y.-M.; Hou, C.-W.; Chou, P.-Y.; Lee, J.-W. Parameters Affecting the Antimicrobial Properties of Cold Atmospheric Plasma Jet. J. Clin. Med. 2019, 8, 1930. [Google Scholar] [CrossRef]
- Jang, J.Y.; Hong, Y.J.; Lim, J.; Choi, J.S.; Choi, E.H.; Kang, S.; Rhim, H. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials 2018, 156, 258–273. [Google Scholar] [CrossRef]
- Cornell, K.A.; White, A.; Croteau, A.; Carlson, J.; Kennedy, Z.; Miller, D.; Provost, M.; Goering, S.; Plumlee, D.; Browning, J. Fabrication and Performance of a Multidischarge Cold-Atmospheric Pressure Plasma Array. IEEE Trans. Plasma Sci. 2021, 49, 1388–1395. [Google Scholar] [CrossRef]
- Puliyalil, H.; Cvelbar, U. Selective Plasma Etching of Polymeric Substrates for Advanced Applications. Nanomaterials 2016, 6, 108. [Google Scholar] [CrossRef]
- Pereira, S.; Pinto, E.; Ribeiro, P.A.; Sério, S. Study of a Cold Atmospheric Pressure Plasma jet device for indirect treatment of Squamous Cell Carcinoma. Clin. Plasma Med. 2019, 13, 9–14. [Google Scholar] [CrossRef]
- Bosch, L.T.; Habedank, B.; Siebert, D.; Mrotzek, J.; Viöl, W. Cold Atmospheric Pressure Plasma Comb—A Physical Approach for Pediculosis Treatment. Int. J. Environ. Res. Public Health 2019, 16, 19. [Google Scholar] [CrossRef]
- Jampa-ngern, S.; Viravaidya-Pasuwat, K.; Suvanasuthi, S.; Khantachawana, A. Effect of laser diode light irradiation on growth capability of human hair follicle dermal papilla cells. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, Jeju Island, Republic of Korea, 11–15 July 2017; pp. 3592–3595. [Google Scholar] [CrossRef]
- Balzer, J.; Demir, E.; Kogelheide, F.; Fuchs, P.C.; Stapelmann, K.; Opländer, C. Cold atmospheric plasma (CAP) differently affects migration and differentiation of keratinocytes via hydrogen peroxide and nitric oxide-related products. Clin. Plasma Med. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Boeckmann, L. Molecular mechanisms of the efficacy of cold atmospheric pressure plasma (CAP) in cancer treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef]
- Racka-Szmidt, K.; Stonio, B.; Żelazko, J.; Filipiak, M.; Sochacki, M. A Review: Inductively Coupled Plasma Reactive Ion Etching of Silicon Carbide. Materials 2021, 15, 123. [Google Scholar] [CrossRef]
- Kos, S.; Blagus, T.; Cemazar, M.; Filipic, G.; Sersa, G.; Cvelbar, U.; Yousfi, M. Safety aspects of atmospheric pressure helium plasma jet operation on skin: In vivo study on mouse skin. PLoS ONE 2017, 12, e0174966. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J.; Koutsopoulos, S. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef]
- Okebiorun, M.O.; Oberbeck, C.; Waite, C.; Clark, S.; Alomar, Z.; Miller, D.; Cornell, K.; Browning, J. Autofluorescence-Guided Removal of Bacterial Biofilms from Tissues Using Cold Atmospheric Pressure Plasma (CAP). IEEE Trans. Radiat. Plasma Med. Sci. 2024, 8, 990–996. [Google Scholar] [CrossRef]
- Browning, J.; Cornell, K. Plasma Scalpel for Selective Removal of Microbes and Microbial Biofilms. US Patent 1187198, 2024. Available online: https://www.patentdigest.org/patent/?patent_id=11871978 (accessed on 8 July 2025).
- Okebiorun, M.; Waite, C.; May, H.; Miller, D.; Cornell, K.; Browning, J. Wound-Based Bacterial Biofilm Imaging for Selective Cold Atmospheric Pressure Plasma Treatment. In Proceedings of the 2021 IEEE International Conference on Plasma Science (ICOPS), Lake Tahoe, NV, USA, 12–16 September 2021. [Google Scholar] [CrossRef]
- Malecha, K.; Golonka, L.J. Microchannel fabrication process in LTCC ceramics. Microelectron. Reliab. 2008, 48, 866–871. [Google Scholar] [CrossRef]
- Cornell, K.A.; Benfield, K.; Berntsen, T.; Clingerman, J.; Croteau, A.; Goering, S.; Moyer, D.; Provost, M.; White, A.; Plumlee, D.; et al. A Cold Atmospheric Pressure Plasma Discharge Device Exerts Antimicrobial Effects. Int. J. Latest Trends Eng. Technol. 2020, 15, 36–41. [Google Scholar] [PubMed Central]
- Borremans, A.; Lenaerts, S.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Biofilm Formation by Meat-Borne Pseudomonas fluorescens on Stainless Steel and Its Resistance to Sanitizers. Food Control 2018, 91, 397–403. [Google Scholar] [CrossRef]
- Cruz, K.L.; de Souza, A. Characterization of Biofilm Production by Pseudomonas fluorescens Isolated from Refrigerated Raw Buffalo Milk. J. Food Sci. Technol. 2019, 56, 4595–4604. [Google Scholar] [CrossRef]
- Chen, X.; Thomsen, T.R.; Winkler, H.; Xu, Y. Influence of Biofilm Growth Age, Media, Antibiotic Concentration and Exposure Time on Staphylococcus aureus and Pseudomonas aeruginosa Biofilm Removal In Vitro. BMC Microbiol. 2020, 20, 264. [Google Scholar] [CrossRef]
- Angarano, V.; Smet, C.; Akkermans, S.; Akritidou, T.; Huyck, B.; Chieffi, A.; Van Impe, J.F.M. A Reproducible Method for Growing Biofilms on Polystyrene Surfaces: Biomass and Bacterial Viability Evolution of Pseudomonas fluorescens and Staphylococcus epidermidis. Appl. Sci. 2020, 10, 4544. [Google Scholar] [CrossRef]
- Calhoun, C.; Geornaras, I.; Zhang, P. Pseudomonas in Meat Processing Environments. Foods 2025, 14, 1615. [Google Scholar] [CrossRef]
- Hou, J.; Wang, C.; Rozenbaum, R.T.; Gusnaniar, N.; de Jong, E.D.; Woudstra, W.; Geertsema-Doornbusch, G.I.; Atema-Smit, J.; Sjollema, J.; Ren, Y.; et al. Bacterial Density and Biofilm Structure Determined by Optical Coherence Tomography. Sci. Rep. 2019, 9, 9794. [Google Scholar] [CrossRef]
- Murga, R.; Stewart, P.S.; Daly, D. Quantitative analysis of biofilm thickness variability. Biotechnol. Bioeng. 1995, 45, 503–510. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6. Available online: http://www.rroij.com/open-access/quantitative-and-qualitative-assessment-methods-for-biofilm-growth-a-minireview-.pdf (accessed on 8 July 2025).
- Sun, Y.; Ogawa, R.; Xiao, B.; Feng, Y.; Wu, Y.; Chen, L.; Gao, X.; Chen, H. Antimicrobial photodynamic therapy in skin wound healing: A systematic review of animal studies. Int. Wound J. 2020, 17, 285–299. [Google Scholar] [CrossRef]
- de Melo, W.C.M.A.; Celiešiūtė-Germanienė, R.; Šimonis, P.; Stirkė, A. Antimicrobial Photodynamic Therapy (aPDT) for Biofilm Treatments. Possible Synergy Between aPDT and Pulsed Electric Fields. Virulence 2021, 12, 2247–2272. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Liu, Y.; Long, S.; Wang, H.; Wang, Y. Biofilm Therapy for Chronic Wounds. Int. Wound J. 2024, 21, e14667. [Google Scholar] [CrossRef]
- Matthes, R.; Jablonowski, L.; Pitchika, V.; Holtfreter, B.; Eberhard, C.; Seifert, L.; Gerling, T.; Scholten, L.V.; Schlüter, R.; Kocher, T. Efficiency of Biofilm Removal by Combination of Water Jet and Cold Plasma: An In-Vitro Study. BMC Oral Health 2022, 22, 157. [Google Scholar] [CrossRef]
- Matthes, R.; Jablonowski, L.; Miebach, L.; Pitchika, V.; Holtfreter, B.; Eberhard, C.; Seifert, L.; Gerling, T.; Schlüter, R.; Kocher, T.; et al. In-Vitro Biofilm Removal Efficacy Using Water Jet in Combination with Cold Plasma Technology on Dental Titanium Implants. Int. J. Mol. Sci. 2023, 24, 1606. [Google Scholar] [CrossRef] [PubMed]
- Abu Rached, N.; Kley, S.; Storck, M.; Meyer, T.; Stücker, M. Cold Plasma Therapy in Chronic Wounds—A Multicenter, Randomized Controlled Clinical Trial (Plasma on Chronic Wounds for Epidermal Regeneration Study): Preliminary Results. J. Clin. Med. 2023, 12, 5121. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okebiorun, M.; Miller, D.; Cornell, K.A.; Browning, J. Trypan Blue Image-Guided Removal of Surface-Based Bacterial Biofilms from Chicken Tissue Using Cold Atmospheric Pressure Plasma. Plasma 2025, 8, 34. https://doi.org/10.3390/plasma8030034
Okebiorun M, Miller D, Cornell KA, Browning J. Trypan Blue Image-Guided Removal of Surface-Based Bacterial Biofilms from Chicken Tissue Using Cold Atmospheric Pressure Plasma. Plasma. 2025; 8(3):34. https://doi.org/10.3390/plasma8030034
Chicago/Turabian StyleOkebiorun, Michael, Dalton Miller, Kenneth A. Cornell, and Jim Browning. 2025. "Trypan Blue Image-Guided Removal of Surface-Based Bacterial Biofilms from Chicken Tissue Using Cold Atmospheric Pressure Plasma" Plasma 8, no. 3: 34. https://doi.org/10.3390/plasma8030034
APA StyleOkebiorun, M., Miller, D., Cornell, K. A., & Browning, J. (2025). Trypan Blue Image-Guided Removal of Surface-Based Bacterial Biofilms from Chicken Tissue Using Cold Atmospheric Pressure Plasma. Plasma, 8(3), 34. https://doi.org/10.3390/plasma8030034





