Investigating the Effects of Gliding Arc Plasma Discharge’s Thermal Characteristic and Reactive Chemistry on Aqueous PFOS Mineralization
Abstract
1. Introduction
2. Materials and Methods
2.1. Gliding Arc Plasma (GAP) Discharge
2.2. Plasma Water Treatment System
2.3. Aqueous PFAS (PFOS) Preparation
2.4. Analytical Methods
2.5. Plasma Gas Temperature Estimation
2.6. Thermal Imaging
3. Results
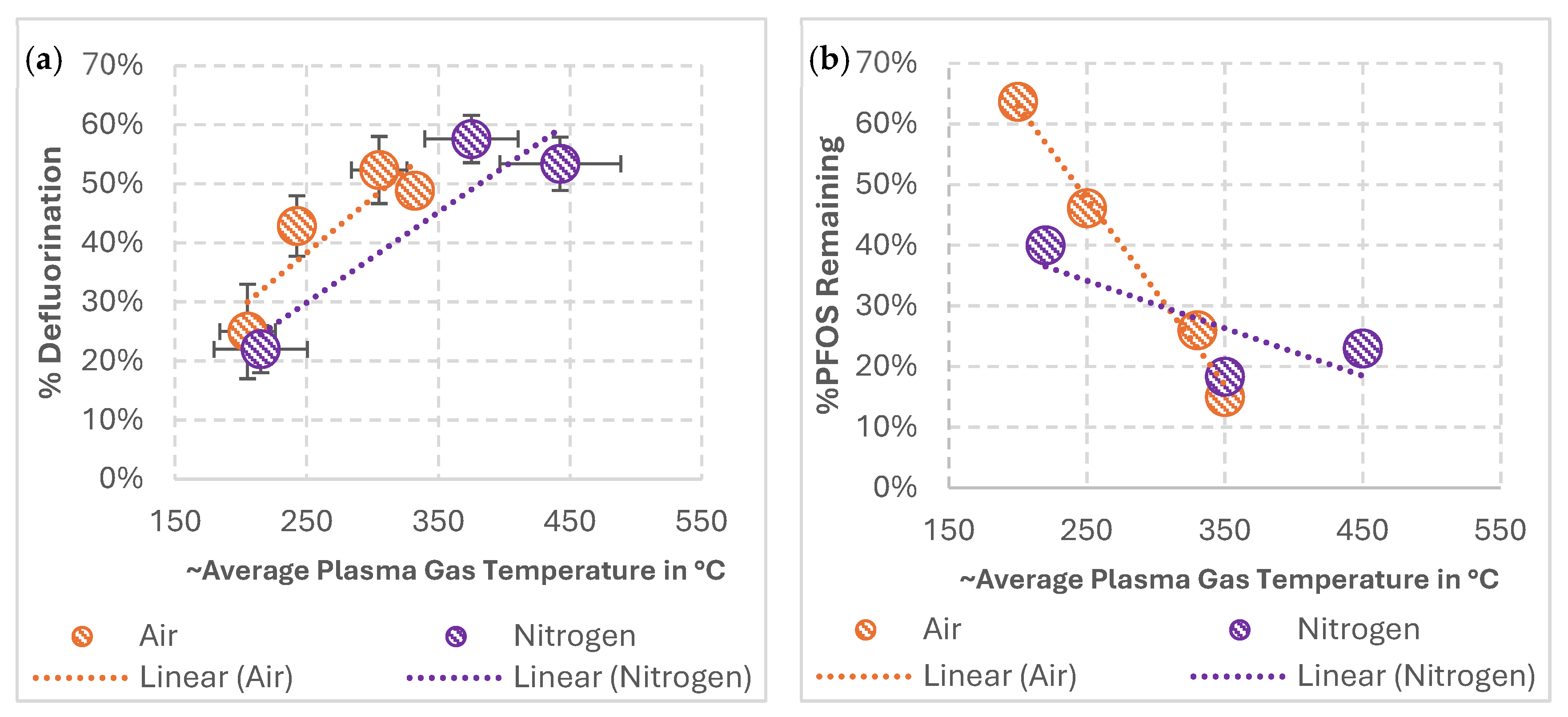
3.1. Impact of Average Plasma Gas Temperatures on PFOS Mineralization during Treatments in Air and Nitrogen GAP Discharges
3.2. Role Played by GAP Discharge on PFOS Mineralization: Purely Thermal or Does Plasma Reactive Chemistry Affect PFOS Mineralization
3.3. Effects of Different Plasma Reactive Chemistries on PFOS Mineralization
4. Discussion
4.1. Impact of Average Plasma Gas Temperatures on PFOS Mineralization
4.2. Role Played by GAP Discharge on PFOS Mineralization
4.3. Effects of Different Plasma Reactive Chemistries on PFOS Mineralization
4.4. Discussions Regarding This Study and Recommendations for Future Studies
5. Conclusions
- Treatments with increased plasma gas temperatures in air and nitrogen GAP discharges led to increased PFOS mineralization.
- GAP-based PFOS mineralization is not a pure thermal process, as gas temperatures similar to plasma gas temperatures but without the presence of plasma reactive species failed to mineralize PFOS.
- Treatments with argon GAP discharges were able to achieve higher PFOS mineralization relative to treatments with air GAP discharges at similar gas temperatures, indicating the involvement of plasma reactive species in PFOS mineralization.
- GAP discharge in air was able to mineralize PFOS at relatively lower gas temperatures than GAP discharge in nitrogen gas, indicating the possible involvement of reactive oxygen and reactive nitrogen species in PFOS mineralization.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel Treatment Technologies for PFAS Compounds: A Critical Review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Nzeribe, B.N.; Crimi, M.; Mededovic Thagard, S.; Holsen, T.M. Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Hayashi, R.; Obo, H.; Takeuchi, N.; Yasuoka, K. Decomposition of Perfluorinated Compounds in Water by DC Plasma within Oxygen Bubbles. Electr. Eng. Jpn. 2015, 190, 9–16. [Google Scholar] [CrossRef]
- Verma, S.; Lee, T.; Sahle-Demessie, E.; Ateia, M.; Nadagouda, M.N. Recent Advances on PFAS Degradation via Thermal and Nonthermal Methods. Chem. Eng. J. Adv. 2023, 13, 100421. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced Oxidation/Reduction Processes Treatment for Aqueous Perfluorooctanoate (PFOA) and Perfluorooctanesulfonate (PFOS)—A Review of Recent Advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Austin, C.; Li, J.; Moore, S.; Purohit, A.; Pinkard, B.R.; Novosselov, I.V. Destruction and Defluorination of PFAS Matrix in Continuous-Flow Supercritical Water Oxidation Reactor: Effect of Operating Temperature. Chemosphere 2023, 327, 138358. [Google Scholar] [CrossRef]
- Podder, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.-B.; Goel, R. Per and Poly-Fluoroalkyl Substances (PFAS) as a Contaminant of Emerging Concern in Surface Water: A Transboundary Review of Their Occurrences and Toxicity Effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, N.; Kumar Yadav, A.; Singh, R.; Kumar, K. Per-and Polyfluoroalkyl Substances (PFAS) as a Health Hazard: Current State of Knowledge and Strategies in Environmental Settings across Asia and Future Perspectives. Chem. Eng. J. 2023, 475, 145064. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Board on Population Health and Public Health Practice; Board on Environmental Studies and Toxicology; Committee on the Guidance on PFAS Testing and Health Outcomes. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up; Consensus Study Report; National Academies Press: Washington, DC, USA, 2022; ISBN 978-0-309-48244-8. [Google Scholar]
- Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Kazwini, T.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated Review on Emerging Technologies for PFAS Contaminated Water Treatment. Chem. Eng. Res. Des. 2022, 182, 667–700. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of Currently Available PFAS Remediation Technologies in Water: A Review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Kah, M.; Oliver, D.; Kookana, R. Sequestration and Potential Release of PFAS from Spent Engineered Sorbents. Sci. Total Environ. 2021, 765, 142770. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Bezerra de Souza, B.; Casarini, M.M.; Kewalramani, J.A. A Review of PFAS Destruction Technologies. Int. J. Environ. Res. Public Health 2022, 19, 16397. [Google Scholar] [CrossRef] [PubMed]
- Hippler, R.; Schoenbach, K.H.; Kersten, H.; Schmidt, M. Low Temperature Plasmas: Fundamentals, Technologies, and Techniques; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Fridman, A.; Nester, S.; Kennedy, L.A.; Saveliev, A.; Mutaf-Yardimci, O. Gliding Arc Gas Discharge. Prog. Energy Combust. Sci. 1999, 25, 211–231. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-Thermal Plasmas in and in Contact with Liquids. J. Phys. Appl. Phys. 2009, 42, 053001. [Google Scholar] [CrossRef]
- Lewis, A.J.; Joyce, T.; Hadaya, M.; Ebrahimi, F.; Dragiev, I.; Giardetti, N.; Yang, J.; Fridman, G.; Rabinovich, A.; Fridman, A.A.; et al. Rapid Degradation of PFAS in Aqueous Solutions by Reverse Vortex Flow Gliding Arc Plasma. Environ. Sci. Water Res. Technol. 2020, 6, 1044–1057. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-Activated Water: Generation, Origin of Reactive Species and Biological Applications. J. Phys. Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Progress of Organic Wastewater Degradation by Atmospheric Pressure Gliding Arc Plasma Technology: A Review|AIP Advances|AIP Publishing. Available online: https://pubs.aip.org/aip/adv/article/14/3/030702/3269921/Progress-of-organic-wastewater-degradation-by (accessed on 2 April 2024).
- Foster, J.E. Plasma-Based Water Purification: Challenges and Prospects for the Future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Sidnell, T.; Wood, R.J.; Hurst, J.; Lee, J.; Bussemaker, M.J. Sonolysis of Per- and Poly Fluoroalkyl Substances (PFAS): A Meta-Analysis. Ultrason. Sonochem. 2022, 87, 105944. [Google Scholar] [CrossRef]
- Saleem, M.; Tomei, G.; Beria, M.; Marotta, E.; Paradisi, C. Highly Efficient Degradation of PFAS and Other Surfactants in Water with Atmospheric RAdial Plasma (RAP) Discharge. Chemosphere 2022, 307, 135800. [Google Scholar] [CrossRef]
- Sun, S.; Sun, B.; Zhu, X.; Yang, Y.; Liu, H. Defluorination of Per-Fluorinated Compound (PFC) by Microwave Discharge Plasma in Liquid: A Green and Efficient Water Treatment Technology. Sep. Purif. Technol. 2023, 319, 124071. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Lentz, D.; Braun, T. Synthesis of Fluorinated Building Blocks by Transition-Metal-Mediated Hydrodefluorination Reactions. Angew. Chem. Int. Ed. 2013, 52, 3328–3348. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Kinetics and Mechanism of the Sonolytic Conversion of the Aqueous Perfluorinated Surfactants, Perfluorooctanoate (PFOA), and Perfluorooctane Sulfonate (PFOS) into Inorganic Products. J. Phys. Chem. A 2008, 112, 4261–4270. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Shetty, S.; Stritzinger, D.; Bellona, C.; Novosselov, I.V. Destruction of Perfluorooctanesulfonate (PFOS) in a Batch Supercritical Water Oxidation Reactor. Chemosphere 2021, 279, 130834. [Google Scholar] [CrossRef]
- Tachibana, K.; Takeuchi, N.; Yasuoka, K. Reaction Process of Perfluorooctanesulfonic Acid (PFOS) Decomposed by DC Plasma Generated in Argon Gas Bubbles. IEEE Trans. Plasma Sci. 2014, 42, 786–793. [Google Scholar] [CrossRef]
- Saleem, M.; Biondo, O.; Sretenović, G.; Tomei, G.; Magarotto, M.; Pavarin, D.; Marotta, E.; Paradisi, C. Comparative Performance Assessment of Plasma Reactors for the Treatment of PFOA; Reactor Design, Kinetics, Mineralization and Energy Yield. Chem. Eng. J. 2020, 382, 123031. [Google Scholar] [CrossRef]
- Yang, Y.; Cho, Y.I.; Fridman, A. Plasma Discharge in Liquid: Water Treatment and Applications; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-21707-9. [Google Scholar]
- Surace, M.J.; Murillo-Gelvez, J.; Shaji, M.A.; Fridman, A.A.; Rabinovich, A.; McKenzie, E.R.; Fridman, G.; Sales, C.M. Plasma-Assisted Abatement of Per- and Polyfluoroalkyl Substances (PFAS): Thermodynamic Analysis and Validation in Gliding Arc Discharge. Plasma 2023, 6, 419–434. [Google Scholar] [CrossRef]
- Takeuchi, N.; Suzuki, D.; Okada, K.; Oishi, K.; Kodama, S.; Namihira, T.; Wang, D. Discharge Conditions for Efficient and Rapid Decomposition of Perfluorooctane Sulfonic Acid (PFOS) in Water Using Plasma. Int. J. Plasma Environ. Sci. Technol. 2020, 14, e02006. [Google Scholar]
- Rabinovich, A.; Nirenberg, G.; Kocagoz, S.; Surace, M.; Sales, C.; Fridman, A. Scaling Up of Non-Thermal Gliding Arc Plasma Systems for Industrial Applications. Plasma Chem. Plasma Process. 2022, 42, 35–50. [Google Scholar] [CrossRef]
- Weber, N.H.; Delva, C.S.; Stockenhuber, S.P.; Grimison, C.C.; Lucas, J.A.; Mackie, J.C.; Stockenhuber, M.; Kennedy, E.M. Thermal Mineralization of Perfluorooctanesulfonic Acid (PFOS) to HF, CO2, and SO2. Ind. Eng. Chem. Res. 2023, 62, 881–892. [Google Scholar] [CrossRef]
- Zhu, D.; Sun, Z.; Zhang, H.; Zhang, A.; Zhang, Y.; Miruka, A.C.; Zhu, L.; Li, R.; Guo, Y.; Liu, Y. Reactive Nitrogen Species Generated by Gas–Liquid Dielectric Barrier Discharge for Efficient Degradation of Perfluorooctanoic Acid from Water. Environ. Sci. Technol. 2022, 56, 349–360. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Geiger, R.; Polevich, A.; Rabinovich, A.; Gutsol, A.; Fridman, A. On-Board Plasma-Assisted Conversion of Heavy Hydrocarbons into Synthesis Gas. Fuel 2010, 89, 1187–1192. [Google Scholar] [CrossRef]
- Cho, Y.; Wright, K.; Kim, H.; Cho, D.; Rabinovich, A.; Fridman, A. Stretched Arc Discharge in Produced Water. Rev. Sci. Instrum. 2015, 86, 013501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ehn, A.; Gao, J.; Kong, C.; Aldén, M.; Salewski, M.; Leipold, F.; Kusano, Y.; Li, Z. Translational, Rotational, Vibrational and Electron Temperatures of a Gliding Arc Discharge. Opt. Express 2017, 25, 20243. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Li, Z.; Aldén, M.; Ehn, A. Thermal Analysis of a High-Power Glow Discharge in Flowing Atmospheric Air by Combining Rayleigh Scattering Thermometry and Numerical Simulation. J. Phys. Appl. Phys. 2020, 53, 085502. [Google Scholar] [CrossRef]
- Du, C.M.; Sun, Y.W.; Zhuang, X.F. The Effects of Gas Composition on Active Species and Byproducts Formation in Gas–Water Gliding Arc Discharge. Plasma Chem. Plasma Process. 2008, 28, 523–533. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. The Role of Different Plasma Forming Gases on Chemical Species Formed in Plasma Activated Water (PAW) and Their Effect on Its Properties. Phys. Scr. 2022, 97, 065003. [Google Scholar] [CrossRef]
- Rumbach, P.; Bartels, D.M.; Sankaran, R.M.; Go, D.B. The Solvation of Electrons by an Atmospheric-Pressure Plasma. Nat. Commun. 2015, 6, 7248. [Google Scholar] [CrossRef]
- Barkhordari, A.; Ganjovi, A. Technical Characteristics of a DC Plasma Jet with Ar/N2 and O2/N2 Gaseous Mixtures. Chin. J. Phys. 2019, 57, 465–478. [Google Scholar] [CrossRef]
- Khan, M.Y.; So, S.; da Silva, G. Decomposition Kinetics of Perfluorinated Sulfonic Acids. Chemosphere 2020, 238, 124615. [Google Scholar] [CrossRef]
- Winchell, L.J.; Ross, J.J.; Wells, M.J.M.; Fonoll, X.; Norton, J.W., Jr.; Bell, K.Y. Per- and Polyfluoroalkyl Substances Thermal Destruction at Water Resource Recovery Facilities: A State of the Science Review. Water Environ. Res. 2021, 93, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Technical Guidelines. In Global Energy Assessment: Toward a Sustainable Future; Global Energy Assessment Writing Team, Ed.; Cambridge University Press: Cambridge, UK, 2012; pp. 1815–1822. ISBN 978-1-107-00519-8. [Google Scholar]
- Roy, N.C.; Talukder, M.R. Effect of Pressure on the Properties and Species Production in Gliding Arc Ar, O2, and Air Discharge Plasmas. Phys. Plasmas 2018, 25, 093502. [Google Scholar] [CrossRef]
- Groele, J.R.; Sculley, N.; Olson, T.M.; Foster, J.E. An Investigation of Plasma-Driven Decomposition of per- and Polyfluoroalkyl Substances (PFAS) in Raw Contaminated Ground Water. J. Appl. Phys. 2021, 130, 053304. [Google Scholar] [CrossRef]
- Oh, J.-S.; Szili, E.J.; Ogawa, K.; Short, R.D.; Ito, M.; Furuta, H.; Hatta, A. UV–Vis Spectroscopy Study of Plasma-Activated Water: Dependence of the Chemical Composition on Plasma Exposure Time and Treatment Distance. Jpn. J. Appl. Phys. 2017, 57, 0102B9. [Google Scholar] [CrossRef]
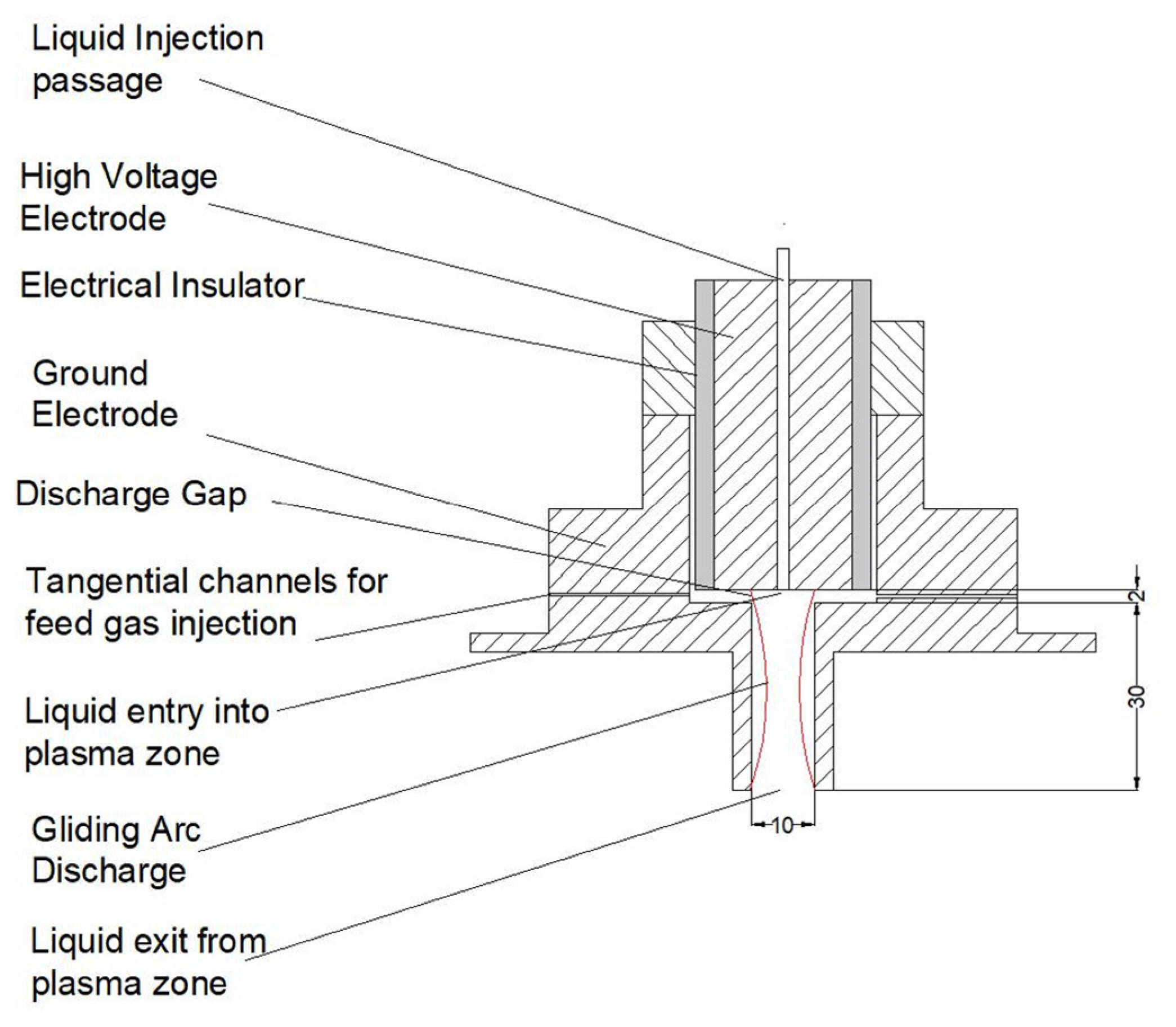




| Feed Gas | Feed Gas Flow Rate (SCFH) | Voltage (kV) | Current (mA) | Power (W) |
|---|---|---|---|---|
| Air | 80 | 1.6 | 300 | 480 |
| Feed Gas | Feed Gas Flow Rate (SCFH) | Voltage (kV) | Current (mA) | Power (W) |
|---|---|---|---|---|
| Air | 50 | 1.3 | 100 | 130 |
| Argon | 50 | 0.75 | 100 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaji, M.A.; Surace, M.J.; Rabinovich, A.; Sales, C.M.; Fridman, G.; McKenzie, E.R.; Fridman, A. Investigating the Effects of Gliding Arc Plasma Discharge’s Thermal Characteristic and Reactive Chemistry on Aqueous PFOS Mineralization. Plasma 2024, 7, 705-720. https://doi.org/10.3390/plasma7030036
Shaji MA, Surace MJ, Rabinovich A, Sales CM, Fridman G, McKenzie ER, Fridman A. Investigating the Effects of Gliding Arc Plasma Discharge’s Thermal Characteristic and Reactive Chemistry on Aqueous PFOS Mineralization. Plasma. 2024; 7(3):705-720. https://doi.org/10.3390/plasma7030036
Chicago/Turabian StyleShaji, Mobish A., Mikaela J. Surace, Alexander Rabinovich, Christopher M. Sales, Gregory Fridman, Erica R. McKenzie, and Alexander Fridman. 2024. "Investigating the Effects of Gliding Arc Plasma Discharge’s Thermal Characteristic and Reactive Chemistry on Aqueous PFOS Mineralization" Plasma 7, no. 3: 705-720. https://doi.org/10.3390/plasma7030036
APA StyleShaji, M. A., Surace, M. J., Rabinovich, A., Sales, C. M., Fridman, G., McKenzie, E. R., & Fridman, A. (2024). Investigating the Effects of Gliding Arc Plasma Discharge’s Thermal Characteristic and Reactive Chemistry on Aqueous PFOS Mineralization. Plasma, 7(3), 705-720. https://doi.org/10.3390/plasma7030036







