Recent Developments in the Use of Plasma in Medical Applications
Abstract
1. Introduction
2. Coating of Medical Devices
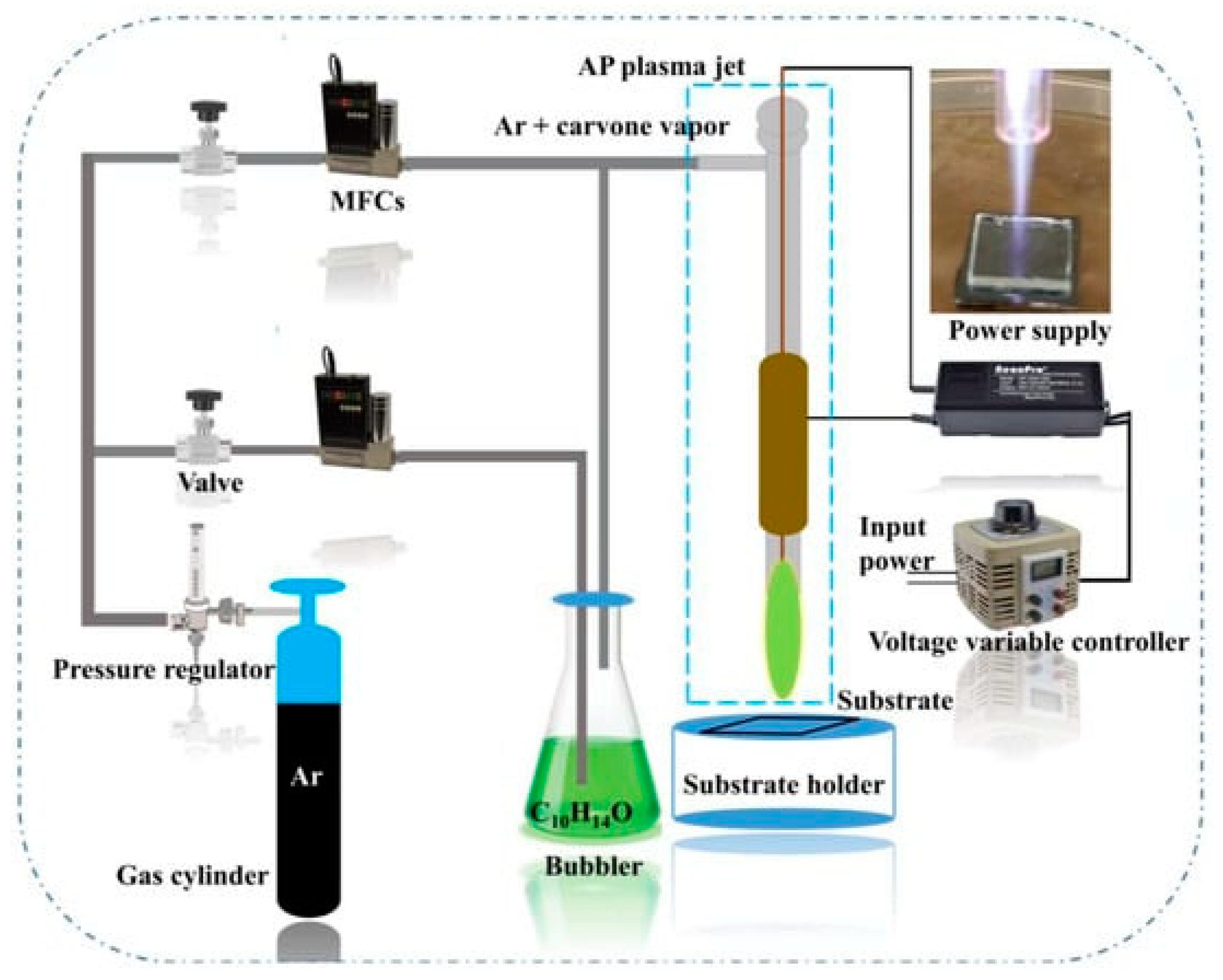
2.1. Deposition of Antimicrobial Films
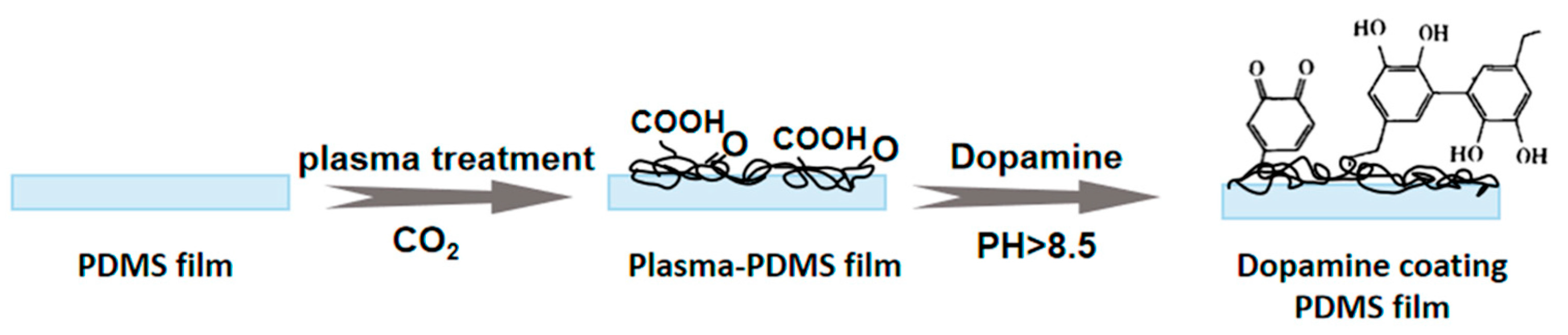
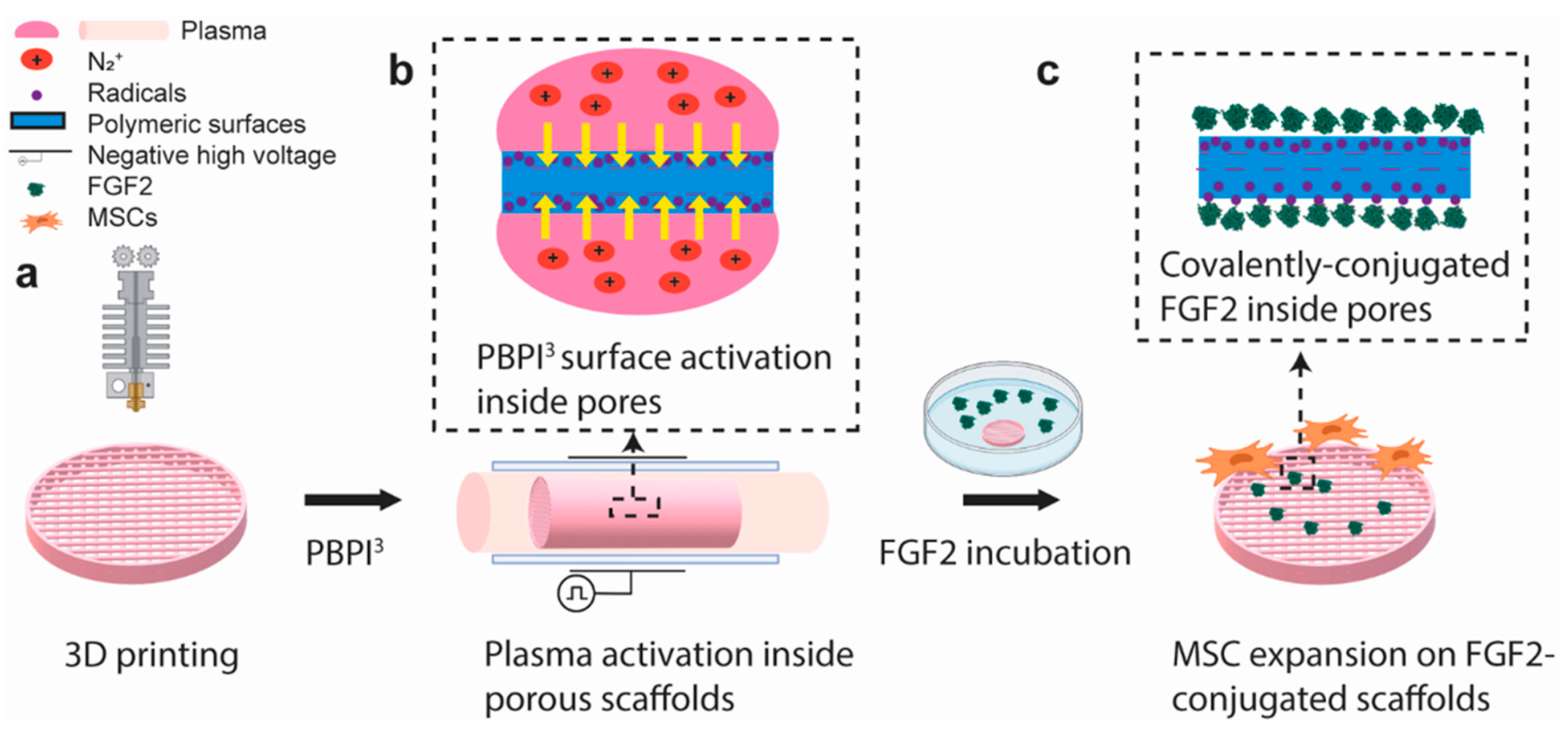
2.2. Deposition of Biocompatible and Adhesive Coatings
3. Plasma as a Therapeutic Intervention
3.1. Direct Application of Plasma to Tissue
3.2. Combinations of Plasma with Traditional Pharmaceuticals
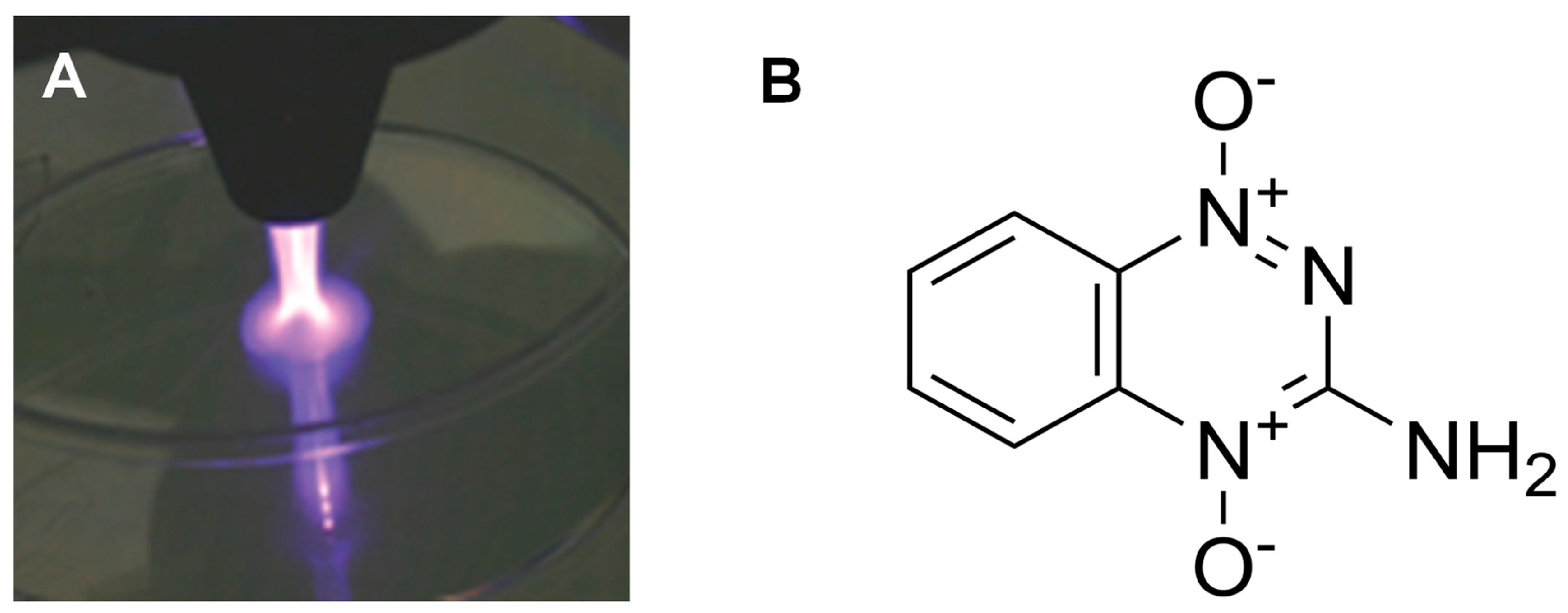
3.3. Interactions of Plasma with Liquids
4. Decontamination of Unwanted Pharmaceutical Active Ingredients
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gildersleeve, E.J.; Vaßen, R. Thermally Sprayed Functional Coatings and Multilayers: A Selection of Historical Applications and Potential Pathways for Future Innovation. J. Therm. Spray Technol. 2023, 32, 778–817. [Google Scholar] [CrossRef] [PubMed]
- Hollahan, J.R. Rare gas plasma polymerization of benzene at −196 °C. Makromol. Chem. 1972, 154, 303–308. [Google Scholar] [CrossRef]
- Yasuda, H. Plasma Polymerization; Academic Press: Orlando, FL, USA, 1985. [Google Scholar]
- Pierson, H.O. Handbook of Chemical Vapour Deposition; Noyes Publication: New York, NY, USA, 1999. [Google Scholar]
- Wróbel, A.M.; Walkiewicz-Pietrzykowska, A.; Klemberg-Sapieha, J.E.; Nakanishi, Y.; Aoki, T.; Hatanaka, Y. Remote Hydrogen Plasma Chemical Vapor Deposition from (Dimethylsilyl)(trimethylsilyl)methane. 1. Kinetics of the Process; Chemical and Morphological Structure of Deposited Silicon−Carbon Films. Chem. Mater. 2003, 15, 1749–1756. [Google Scholar] [CrossRef]
- Lopez, G.P.; Ratner, B.D. Plasma Polymerization and Plasma Interactions with Polymeric Materials: Proceedings of the Symposium on Plasma Polymerization and Plasma Interactions with Polymeric Materials, Held at the ACS 199th National Meeting in Boston, Massachusetts, April 1990; Wiley: Hoboken, NJ, USA, 1990; ISBN 978-0-471-54362-6. [Google Scholar]
- Han, L.M.; Rajeshwar, K.; Timmons, R.B. Film Chemistry Control and Electrochemical Properties of Pulsed Plasma Polymerized Ferrocene and Vinylferrocene. Langmuir 1997, 13, 5941–5950. [Google Scholar] [CrossRef]
- Ryan, M.E.; Hynes, A.M.; Badyal, J.P.S. Pulsed Plasma Polymerization of Maleic Anhydride. Chem. Mater. 1996, 8, 37–42. [Google Scholar] [CrossRef]
- Coulson, S.R.; Woodward, I.S.; Badyal, J.P.S.; Brewer, S.A.; Willis, C. Plasmachemical Functionalization of Solid Surfaces with Low Surface Energy Perfluorocarbon Chains. Langmuir 2000, 16, 6287–6293. [Google Scholar] [CrossRef]
- Herbert, P.A.F.; O’Neill, L.; Jaroszyńska-Wolińska, J. Soft Plasma Polymerization of Gas State Precursors from an Atmospheric Pressure Corona Plasma Discharge. Chem. Mater. 2009, 21, 4401–4407. [Google Scholar] [CrossRef]
- Ward, L.J.; Schofield, W.C.E.; Badyal, J.P.S.; Goodwin, A.J.; Merlin, P.J. Atmospheric Pressure Plasma Deposition of Structurally Well-Defined Polyacrylic Acid Films. Chem. Mater. 2003, 15, 1466–1469. [Google Scholar] [CrossRef]
- Ward, L.J.; Schofield, W.C.E.; Badyal, J.P.S.; Goodwin, A.J.; Merlin, P.J. Atmospheric Pressure Glow Discharge Deposition of Polysiloxane and SiOx Films. Langmuir 2003, 19, 2110–2114. [Google Scholar] [CrossRef]
- Tatoulian, M.; Arefi-Khonsari, F.; Borra, J.-P. Deposition of Organic Coatings at Atmospheric Pressure from Liquid Precursors. Plasma Process. Polym. 2007, 4, 360–369. [Google Scholar] [CrossRef]
- O’Hare, L.-A.; O’Neill, L.; Goodwin, A.J. Anti-microbial coatings by agent entrapment in coatings deposited via atmospheric pressure plasma liquid deposition. Surf. Interface Anal. 2006, 38, 1519–1524. [Google Scholar] [CrossRef]
- Pignatelli, D.; Sardella, E.; Palumbo, F.; Lo Porto, C.; Taccola, S.; Greco, F.; Mattoli, V.; Favia, P. Plasma assisted deposition of free-standing nanofilms for biomedical applications. Plasma Process. Polym. 2016, 13, 1224–1229. [Google Scholar] [CrossRef]
- Heyse, P.; Roeffaers, M.B.J.; Paulussen, S.; Hofkens, J.; Jacobs, P.A.; Sels, B.F. Protein Immobilization Using Atmospheric-Pressure Dielectric-Barrier Discharges: A Route to a Straightforward Manufacture of Bioactive Films. Plasma Process. Polym. 2008, 5, 186–191. [Google Scholar] [CrossRef]
- Da Ponte, G.; Sardella, E.; Fanelli, F.; Paulussen, S.; Favia, P. Atmospheric Pressure Plasma Deposition of Poly Lactic Acid-Like Coatings with Embedded Elastin. Plasma Process. Polym. 2014, 11, 345–352. [Google Scholar] [CrossRef]
- Malinowski, S.; Herbert, P.A.F.; Rogalski, J.; Jaroszyńska-Wolińska, J. Laccase Enzyme Polymerization by Soft Plasma Jet for Durable Bioactive Coatings. Polymers 2018, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Los, A.; Ziuzina, D.; Boehm, D.; Bourke, P.; Al, E. Efficacy of Cold Plasma for Direct Deposition of Antibiotics as a Novel Approach for Localized Delivery and Retention of Effect. Front. Cell. Infect. Microbiol. 2019, 9, 428. [Google Scholar] [CrossRef]
- O’sullivan, D.; McArdle, H.; O’Reilly, J.-A.; O’Kennedy, R.J.; Forster, R.; O’Neill, L. Plasma deposition of collagen for cell-culture applications. Plasma Process. Polym. 2020, 17, 1900147. [Google Scholar] [CrossRef]
- Global Medical Device Coating Market Size Analysis Report. Available online: https://www.bccresearch.com/market-research/healthcare/medical-device-coatings-report.html (accessed on 9 January 2024).
- Dao, A.; Gaitanos, C.; Kamble, S.; Sharifahmadian, O.; Tan, R.; Wise, S.G.; Cheung, T.L.Y.; Bilek, M.M.M.; Savage, P.B.; Schindeler, A.; et al. Antibacterial Plasma Polymer Coatings on 3D Materials for Orthopedic Applications. Adv. Mater. Interfaces 2024, 11, 2300063. [Google Scholar] [CrossRef]
- Egghe, T.; Morent, R.; Hoogenboom, R.; Geyter, N.D. Substrate-independent and widely applicable deposition of antibacterial coatings. Trends Biotechnol. 2023, 41, 63–76. [Google Scholar] [CrossRef]
- Baculi, R.Q.; Malapit, G.M.; Abayao, L.E. Atmospheric pressure plasma deposition of silver nanoparticles on bark fabric for bacterial growth inhibition. J. Text. Inst. 2023, 114, 142–150. [Google Scholar] [CrossRef]
- Loesch-Zhang, A.; Geissler, A.; Biesalski, M. Plasma polymerization of biogenic precursors. Plasma Process. Polym. 2023, 20, e2300016. [Google Scholar] [CrossRef]
- Prasad, K.; Sasi, S.; Weerasinghe, J.; Levchenko, I.; Bazaka, K. Enhanced Antimicrobial Activity through Synergistic Effects of Cold Atmospheric Plasma and Plant Secondary Metabolites: Opportunities and Challenges. Molecules 2023, 28, 7481. [Google Scholar] [CrossRef]
- Lu, M.; Ding, L.; Zhong, T.; Dai, Z. Improving Hydrophilicity and Adhesion of PDMS through Dopamine Modification Assisted by Carbon Dioxide Plasma. Coatings 2023, 13, 126. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, T.; Li, B.; Li, J. Surface modifications of zirconia with plasma pretreatment and polydopamine coating to enhance the bond strength and durability between zirconia and titanium. Dent. Mater. J. 2023, 42, 449–457. [Google Scholar] [CrossRef]
- Saitaer, X.; Sanbhal, N.; Qiao, Y.; Li, Y.; Gao, J.; Brochu, G.; Guidoin, R.; Khatri, A.; Wang, L. Polydopamine-Inspired Surface Modification of Polypropylene Hernia Mesh Devices via Cold Oxygen Plasma: Antibacterial and Drug Release Properties. Coatings 2019, 9, 164. [Google Scholar] [CrossRef]
- Su, T.-L.; Chen, T.-P.; Liang, J. Green in-situ synthesis of silver coated textiles for wide hygiene and healthcare applications. Colloids Surf. Physicochem. Eng. Asp. 2023, 657, 130506. [Google Scholar] [CrossRef]
- Gallingani, T.; Resca, E.; Dominici, M.; Gavioli, G.; Laurita, R.; Liguori, A.; Mari, G.; Ortolani, L.; Pericolini, E.; Sala, A.; et al. A new strategy to prevent biofilm and clot formation in medical devices: The use of atmospheric non-thermal plasma assisted deposition of silver-based nanostructured coatings. PLoS ONE 2023, 18, e0282059. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.T.L.; Gelamo, R.V.; Mateus Santos Obata, M.; de Andrade Silva, L.E.; da Silva, M.V.; de Oliveira, C.J.F.; da Silva, B.P.; Aoki, I.V.; Moreto, J.A.; Slade, N.B.L. Exploring the functionalization of Ti-6Al-4V alloy with the novel antimicrobial peptide JIChis-2 via plasma polymerization. Biofouling 2023, 39, 47–63. [Google Scholar] [CrossRef]
- Vesel, A. Deposition of Chitosan on Plasma-Treated Polymers—A Review. Polymers 2023, 15, 1109. [Google Scholar] [CrossRef]
- Ho, K.-N.; Chen, L.-W.; Kuo, T.-F.; Chen, K.-S.; Lee, S.-Y.; Wang, S.-F. Surface modification of zirconia ceramics through cold plasma treatment and the graft polymerization of biomolecules. J. Dent. Sci. 2023, 18, 73–80. [Google Scholar] [CrossRef]
- da Silva Bullmann, M.; de Castro, V.V.; Coutinho, D.A.K.; Lopes, F.C.; Maurmann, N.; Pereira, M.B.; Rodrigues, M.; Pranke, P.; Ferraz, M.P.; Lopes, M.A.; et al. Eucalyptus globulus essential oil thin film polymerized by cold plasma on Ti6Al4V: Sterilization effect, antibacterial activity, adhesion, and viability of mesenchymal stem cells. Plasma Process. Polym. 2023, 20, e2300075. [Google Scholar] [CrossRef]
- Kayaian, M.-R.; Hawker, M.J. Using 1,8-cineole plasma with both pulsed and continuous depositions to modify commercially available wound dressing materials. Biointerphases 2023, 18, 051002. [Google Scholar] [CrossRef]
- Marzoug, H.N.B.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Khouja, M.L.; Bouajila, J. Eucalyptus oleosa Essential Oils: Chemical Composition and Antimicrobial and Antioxidant Activities of the Oils from Different Plant Parts (Stems, Leaves, Flowers and Fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Sakthi Kumar, D.; Nakamura, K.; Nishiyama, S.; Noguchi, H.; Ishii, S.; Kashiwagi, K.; Yoshida, Y. Electrical and optical properties of plasma polymerized eucalyptus oil films. J. Appl. Polym. Sci. 2003, 90, 1102–1107. [Google Scholar] [CrossRef]
- Masood, A.; Ahmed, N.; Razip Wee, M.F.M.; Patra, A.; Mahmoudi, E.; Siow, K.S. Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity. Polymers 2023, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Ahmed, N.; Shahid, F.; Mohd Razip Wee, M.F.; Patra, A.; Siow, K.S. Atmospheric Pressure Plasma Polymerization of Carvone: A Promising Approach for Antimicrobial Coatings. Coatings 2023, 13, 1112. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Zulkiflee, I.; Mohd Razip Wee, M.F.; Masood, A.; Siow, K.S.; Motta, A.; Fauzi, M.B. Plasma-Polymerised Antibacterial Coating of Ovine Tendon Collagen Type I (OTC) Crosslinked with Genipin (GNP) and Dehydrothermal-Crosslinked (DHT) as a Cutaneous Substitute for Wound Healing. Materials 2023, 16, 2739. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.O.M.; Rao, X.; García, A.F.; Vega, C.G.; Canche, C.N.A.; López, J.A.G.; Pérez, A.S.L.; Medina, M.D.D.; Gaona, C.G.C.; Céspedes, R.I.N.; et al. Cold plasma copolymer with antimicrobial activity deposited on three different substrates. Polímeros 2023, 33, e20230038. [Google Scholar] [CrossRef]
- Shabani, H.; Dezhpour, A.; Jafari, S.; Moghaddam, M.J.M.; Nilkar, M. Antimicrobial activity of cold atmospheric-pressure argon plasma combined with chicory (Cichorium intybus L.) extract against P. aeruginosa and E. coli biofilms. Sci. Rep. 2023, 13, 9441. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Mohseni, M.; Naseripour, M.; Mirzaei, M.; Bagherzadeh, K.; Alemezadeh, S.A.; Mehravi, B. Synthesis and evaluation of modified lens using plasma treatment containing timolol-maleate loaded lauric acid-decorated chitosan-alginate nanoparticles for glaucoma. J. Biomater. Sci. Polym. Ed. 2023, 34, 1793–1812. [Google Scholar] [CrossRef]
- Wulandari, E.; Bilimoria, K.; Krasowska, M.; Al-Bataineh, S.; Beattie, D.; Gillam, T.; Ge, W.; Whittle, J.D.; Wong, E.H.H.; Blencowe, A. Nanoscale iodophoric poly(vinyl amide) coatings for the complexation and release of iodine for antimicrobial surfaces. Appl. Surf. Sci. 2023, 641, 158422. [Google Scholar] [CrossRef]
- Machková, A.; Vaňková, E.; Obrová, K.; Fürhacker, P.; Košutová, T.; Lion, T.; Hanuš, J.; Scholtz, V. Silver nanoparticles with plasma-polymerized hexamethyldisiloxane coating on 3D printed substrates are non-cytotoxic and effective against respiratory pathogens. Front. Microbiol. 2023, 14, 1217617. [Google Scholar] [CrossRef]
- Strudwick, X.L.; Whittle, J.D.; Cowin, A.J.; Smith, L.E. Plasma-Functionalised Dressings for Enhanced Wound Healing. Int. J. Mol. Sci. 2023, 24, 797. [Google Scholar] [CrossRef]
- Rout, B.; Girard-Lauriault, P.-L. Cell Attachment and Laminin Immobilization on Hydrogels Coated by Plasma Deposited Nitrogen, Oxygen or Sulfur Based Organic Thin Films. Plasma Chem. Plasma Process. 2023, 43, 709–736. [Google Scholar] [CrossRef]
- Solovieva, A.O.; Sitnikova, N.A.; Nimaev, V.V.; Koroleva, E.A.; Manakhov, A.M. PRP of T2DM Patient Immobilized on PCL Nanofibers Stimulate Endothelial Cells Proliferation. Int. J. Mol. Sci. 2023, 24, 8262. [Google Scholar] [CrossRef] [PubMed]
- Aung, L.M.; Lin, J.C.-Y.; Salamanca, E.; Wu, Y.-F.; Pan, Y.-H.; Teng, N.-C.; Huang, H.-M.; Sun, Y.-S.; Chang, W.-J. Functionalization of zirconia ceramic with fibronectin proteins enhanced bioactivity and osteogenic response of osteoblast-like cells. Front. Bioeng. Biotechnol. 2023, 11, 1159639. [Google Scholar] [CrossRef]
- Le, D.; Pan, J.; Xing, H. The Cell Adhesion and Proliferation Enhancement Impact of Low-Temperature Atmospheric Pressure Plasma-Polymerized Heptylamine on the Surface of Ti6Al4V Alloy. Materials 2023, 16, 6450. [Google Scholar] [CrossRef]
- Mills, S.J.; Kirby, G.T.; Hofma, B.R.; Smith, L.E.; Statham, P.; Vaes, B.; Ting, A.E.; Short, R.; Cowin, A.J. Delivery of multipotent adult progenitor cells via a functionalized plasma polymerized surface accelerates healing of murine diabetic wounds. Front. Bioeng. Biotechnol. 2023, 11, 1213021. [Google Scholar] [CrossRef] [PubMed]
- Laghi, G.; Franco, D.; Condorelli, G.G.; Gallerani, R.; Guglielmino, S.; Laurita, R.; Morganti, D.; Traina, F.; Conoci, S.; Gherardi, M. Control strategies for atmospheric pressure plasma polymerization of fluorinated silane thin films with antiadhesive properties. Plasma Process. Polym. 2023, 20, 2200194. [Google Scholar] [CrossRef]
- de los Arcos, T.; Awakowicz, P.; Böke, M.; Boysen, N.; Brinkmann, R.P.; Dahlmann, R.; Devi, A.; Eremin, D.; Franke, J.; Gergs, T.; et al. PECVD and PEALD on polymer substrates (Part II): Understanding and tuning of barrier and membrane properties of thin films. arXiv 2023. [Google Scholar] [CrossRef]
- Kelarová, Š.; Přibyl, R.; Homola, V.; Polčák, J.; Charvátová Campbell, A.; Havlíček, M.; Vrchovecká, K.; Václavik, R.; Zábranský, L.; Buršíková, V. Influence of the argon ratio on the structure and properties of thin films prepared using PECVD in TMSAc/Ar mixtures. Vacuum 2023, 207, 111634. [Google Scholar] [CrossRef]
- Coulson, S.R.; Woodward, I.S.; Badyal, J.P.S.; Brewer, S.A.; Willis, C. Ultralow Surface Energy Plasma Polymer Films. Chem. Mater. 2000, 12, 2031–2038. [Google Scholar] [CrossRef]
- Burmeister, N.; Vollstedt, C.; Kröger, C.; Friedrich, T.; Scharnagl, N.; Rohnke, M.; Zorn, E.; Wicha, S.G.; Streit, W.R.; Maison, W. Zwitterionic surface modification of polyethylene via atmospheric plasma-induced polymerization of (vinylbenzyl-)sulfobetaine and evaluation of antifouling properties. Colloids Surf. B Biointerfaces 2023, 224, 113195. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.; Leclerc, A.; Arditi, W.; Calzuola, S.T.; Feaugas, T.; Roy, E.; Perrault, C.M.; Porrini, C.; Bechelany, M. Challenge of material haemocompatibility for microfluidic blood-contacting applications. Front. Bioeng. Biotechnol. 2023, 11, 1249753. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, R.; Becker, K.H.; Weltmann, K.-D. Barrier Discharges in Science and Technology Since 2003: A Tribute and Update. Plasma Chem. Plasma Process. 2023, 43, 1303–1334. [Google Scholar] [CrossRef]
- Yu, H.; Yu, Y.; Li, C.; Feng, L. Surface Preparation and Cytocompatibility of Three-Dimensional Printed Fully Degraded Coronary Stents Using the Plasma Polymerization Technology. Sci. Adv. Mater. 2023, 15, 67–78. [Google Scholar] [CrossRef]
- Rezaei, H.; Matin, A.A.; Mohammadnejad, M. Cold atmospheric plasma treated 3D printed polylactic acid film; application in thin film solid phase microextraction of anticancer drugs. Talanta 2024, 266, 125064. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Anoop, A.G.; Thakur, A.; Mohanta, G.C.; Kumar, P. Strategies To Modify the Surface and Bulk Properties of 3D-Printed Solid Scaffolds for Tissue Engineering Applications. ACS Omega 2023, 8, 5139–5156. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Torres, M.; Fucile, P.; Sinha, R.; Mota, C.; Moroni, L. Boosting bone regeneration using augmented melt-extruded additive-manufactured scaffolds. Int. Mater. Rev. 2023, 68, 755–785. [Google Scholar] [CrossRef]
- Lotz, O.; McKenzie, D.R.; Bilek, M.M.; Akhavan, B. Biofunctionalized 3D printed structures for biomedical applications: A critical review of recent advances and future prospects. Prog. Mater. Sci. 2023, 137, 101124. [Google Scholar] [CrossRef]
- Zhang, A.; Wong, J.K.U.; Redzikultsava, K.; Baldry, M.; Alavi, S.K.H.; Wang, Z.; van Koten, E.; Weiss, A.; Bilek, M.; Yeo, G.C.; et al. A cost-effective and enhanced mesenchymal stem cell expansion platform with internal plasma-activated biofunctional interfaces. Mater. Today Bio 2023, 22, 100727. [Google Scholar] [CrossRef] [PubMed]
- Miri, L.; Irani, S.; Pezeshki-Modaress, M.; Daemi, H.; Atyabi, S.M. Guiding mesenchymal stem cells differentiation into chondrocytes using sulfated alginate/cold atmospheric plasma modified polycaprolactone nanofibrous scaffold. Polym. Bull. 2023, 80, 8845–8860. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, F.; Chong, M.S.K. Surface Modification of Tissue Engineering Scaffolds. In Polymeric Biomaterials for Tissue Regeneration: From Surface/Interface Design to 3D Constructs; Gao, C., Ed.; Springer Nature: Singapore, 2023; pp. 227–264. ISBN 978-981-9969-48-7. [Google Scholar]
- Asadian, M.; Tomasina, C.; Onyshchenko, Y.; Chan, K.V.; Norouzi, M.; Zonderland, J.; Camarero-Espinosa, S.; Morent, R.; De Geyter, N.; Moroni, L. The role of plasma-induced surface chemistry on polycaprolactone nanofibers to direct chondrogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. A 2024, 112, 210–230. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarshirazi, S.; Ghobeira, R.; Egghe, T.; De Geyter, N.; Declercq, H.; Morent, R. New plasma-assisted polymerization/activation route leading to a high density primary amine silanization of PCL/PLGA nanofibers for biomedical applications. Appl. Surf. Sci. 2023, 640, 158380. [Google Scholar] [CrossRef]
- Seemann, S.; Dubs, M.; Koczan, D.; Salapare, H.S.; Ponche, A.; Pieuchot, L.; Petithory, T.; Wartenberg, A.; Staehlke, S.; Schnabelrauch, M.; et al. Response of Osteoblasts on Amine-Based Nanocoatings Correlates with the Amino Group Density. Molecules 2023, 28, 6505. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.C.; Nagay, B.E.; Dini, C.; Borges, M.H.R.; Miranda, L.F.B.; Cordeiro, J.M.; Souza, J.G.S.; Sukotjo, C.; Cruz, N.C.; Barão, V.A.R. The race for the optimal antimicrobial surface: Perspectives and challenges related to plasma electrolytic oxidation coating for titanium-based implants. Adv. Colloid Interface Sci. 2023, 311, 102805. [Google Scholar] [CrossRef] [PubMed]
- Fattah-alhosseini, A.; Molaei, M. A review of functionalizing plasma electrolytic oxidation (PEO) coatings on titanium substrates with laser surface treatments. Appl. Surf. Sci. Adv. 2023, 18, 100506. [Google Scholar] [CrossRef]
- Moreno, L.; Wang, C.; Lamaka, S.V.; Zheludkevich, M.L.; Rodríguez-Hernández, J.; Arrabal, R.; Matykina, E. Ciprofloxacin Release and Corrosion Behaviour of a Hybrid PEO/PCL Coating on Mg3Zn0.4Ca Alloy. J. Funct. Biomater. 2023, 14, 65. [Google Scholar] [CrossRef]
- Farshid, S.; Kharaziha, M.; Atapour, M. A self-healing and bioactive coating based on duplex plasma electrolytic oxidation/polydopamine on AZ91 alloy for bone implants. J. Magnes. Alloys 2023, 11, 592–606. [Google Scholar] [CrossRef]
- Wang, R.; Shen, J.; Ma, Y.; Qin, X.; Qin, X.; Yang, F.; Ostrikov, K.; Zhang, Q.; He, J.; Zhong, X. Cancer-targeting carbon quantum dots synthesized by plasma electrochemical method for red-light-activated photodynamic therapy. Plasma Process. Polym. 2024, 21, e2300174. [Google Scholar] [CrossRef]
- Sladek, R.E.J.; Stoffels, E. Deactivation of Escherichia coli by the plasma needle. J. Phys. Appl. Phys. 2005, 38, 1716. [Google Scholar] [CrossRef]
- Sosnin, E.A.; Stoffels, E.; Erofeev, M.V.; Kieft, I.E.; Kunts, S.E. The effects of UV irradiation and gas plasma treatment on living mammalian cells and bacteria: A comparative approach. IEEE Trans. Plasma Sci. 2004, 32, 1544–1550. [Google Scholar] [CrossRef]
- Laroussi, M.; Tendero, C.; Lu, X.; Alla, S.; Hynes, W.L. Inactivation of Bacteria by the Plasma Pencil. Plasma Process. Polym. 2006, 3, 470–473. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric-Pressure Plasma on Bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Weltmann, K.-D.; von Woedtke, T. Basic requirements for plasma sources in medicine. Eur. Phys. J. Appl. Phys. 2011, 55, 13807. [Google Scholar] [CrossRef]
- Yasuda, H.; Hashimoto, M.; Rahman, M.M.; Takashima, K.; Mizuno, A. States of Biological Components in Bacteria and Bacteriophages during Inactivation by Atmospheric Dielectric Barrier Discharges. Plasma Process. Polym. 2008, 5, 615–621. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Bekeschus, S.; Tanaka, H.; Lin, A.; Choi, E.H. Plasma Medicine Technologies. Appl. Sci. 2021, 11, 4584. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef]
- Lotfi, M.; Khani, M.; Shokri, B. A Review of Cold Atmospheric Plasma Applications in Dermatology and Aesthetics. Plasma Med. 2023, 13, 39–63. [Google Scholar] [CrossRef]
- Chupradit, S.; Widjaja, G.; Radhi Majeed, B.; Kuznetsova, M.; Ansari, M.J.; Suksatan, W.; Turki Jalil, A.; Ghazi Esfahani, B. Recent advances in cold atmospheric plasma (CAP) for breast cancer therapy. Cell Biol. Int. 2023, 47, 327–340. [Google Scholar] [CrossRef]
- Gelbrich, N.; Miebach, L.; Berner, J.; Freund, E.; Saadati, F.; Schmidt, A.; Stope, M.; Zimmermann, U.; Burchardt, M.; Bekeschus, S. Medical gas plasma augments bladder cancer cell toxicity in preclinical models and patient-derived tumor tissues. J. Adv. Res. 2023, 47, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Nejat, F.; Jadidi, K.; Eghtedari, S.; Nabavi, N. Sublimation of Benign Conjunctival Nevi Using Plasma-Assisted Noninvasive Surgery: A Clinical Case Series. Iran. J. Med. Sci. 2023, 48, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Yoon, H.K.; Jung, C.J.; Jo, S.Y.; Hwang, S.G.; Lee, H.J.; Lee, W.J.; Chang, S.E.; Won, C.H. Cold Plasma Treatment Promotes Full-thickness Healing of Skin Wounds in Murine Models. Int. J. Low. Extrem. Wounds 2023, 22, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ricky, S.; Lim, T.H.; Kim, H.; Lee, E.J.; Song, Y.; Lee, S.; Jang, Y. An Atmospheric Plasma Jet Induces Expression of Wound Healing Genes in Progressive Burn Wounds in a Comb Burn Rat Model: A Pilot Study. J. Burn Care Res. 2023, 44, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; von Kohout, M.; Zoric, A.; Fuchs, P.C.; Schiefer, J.L.; Opländer, C. Can Cold Atmospheric Plasma Be Used for Infection Control in Burns? A Preclinical Evaluation. Biomedicines 2023, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Liu, D.; He, D.; Lu, X.; Ostrikov, K. Plasma Scalpels: Devices, Diagnostics, and Applications. Biomedicines 2022, 10, 2967. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Prakash, G.V.; Mohapatra, S.; Kar, S.; Bhatt, S.; Gautam, H.; Singh, G.; Kapil, A.; Das, B.K.; Sood, S.; et al. Antimicrobial Efficacy of Argon Cold Atmospheric Pressure Plasma Jet on Clinical Isolates of Multidrug-Resistant ESKAPE Bacteria. IEEE Trans. Radiat. Plasma Med. Sci. 2023, 7, 421–428. [Google Scholar] [CrossRef]
- Maybin, J.-A.; Thompson, T.P.; Flynn, P.B.; Skvortsov, T.; Hickok, N.J.; Freeman, T.A.; Gilmore, B.F. Cold atmospheric pressure plasma-antibiotic synergy in Pseudomonas aeruginosa biofilms is mediated via oxidative stress response. Biofilm 2023, 5, 100122. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Han, Q. Mechanisms of bacterial inhibition and tolerance around cold atmospheric plasma. Appl. Microbiol. Biotechnol. 2023, 107, 5301–5316. [Google Scholar] [CrossRef]
- Hu, S.; Fu, Y.; Xue, M.; Lan, Y.; Xi, W.; Xu, Z.; Han, W.; Wu, D.; Cheng, C. Simultaneous removal of antibiotic-resistant Escherichia coli and its resistance genes by dielectric barrier discharge plasma. Environ. Res. 2023, 231, 116163. [Google Scholar] [CrossRef]
- Algammal, A.; Hetta, H.F.; Mabrok, M.; Behzadi, P. Editorial: Emerging multidrug-resistant bacterial pathogens “superbugs”: A rising public health threat. Front. Microbiol. 2023, 14, 1135614. [Google Scholar] [CrossRef] [PubMed]
- Abu Rached, N.; Kley, S.; Storck, M.; Meyer, T.; Stücker, M. Cold Plasma Therapy in Chronic Wounds—A Multicenter, Randomized Controlled Clinical Trial (Plasma on Chronic Wounds for Epidermal Regeneration Study): Preliminary Results. J. Clin. Med. 2023, 12, 5121. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, J.; Wang, L.; Liu, J.; Fu, C.; Yang, X.; Zhang, S.; Li, X.; Luo, S.; Yang, C. Basic research and clinical exploration of cold atmospheric plasma for skin wounds. Bioeng. Transl. Med. 2023, 8, e10550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Zhao, F.; Song, K.; Nie, L.; Liu, D.; Lu, X. Plasma-activated ethanol solution and it’s decontamination effect. High Volt. 2023, 8, 833–840. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Ziuzina, D.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Cold Plasma Inactivation of Bacterial Biofilms and Reduction of Quorum Sensing Regulated Virulence Factors. PLoS ONE 2015, 10, e0138209. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Singer, D.; Potlitz, F.; Nasri, Z.; von Woedtke, T.; Link, A.; Bekeschus, S.; Wende, K. Cold Physical Plasma-Mediated Fenretinide Prodrug Activation Confers Additive Cytotoxicity in Epithelial Cells. Antioxidants 2023, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro Lopes, B.; O’Neill, L.; Bourke, P.; Boehm, D. Combined Effect of Plasma-Activated Water and Topotecan in Glioblastoma Cells. Cancers 2023, 15, 4858. [Google Scholar] [CrossRef] [PubMed]
- Yehl, M.; Kucharski, D.; Eubank, M.; Gulledge, B.; Rayan, G.; Uddin, M.G.; Remmers, G.; Kandel, E.S.; DuFaux, D.P.; Hutcherson, T.C.; et al. The Development of Nonthermal Plasma and Tirapazamine as a Novel Combination Therapy to Treat Melanoma In Situ. Cells 2023, 12, 2113. [Google Scholar] [CrossRef]
- Pavlik, T.; Gudkova, V.; Razvolyaeva, D.; Pavlova, M.; Kostukova, N.; Miloykovich, L.; Kolik, L.; Konchekov, E.; Shimanovskii, N. The Role of Autophagy and Apoptosis in the Combined Action of Plasma-Treated Saline, Doxorubicin, and Medroxyprogesterone Acetate on K562 Myeloid Leukaemia Cells. Int. J. Mol. Sci. 2023, 24, 5100. [Google Scholar] [CrossRef]
- Dezhpour, A.; Ghafouri, H.; Jafari, S.; Nilkar, M. Effects of cold atmospheric-pressure plasma in combination with doxorubicin drug against breast cancer cells in vitro and in vivo. Free Radic. Biol. Med. 2023, 209, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Kniazeva, V.; Tzerkovsky, D.; Baysal, Ö.; Kornev, A.; Roslyakov, E.; Kostevitch, S. Adjuvant composite cold atmospheric plasma therapy increases antitumoral effect of doxorubicin hydrochloride. Front. Oncol. 2023, 13, 1171042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mang, X.; Li, D.; Wang, Z.; Chen, Y.; Cai, Z.; Tan, F. Cold atmospheric plasma sensitizes head and neck cancer to chemotherapy and immune checkpoint blockade therapy. Redox Biol. 2024, 69, 102991. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhao, L.; Zhang, X.; Chu, Z.; Zhou, T.; Zhang, Y.; Geng, S.; Guo, K. Cold atmospheric plasma sensitizes melanoma cells to targeted therapy agents in vitro. J. Biophotonics 2024, 17, e202300356. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, T.; Kostukova, N.; Pavlova, M.; Gusein-Zade, N.; Shimanovskii, N. Modulation of the Effect of Doxorubicin and Medroxyprogesterone Acetate on Cytokine and Oxidant Activity of Human Leukocytes by Hanks Cold Plasma-Treated Solution. Plasma Med. 2023, 13, 13–27. [Google Scholar] [CrossRef]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Lv, Y.; Nie, L.; Li, X.; Liu, J.; Lu, X. Comparative study of touchable plasma devices on transdermal drug delivery. Plasma Process. Polym. 2023, 20, e2200216. [Google Scholar] [CrossRef]
- Wu, E.; Nie, L.; Liu, D.; Lu, X.; Ostrikov, K. Plasma poration: Transdermal electric fields, conduction currents, and reactive species transport. Free Radic. Biol. Med. 2023, 198, 109–117. [Google Scholar] [CrossRef]
- Sreedevi, P.R.; Suresh, K. Cold atmospheric plasma mediated cell membrane permeation and gene delivery-empirical interventions and pertinence. Adv. Colloid Interface Sci. 2023, 320, 102989. [Google Scholar] [CrossRef]
- Vijayarangan, V.; Dozias, S.; Heusèle, C.; Jeanneton, O.; Nizard, C.; Pichon, C.; Pouvesle, J.M.; Stancampiano, A.; Robert, E. Boost of cosmetic active ingredient penetration triggered and controlled by the delivery of kHz plasma jet on human skin explants. Front. Phys. 2023, 11, 73349. [Google Scholar] [CrossRef]
- Fang, T.; Cao, X.; Shen, B.; Chen, Z.; Chen, G. Injectable cold atmospheric plasma-activated immunotherapeutic hydrogel for enhanced cancer treatment. Biomaterials 2023, 300, 122189. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, L.; Zhao, P.; Jing, X.; Zhang, F.; Niyazi, G.; Li, T.; Qi, Y.; Yan, J.; Jia, Y.; et al. Bactericidal effects of plasma-activated saline prepared by surface dielectric barrier discharge with different dielectric layers and working gases. Plasma Process. Polym. 2023, 20, 2200110. [Google Scholar] [CrossRef]
- Hummert, M.; Leenders, P.; Mellmann, A.; Becker, K.; Kuczius, T. Generation of Plasma-Activated Fluids for Successful Disinfection of Pseudomonas aeruginosa in Liquid Environments and Determination of Microbial Damage. Plasma 2023, 6, 699–713. [Google Scholar] [CrossRef]
- Lin, J.; Liu, D.; Zhang, J.; Zhou, R.; Rong, M.; Ostrikov, K. Insights into reactivity and bactericidal effects of water activated by He and Ar plasma jets. Plasma Process. Polym. 2023, 20, 2200173. [Google Scholar] [CrossRef]
- von Woedtke, T.; Gabriel, G.; Schaible, U.E.; Bekeschus, S. Oral SARS-CoV-2 reduction by local treatment: A plasma technology application? Plasma Process. Polym. 2023, 20, 2200196. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Wu, Y.; Bashir, M.A.; Shao, C.; Huang, Q. Application of NaCl in Cold Atmospheric Plasma Jet and Plasma-Activated Solution to Enhance Virus Inactivation. Plasma Med. 2023, 13, 47–63. [Google Scholar] [CrossRef]
- Armenise, V.; Veronico, V.; Cosmai, S.; Benedetti, D.; Gristina, R.; Favia, P.; Fracassi, F.; Sardella, E. The effect of different cold atmospheric plasma sources and treatment modalities on the generation of reactive oxygen and nitrogen species in water. Plasma Process. Polym. 2023, 20, 2200182. [Google Scholar] [CrossRef]
- Shaji, M.; Rabinovich, A.; Surace, M.; Sales, C.; Fridman, A. Physical Properties of Plasma-Activated Water. Plasma 2023, 6, 45–57. [Google Scholar] [CrossRef]
- Wong, K.S.; Chew, N.S.L.; Low, M.; Tan, M.K. Plasma-Activated Water: Physicochemical Properties, Generation Techniques, and Applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Liu, Z.; Tantai, X.; Wang, S.; Pang, B.; Gao, Y.; Zhang, F.; Zhao, X.; Xu, D.; Soni, A.; Mai-Prochnow, A.; et al. Controlling plasma-activated solution chemistry for targeted cancer cell death. Plasma Process. Polym. 2023, 20, e2300029. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, H.; Qi, Z.; Liu, D. Biological and Chemical Reactivities of Plasma-Activated Water Prepared at Different Temperatures. Plasma Chem. Plasma Process. 2024, 44, 393–410. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, T.; Zhou, R. Pulsed Discharges for Water Activation and Plasma-Activated Water Production. In Pulsed Discharge Plasmas: Characteristics and Applications; Shao, T., Zhang, C., Eds.; Springer Series in Plasma Science and Technology; Springer Nature: Singapore, 2023; pp. 325–347. ISBN 978-981-9911-41-7. [Google Scholar]
- Veronico, V.; Morelli, S.; Piscioneri, A.; Gristina, R.; Casiello, M.; Favia, P.; Armenise, V.; Fracassi, F.; De Bartolo, L.; Sardella, E. Anticancer Effects of Plasma-Treated Water Solutions from Clinically Approved Infusion Liquids Supplemented with Organic Molecules. ACS Omega 2023, 8, 33723–33736. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wei, Z.; Huang, Y.; Xu, H.; Li, B.; Zhang, H.; Xie, K.; Shao, M. Enhancement of the drug sensitization of cancer cells by plasma-activated saline. Plasma Process. Polym. 2023, 20, e2300001. [Google Scholar] [CrossRef]
- Xu, S.; Jing, X.; Zhang, J.; Liu, D.; Zhang, H.; Wang, Z.; Chen, M.; Zhou, R.; Xu, Y.; Xu, H.; et al. Anticancer effects of DBD plasma-activated saline within different discharge modes. J. Phys. Appl. Phys. 2023, 56, 345205. [Google Scholar] [CrossRef]
- Kutasi, K.; Bencs, L.; Tóth, Z.; Milošević, S. The role of metals in the deposition of long-lived reactive oxygen and nitrogen species into the plasma-activated liquids. Plasma Process. Polym. 2023, 20, 2200143. [Google Scholar] [CrossRef]
- Guo, L.; Niyazi, G.; Huang, L.; Zhao, P.; Wang, Z.; Lin, J.; Li, T.; Li, G.; Song, L.; Liu, D.; et al. Inactivation effects of the mist nebulized with plasma-activated air on Pseudomonas aeruginosa through the simulated respiratory tract. Plasma Process. Polym. 2023, 20, e2200204. [Google Scholar] [CrossRef]
- Saadawy, H.; Fathi, E.M.; Elsayed, I.; Anzour, J.S.; Zaki, A.; El Shaer, M.; Emara, M. Treatment of hepatocellular carcinoma with in situ generated plasma-activated air-driven water mist. Plasma Process. Polym. 2023, 20, e2200234. [Google Scholar] [CrossRef]
- Chakraborty, A.; Adhikary, S.; Bhattacharya, S.; Dutta, S.; Chatterjee, S.; Banerjee, D.; Ganguly, A.; Rajak, P. Pharmaceuticals and Personal Care Products as Emerging Environmental Contaminants: Prevalence, Toxicity, and Remedial Approaches. ACS Chem. Health Saf. 2023, 30, 362–388. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; de A. Dutra, A.R.; Alves, T.C.; Bianchet, R.T.; Rambo, A.A.; Debacher, N.A. Evaluation of antimicrobial sensitivity to tetracycline exposed to non-thermal plasma. Quím. Nova 2023, 46, 236–240. [Google Scholar] [CrossRef]
- LU, F.; ZHOU, J.; WU, Z. Degradation of antibiotic contaminants from water by gas–liquid underwater discharge plasma. Plasma Sci. Technol. 2023, 25, 035506. [Google Scholar] [CrossRef]
- El Shaer, M.; Eldaly, M.; Heikal, G.; Sharaf, Y.; Diab, H.; Mobasher, M.; Rousseau, A. Antibiotics Degradation and Bacteria Inactivation in Water by Cold Atmospheric Plasma Discharges Above and Below Water Surface. Plasma Chem. Plasma Process. 2020, 40, 971–983. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; David, V.; Medvedovici, A.; Bradu, C.; Parvulescu, V.I. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011, 45, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
- Terefinko, D.; Caban, M.; Motyka-Pomagruk, A.; Babinska, W.; Pohl, P.; Jamroz, P.; Cyganowski, P.; Sledz, W.; Lojkowska, E.; Stepnowski, P.; et al. Removal of clinically significant antibiotics from aqueous solutions by applying unique high-throughput continuous-flow plasma pencil and plasma brush systems. Chem. Eng. J. 2023, 452, 139415. [Google Scholar] [CrossRef]
- Liang, C.; Fang, C.; Wang, H.; Bashir, M.A.; Huang, Q. Removal of Ampicillin Using Cold Atmospheric-Pressure Plasma Jet and Its Plasma-Activated Water. Plasma Med. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Bilea, F.; Tian, T.; Magureanu, M.; Rabat, H.; Antoissi, M.-A.; Aubry, O.; Hong, D. Removal of a mixture of antibiotics in water using nonthermal plasma. Plasma Process. Polym. 2023, 20, 2300020. [Google Scholar] [CrossRef]
- Hu, S.; Yan, W.; Yu, J.; Zhu, B.; Lan, Y.; Xi, W.; Xu, Z.; Han, W.; Cheng, C. Degradation of sulfamethoxazole in water by dielectric barrier discharge plasma jet: Influencing parameters, degradation pathway, toxicity evaluation. Plasma Sci. Technol. 2023, 25, 035510. [Google Scholar] [CrossRef]
- Fang, C.; Shao, C.; Wang, S.; Wu, Y.; Liu, C.; Huang, Q. Simultaneous removal of levofloxacin and sulfadiazine in water by dielectric barrier discharge (DBD) plasma: Enhanced performance and degradation mechanism. Process Saf. Environ. Prot. 2023, 171, 459–469. [Google Scholar] [CrossRef]
- Nandy, N.; Pasupathi, A.; Subramaniam, Y.; Nachimuthu, S. Eliminating ciprofloxacin antibiotic contamination from water with a novel submerged thermal plasma technology. Chemosphere 2023, 326, 138470. [Google Scholar] [CrossRef]
- Mosaka, T.B.M.; Unuofin, J.O.; Daramola, M.O.; Tizaoui, C.; Iwarere, S.A. Inactivation of antibiotic-resistant bacteria and antibiotic-resistance genes in wastewater streams: Current challenges and future perspectives. Front. Microbiol. 2023, 13, 1100102. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.; Li, J.; Xu, P.; Yang, C.; Wang, P.; Zhang, G. Introduction of oxygen vacancy to Bi2Mn4O10 supported by nickel foam for 1O2 dominated metronidazole degradation under dielectric barrier discharge plasma. Appl. Catal. B Environ. 2023, 328, 122518. [Google Scholar] [CrossRef]
- Sarangapani, C.; Ziuzina, D.; Behan, P.; Boehm, D.; Gilmore, B.F.; Cullen, P.J.; Bourke, P. Degradation kinetics of cold plasma-treated antibiotics and their antimicrobial activity. Sci. Rep. 2019, 9, 3955. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, F.; O’Neill, L.; Bourke, P. Recent Developments in the Use of Plasma in Medical Applications. Plasma 2024, 7, 284-299. https://doi.org/10.3390/plasma7020016
O’Neill F, O’Neill L, Bourke P. Recent Developments in the Use of Plasma in Medical Applications. Plasma. 2024; 7(2):284-299. https://doi.org/10.3390/plasma7020016
Chicago/Turabian StyleO’Neill, Fiona, Liam O’Neill, and Paula Bourke. 2024. "Recent Developments in the Use of Plasma in Medical Applications" Plasma 7, no. 2: 284-299. https://doi.org/10.3390/plasma7020016
APA StyleO’Neill, F., O’Neill, L., & Bourke, P. (2024). Recent Developments in the Use of Plasma in Medical Applications. Plasma, 7(2), 284-299. https://doi.org/10.3390/plasma7020016





