Tailoring Black TiO2 Thin Films: Insights from Hollow Cathode Hydrogen Plasma Treatment Duration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pristine Anatase TiO2 Thin Films Growth
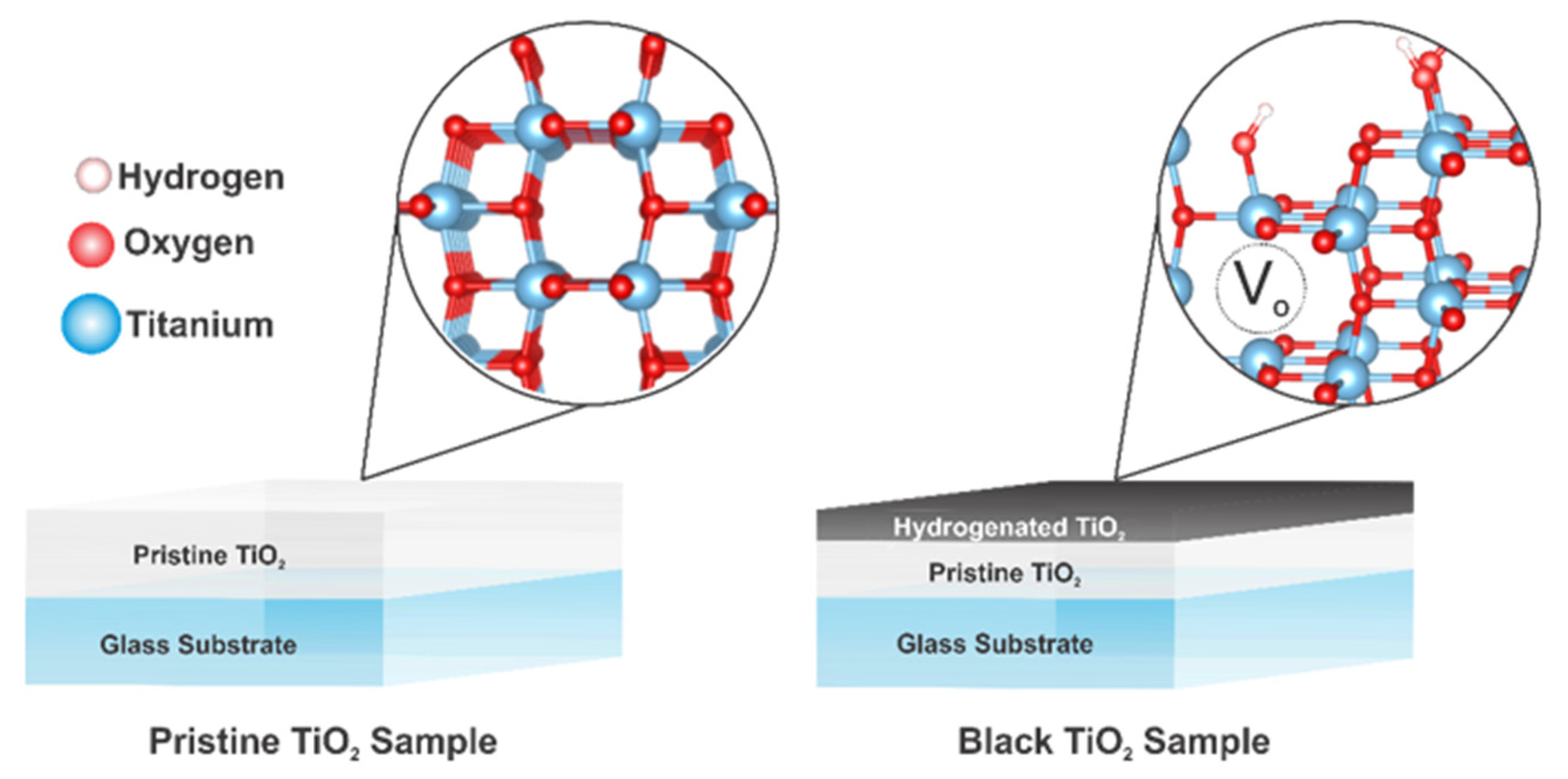
2.3. Hydrogenation Process
2.4. Thin Film Characterization
2.4.1. Morphological Analysis
2.4.2. Crystalline Structure and Chemical Analysis
2.4.3. Optical and Electric Analysis
2.4.4. Wettability and Surface Energy Analysis
2.4.5. Photocatalytic Activity Evaluation
3. Results and Discussion
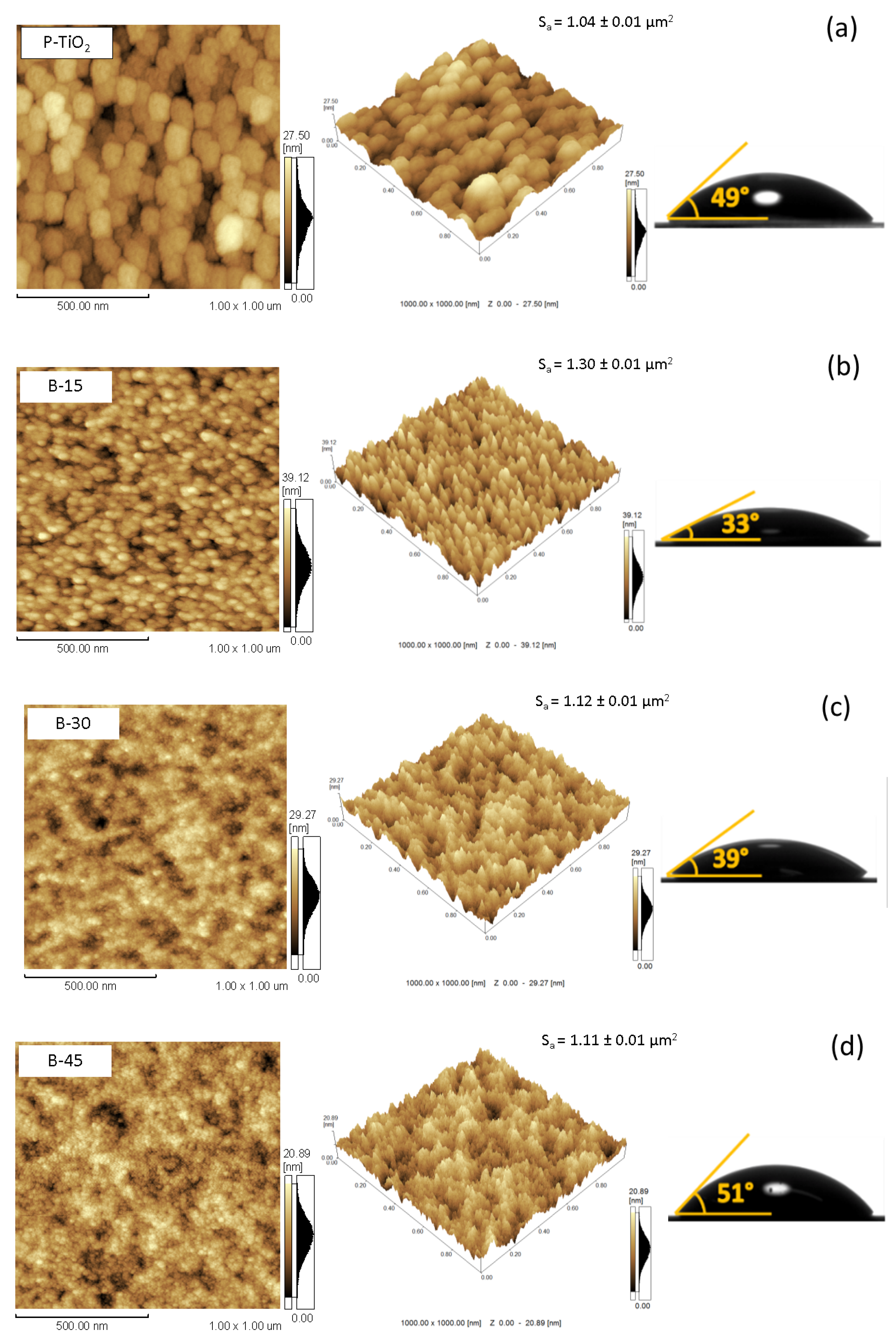
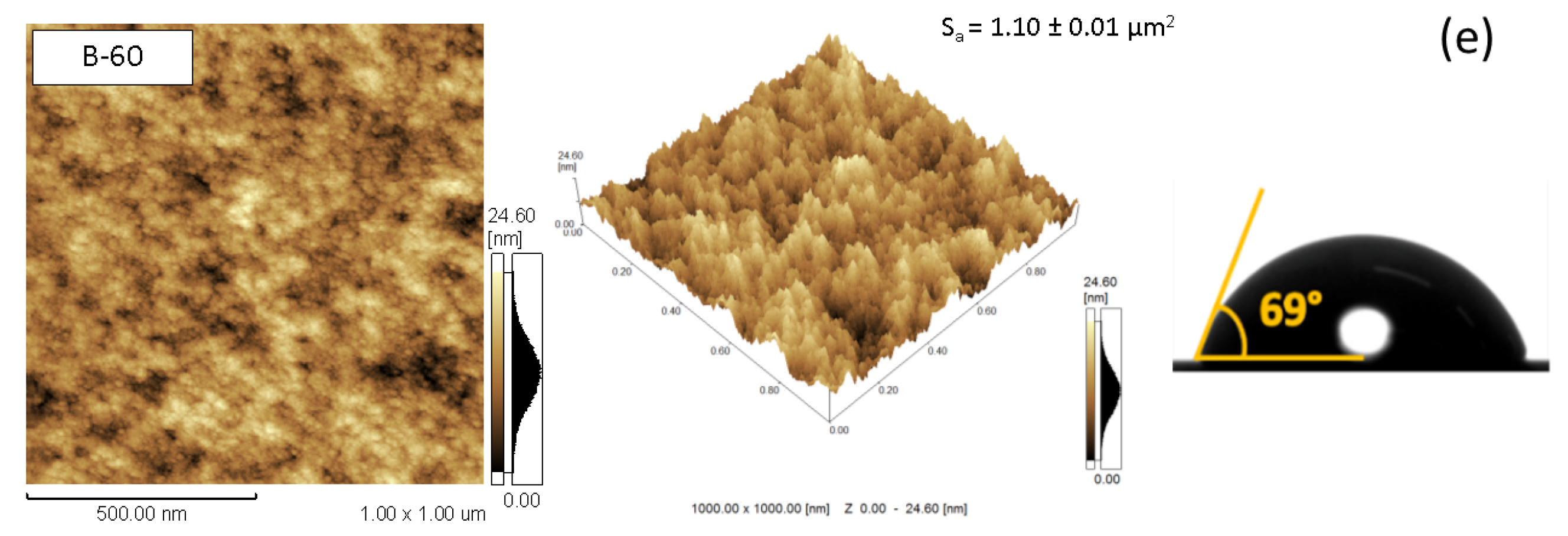
3.1. Morphological Characteristics
3.2. Wettability and Surface Energy Characteristics
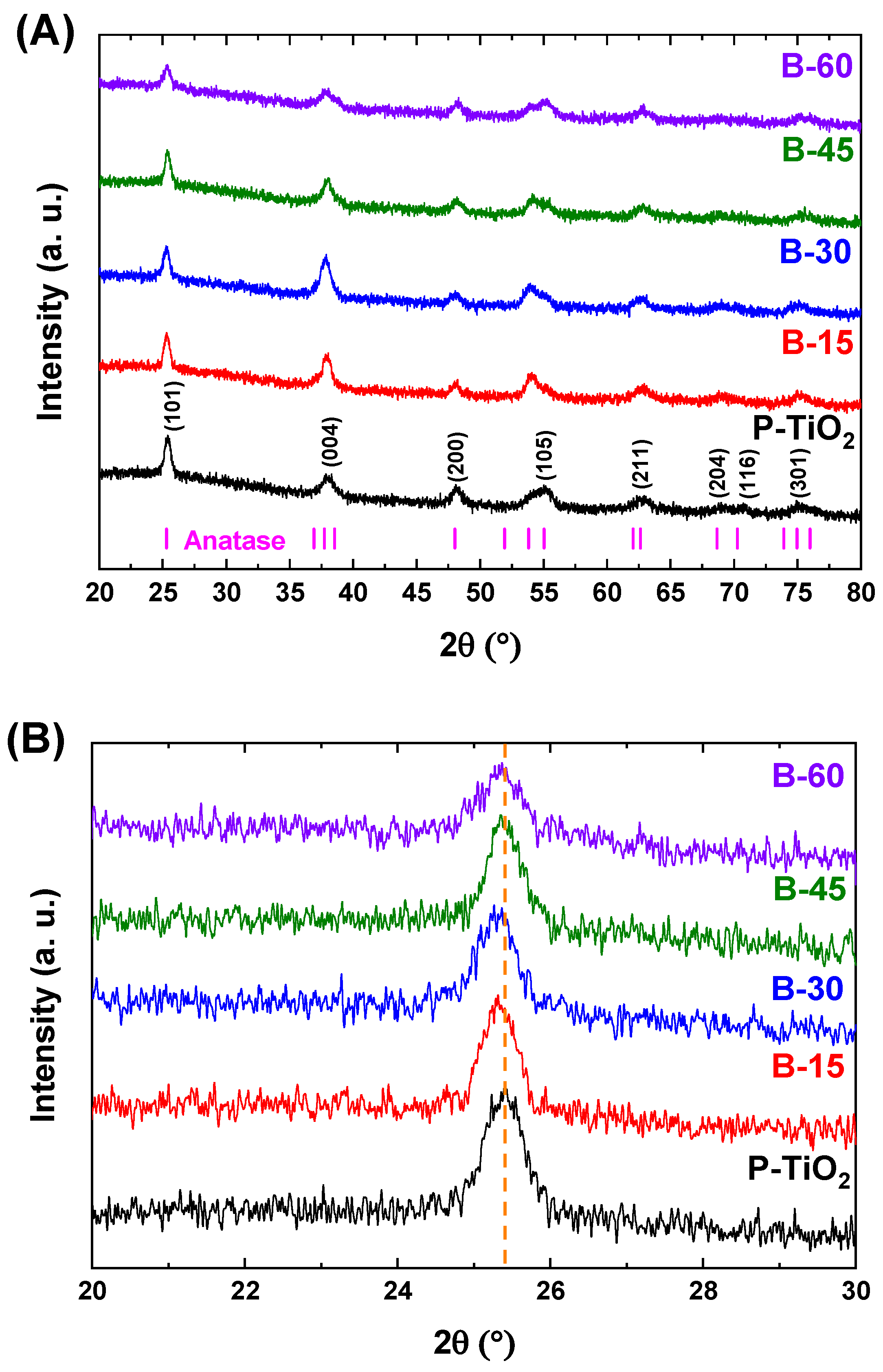
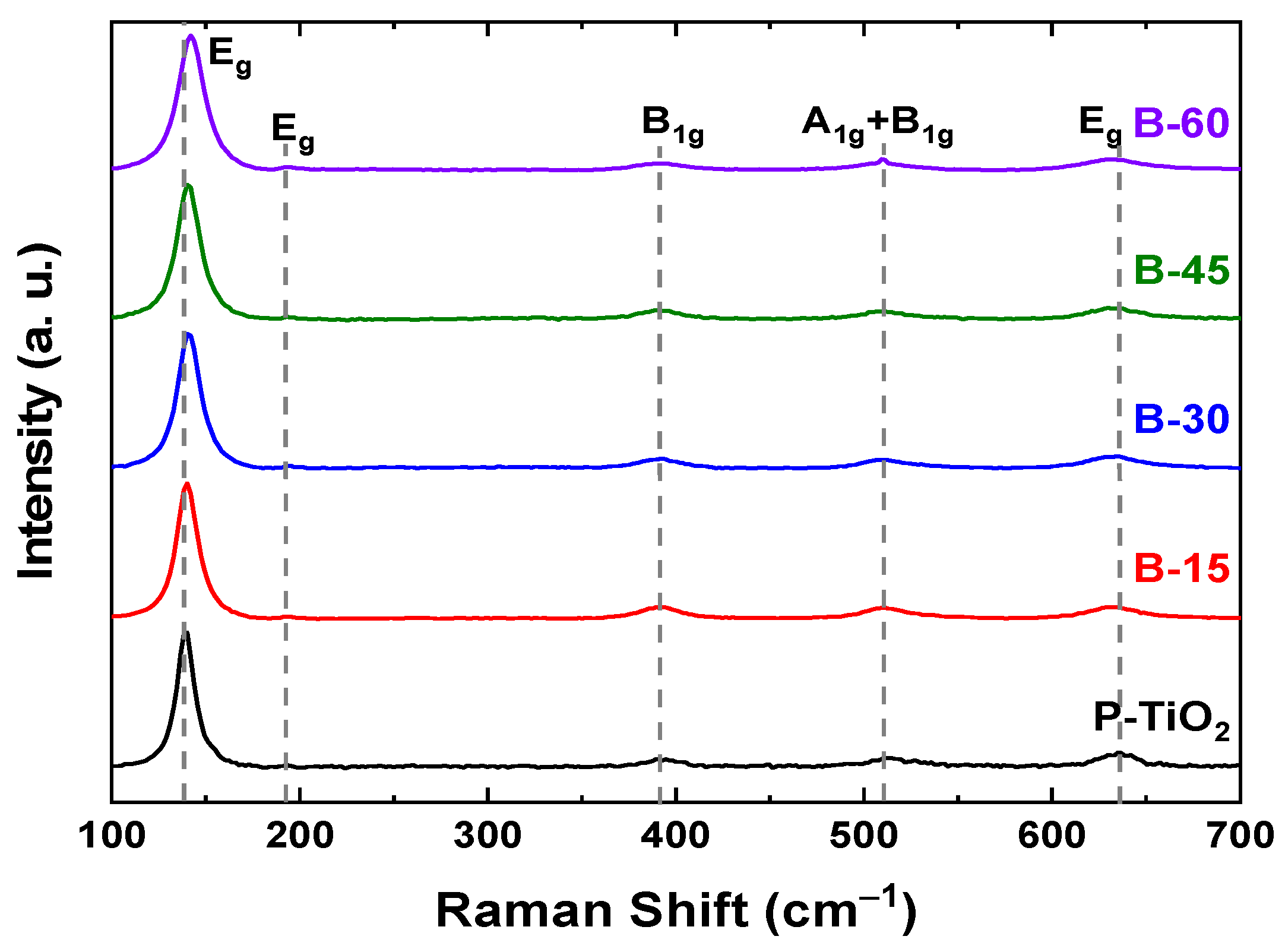
3.3. Microstructural Characteristics
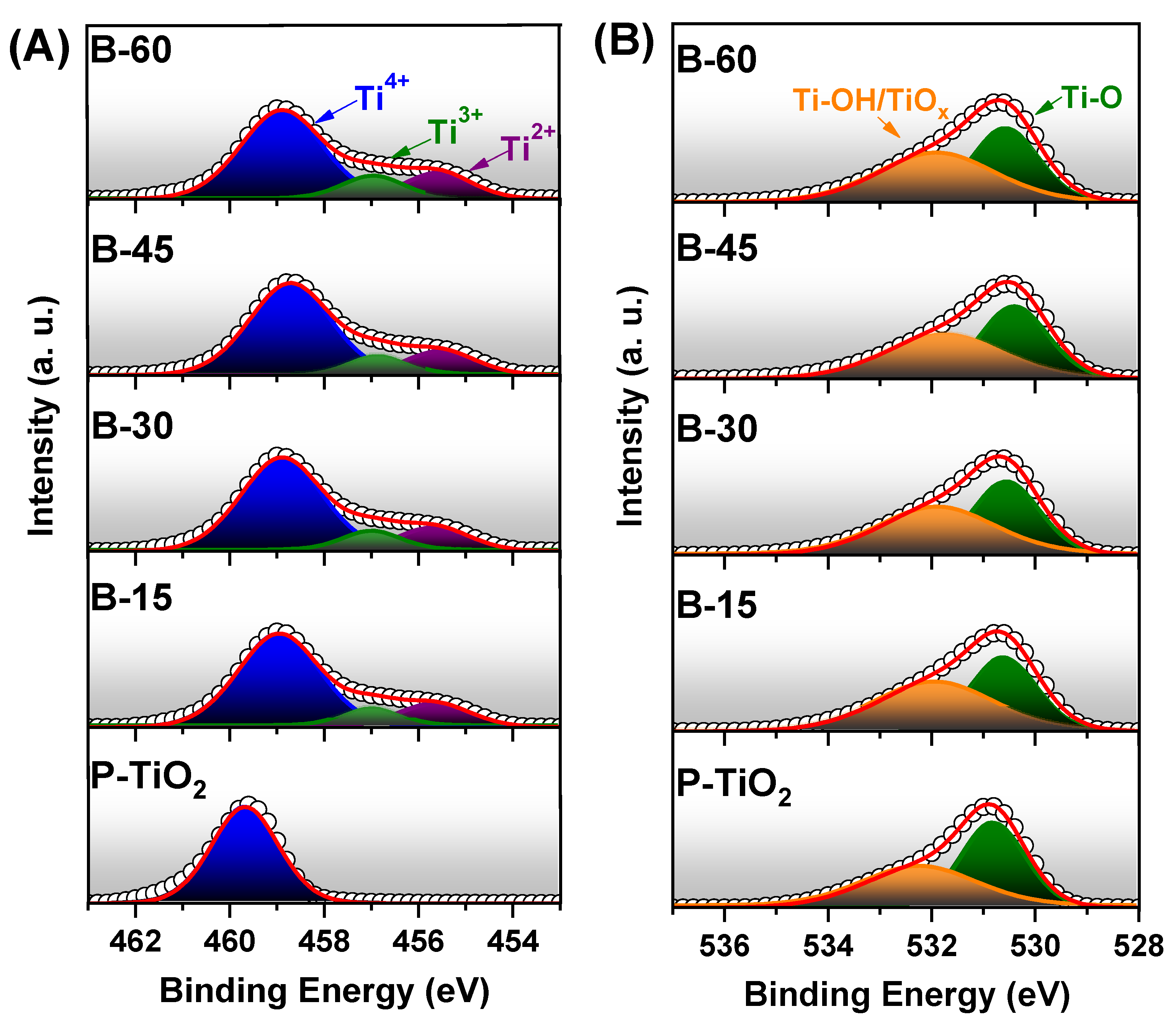
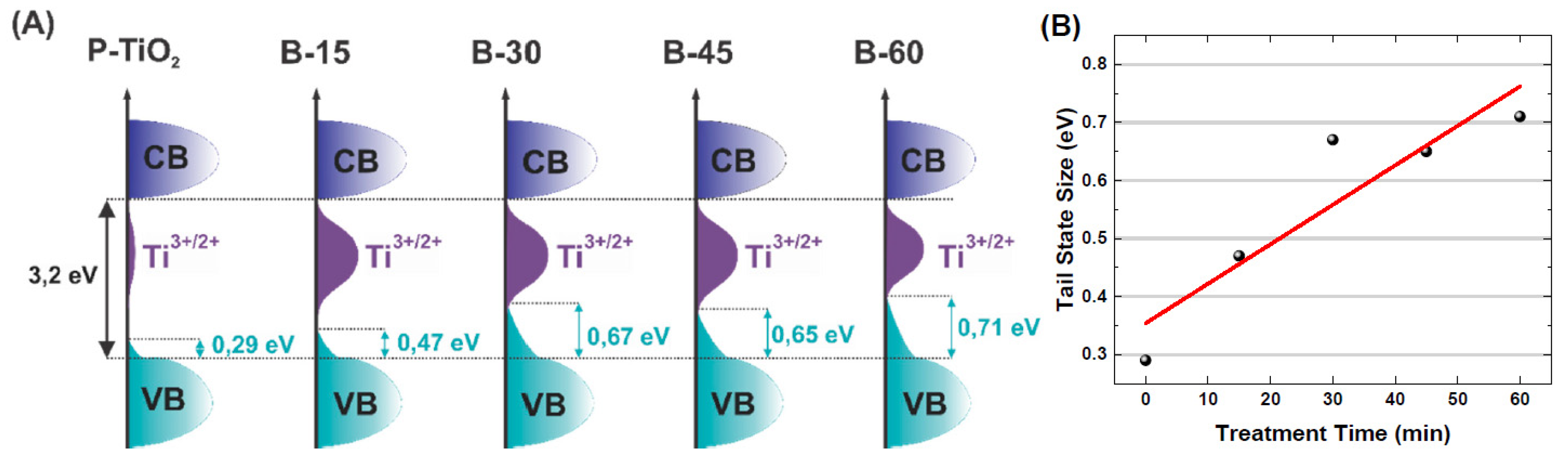
3.4. Chemical Characteristics
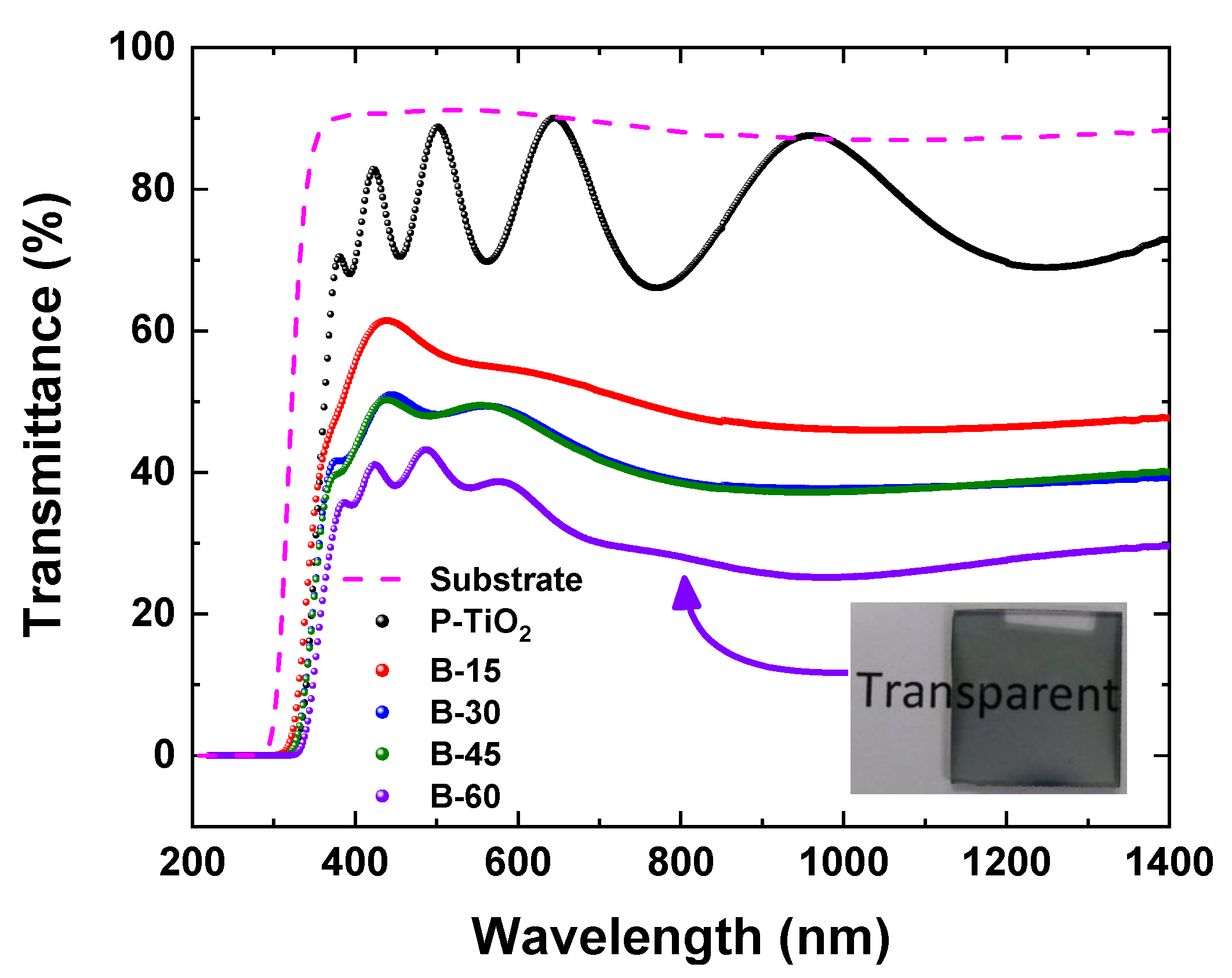
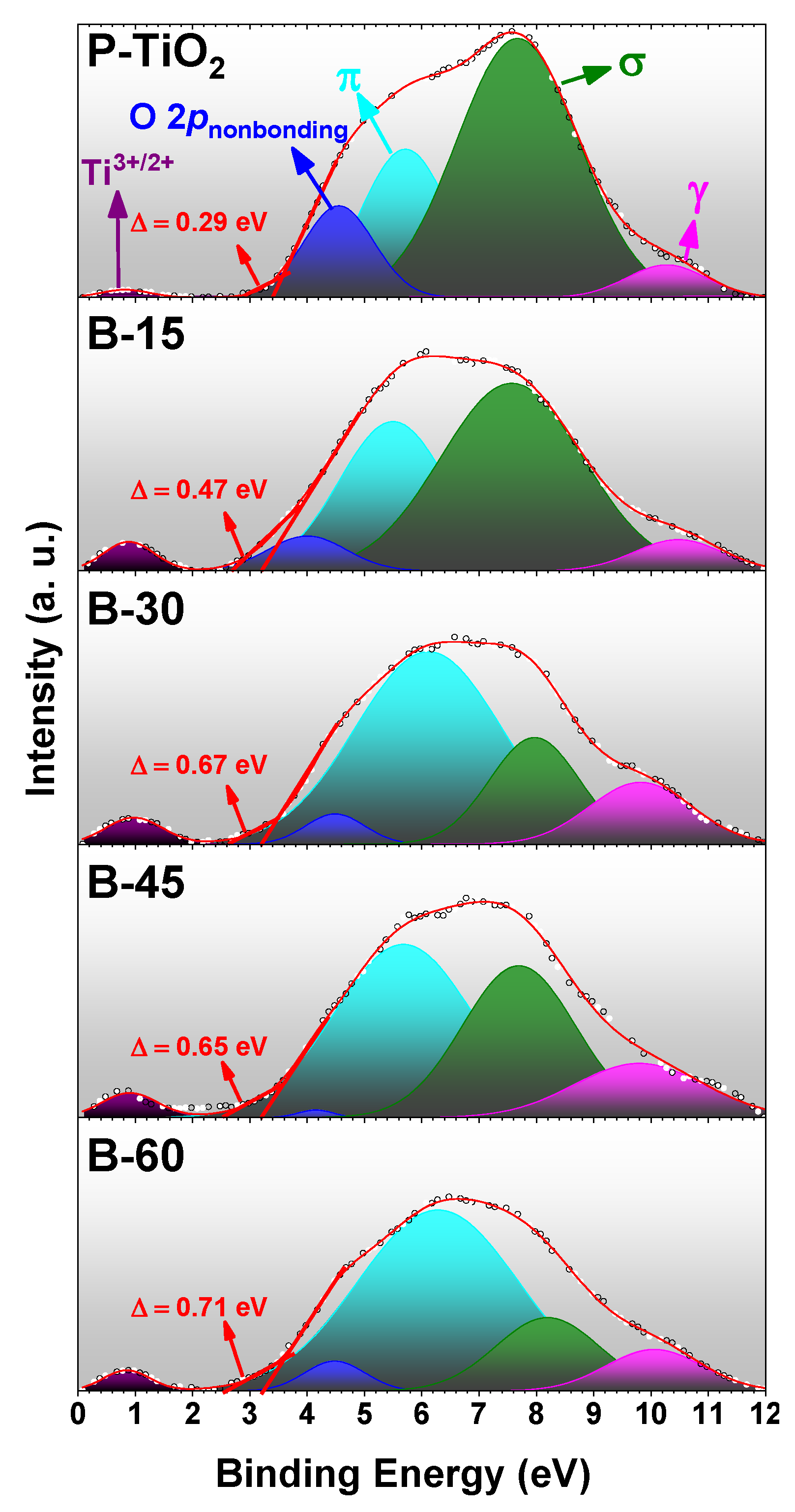
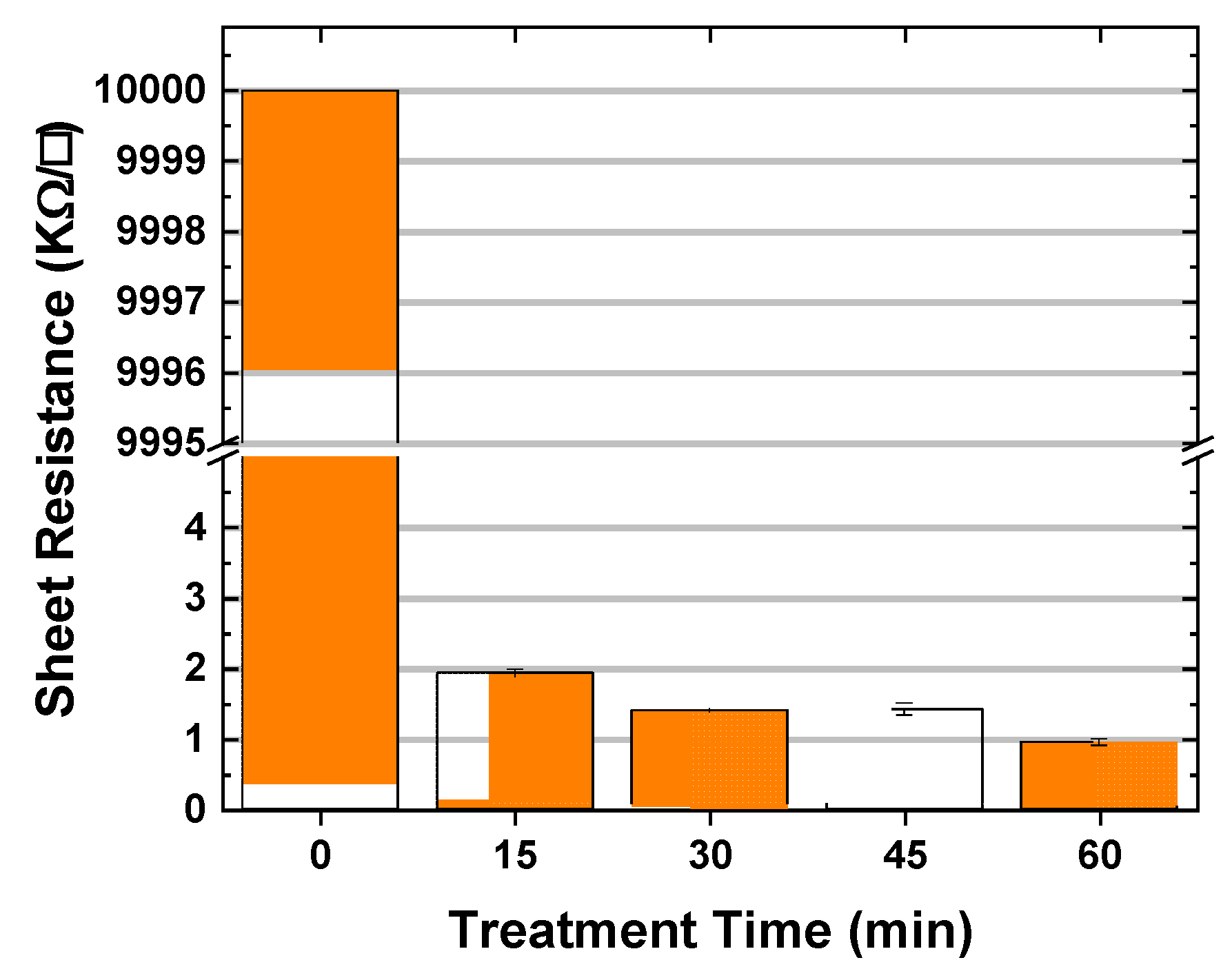
3.5. Optical and Electric Characteristics
3.6. Photocatalytic Degradation of Methylene Blue Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solak, E.K.; Irmak, E. Advances in organic photovoltaic cells: A comprehensive review of materials, technologies, and performance. RSC Adv. 2023, 19, 12244–12269. [Google Scholar] [CrossRef]
- Querebillo, C.J. A Review on Nano Ti-Based Oxides for Dark and Photocatalysis: From Photoinduced Processes to Bioimplant Applications. Nanomaterials 2023, 13, 982. [Google Scholar] [CrossRef]
- Wang, Y.; Suzuki, H.; Xie, J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.S.; Fraga, M.A.; Santos, L.V.; Massi, M.; Maciel, H.S. Nanostructured Thin Films Based on TiO2 and/or SiC for Use in Photoelectrochemical Cells: A Review of the Material Characteristics, Synthesis and Recent Applications. Mater. Sci. Semicond. Process. 2015, 29, 56–68. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Peng, C.; Xiang, Y.; Chen, H. Enhanced Photocatalytic Hydrogen Production over Conjugated Polymer/Black TiO2 Hybrid: The Impact of Constructing Active Defect States. Appl. Surf. Sci. 2019, 465, 288–296. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.M.; Lu, G.Q. Titania-Based Photocatalysts—Crystal Growth, Doping and Heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Nair, R.V.; Gayathri, P.K.; Gummaluri, V.S.; Nambissan, P.M.G.; Vijayan, C. Large Bandgap Narrowing in Rutile TiO2 Aimed towards Visible Light Applications and Its Correlation with Vacancy-Type Defects History and Transformation. J. Phys. D Appl. Phys. 2018, 51, 045107. [Google Scholar] [CrossRef]
- Xu, J.; Huang, J.; Zhang, S.; Hong, Z.; Huang, F. Understanding the Surface Reduction of Nano Rutile and Anatase: Selective Breaking of Ti-O Bonds. Mater. Res. Bull. 2020, 121, 110617. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 Nanomaterials: A Review of Recent Advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Godoy, A., Jr.; Pereira, A.; Gomes, M.; Fraga, M.; Pessoa, R.; Leite, D.; Petraconi, G.; Nogueira, A.; Wender, H.; Miyakawa, W.; et al. Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts 2020, 10, 282. [Google Scholar] [CrossRef]
- Nair, P.R.; Ramirez, C.R.S.; Pinilla, M.A.G.; Krishnan, B.; Avellaneda, D.A.; Pelaes, R.F.C.; Shaji, S. Black titanium dioxide nanocolloids by laser irradiation in liquids for visible light photo-catalytic/electrochemical applications. Appl. Surf. Sci. 2023, 623, 157096. [Google Scholar] [CrossRef]
- Hu, B.; Liang, C.-H.; Yang, F.; Chen, J.; Jin, W.; Xing, H.; Cai, L.; Yang, S.; Liu, K.Z.; Kleyn, A.W.; et al. TiO2 Powder Modified by Plasma Afterglow: A Correlation between Active Species, Microstructure, and Optical Properties. Mater. Lett. 2020, 268, 127577. [Google Scholar] [CrossRef]
- Luminita, A.; Alexandru, E. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. 2020, 8, 565489. [Google Scholar] [CrossRef]
- Jian, J.; Sun, Y.; Chen, F.; Wu, R.; Wei, S. Black and Yellow Anatase Titania Formed by (H,N)-Doping: Strong Visible-Light Absorption and Enhanced Visible-Light Photocatalysis. Dalt. Trans. 2014, 44, 1534–1538. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, B.; Yang, J.; Wu, J.; Zhai, W.; Yang, B.; Liu, M. Rapid Preparation of TiO2-x and Its Photocatalytic Oxidation for Arsenic Adsorption under Visible Light. Langmuir 2020, 36, 3853–3861. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-Doped Black Titania with Very High Solar Absorption and Excellent Photocatalysis Enhanced by Localized Surface Plasmon Resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Z.; Zhou, Q.; Meng, B.; Meng, X.; Qiu, J. Highly Efficient Low-Temperature Plasma-Assisted Modification of TiO2 Nanosheets with Exposed {001} Facets for Enhanced Visible-Light Photocatalytic Activity. Chem. A Eur. J. 2014, 20, 14763–14770. [Google Scholar] [CrossRef]
- Hu, L.; Song, X.; Shan, X.; Zhao, X.; Guo, F.; Xiao, P. Visible Light-Activated Self-Recovery Hydrophobic CeO2/Black TiO2 Coating Prepared Using Air Plasma Spraying. ACS Appl. Mater. Interfaces 2019, 11, 37209–37215. [Google Scholar] [CrossRef]
- Zhai, M.; Liu, Y.; Huang, J.; Wang, Y.; Chen, K.; Fu, Y.; Li, H. Efficient Suspension Plasma Spray Fabrication of Black Titanium Dioxide Coatings with Visible Light Absorption Performances. Ceram. Int. 2019, 45, 930–935. [Google Scholar] [CrossRef]
- Pylnev, M.; Wong, M.S. Comparative Study of Photocatalytic Deactivation of Pure and Black Titania Thin Films. J. Photochem. Photobiol. A Chem. 2019, 378, 125–130. [Google Scholar] [CrossRef]
- Islam, S.Z.; Reed, A.; Nagpure, S.; Wanninayake, N.; Browning, J.F.; Strzalka, J.; Kim, D.Y.; Rankin, S.E. Hydrogen Incorporation by Plasma Treatment Gives Mesoporous Black TiO2 Thin Films with Visible Photoelectrochemical Water Oxidation Activity. Microporous Mesoporous Mater. 2018, 261, 35–43. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A Hydrogenated Black TiO2 Coating with Excellent Effects for Photothermal Therapy of Bone Tumor and Bone Regeneration. Mater. Sci. Eng. C 2019, 102, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Hao, B.; Wang, D.; Chen, G.; Markweg, E.; Albrecht, A.; Schaaf, P. Understanding the Fast Lithium Storage Performance of Hydrogenated TiO2 Nanoparticles. J. Mater. Chem. A 2013, 1, 14507. [Google Scholar] [CrossRef]
- Teng, F.; Li, M.; Gao, C.; Zhang, G.; Zhang, P.; Wang, Y.; Chen, L.; Xie, E. Preparation of Black TiO2 by Hydrogen Plasma Assisted Chemical Vapor Deposition and Its Photocatalytic Activity. Appl. Catal. B Environ. 2014, 148–149, 339–343. [Google Scholar] [CrossRef]
- Ren, W.; Yan, Y.; Zeng, L.; Shi, Z.; Gong, A.; Schaaf, P.; Wang, D.; Zhao, J.; Zou, B.; Yu, H.; et al. A Near Infrared Light Triggered Hydrogenated Black TiO2 for Cancer Photothermal Therapy. Adv. Healthc. Mater. 2015, 4, 1526–1536. [Google Scholar] [CrossRef]
- Godoy, A.; Carlucci, F.G.; Leite, D.M.G.; Miyakawa, W.; Pereira, A.L.J.; Massi, M.; da Silva Sobrinho, A.S. Plasma Nanotexturing of Amorphous Carbon Films by Reactive Ion Etching. Surf. Coatings Technol. 2018, 354, 153–160. [Google Scholar] [CrossRef]
- Duarte, D.A.; Massi, M.; Da Silva Sobrinho, A.S. Comparison between Conventional and Hollow Cathode Magnetron Sputtering Systems on the Growing of Titanium Dioxide Thin Films: A Correlation between the Gas Discharge and Film Formation. EPJ Appl. Phys. 2011, 54, 20801. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore Suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Axis, K. Peak Fitting in XPS; Casa XPS: Pasadena, CA, USA, 2006. [Google Scholar]
- Gomes, L.E.; Da Silva, M.F.; Gonçalves, R.V.; Machado, G.; Alcantara, G.B.; Caires, A.R.L.; Wender, H. Synthesis and Visible-Light-Driven Photocatalytic Activity of Ta4+ Self-Doped Gray Ta2O5 Nanoparticles. J. Phys. Chem. C 2018, 122, 6014–6025. [Google Scholar] [CrossRef]
- Nogueira, A.C.; Gomes, L.E.; Ferencz, J.A.P.; Rodrigues, J.E.F.S.; Goncąlves, R.V.; Wender, H. Improved Visible Light Photoactivity of CuBi2O4/CuO Heterojunctions for Photodegradation of Methylene Blue and Metronidazole. J. Phys. Chem. C 2019, 123, 25680–25690. [Google Scholar] [CrossRef]
- Baik, S.J.; Jang, J.H.; Lee, C.H.; Cho, W.Y.; Lim, K.S. Highly Textured and Conductive Undoped ZnO Film Using Hydrogen Post-Treatment. Appl. Phys. Lett. 1997, 70, 3516–3518. [Google Scholar] [CrossRef]
- Wang, F.-H.; Chao, J.-C.; Liu, H.-W.; Liu, F.-J. Physical Properties of TiO2-Doped Zinc Oxide Thin Films: Influence of Plasma Treatment in H2 and/or Ar Gas Ambient. Vacuum 2017, 140, 155–160. [Google Scholar] [CrossRef]
- Han, J.; Cheng, Y.; Tu, W.; Zhan, T.-Y.; Cheng, Y. The Black and White Coatings on Ti-6Al-4V Alloy or Pure Titanium by Plasma Electrolytic Oxidation in Concentrated Silicate Electrolyte. Appl. Surf. Sci. 2018, 428, 684–697. [Google Scholar] [CrossRef]
- Hannula, M.; Ali-Löytty, H.; Lahtonen, K.; Sarlin, E.; Saari, J.; Valden, M. Improved Stability of Atomic Layer Deposited Amorphous TiO2 Photoelectrode Coatings by Thermally Induced Oxygen Defects. Chem. Mater. 2018, 30, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Lepcha, A.; Maccato, C.; Mettenbörger, A.; Andreu, T.; Mayrhofer, L.; Walter, M.; Olthof, S.; Ruoko, T.-P.; Klein, A.; Moseler, M.; et al. Electrospun Black Titania Nanofibers: Influence of Hydrogen Plasma-Induced Disorder on the Electronic Structure and Photoelectrochemical Performance. J. Phys. Chem. C 2015, 119, 18835–18842. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, H.; Wang, W.; Zhang, Y.; Li, Z.; Wu, X.; Yu, X.; Zhou, W. Fabrication of 3D Mesoporous Black TiO2/MoS2/TiO2 Nanosheets for Visible-Light-Driven Photocatalysis. ChemSusChem 2016, 9, 1118–1124. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Watthanaphanit, A.; Ishizaki, T.; Saito, N. Water-Plasma-Assisted Synthesis of Black Titania Spheres with Efficient Visible-Light Photocatalytic Activity. Phys. Chem. Chem. Phys. 2015, 17, 13794–13799. [Google Scholar] [CrossRef]
- Kibasomba, P.M.; Dhlamini, S.; Maaza, M.; Liu, C.P.; Rashad, M.M.; Rayan, D.A.; Mwakikunga, B.W. Strain and Grain Size of TiO2 Nanoparticles from TEM, Raman Spectroscopy and XRD: The Revisiting of the Williamson-Hall Plot Method. Results Phys. 2018, 9, 628–635. [Google Scholar] [CrossRef]
- Tudu, B.K.; Gupta, V.; Kumar, A.; Sinhamahapatra, A. Freshwater Production via Efficient Oil-Water Separation and Solar-Assisted Water Evaporation Using Black Titanium Oxide Nanoparticles. J. Colloid Interface Sci. 2020, 566, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, B.; Xue, J.; Wu, J.; Chang, J.; Wu, C. Defective Black Nano-Titania Thermogels for Cutaneous Tumor-Induced Therapy and Healing. Nano Lett. 2019, 19, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Saied, S.; Sullivan, J.; Choudhury, T.; Pearce, C. A Comparison of Ion and Fast Atom Beam Reduction in TiO2. Vacuum 1988, 38, 917–922. [Google Scholar] [CrossRef]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black Hydroxylated Titanium Dioxide Prepared via Ultrasonication with Enhanced Photocatalytic Activity. Sci. Rep. 2015, 5, srep11712. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and N-Type Doped TiO2: Nature of Ti3+ Species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]

| Sample | Thickness (nm) | Surface Energy (mN/m) |
|---|---|---|
| P-TiO2 B-15 B-30 B-45 B-60 | 342 ± 47 309 ± 10 291 ± 17 265 ± 21 202 ± 12 | 61.3 ± 0.4 70.0 ± 0.2 67.0 ± 0.2 60.0 ± 0.3 47.0 ± 0.4 |
| Sample | Eg Peak Center (cm−1) | Eg FWHM (cm−1) |
|---|---|---|
| P-TiO2 B-15 B-30 B-45 B-60 | 139.44 140.26 141.15 140.88 142.29 | 10.77 13.40 14.87 17.32 16.18 |
| Sample | Ti 2p3/2 | O 1s | |
|---|---|---|---|
| Ti3+/2+ | Ti-OH/TiOx | Ti-O | |
| P-TiO2 B-15 B-30 B-45 B-60 | 0 26.41 26.87 27.76 30.46 | 45.47 52.98 52.52 52.13 53.23 | 54.53 47.02 47.48 47.87 46.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy-Junior, A.; Pereira, A.; Damasceno, B.; Horta, I.; Gomes, M.; Leite, D.; Miyakawa, W.; Baldan, M.; Massi, M.; Pessoa, R.; et al. Tailoring Black TiO2 Thin Films: Insights from Hollow Cathode Hydrogen Plasma Treatment Duration. Plasma 2023, 6, 362-378. https://doi.org/10.3390/plasma6020025
Godoy-Junior A, Pereira A, Damasceno B, Horta I, Gomes M, Leite D, Miyakawa W, Baldan M, Massi M, Pessoa R, et al. Tailoring Black TiO2 Thin Films: Insights from Hollow Cathode Hydrogen Plasma Treatment Duration. Plasma. 2023; 6(2):362-378. https://doi.org/10.3390/plasma6020025
Chicago/Turabian StyleGodoy-Junior, Armstrong, André Pereira, Barbara Damasceno, Isabela Horta, Marcilene Gomes, Douglas Leite, Walter Miyakawa, Maurício Baldan, Marcos Massi, Rodrigo Pessoa, and et al. 2023. "Tailoring Black TiO2 Thin Films: Insights from Hollow Cathode Hydrogen Plasma Treatment Duration" Plasma 6, no. 2: 362-378. https://doi.org/10.3390/plasma6020025
APA StyleGodoy-Junior, A., Pereira, A., Damasceno, B., Horta, I., Gomes, M., Leite, D., Miyakawa, W., Baldan, M., Massi, M., Pessoa, R., & Sobrinho, A. d. S. (2023). Tailoring Black TiO2 Thin Films: Insights from Hollow Cathode Hydrogen Plasma Treatment Duration. Plasma, 6(2), 362-378. https://doi.org/10.3390/plasma6020025








