Plasma Activation as a Powerful Tool for Selective Modification of Cellulose Fibers towards Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Cellulose Treatment
2.3. Sample Characterization
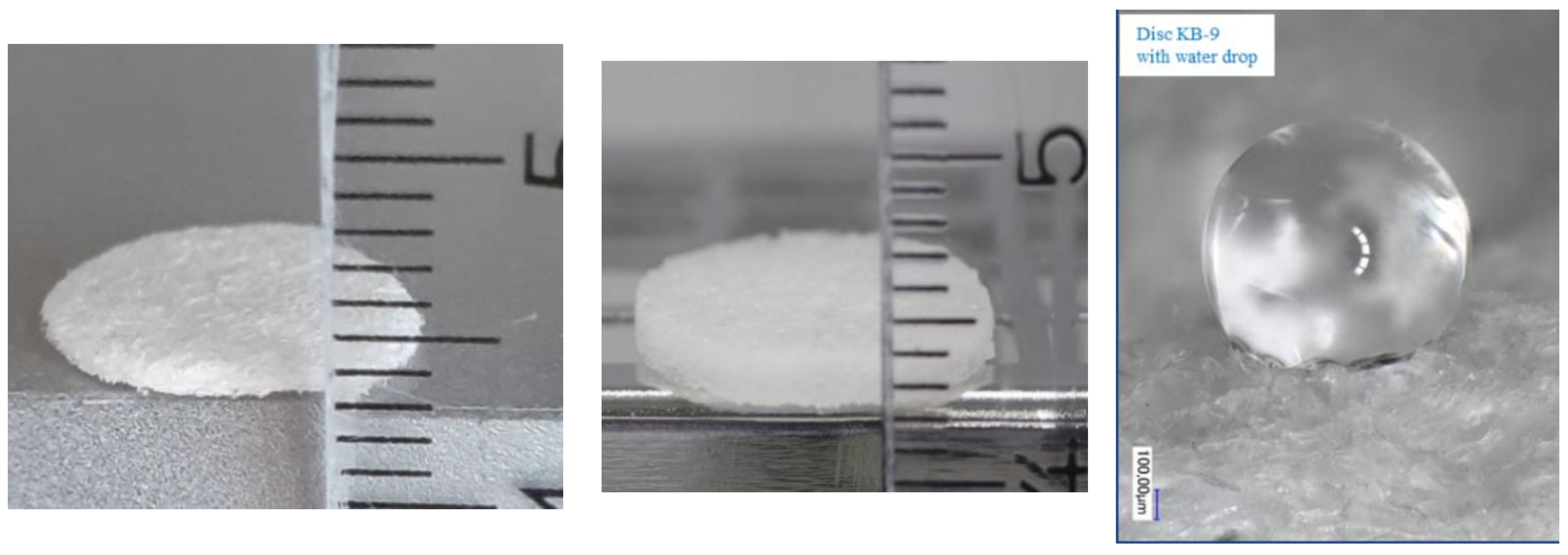
2.4. Swelling Test
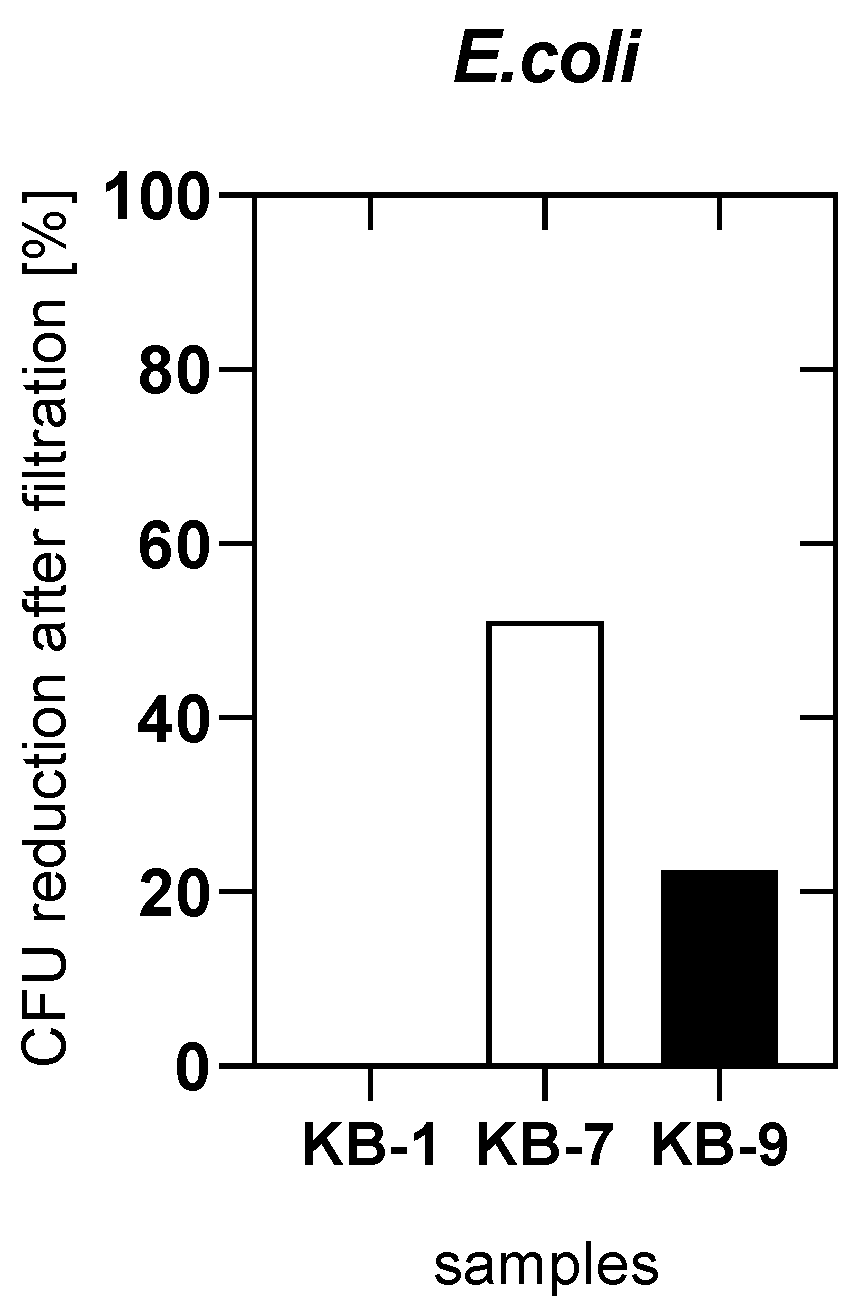
2.5. Antibacterial Test
3. Results
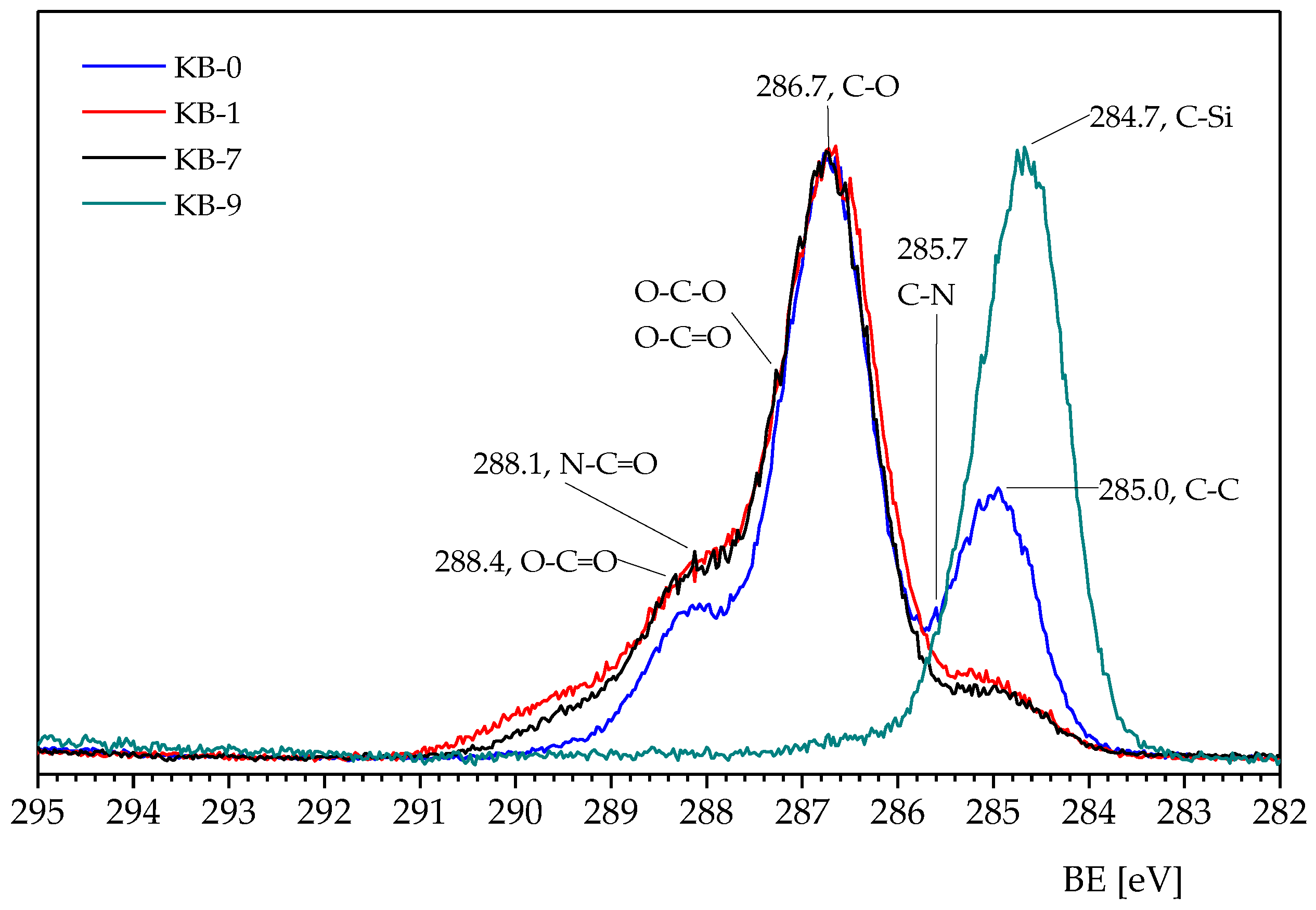
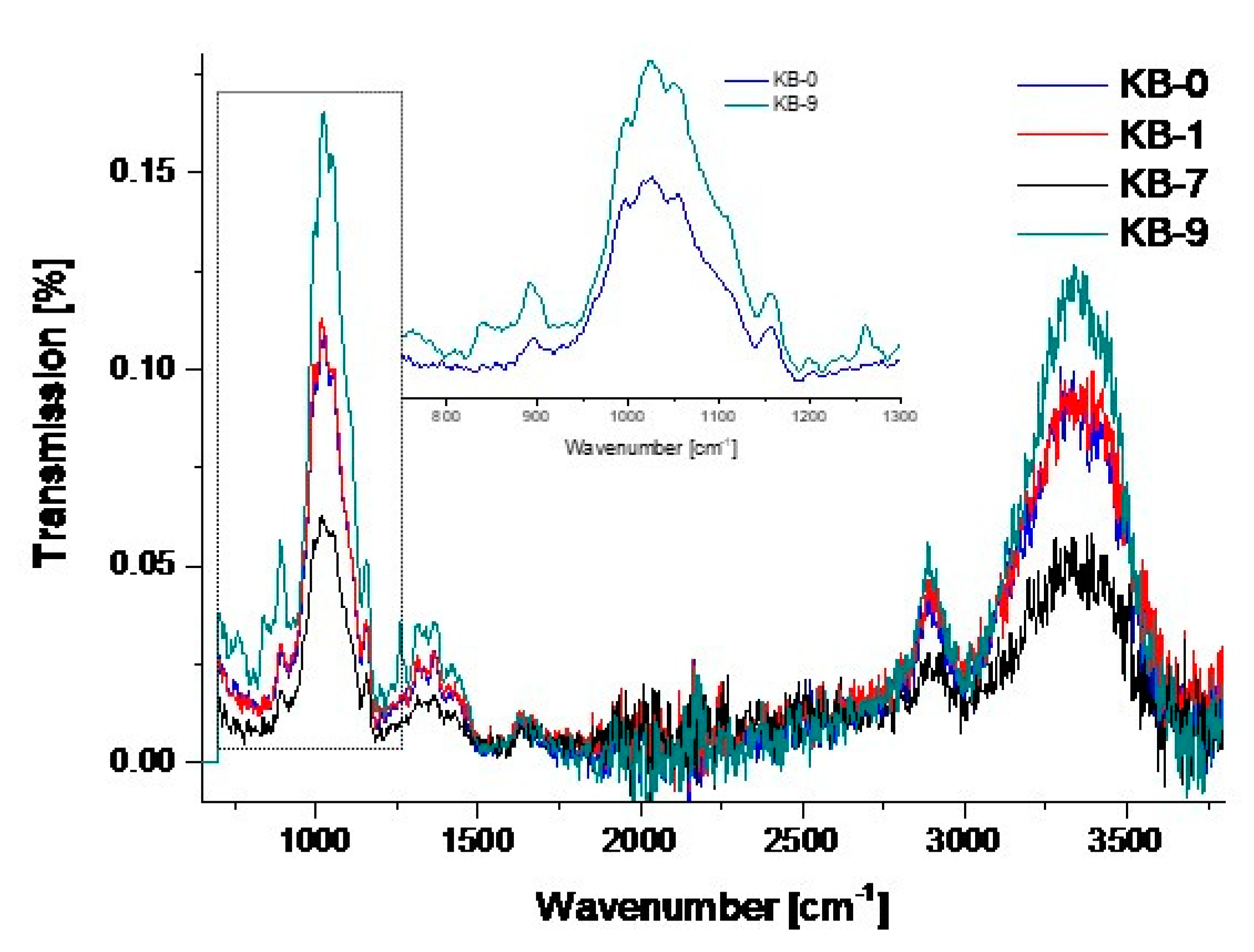
3.1. Chemical Modifications
3.2. Analyses
3.3. Test of E. coli Adsorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Hoenich, N. Cellulose for Medical Applications: Past, Present and Future. BioResources 2006, 1, 270–280. [Google Scholar] [CrossRef]
- Fazeli, M.; Florez, R.; Simão, A. Improvement in adhesion of cellulose fibers to the thermoplastic starch matrix by plasma treatment modification. Compos. Part B Eng. 2019, 163, 207–216. [Google Scholar] [CrossRef]
- Yang, J.; Pu, Y.; Miao, D.; Ning, X. Fabrication of Durably Superhydrophobic Cotton Fabrics by Atmospheric Pressure Plasma Treatment with a Siloxane Precursor. Polymers 2018, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.; Cristelo, C.; Silvestre, S.; Fortunato, E.; Sousa, A.; Alves, A.; Correia, D.M.; Lanceros-Mendez, S.; Gama, M. Hydrophobic modification of bacterial cellulose using oxygen plasma treatment and chemical vapor deposition. Cellulose 2020, 1–14. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R. Cold plasma surface modification of biodegradable polymer biomaterials. In Biomaterials for Bone Regeneration, 1st ed.; Dubruel, P., Van Vlierberghe, S., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 202–224. [Google Scholar]
- Tiwari, S.; Bijwe, J. Surface Treatment of Carbon Fibers—A Review. Procedia Technol. 2014, 14, 505–512. [Google Scholar] [CrossRef]
- Thakur, M.K.; Gupta, R.K.; Thakur, V.K. Surface modification of cellulose using silane coupling agent. Carbohydr. Polym. 2014, 111, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sautter, K.; Larsen, A.M.; Findley, D.A.; Davis, R.C.; Samha, H.; Linford, M.R. Chemical Vapor Deposition of Three Aminosilanes on Silicon Dioxide: Surface Characterization, Stability, Effects of Silane Concentration, and Cyanine Dye Adsorption. Langmuir 2010, 26, 14648–14654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.; Li, M.; Wu, X.; Cheng, G. Homogeneous hydroxyethylation of cellulose in NaOH/urea aqueous solution. Polym. Bull. 2005, 53, 243–248. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
| Sample | Process Gas | Gas Flow/Flow Time | Pressure (bar) | Chamber Vacuum (mbar) | Plasma Power (W) |
|---|---|---|---|---|---|
| KB-1 | N2 | 5 sccm 2 min | max. 1.5 | 0.20 | 150 |
| KB-7 | O2 | 5 sccm 2 min | max. 1.5 | 0.20 | 150 |
| KB-9 | O2 | 5 sccm 2 min | max. 1.5 | 0.20 | 150 |
| Sample | C (at %) | N (at %) | O (at %) | Si (at %) |
|---|---|---|---|---|
| KB-0 | 65.2 ± 0.3 | 34.8 ± 0.2 | ||
| KB-1 | 55.9 ± 0.3 | 4.4 ± 0.02 | 39.5 ± 0.2 | |
| KB-7 | 56.7 ± 0.3 | 42.6 ± 0.2 | ||
| KB-9 | 34.6 ± 0.2 | 34.0 ± 0.2 | 31.3 ± 0.2 |
| Sample | Weight after Swelling (g) | Number of Samples |
|---|---|---|
| KB-0 | 0.26 ± 0.02 | 16 |
| KB-1 | 0.23 ± 0.05 | 10 |
| KB-7 | 0.24 ± 0.03 | 10 |
| KB-9 | 0.040 ± 0.002 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauger, O.; Westphal, S.; Klöpzig, S.; Krüger-Genge, A.; Müller, W.; Storsberg, J.; Bohrisch, J. Plasma Activation as a Powerful Tool for Selective Modification of Cellulose Fibers towards Biomedical Applications. Plasma 2020, 3, 196-203. https://doi.org/10.3390/plasma3040015
Mauger O, Westphal S, Klöpzig S, Krüger-Genge A, Müller W, Storsberg J, Bohrisch J. Plasma Activation as a Powerful Tool for Selective Modification of Cellulose Fibers towards Biomedical Applications. Plasma. 2020; 3(4):196-203. https://doi.org/10.3390/plasma3040015
Chicago/Turabian StyleMauger, Olivia, Sophia Westphal, Stefanie Klöpzig, Anne Krüger-Genge, Werner Müller, Joachim Storsberg, and Jörg Bohrisch. 2020. "Plasma Activation as a Powerful Tool for Selective Modification of Cellulose Fibers towards Biomedical Applications" Plasma 3, no. 4: 196-203. https://doi.org/10.3390/plasma3040015
APA StyleMauger, O., Westphal, S., Klöpzig, S., Krüger-Genge, A., Müller, W., Storsberg, J., & Bohrisch, J. (2020). Plasma Activation as a Powerful Tool for Selective Modification of Cellulose Fibers towards Biomedical Applications. Plasma, 3(4), 196-203. https://doi.org/10.3390/plasma3040015





