Design and Medical Effects of a Vaginal Cleaning Device Generating Plasma-Activated Water with Antimicrobial Activity on Bacterial Vaginosis
Abstract
1. Introduction
2. Materials and Methods
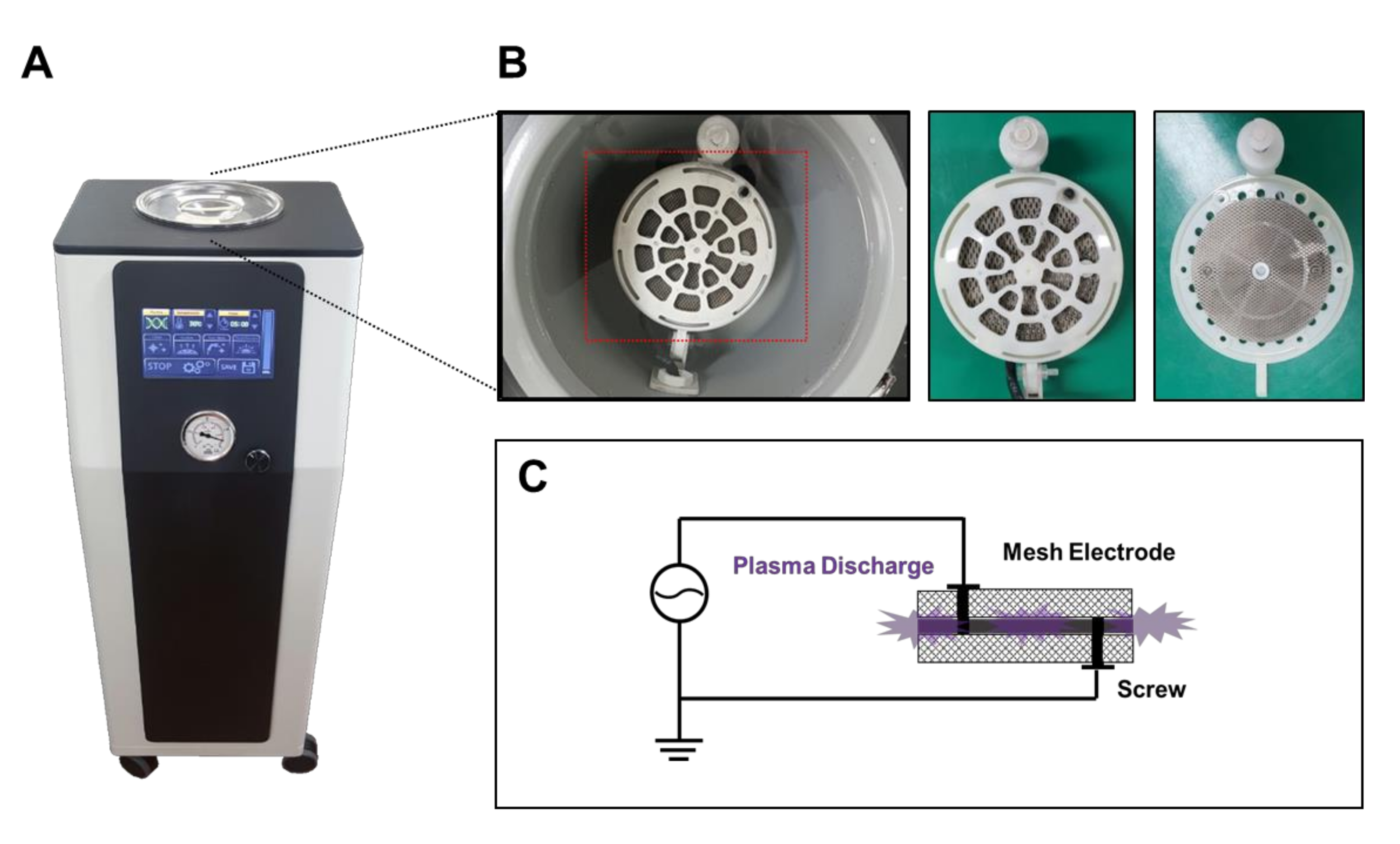
2.1. Device Design for Bacterial Vaginosis
2.2. Device Construction for Bacterial Vaginosis
3. Results and Discussion
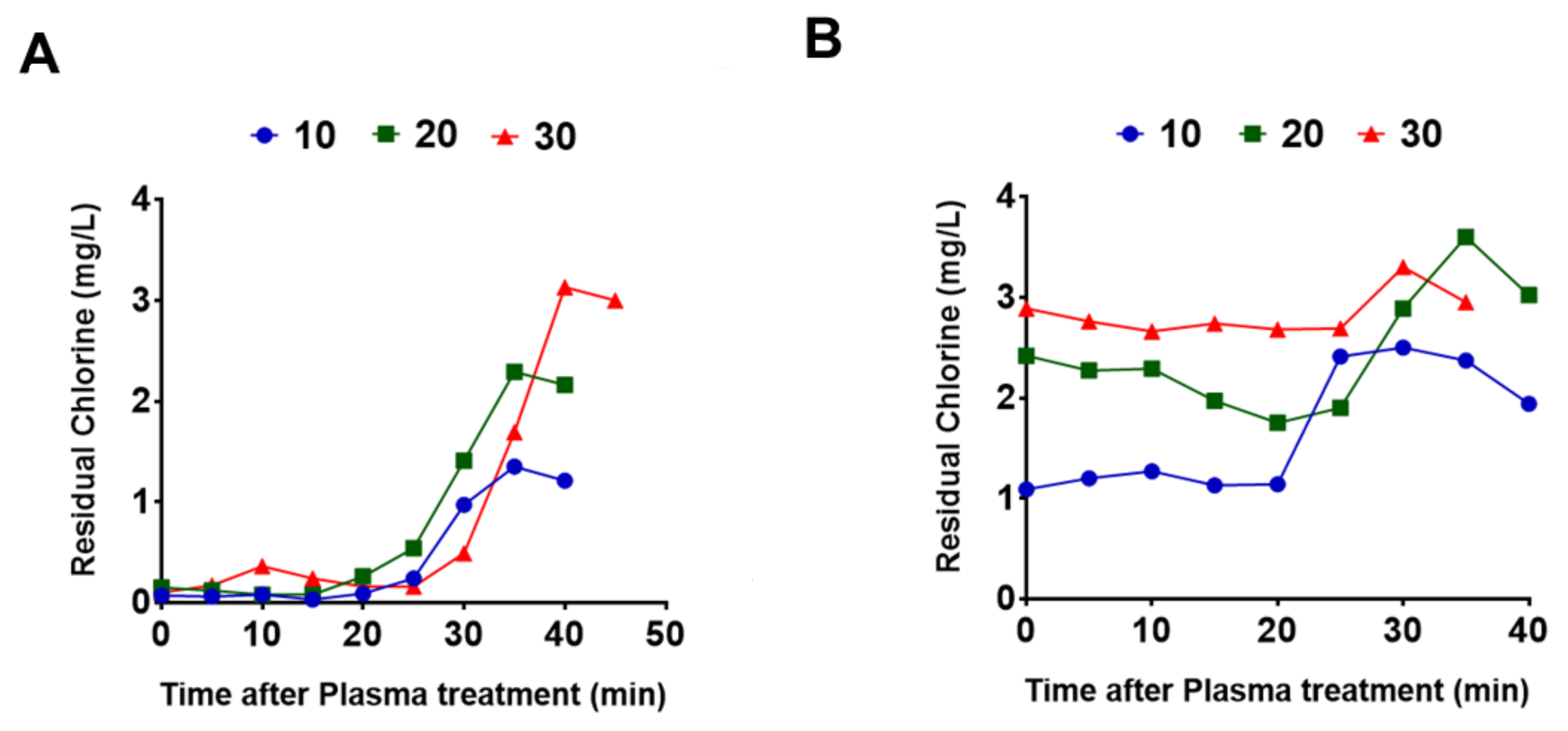
3.1. Performance Test
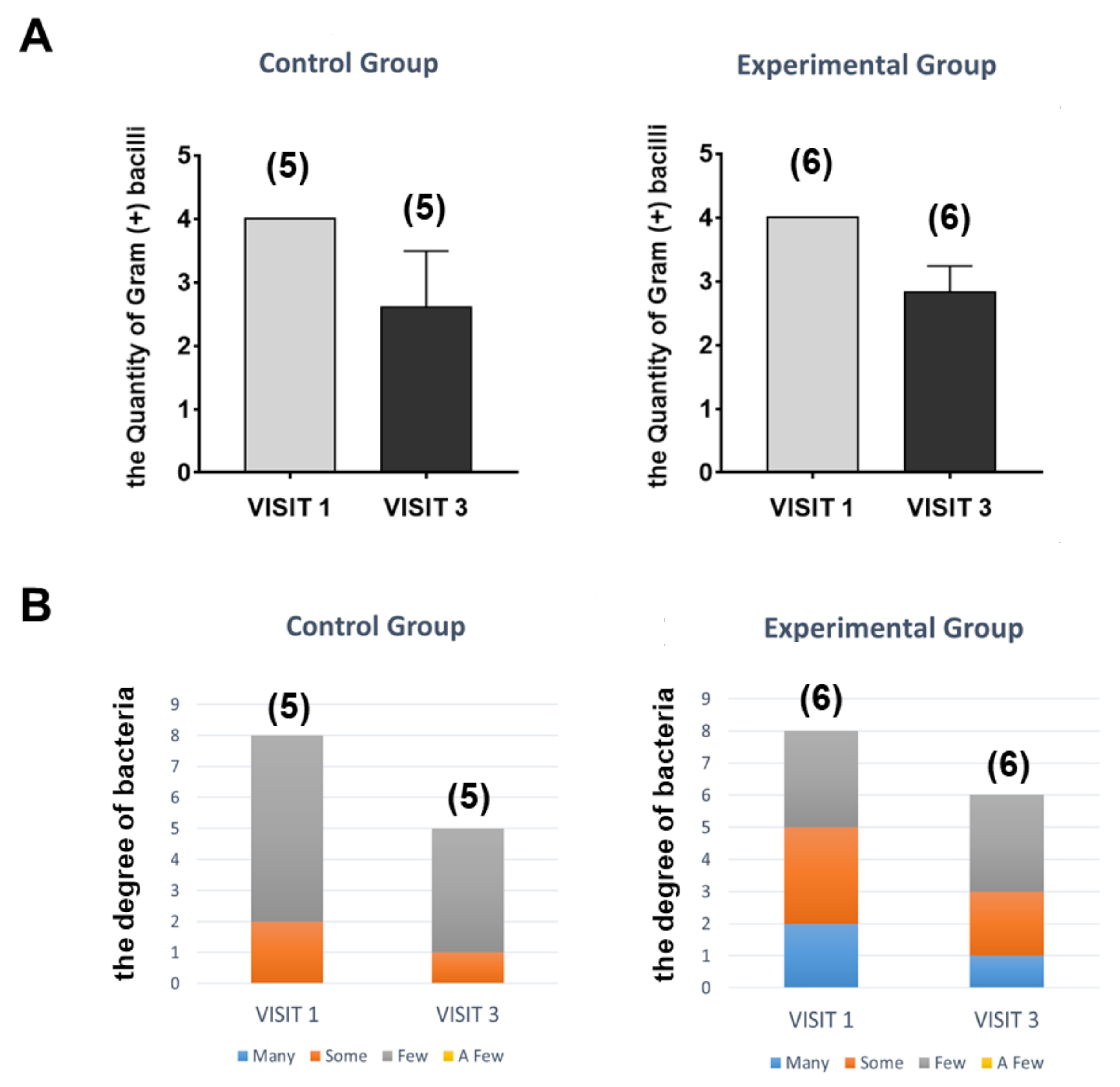
3.2. Clinical Design and Results to Assess the Safety and Effectiveness of the Device
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coudray, M.S.; Madhivanan, P. Bacterial vaginosis-A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, P.; Rizzolo, D. Bacterial vaginosis: A practical review. JAAPA 2017, 30, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Haefner, H.K. Current evaluation and management of vulvovaginitis. Clin. Obstet. Gynecol. 1999, 42, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef]
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.S. Vaginal Microbiomes Associated With Aerobic Vaginitis and Bacterial Vaginosis. Front Public Health 2018, 6, 78. [Google Scholar] [CrossRef]
- Mendling, W. Vaginal Microbiota. Adv. Exp. Med. Biol. 2016, 902, 83–93. [Google Scholar] [CrossRef]
- Tomas, M.; Palmeira-de-Oliveira, A.; Simoes, S.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Bacterial vaginosis: Standard treatments and alternative strategies. Int. J. Pharm. 2020, 587, 119659. [Google Scholar] [CrossRef]
- Wewalka, G.; Stary, A.; Bosse, B.; Duerr, H.E.; Reimer, K. Efficacy of povidone-iodine vaginal suppositories in the treatment of bacterial vaginosis. Dermatology 2002, 204 (Suppl. S1), 79–85. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schafer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Setsuhara, Y. Low-temperature atmospheric-pressure plasma sources for plasma medicine. Arch. Biochem. Biophys. 2016, 605, 3–10. [Google Scholar] [CrossRef]
- Brany, D.; Dvorska, D.; Halasova, E.; Skovierova, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Ma, S.H.; Hong, Y.C.; Choi, M.C. Effects of pulsed and continuous wave discharges of underwater plasma on Escherichia coli. Sep. Purif. Technol. 2018, 193, 351–357. [Google Scholar] [CrossRef]
- Hong, Y.C.; Park, H.J.; Lee, B.J.; Kang, W.S.; Uhm, H.S. Plasma formation using a capillary discharge in water and its application to the sterilization of E. coli. Phys. Plasmas 2010, 17, 053502. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxid. Med. Cell Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Mori, M.; Hamamoto, A.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Tachibana, S.; Ikehara, T.; Nakaya, Y.; Akutagawa, M.; Kinouchi, Y. Development of a new water sterilization device with a 365 nm UV-LED. Med. Biol. Eng. Comput. 2007, 45, 1237–1241. [Google Scholar] [CrossRef]
- Faught, B.M.; Reyes, S. Characterization and Treatment of Recurrent Bacterial Vaginosis. J. Womens Health 2019, 28, 1218–1226. [Google Scholar] [CrossRef]
- Brosseau, G.; Page, N.; de Jaham, C.; Del Castillo, J.R.E. Medical honey for canine nasal intertrigo: A randomized, blinded, placebo-controlled, adaptive clinical trial to support antimicrobial stewardship in veterinary dermatology. PLoS ONE 2020, 15, e0235689. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, W.J.; Hong, G.S.; Shim, W.S. Red ginseng extract blocks histamine-dependent itch by inhibition of H1R/TRPV1 pathway in sensory neurons. J. Ginseng Res. 2015, 39, 257–264. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, M.; Hwang, S.W. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J. Neuroinflamm. 2020, 17, 30. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Wholey, W.Y.; Jakob, U. Bacterial responses to reactive chlorine species. Annu. Rev. Microbiol. 2013, 67, 141–160. [Google Scholar] [CrossRef] [PubMed]

| Patients | STD (VISIT 1) | Culture (VISIT 1) | Culture (VISIT 2) | Culture (VISIT 3) | Culture (VISIT 4) | |
|---|---|---|---|---|---|---|
| 1 | KJY 01880498 | M. hominis (-) G. vaginali (-) | Gram(+) bacilli 4+ Gram(-) bacilli 2+ | Gram (+) bacilli 1+ | Gram (+) bacilli 1+ Gram (+) cocci pairs & chains 3+ Gram (-) bacilli 1+ | Gram (+) bacilli 1+ Gram (+) cocci pairs & chains 1+ Gram (-) bacilli 2+ |
| Enterobacter cloacae complex Some | No gowth for 48hrs | Escherichia coli few | Escherichia coli Many | |||
| 2 | JSY 02331373 | M. hominis (-) G. vaginali (+) C. trachoma(+) C. albicans(+) | Gram(+) bacilli 4+ Gram (+) cocci pairs & chains 2+ Yeast-like organism 1+ | Gram(+) bacilli 1+ Gram (+) cocci pairs & chains 1+ Yeast-like organism 1+ | Gram(+) bacilli 3+ Gram (+) cocci pairs & chains 4+ Yeast-like organism 1+ | Gram(+) bacilli 4+ Gram (+) cocci pairs & chains 2+ Yeast-like organism 1+ |
| Candida albicans Few Gram positive cocci Few Lactobacillus sp. Few | Yeast Few Non spore-forming Gram (+) bacilli Few | Candida albicans Some | Yeast Few Gram positive cocci Few Non spore-forming Gram (+) bacilli Some | |||
| 3 | HYS 02702448 | M. hominis (-) G. vaginali (-) | Gram(-) bacilli 4+ Gram(+) bacilli 4+ | Gram(+) bacilli 3+ | Gram(+) bacilli 3+ | Gram (+) bacilli 4+ |
| Excherichia coli Some | Lactobacillus sp. Few | Lactobacillus sp. Few | Non spore-forming Gram (+) bacilli Few | |||
| 4 | HMJ 02720811 | M. hominis (-) G. vaginali (-) | Gram (+) bacilli 4+ Yeast-like organism 2+ | Gram (+) bacilli 3+ | Gram (+) bacilli 3+ Gram (+) cocci pairs & chains 1+ | Gram (+) bacilli 2+ Gram (+) cocci pairs & chains 4+ Gram (-) bacilli 4+ |
| Non spore forming Gram (+) bacilli Few Candida albicans Few | Lactobacillus sp. Few | Lactobacillus sp. Few | Enterobacter aerogenes Many | |||
| 5 | KKM 01398837 | M. hominis (-) G. vaginali (-) | Gram (+) bacilli 4+ Gram (+) cocci pairs & chains 1+ | Gram (+) bacilli 4+ | Gram (+) bacilli 3+ | Gram (+) bacilli 4+ |
| Streptococcus-agalactiae-(Group B) Few | Non spore-forming Gram (+) bacilli Few | Non spore-forming Gram (+) bacilli Few | Non spore-forming Gram (+) bacilli Few |
| Patients | STD (VISIT 1) | Culture (VISIT 1) | Culture (VISIT 2) | Culture (VISIT 3) | Culture (VISIT 4) | |
|---|---|---|---|---|---|---|
| 1 | NTI 02759285 | M. hominis (-) G. vaginali (+) | Gram (+) bacilli 4+ Yeast-like organism 1+ | Gram (+) bacilli 4+ | Gram (+) bacilli 3+ | Gram (+) bacilli 4+ Gram (+) cocci pairs & chains 1+ Gram (-) bacilli 4+ |
| Candida albicans Some | Yeast Few Non spore-forming Gram(+) bacilli Few | Non spore-forming Gram(+) bacilli Few | Escherichia coli Some | |||
| 2 | PKH 01652805 | M. hominis (-) G. vaginali (+) C. trachoma weakly (+) C. albicans (+) | Gram (+) bacilli 4+ Gram (+) cocci pairs & chains 2+ Gram (-) bacilli 1+ Yeast-like organism 1+ | Gram (+) bacilli 4+ Gram (+) cocci pairs & chains 2+ | Gram (+) bacilli 3+ Gram (+) cocci pairs & chains 2+ | Gram (+) bacilli 3+ Gram (+) cocci pairs & chains 1+ Yeast-like organism 1+ |
| Candida albicans Few | Candida albicans Few Lactobacillus sp. Some | Gram positive cocci Many Non spore-forming Gram(+) bacilli SomeYeast Few | Gram positive cocci Few Non spore-forming Gram (+) cilli Some Yeast Some | |||
| 3 | SSY 01464025 | M. hominis (-) G. vaginali (+) | Gram(+) bacilli 4+ Gram(-) bacilli 4+ Gram(+) colli pairs & chains 1+ | Gram(+) bacilli 1+ | Gram(+) bacilli 3+ | Gram(+) bacilli 4+ |
| Escherichia coli Many | Non spore-forming Gram (+) bacillli Few | Lactobacillus sp. some | Lactobacillus sp. some | |||
| 4 | JSS 01653482 | M. hominis (-) G. vaginali (+) | Gram (+) bacilli 4+ | Gram (+) bacilli 4+ | Gram (+) bacilli 3+ | Gram (+) bacilli 3+ |
| Lactobacillus sp. Some Gram positive cocci Few | Lactobacillus sp. Few | Lactobacillus sp. Few | Lactobacillus sp. Few | |||
| 5 | LHJ 02727501 | M. hominis (-) G. vaginali (+) C. trahoma (+) | Gram (+) bacilli 4+ Gram (+) cocci pairs & chains 1+ | Gram (+) bacilli 4+ Gram (+) cocci pairs & chains 1+ | Gram (+) bacilli 3+ Gram (+) cocci pairs & chains 1+ | Gram (+) bacilli 3+ |
| Streptococcus agalactiae-(Group B) Some | Streptococcus agalactiae-(Group B) Few | Streptococcus agalactiae-(Group B) Few | Non spore-forming Gram(+) bacilli Few | |||
| 6 | HJY 01617842 | M. hominis (-) G. vaginali (-) | Gram (+) biacilli 4+ Gram (+) cocci pairs & chains 3+ | Gram (+) biacilli 3+ Gram (+) cocci pairs & chains 1+ | Gram (+) biacilli 2+ | Gram (+) biacilli 3+ |
| Gram negative bacilli Few Gram positive cocci Many | Lactobacillus sp. Few | Lactobacillus sp. Few | Lactobacillus sp. Some Gram positive cocci Few |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.; Jeon, H.; Wang, G.Y.; Kim, H.K.; Kim, J.-H.; Ahn, D.K.; Choi, J.S.; Jang, Y. Design and Medical Effects of a Vaginal Cleaning Device Generating Plasma-Activated Water with Antimicrobial Activity on Bacterial Vaginosis. Plasma 2020, 3, 204-213. https://doi.org/10.3390/plasma3040016
Hwang Y, Jeon H, Wang GY, Kim HK, Kim J-H, Ahn DK, Choi JS, Jang Y. Design and Medical Effects of a Vaginal Cleaning Device Generating Plasma-Activated Water with Antimicrobial Activity on Bacterial Vaginosis. Plasma. 2020; 3(4):204-213. https://doi.org/10.3390/plasma3040016
Chicago/Turabian StyleHwang, Yuan, Hyanghee Jeon, Geon Yeoung Wang, Hyung Kyu Kim, Jun-Hyun Kim, Dong Keun Ahn, Joong Sub Choi, and Yongwoo Jang. 2020. "Design and Medical Effects of a Vaginal Cleaning Device Generating Plasma-Activated Water with Antimicrobial Activity on Bacterial Vaginosis" Plasma 3, no. 4: 204-213. https://doi.org/10.3390/plasma3040016
APA StyleHwang, Y., Jeon, H., Wang, G. Y., Kim, H. K., Kim, J.-H., Ahn, D. K., Choi, J. S., & Jang, Y. (2020). Design and Medical Effects of a Vaginal Cleaning Device Generating Plasma-Activated Water with Antimicrobial Activity on Bacterial Vaginosis. Plasma, 3(4), 204-213. https://doi.org/10.3390/plasma3040016






