Modelling of an Atmospheric–Pressure Air Glow Discharge Operating in High–Gas Temperature Regimes: The Role of the Associative Ionization Reactions Involving Excited Atoms
Abstract
:1. Introduction
2. Modeling of an Atmospheric-Pressure Air Glow Discharge Operated in High-Gas-Temperature Regimes
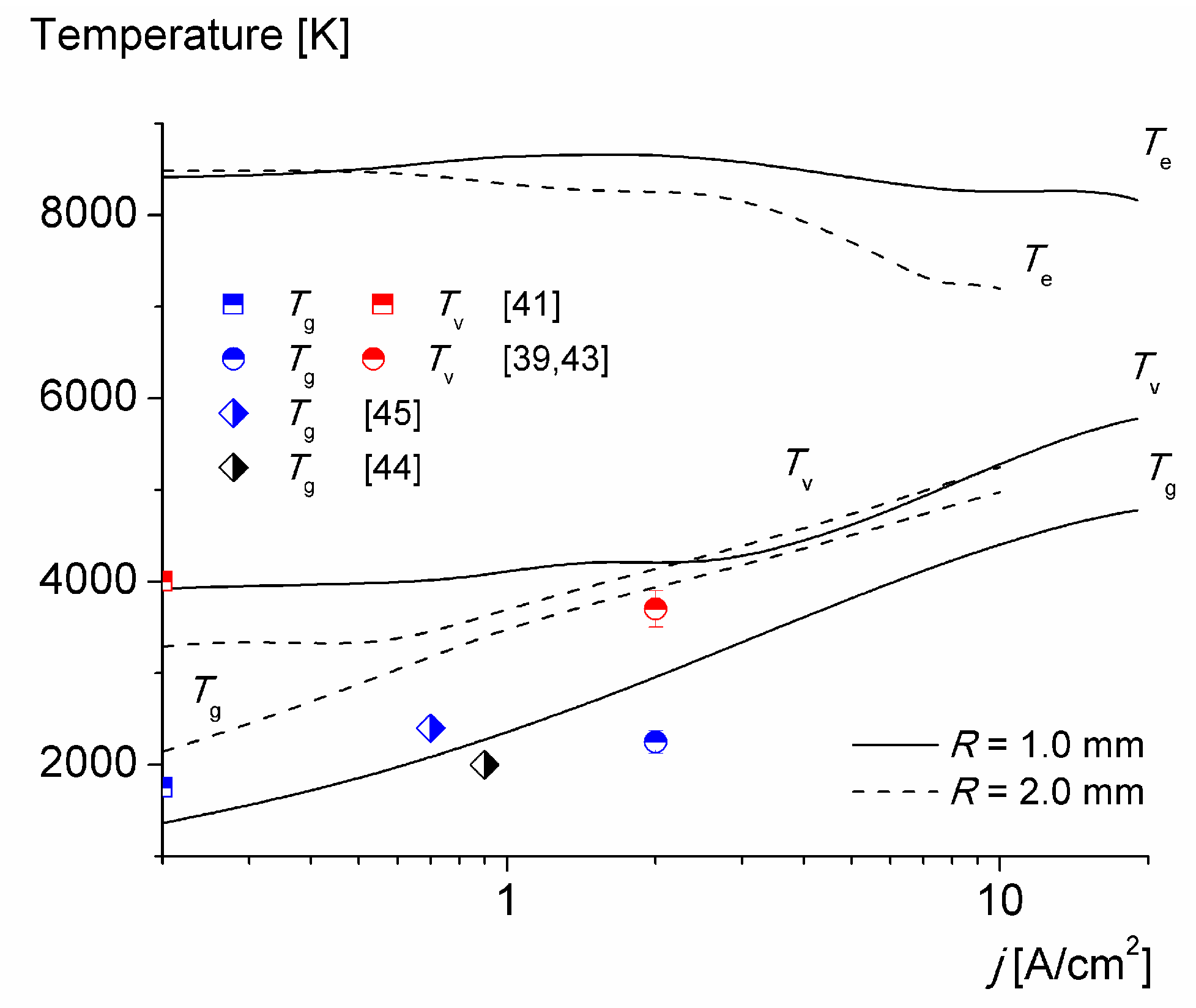
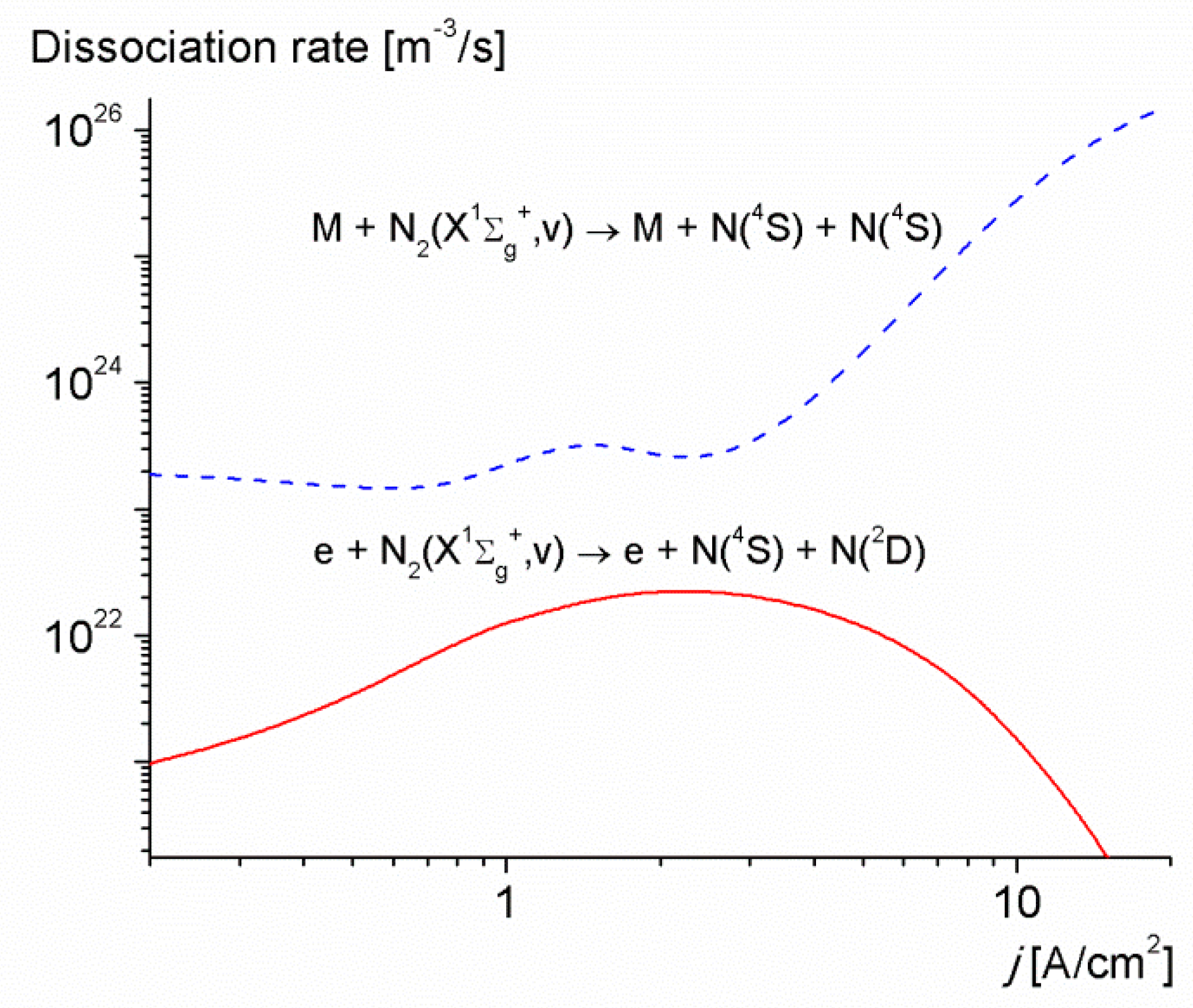
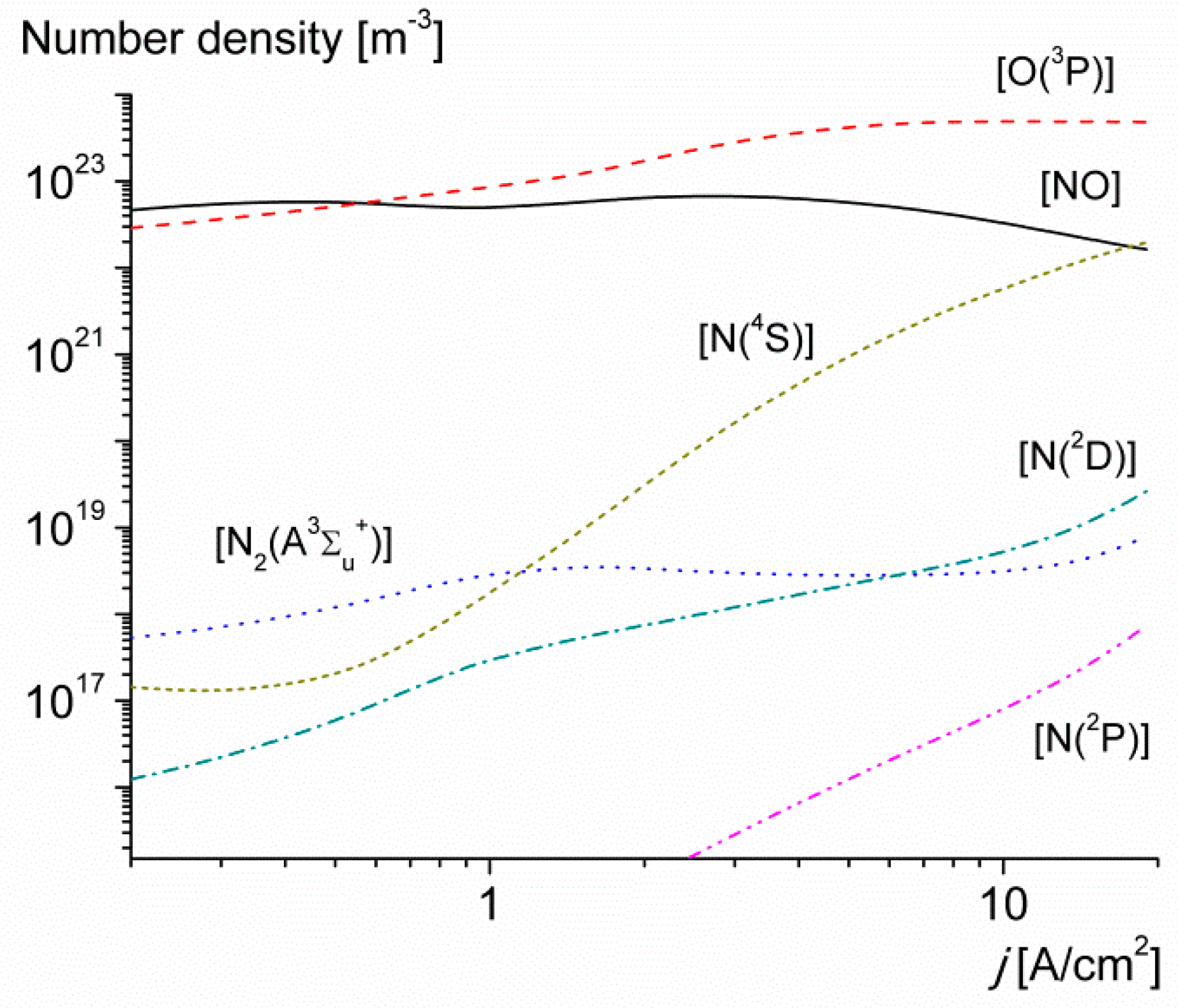
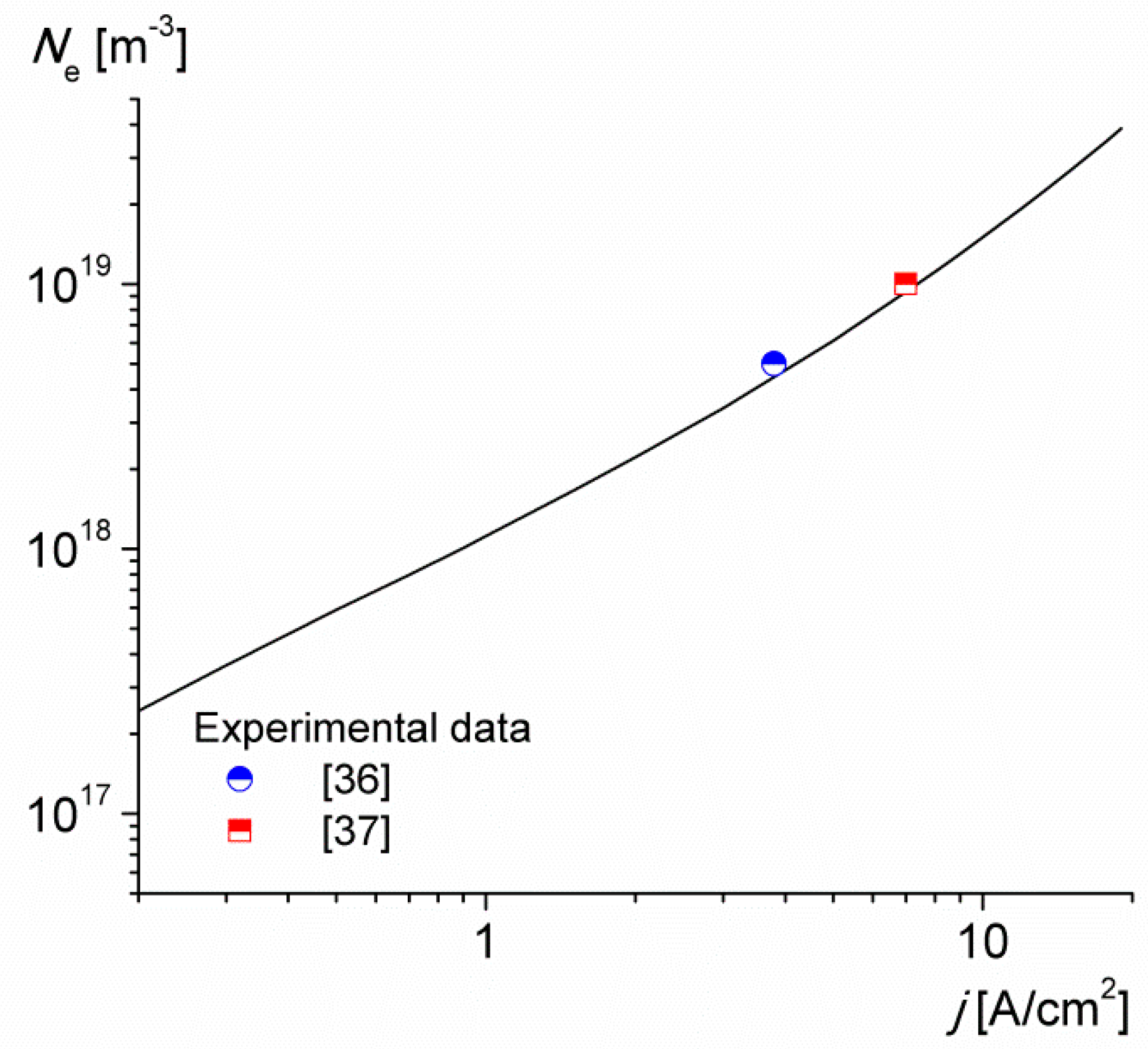
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Rj | Reaction | Rate Coefficient (m3/s or m6/s) | Reference |
|---|---|---|---|
| Electron-Impact Processes | |||
| R1 | e + N2(X) → e + e + N2+ | k1 = f (E/N) | [49,50] |
| R2 | e + O2 → e + e + O2+ | k2 = f (E/N) | [49,50] |
| R3 | e + NO → e + e + NO+ | k3 = f (E/N) | [49,50] |
| R4 | e + O(3P) → e + e + O+ | k4 = f (E/N) | [49,50] |
| R5 | e + N2(X) → e + N2* (∆E = 13 eV) e + N(4S) + N(2D) | k5 = f (E/N) | [49,50] |
| R6 | e + O2 → e + O2* (∆E = 6.0 eV) e + O(3P) + O(3P) + 0.8 eV | k6 = f (E/N) | [49,50] |
| R7 | e + O2 → e + O2 (∆E = 8.4 eV) e + O(3P) + O(1D) + 1.26 eV | k7 = f (E/N) | [49,50] |
| R8 | e + O2 → e + O2 (∆E = 9.97 eV) e + O(3P) + O(1S) + 0.6 eV | k8 = f (E/N) | [49,50] |
| R9 | e + N2(X) → e + N2(A) | k9 = f (E/N) | [49,50] |
| R10 | e + N2(X) → e + N2(B) | k10 = f (E/N) | [49,50] |
| R11 | e + N2(X) → e + N2(a’) | k11 = f (E/N) | [49,50] |
| R12 | e + N2(X) → e + N2(C) | k12 = f (E/N) | [49,50] |
| Associative Ionization | |||
| R13 | N(4S) + O(3P) → NO+ + e | k13 = 5 × 10−17 Tg−0.5 e(−32500/Tg) | [51] |
| R14 | N(4S) + O(1S) → NO+ + e | k14 = (1–3) × 10−17 (Tg/300)1/6 | [18] |
| R15 | N(4S) + O(1D) → NO+ + e | k15 = 3.1 × 10−25 Tg0.5 (9287 + 2Tg) e(−9287/Tg) | [52] |
| R16 | N(2D) + O(3P) → NO+ + e | k16 = 1.3 × 10−24 Tg0.5 (4411 + 2Tg) e(−4411/Tg) | [20,21] |
| R17 | N(2D) + N(2P) → N2+ + e | k17 = 1.9 × 10−21 Tg0.98 | [53] |
| [1 − e(−3129/Tg)] −1 | |||
| R18 | N(2P) + O(3P) → NO+ + e | k18 = (1–3) × 10−17 (Tg/300)1/6 | [18] |
| R19 | N(2P) + N(2P) → N2+ + e | k19 = 3.2 × 10−21 Tg0.98 | [53] |
| [1 − e(−3129/Tg)] −1 | |||
| Penning Ionization | |||
| R20 | N2(A) + N2(a’) → N2+ + N2(X) + e | k20 = 5 × 10−17 | [54] |
| R21 | N2(a’) + N2(a’) → N2+ + N2(X) + e | k21 = 2 × 10−16 | [54] |
| Dissociative electron–Ion Recombination | |||
| R22 | e + NO+ → N(4S) + O(3P) | k22 = 0.05 × 1.5 × 10−11 Te−0.65 | [55,56] |
| k22 = 0.05 ×1.1 × 10−8 Te−1.5 | [48] | ||
| R23 | e + NO+ → N(2D) + O(3P) | k23 = 0.95 × 1.5 × 10−11 Te−0.65 | [55,56] |
| k23 = 0.95 × 1.1 × 10−8 Te−1.5 | [56,48] | ||
| R24 | e + N2+ → N(4S) + N(2D) | k24 = 0.46 × 2.0 × 10−13 (300/Te)0.5 | [22,57] |
| R25 | e + N2+ → N(4S) + N(2P) | k25 = 0.08 × 2.0 × 10−13 (300/Te)0.5 | [22,57] |
| R26 | e + N2+ → N(2D) + N(2D) | k26 = 0.46 × 2.0 × 10−13 (300/Te)0.5 | [22,57] |
| R27 | e + O2+ → O(3P) + O(3P) | k27 = 0.32 × 2.0 × 10−13 (300/Te) | [22,57] |
| R28 | e + O2+ → O(3P) + O(1D) | k28 = 0.43 × 2.0 × 10−13 (300/Te) | [22,57] |
| R29 | e + O2+ → O(1D) + O(1D) | k29 = 0.20 × 2.0 × 10−13 (300/Te) | [22,57] |
| R30 | e + O2+ → O(1D) + O(1S) | k30 = 0.05 × 2.0 × 10−13 (300/Te) | [22,57] |
| Three Body Electron–Ion Recombination | |||
| R31 | e + e + O+ → e + O(3P) | k31 = 1.0 × 10−31 (300/Te)4.5 | [22] |
| Thermal Dissociation/Three-Body Recombination | |||
| R32 | N2(X) + M → N(4S) + N(4S) + M | k32 = 5 × 10−14 e(−113200/Tg) | [10] |
| M = N2(X), O2, and NO | [1 − e(−3354/Tg)] | ||
| R33 | N2(X) + M → N(4S) + N(4S) + M | k33 = 1.1 × 10−13 e(−113200/Tg) | [10] |
| M = N(4S) and O(3P) | [1 − e(−3354/Tg)] | ||
| R34 | N(4S) + N(4S) + M → N2(X) + M M = N2(X), O2, NO, O(3P), N(4S) | k34 = 8.27 × 10−46 e(500/Tg) | [10] |
| R35 | O2(X) + M → O(3P) + O(3P) + M M = O2 | k35 = 3.7 × 10−14 e(−59380/Tg) [1 − e(−2240/Tg)] | [10] |
| R36 | O2(X) + M → O(3P) + O(3P) + M M = O(3P) | k36 = 1.3 × 10−13 e(−59380/Tg) [1 − e(−2240/Tg)] | [10] |
| R37 | O2(X) + M → O(3P) + O(3P) + M M = N2(X), N(4S), and NO | k37 = 9.3 × 10−15 e(−59380/Tg) [1 − e(−2240/Tg)] | [10] |
| R38 | O(3P) + O(3P) + M → O2(X) + M M = N2(X) | k38 = 2.76 × 10−46 e(720/Tg) | [10] |
| R39 | O(3P) + O(3P) + M → O2(X) + M M = O2 | k39 = 2.45 × 10−43 Tg−0.63 | [10] |
| R40 | O(3P) + O(3P) + M → O2(X) + M M = O(3P) | k40 = 8.8 × 10−43 Tg−0.63 | [10] |
| R41 | NO + M → N(4S) + O(3P) + M M = N2(X) and O2 | k41 = 8.7 × 10−15 e(−76000/Tg) | [10] |
| R42 | NO + M → N(4S) + O(3P) + M M = O(3P) and NO | k42 = 1.7 × 10−13 e(−76000/Tg) | [10] |
| R43 | N(4S) + O(3P) + M → NO(X) + M M = N2(X), O2, NO, and O(3P) | k43 = 1.76 × 10−43 Tg−0.5 | [10] |
| Chemical Reactions | |||
| R44 | N2(A) + O2 → N2(X) + 2 O(3P) + 1.1 eV | k44 = 1.7 × 10−18 | [58] |
| R45 | N2(A) + O2 → N2(X) + O2(b) | k45 = 7.5 × 10−19 | [58] |
| R46 | N2(A) + N2(A) → N2(X) + N2(B) | k46 = 7.7 × 10−17 | [59] |
| R47 | N2(A) + N2(A) → N2(X) + N2(C) | k47 = 1.6 × 10−16 | [59] |
| R48 | N2(A) + O(3P) → N2(X) + O(1S) | k48 = 2.1 × 10−17 | [22] |
| R49 | N2(A) + O(3P) → NO + N(2D) | k49 = 7.0 × 10−18 | [22] |
| R50 | N2(A) + N(4S) → N2(X) + N(2P) | k50 = 5.0 × 10−17 | [60] |
| R51 | N2(A) + NO → N2(X) + NO | k51 = 6.4 × 10−17 | [58] |
| R52 | N2(B) + O2 → N2(X) + 2 O(3P) | k52 = 3.0 × 10−16 | [22] |
| R53 | N2(B) + N2(X) → N2(X) + N2(A) | k53 = 1.0 × 10−17 | [60] |
| R54 | N2(a’) + O2 → N2(X) + O(3P) + O(1D) + 1.4 eV | k54 = 2.8 × 10−17 | [22] |
| R55 | N2(a’) + N2(X) → N2(X) + N2(B) | k55 = 2.0 × 10−19 | [22] |
| R56 | N2(a’) + O(3P) → NO + N(2D) | k56 = 3.0 × 10−16 | [61] |
| R57 | N2(a’) + NO → N(4S) + O(3P) + N2(X) | k57 = 3.6 × 10−16 | [62] |
| R58 | N2(C) + O2 → N2(X) + 2O(3P) | k58 = 2.5 × 10−16 | [63] |
| R59 | N2(C) + N2(X) → N2(X) + N2(B) | k59 = 1.0 × 10−17 | [63] |
| R60 | N2(C) → N2(B) + hυ | k60 = 2.4 × 107 s−1 | [22] |
| R61 | N(4S) + NO → O(3P) + N2(X) | k61 = 1.0 × 10−18 Tg0.5 | [22] |
| R62 | N(4S) + O2 → O(3P) + NO | k62 = 1.1 × 10−20 Tg e(−3150/Tg) | [22] |
| R63 | N(2D) + N2(X) → N(4S) + N2(X) | k63 = 1.7 × 10−20 | [58] |
| R64 | N(2D) + O(3P) → N(4S) + O(3P) | k64 = 1.4 × 10−18 | [58] |
| R65 | N(2D) + O2 → NO + O(3P) | k65 = 2.4 × 10−18 e(−185/Tg) | [58] |
| R66 | N(2D) + O2 → NO + O(1D) | k66 = 7.3 × 10−18 e(−185/Tg) | [58] |
| R67 | N(2D) + NO → N2(X) + O(1S) | k67 = 6.0 × 10−17 | [58] |
| R68 | N(2P) + N(4S) → N(2D) + N(4S) | k68 = 1.8 × 10−18 | [22] |
| R69 | N(2P) + O(3P) → N(2D) + O(3P) | k69 = 1.0 × 10−18 | [60] |
| R70 | N(2P) + O2 → NO + O(3P) | k70 = 2.5 × 10−18 | [58] |
| R71 | N(2P) + NO → N2(X) + O(3P) | k71 = 2.9 × 10−17 | [58] |
| R72 | O(3P) + N2(X) → N(4S) + NO | k72 = 1.3 × 10−16 e(−38000/Tg) | [10] |
| R73 | O(3P) + NO → N(4S) + O2 | k73 = 2.5 × 10−21 Tg e(−19500/Tg) | [10] |
| R74 | O(1D) + O(3P) → O(3P) + O(3P) | k74 = 8.0 × 10−18 | [22] |
| R75 | O(1D) + O2 → O(3P) + O2(b) | k75 = 3.2 × 10−17 e(67/Tg) | [22] |
| R76 | O(1D) + N2(X) → O(3P) + N2(X) + 1.4 eV | k76 = 1.8 × 10−17 e(107/Tg) | [22] |
| R77 | O(1S) + O(3P) → O(1D) + O(3P) | k77 = 5.0 × 10−17 e(−301/Tg) | [22] |
| R78 | O(1S) + O2 → O2 + O(3P) | k78 = 3.0 × 10−18 e(−850/Tg) | [22] |
| R79 | O(1S) + O2 → O2 + O(1D) | k79 = 1.3 × 10−18 e(−850/Tg) | [22] |
| R80 | O(1S) + N(4S) → O(3P) + N(2P) | k80 = 1.0 × 10−18 | [60] |
| R81 | O(1S) + NO → O(3P) + NO | k81 = 1.8 × 10−16 | [22] |
| R82 | O(1S) + NO → O(1D) + NO | k82 = 3.2 × 10−16 | [22] |
| Electron Attachment and Detachment | |||
| R83 | e + O2 + O2 → O2− + O2 | k83 = 1.4 × 10−41 (300/Te) e(−660/Tg) e[700 (Te − Tg)/(Te Tg)] | [22] |
| R84 | e + O2 → O− + O(3P) | k84 = f(E/N) | [49,50] |
| R85 | O2− + O2 → O2 + O2 + e | k85 = 2.7 × 10−16 (Tg/300)0.5 e(−5590/Tg) | [22] |
| R86 | O2− + O(3P) → O3 + e | k86 = 1.5 × 10−16 | [22] |
| R87 | O− + N2(X) → N2O + e | k87 = 9.0 × 10−19 | [22] |
| R88 | O− + O(3P) → O2 + e | k88 = 5.0 × 10−16 | [22] |
| R89 | O− + NO → NO2 + e | k89 = 2.6 × 10−16 | [22] |
| R90 | O3− + O(3P) → O2 + O2 + e | k90 = 3.0 × 10−16 | [22] |
| Ion Conversi on | |||
| R91 | O− + O2(X) + M → O3− + M M = N2(X),O2 | k91 = 1.1 × 10−42 (300/Tg) | [22] |
| R92 | O+ + N2(X) → NO+ + N(4S) | k92 = (1.5 − 2.0 × 10−3 Tg + 9.56 × 10−7 Tg2) × 10−18 | [60] |
| R93 | N2+ + O2(X) → N2(X) + O2+ | k93 = 6 × 10−17 (300/Tg)0.5 | [22] |
| R94 | N2+ + O(3P) → N2(X) + O+ | k94 = 1.0 × 10−17 (300/Tg)0.2 | [22] |
| R95 | N2+ + O(3P) → NO+ + N(4S) | k95 = 0.95 × 1.3 × 10−16 (300/Tg)0.5 | [22,64] |
| R96 | N2+ + O(3P) → NO+ + N(2D) | k96 = 0.05 × 1.3 × 10−16 (300/Tg)0.5 | [22,64] |
| R97 | O2+ + NO → NO+ + O2 | k97 = 6.3 × 10−16 | [60] |
| Ion–Ion Recombination | |||
| R98 | X− + Y+ → X + Y X− = O−, O2−, and O3− Y+ = N2+, O2+, NO+, and O+ | k98 = 2.0 × 10−13 (300/Tg)0.5 | [22] |
References
- Naidis, G.V. Simulation of streamer-to-spark transition in short non-uniform air gaps. J. Phys. D Appl. Phys. 1999, 32, 2649–2654. [Google Scholar] [CrossRef]
- Naidis, G.V. Dynamics of streamer breakdown of short non-uniform air gaps. J. Phys. D Appl. Phys. 2005, 38, 3889–3893. [Google Scholar] [CrossRef]
- Prevosto, L.; Kelly, H.; Mancinelli, B.; Chamorro, J.C.; Cejas, E. On the physical processes ruling an atmospheric pressure air glow discharge operating in an intermediate current regime. Phys. Plasmas 2015, 22, 023504. [Google Scholar] [CrossRef] [Green Version]
- Benilov, M.S.; Naidis, G.V. Modelling of low-current discharges in atmospheric-pressure air taking account of non-equilibrium effects. J. Phys. D Appl. Phys. 2003, 36, 1834–1841. [Google Scholar] [CrossRef] [Green Version]
- Naidis, G.V. Simulation of convection-stabilized low-current glow and arc discharges in atmospheric-pressure air. Plasma Sources Sci. Technol. 2007, 16, 297–303. [Google Scholar] [CrossRef]
- Xaubet, M.; Giuliani, L.; Grondona, D.; Minotti, F. Experimental and theoretical study of an atmospheric air plasma-jet. Phys. Plasmas 2017, 24, 013502. [Google Scholar] [CrossRef]
- Laux, C.O.; Yu, L.; Packan, D.M.; Gessman, R.J.; Pierrot, L.; Kruger, C.H.; Zare, R.N. Ionization Mechanisms in Two-Temperature Air Plasmas. In Proceedings of the 30th Plasmadynamic and Lasers Conference, Norfolk, VA, USA, 28 June–1 July 1999. [Google Scholar]
- Benilov, M.S.; Naidis, G.V. Modelling of discharges in a flow of preheated air. Plasma Sources Sci. Technol. 2005, 16, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Popov, N.A. Simulations of a longitudinal glow discharge in a hot air flow at atmospheric pressure. Plasma Phys. Rep. 2006, 32, 237–245. [Google Scholar] [CrossRef]
- Aleksandrov, N.L.; Bazelyan, E.M.; Kochetov, I.V.; Dyatko, N.A. The ionization kinetics and electric field in the leader channel in long air gaps. J. Phys. D Appl. Phys. 1997, 30, 1616–1624. [Google Scholar] [CrossRef]
- Popov, N.A. Formation and Development of a Leader Channel in Air. Plasma Phys. Rep. 2003, 29, 695–708. [Google Scholar] [CrossRef]
- da Silva, C.L.; Pasko, V.P. Dynamics of streamer-to-leader transition at reduced air densities and its implications for propagation of lightning leaders and gigantic jets. J. Geophys. Res. 2013, 118, 13561–13590. [Google Scholar] [CrossRef]
- Aleksandrov, N.L.; Bazelyan, E.M. Ionization processes in spark discharge plasmas. Plasma Sources Sci. Technol. 1999, 8, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrov, N.L.; Bazelyan, E.M.J. The mechanism of re-breakdown within a post-arc channel in long non-uniform air gaps. Phys. D Appl. Phys. 1998, 31, 1343–1351. [Google Scholar] [CrossRef]
- Aleksandrov, N.L.; Bazelyan, E.M.; Konchakov, A.M. Plasma Parameters in the Channel of a Long Leader in Air. Plasma Phys. Rep. 2001, 27, 875–885. [Google Scholar] [CrossRef]
- Mankelevich, Y.A.; Pal, A.F.; Popov, N.A.; Rakhimova, T.V.; Filippov, A.V. Current Dynamics and Mechanisms for the Instability of a Non-Self-Sustained Glow Discharge in Nitrogen. Plasma Phys. Rep. 2001, 27, 979–989. [Google Scholar] [CrossRef]
- Cejas, E.; Mancinelli, B.R.; Prevosto, L. Glow Discharge in a High-Velocity Air Flow: The Role of the Associative Ionization Reactions Involving Excited Atoms. Materials 2019, 12, 2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernyi, G.G.; Losev, S.A.; Macheret, S.O.; Potapkin, B.V. Physical and Chemical Processes in Gas Dynamics: Cross Sections and Rate Constants Vol. 1; AIAA: Reston, VA, USA, 2002; pp. 237–297. [Google Scholar]
- Piper, L.G. The reactions of N(2P) with O2 and O. J. Chem. Phys. 1993, 98, 8560–8564. [Google Scholar] [CrossRef]
- Golubkov, G.V.; Ozerov, G.K. The Near-Threshold Associative Ionization N(2D) + O(3P)→NO(X1Σ+) + e- Reaction. Doklady Phys. 2014, 59, 122–125. [Google Scholar] [CrossRef]
- Ringer, G.; Gentry, W.R. A merged molecular beam study of the endoergic associative ionization reaction N(2D) + O(3P) → NO+ + e−. J. Chem. Phys. 1979, 71, 1902–1909. [Google Scholar] [CrossRef]
- Kossyi, I.A.; Kostinsky, A.Y.; Matveyev, A.A.; Silakov, V.P. Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures. Plasma Sources Sci. Technol. 1992, 1, 207–220. [Google Scholar] [CrossRef]
- Capitelli, M.; Ferreira, C.M.; Gordiets, B.F.; Osipov, A.I. Plasma Kinetics in Atmospheric Gases; Springer: New York, NY, USA, 2000; pp. 66–154. [Google Scholar]
- Hurlbatt, A.; Gibson, A.R.; Schroter, S.; Bredin, J.; Foote, A.P.S.; Grondein, P.; O’Connell, D.; Gans, T. Concepts, Capabilities, and Limitations of Global Models: A Review. Plasma Process. Polym. 2017, 14, 1600138. [Google Scholar] [CrossRef] [Green Version]
- Boeuf, J.P.; Kunhardt, E.E. Energy balance in a nonequilibrium weakly ionized nitrogen discharge. J. Appl. Phys. 1986, 60, 915–923. [Google Scholar] [CrossRef]
- Breshears, W.D.; Bird, P.F. Effect of Oxygen Atoms on the Vibrational Relaxation of Nitrogen. J. Chem. Phys. 1968, 48, 4768–4773. [Google Scholar] [CrossRef]
- Eckstrom, D.J. Vibrational relaxation of shockheated N2 by atomic oxygen using the ir tracer method. J. Chem. Phys. 1973, 59, 2787–2795. [Google Scholar] [CrossRef]
- McNeal, R.J.; Whitson, M.E.; Cooke, G.R. Temperature Dependence of the Quenching of Vibrationally Excited Nitrogen by Atomic Oxygen. J. Geophys. Res. 1974, 79, 1527–1531. [Google Scholar] [CrossRef]
- Popov, N.A. Investigation of the Mechanism for Rapid Heating of Nitrogen and Air in Gas Discharges. Plasma Phys. Rep. 2001, 27, 886–896. [Google Scholar] [CrossRef]
- Pintassilgo, C.D.; Guerra, V. On the different regimes of gas heating in air plasmas. Plasma Sources Sci. Technol. 2015, 24, 055009. [Google Scholar] [CrossRef]
- D’Angola, A.; Colonna, G.; Bonomo, A.; Bruno, D.; Laricchiuta, A.; Capitelli, M. A phenomenological approach for the transport properties of air plasmas. Eur. Phys. J. D 2012, 66, 205. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Iza, F.; Brandenburg, R. Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef] [Green Version]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Becker, K. 20 years of microplasma research: A status report. Eur. Phys. J. D 2016, 70, 29. [Google Scholar] [CrossRef]
- André, P.; Barinov, Y.A.; Faure, G.; Shkol’nik, S.M. Characteristics of discharge with liquid nonmetallic cathode burning in air flow. J. Phys. D Appl. Phys. 2018, 51, 445202. [Google Scholar] [CrossRef] [Green Version]
- Stark, R.H.; Schoenbach, K.H. Direct current glow discharges in atmospheric air. Appl. Phys. Lett. 1999, 74, 3770–3772. [Google Scholar] [CrossRef] [Green Version]
- Leipold, F.; Stark, R.H.; El–Habachi, A.; Schoenbach, K.H. Electron density measurements in an atmospheric pressure air plasma by means of infrared heterodyne interferometry. J. Phys. D Appl. Phys. 2000, 33, 2268–2273. [Google Scholar] [CrossRef]
- Machala, Z.; Laux, C.O.; Kruger, C.H.; Candler, G.V. Atmospheric Air and Nitrogen DC Glow Discharges with Thermionic Cathodes and Swirl Flow. In Proceedings of the 42nd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 5–8 January 2004. [Google Scholar]
- Machala, Z.; Janda, M.; Hensel, K.; Jedlovský, I.; Leštinská, L.; Foltin, V.; Martišovitš, V.; Morvová, M. Emission spectroscopy of atmospheric pressure plasmas for bio-medical and environmental applications. J. Mol. Spectrosc. 2007, 243, 194–201. [Google Scholar] [CrossRef]
- Lu, X.P.; Leipold, F.; Laroussi, M. Optical and electrical diagnostics of a non-equilibrium air plasma. J. Phys. D Appl. Phys. 2003, 36, 2662. [Google Scholar] [CrossRef]
- Andre, P.; Barinov, Y.; Faure, G.; Kaplan, V.; Lefort, A.; Shkol’nik, S.; Vacher, D. Experimental study of discharge with liquid non-metallic (tap-water) electrodes in air at atmospheric pressure. J. Phys. D: Appl. Phys. 2001, 34, 3456. [Google Scholar] [CrossRef]
- Yu, L.; Laux, C.O.; Packan, D.M.; Kruger, C.H.J. Direct-current glow discharges in atmospheric pressure air plasmas. Appl. Phys. 2002, 91, 2678–2686. [Google Scholar] [CrossRef]
- Machala, Z.; Marode, E.; Laux, C.O.; Kruger, C.H.J. DC Glow Discharges in Atmospheric Pressure Air. Adv. Oxid. Technol. 2004, 7, 133–137. [Google Scholar] [CrossRef]
- Duten, X.; Packan, D.; Yu, L.; Laux, C.O.; Kruger, C.H. DC and Pulsed Glow Discharges in Atmospheric Pressure Air and Nitrogen. IEEE Trans. Plasma Sci. 2002, 30, 178–179. [Google Scholar] [CrossRef]
- Stepaniuk, V.P.; Ioppolo, T.; Ötügen, M.V.; Sheverev, V.A. Measurement of gas temperature and convection velocity profiles in a dc atmospheric glow discharge. J. Appl. Phys. 2007, 102, 123302. [Google Scholar] [CrossRef]
- Akishev, Y.; Grushin, M.; Karalnik, V.; Petryakov, A.; Trushkin, N. On basic processes sustaining constricted glow discharge in longitudinal N2 flow at atmospheric pressure. J. Phys. D Appl. Phys. 2010, 43, 215202. [Google Scholar] [CrossRef]
- Prevosto, L.; Kelly, H.; Mancinelli, B. Modelling of an Atmospheric Pressure Nitrogen Glow Discharge Operating in High-Gas Temperature Regimes. Plasma Chem. Plasma Process. 2016, 36, 973–992. [Google Scholar] [CrossRef]
- Kang, S.W.; Jones, W.L.; Dunn, M.G. Theoretical and Measured Electron-Density Distributions at High Altitudes. AIAA J. 1973, 11, 141–149. [Google Scholar] [CrossRef]
- Hagelaar, G.J.M.; Pitchford, L.C. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci. Technol. 2005, 14, 722–733, Freeware Code BOLSIG + Version 07/2015. Available online: http://www.bolsig.laplace.univ-tlse.fr (accessed on 14 November 2015). [CrossRef]
- SIGLO. Database. Available online: http://www.lxcat.laplace.univ-tlse.fr (accessed on 4 June 2013).
- Lin, S.C.; Teare, J.D. Rate of Ionization behind Shock Waves in Air. II. Theoretical Interpretations. Phys. Fluids 1963, 6, 355–375. [Google Scholar] [CrossRef]
- Le Padellec, A. Partial near Threshold Cross Sections for the Associative Ionization to Form CO+, NO+ and O2+. Phys. Scr. 2005, 71, 621–626. [Google Scholar] [CrossRef]
- Matveyev, A.A.; Silakov, V.P. Theoretical study of the role of ultraviolet radiation of the non-equilibrium plasma in the dynamics of the microwave discharge in molecular nitrogen. Plasma Sources Sci. Technol. 1999, 8, 162–178. [Google Scholar] [CrossRef]
- Brunet, H.; Roca Serra, J. Model for a glow discharge in flowing nitrogen. J. Appl. Phys. 1985, 57, 1574–1581. [Google Scholar] [CrossRef]
- Park, C. A Review of Reaction Rates in High Temperature Air. In Proceedings of the 24th Thermophysics Conference, Buffalo, NY, USA, 12–14 June 1989. [Google Scholar]
- Hellberg, F.; Rosén, S.; Thomas, R.; Neau, A.; Larsson, M.; Petrignani, P.; van der Zande, W.J. Dissociative recombination of NO+: Dynamics of the X1 Σ+ and a3 Σ+ electronic states. J. Chem. Phys. 2003, 118, 6250–6259. [Google Scholar] [CrossRef]
- Florescu, A.I.; Mitchell, J.B.A. Dissociative recombination. Phys. Rep. 2006, 430, 277–374. [Google Scholar]
- Herron, J.T. Evaluated Chemical Kinetics Data for Reactions of N(2D), N(2P), and N2(A3Σu+) in the Gas Phase. J. Phys. Chem. Ref. Data 1999, 28, 1453–1483. [Google Scholar] [CrossRef]
- Piper, L.G. Statetostate N2(A3Σu+) energy pooling reactions. II. The formation and quenching of N2(B3Πg, v′=1–12). J. Chem. Phys. 1988, 88, 6911–6921. [Google Scholar] [CrossRef]
- Gordiets, B.F.; Ferreira, C.M.; Guerra, V.L.; Loureiro, J.M.A.H.; Nahorny, J.; Pagnon, D.; Touzeau, M.; Vialle, M. Kinetic Model of a Low–Pressure N2–O2 Flowing Glow Discharge. IEEE Trans. Plasma Sci. 1995, 23, 750–768. [Google Scholar] [CrossRef]
- Shkurenkov, I.; Burnette, D.; Lempert, W.R.; Adamovich, I.V. Kinetics of excited states and radicals in a nanosecond pulse discharge and afterglow in nitrogen and air. Plasma Sources Sci. Technol. 2014, 23, 065003. [Google Scholar] [CrossRef]
- Piper, L.G. Quenching rate coefficients for N2(a′1Σu-). J. Chem. Phys. 1987, 87, 1625–1629. [Google Scholar] [CrossRef]
- Pancheshnyi, S.V.; Starikovskaia, S.M.; Starikovskii, A.Y. Collisional deactivation of N2(C3Πu, v=0,1,2,3) states by N2, O2, H2 and H2O molecules. Chem. Phys. 2000, 262, 349–357. [Google Scholar] [CrossRef]
- Siskind, D.E.; Barth, D.A.; Cleary, D.D. The Possible Effect of Solar Soft X Rays on Thermospheric N itric Oxide. J. Geophys. Res. 1990, 95, 4311–4317. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cejas, E.; Mancinelli, B.; Prevosto, L. Modelling of an Atmospheric–Pressure Air Glow Discharge Operating in High–Gas Temperature Regimes: The Role of the Associative Ionization Reactions Involving Excited Atoms. Plasma 2020, 3, 12-26. https://doi.org/10.3390/plasma3010003
Cejas E, Mancinelli B, Prevosto L. Modelling of an Atmospheric–Pressure Air Glow Discharge Operating in High–Gas Temperature Regimes: The Role of the Associative Ionization Reactions Involving Excited Atoms. Plasma. 2020; 3(1):12-26. https://doi.org/10.3390/plasma3010003
Chicago/Turabian StyleCejas, Ezequiel, Beatriz Mancinelli, and Leandro Prevosto. 2020. "Modelling of an Atmospheric–Pressure Air Glow Discharge Operating in High–Gas Temperature Regimes: The Role of the Associative Ionization Reactions Involving Excited Atoms" Plasma 3, no. 1: 12-26. https://doi.org/10.3390/plasma3010003
APA StyleCejas, E., Mancinelli, B., & Prevosto, L. (2020). Modelling of an Atmospheric–Pressure Air Glow Discharge Operating in High–Gas Temperature Regimes: The Role of the Associative Ionization Reactions Involving Excited Atoms. Plasma, 3(1), 12-26. https://doi.org/10.3390/plasma3010003






