A New Cold Plasma Jet: Performance Evaluation of Cold Plasma, Hybrid Plasma and Argon Plasma Coagulation
Abstract
:1. Introduction
2. Materials and Methods
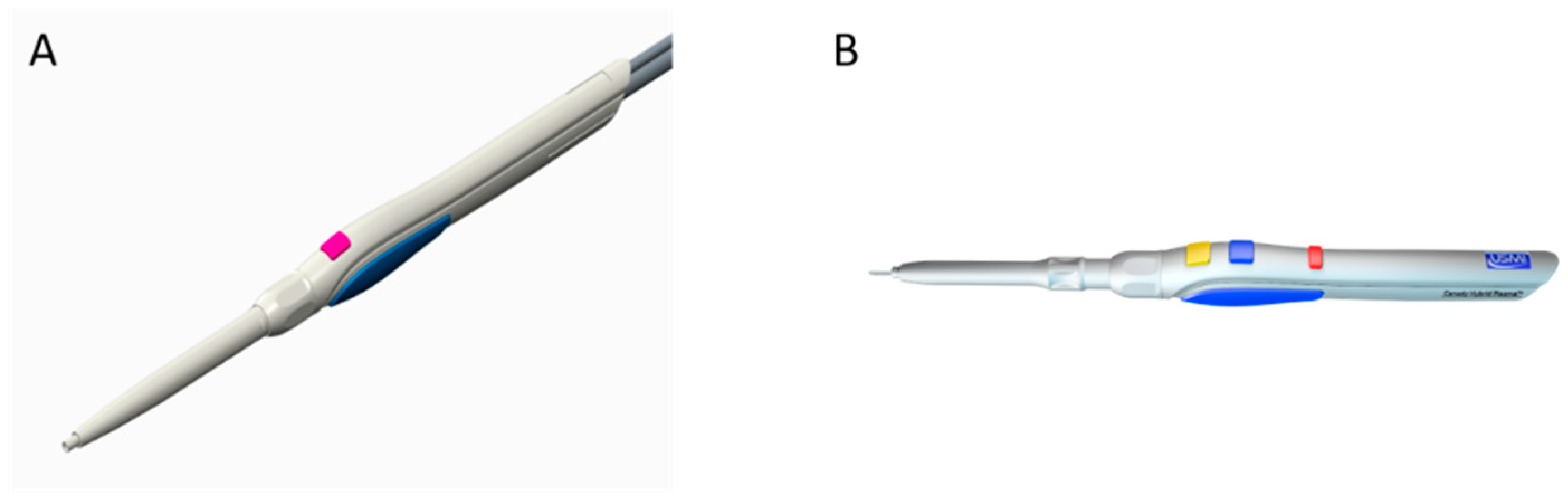
2.1. Canady Plasma® Scalpels
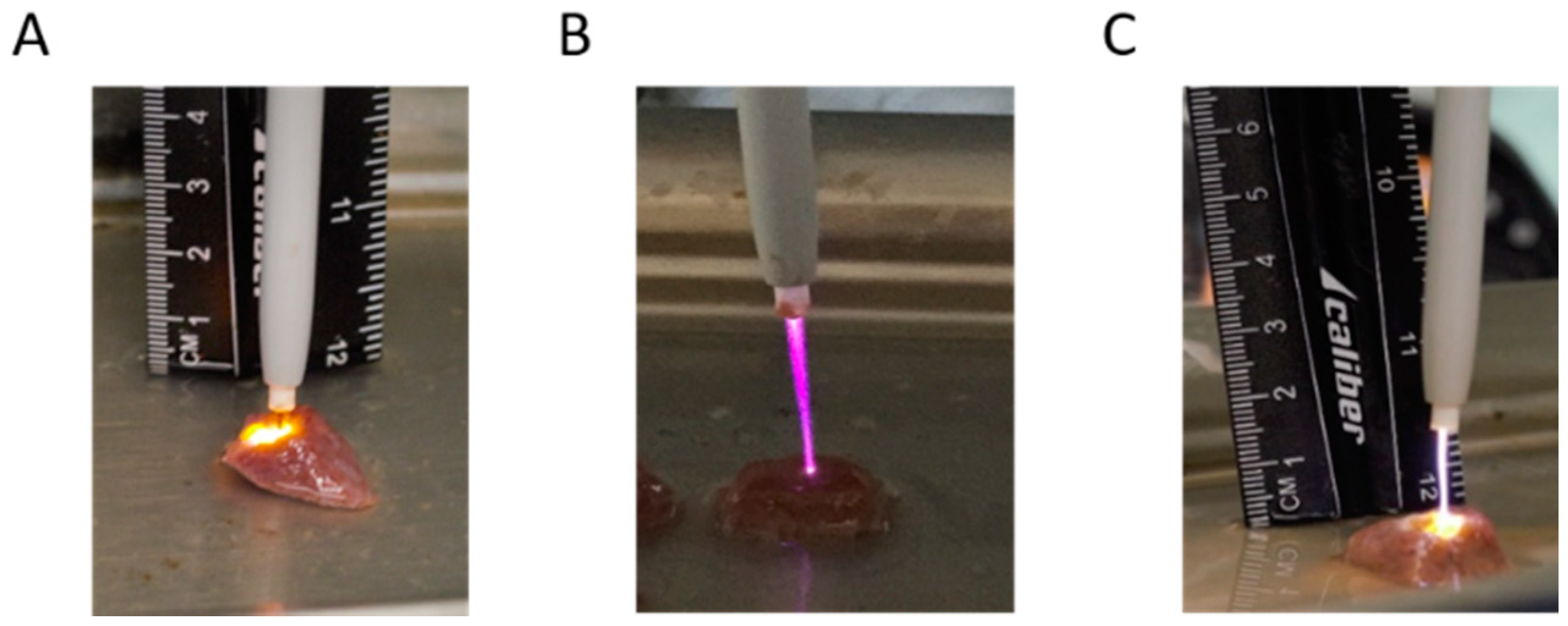
2.1.1. CAP Mode
2.1.2. APC Mode
2.1.3. Hybrid Mode
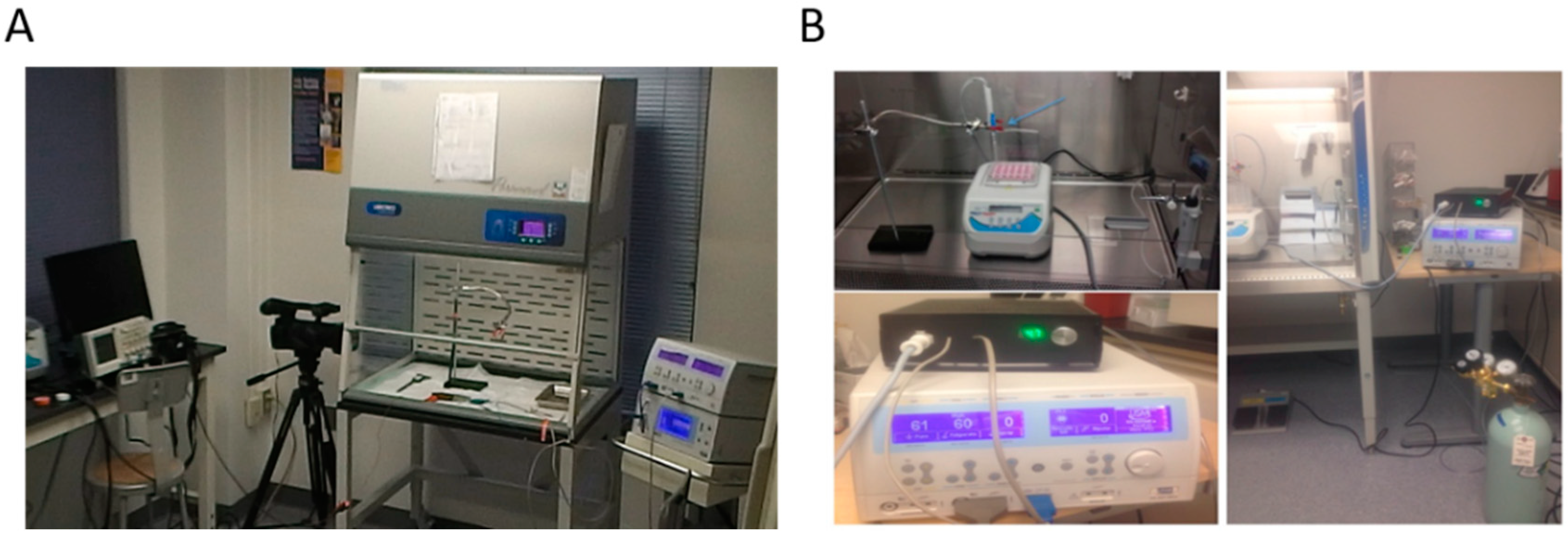
2.2. Preparation and Treatment Conditions of Liver Tissue Samples
2.3. Statistics
3. Results
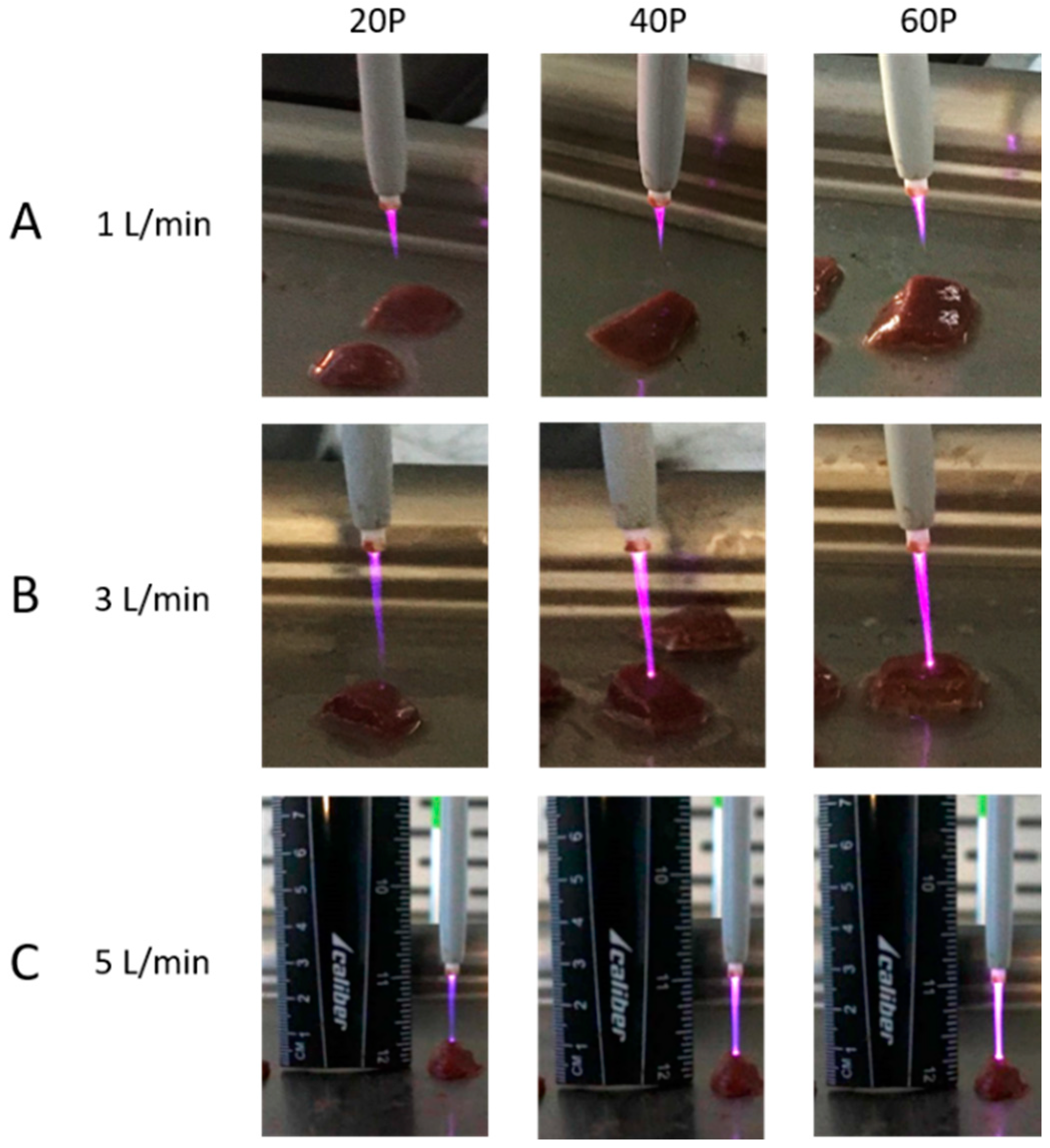
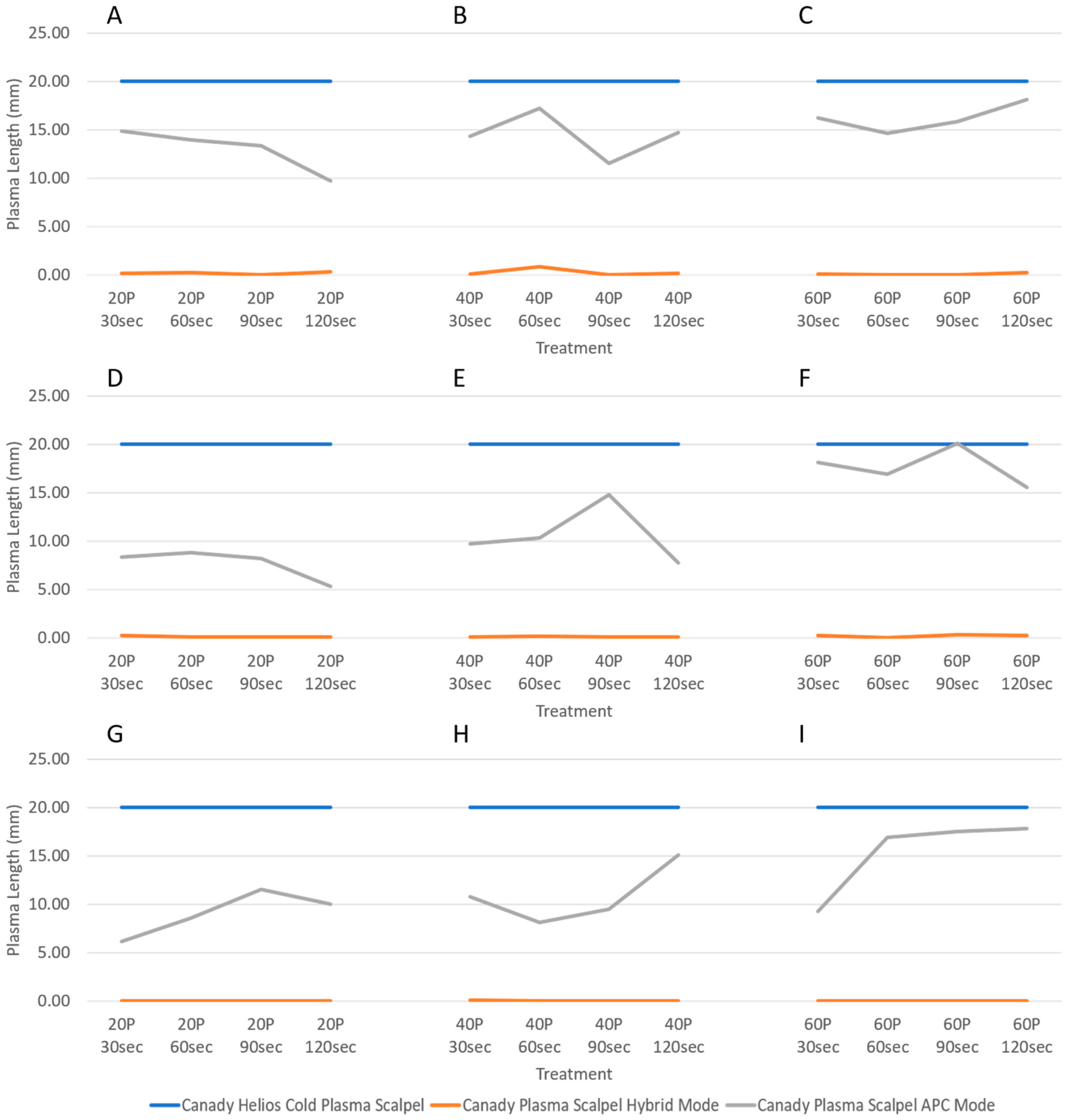
3.1. Plasma Length Measurements
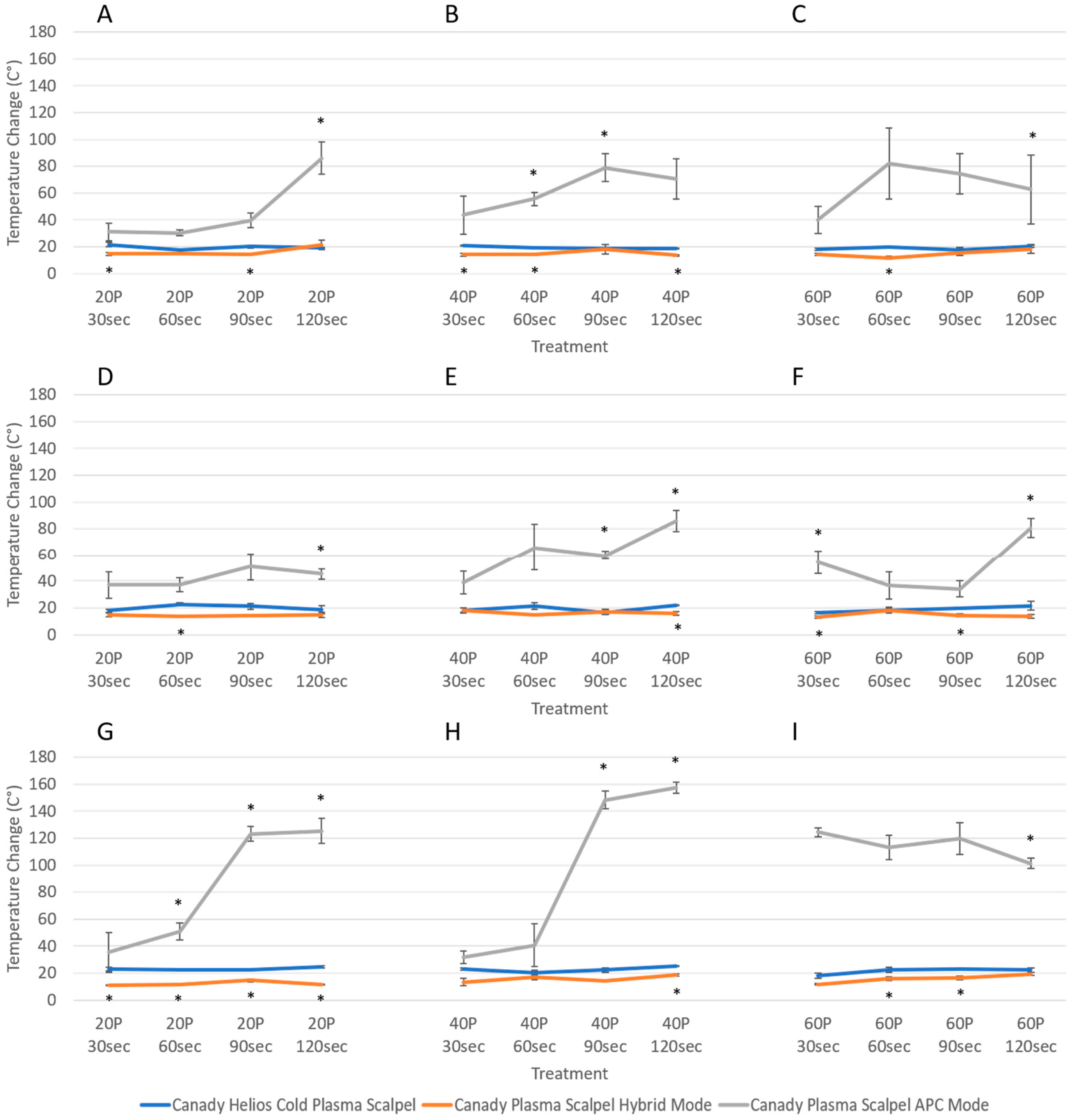
3.2. Temperature Change Measurements
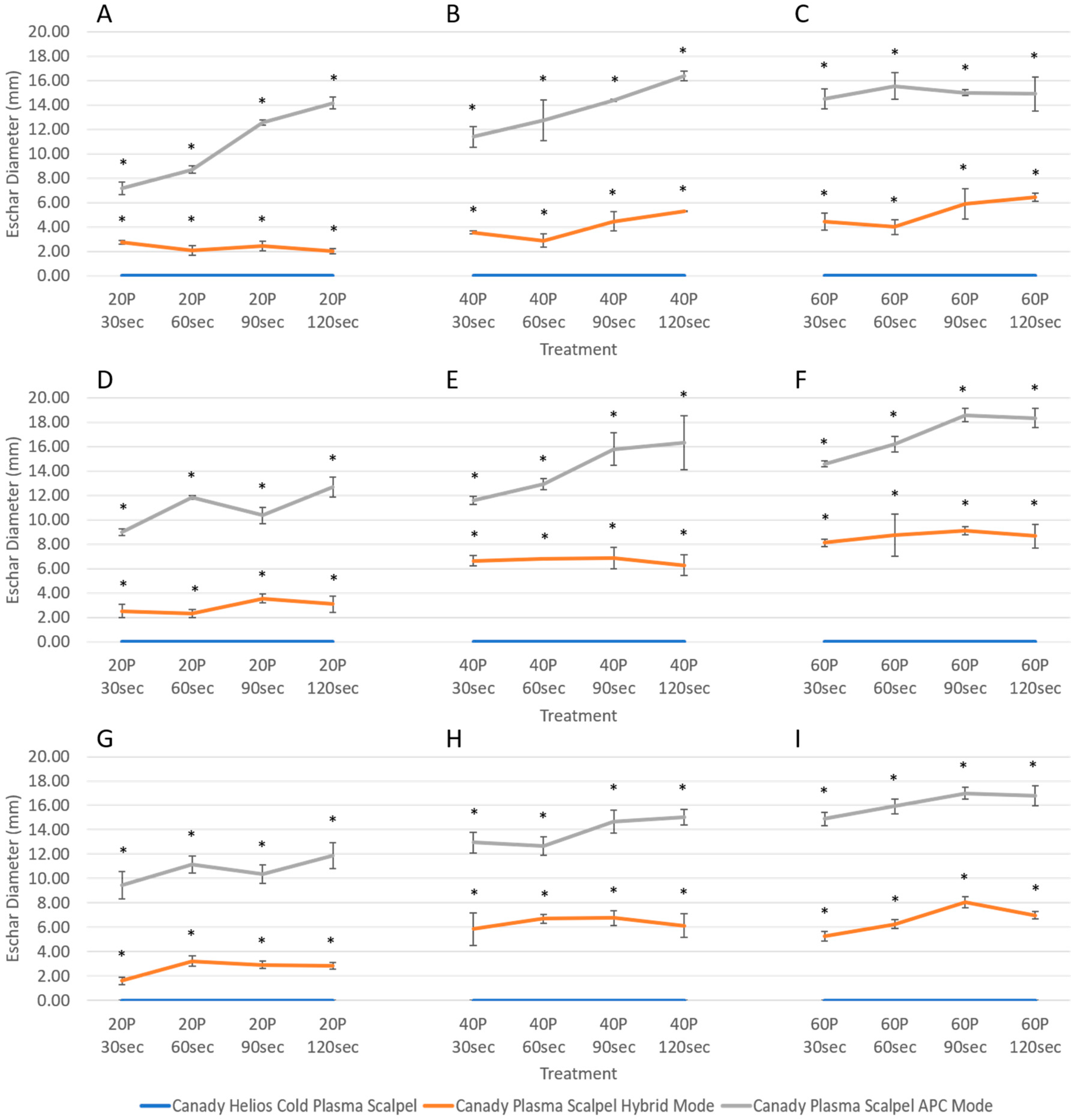
3.3. Variability in Injury Magnitude: Eschar Diameter
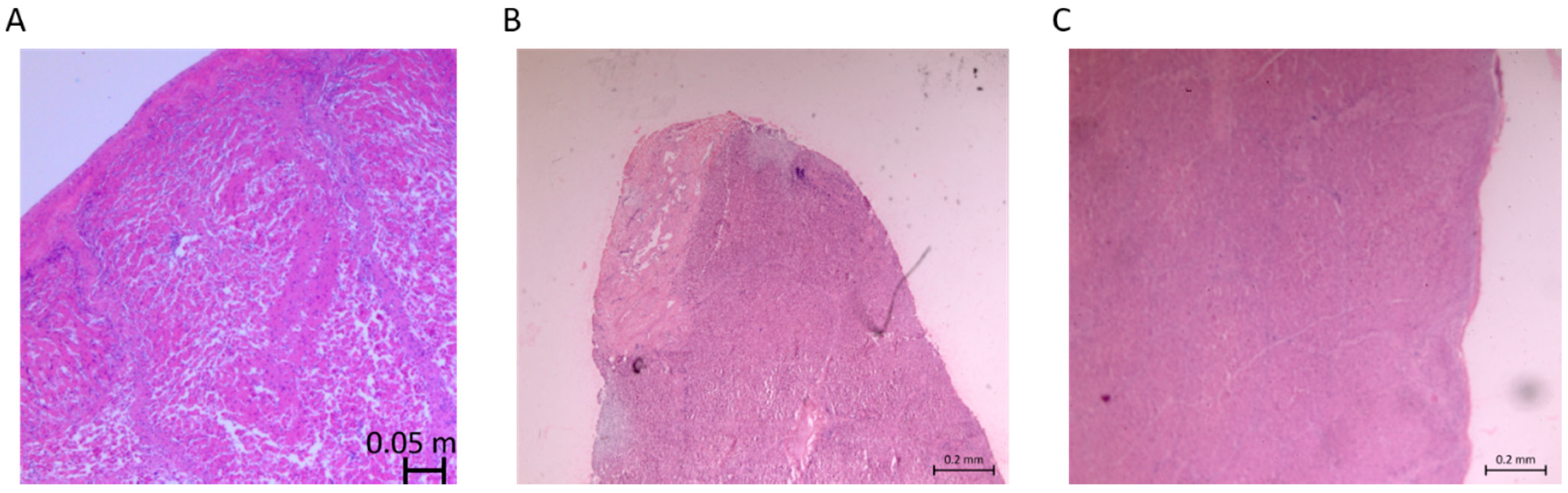
3.4. Variability in Injury Magnitude: Depth of Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Arsonval, A. Action physiologique des courants alternatis a grande frequence. Arch. Physiol. Porm. Pathol. 1893, 5, 780–790. [Google Scholar]
- Bovie, W.T. Electrosurgical Apparatus. U.S. Patent 1,813,902, 14 July 1931. [Google Scholar]
- Cushing, H.; Bovie, W.T. Electrosurgery as an aid to the removal of intracranial tumors. In Surgery, Gynecology and Obstetrics; Surgical Publishing Company: Chicago, IL, USA, 1928. [Google Scholar]
- Morrison, C.F., Jr. Electrosurgical Method and Apparatus for Initiating an Electrical Discharge in an Inert Gas Flow. U.S. Patent 40,404,426, 9 August 1977. [Google Scholar]
- Canady, J. Surgical Coagulation Device. U.S. Patent 5,207,675, 4 May 1993. [Google Scholar]
- Canady, J.; Vieira, E.; Vieira, N.; Wiley, K. System and Method for Electrosurgical Conductive Gas Cutting for Improving Eschar, Sealing Vessels and Tissues. U.S. Patent 2013/0296846, 2 November 2010. [Google Scholar]
- Gjika, E.; Pekker, M.; Shashurin, A.; Shneider, M.; Zhuang, T.; Canady, J.; Keidar, M. The cutting mechanism of the electrosurgical scalpel. J. Phys. D Appl. Phys. 2017, 50, 025401. [Google Scholar] [CrossRef]
- Gjika, E.; Scott, D.; Shashurin, A.; Zhuang, T.; Canady, J.; Keidar, M. Plasma-tissue interactions in argon plasma coagulation: Effects of power and tissue resistance. Plasma Med. 2016, 6, 125–134. [Google Scholar] [CrossRef]
- Canady, J.; Shashurin, A.; Wiley, K.; Fisch, N.J.; Keidar, M. Characterization of plasma parameters and tissue injury produced by plasma electrosurgical systems. Plasma Med. 2013, 3, 279–289. [Google Scholar] [CrossRef]
- Canady, J.; Shashurin, A.; Keidar, M.; Zhuang, T. Integrated Cold Plasma and High Frequency Plasma Electrosurgical System and Method. U.S. Patent 9,999,452, 19 June 2018. [Google Scholar]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Ann Stepp, M.; Srinivasan, P.; Sandler, A.; Trink, B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma jet-generated rons and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Park, J.H.; Jeon, S.N.; Park, B.S.; Choi, E.H.; Attri, P. The action of microsecond-pulsed plasmaactivated media on the inactivation of human lung cancer cells. J. Phys. D App. Phys. 2016, 49, 115401. [Google Scholar] [CrossRef]
- Kumar, N.; Attri, P.; Dewilde, S.; Bogaerts, A. Inactivation of human pancreatic ductal adenocarcinoma with atmospheric plasma treated media and water: A comparative study. J. Phys. D App. Phys. 2018, 51, 255401. [Google Scholar] [CrossRef]
- Rowe, W.; Cheng, X.; Ly, L.; Zhuang, T.; Basadonna, G.; Trink, B.; Keidar, M.; Canady, J. The Canady Helios Cold Plasma Scalpel Significantly Decreases Viability in Malignant Solid Tumor Cells in a Dose-Dependent Manner. Plasma 2018. accepted. [Google Scholar] [CrossRef]
- Cheng, X.; Rowe, W.; Ly, L.; Shashurin, A.; Zhuang, T.; Wigh, S.; Basadonna, G.; Trink, B.; Keidar, M.; Canady, J. Treatment of Triple-negative Breast Cancer Cells with the Canady Cold Plasma Conversion System: Preliminary Results. Plasma 2018. submitted. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, L.; Jones, S.; Shashurin, A.; Zhuang, T.; Rowe, W.; Cheng, X.; Wigh, S.; Naab, T.; Keidar, M.; Canady, J. A New Cold Plasma Jet: Performance Evaluation of Cold Plasma, Hybrid Plasma and Argon Plasma Coagulation. Plasma 2018, 1, 189-200. https://doi.org/10.3390/plasma1010017
Ly L, Jones S, Shashurin A, Zhuang T, Rowe W, Cheng X, Wigh S, Naab T, Keidar M, Canady J. A New Cold Plasma Jet: Performance Evaluation of Cold Plasma, Hybrid Plasma and Argon Plasma Coagulation. Plasma. 2018; 1(1):189-200. https://doi.org/10.3390/plasma1010017
Chicago/Turabian StyleLy, Lawan, Sterlyn Jones, Alexey Shashurin, Taisen Zhuang, Warren Rowe, Xiaoqian Cheng, Shruti Wigh, Tammey Naab, Michael Keidar, and Jerome Canady. 2018. "A New Cold Plasma Jet: Performance Evaluation of Cold Plasma, Hybrid Plasma and Argon Plasma Coagulation" Plasma 1, no. 1: 189-200. https://doi.org/10.3390/plasma1010017
APA StyleLy, L., Jones, S., Shashurin, A., Zhuang, T., Rowe, W., Cheng, X., Wigh, S., Naab, T., Keidar, M., & Canady, J. (2018). A New Cold Plasma Jet: Performance Evaluation of Cold Plasma, Hybrid Plasma and Argon Plasma Coagulation. Plasma, 1(1), 189-200. https://doi.org/10.3390/plasma1010017





