3.1. Deposition Rate of C:H:O Films at Various Conditions
Deposition rates of C:H:O plasma polymer films were characterized under different RF plasma powers in vapours of pure DMC with a constant flow rate of 6 sccm for the two working pressures of 10 and 20 Pa. These two pressure parameters have been selected, since they mainly reveal a difference in ion energies of charged species incident on the substrate surface (roughly factor 2) due to collisions in the plasma sheath, while other parameters can largely be maintained (such as ion flux and energy input in the plasma zone) [
28]. Lower pressures (<10 Pa) result in even stronger ion bombardment supporting ion-assisted ablation reactions, whereas higher pressures (>20 Pa) yield a reduced plasma length. It is thus no longer given that the plasma uniformly fills the entire volume between the electrodes (5 cm gap) of the symmetric plasma reactor affecting the energy available per particle in the plasma [
29]. The selection of the pressure range is thus reactor dependent. As can be seen in
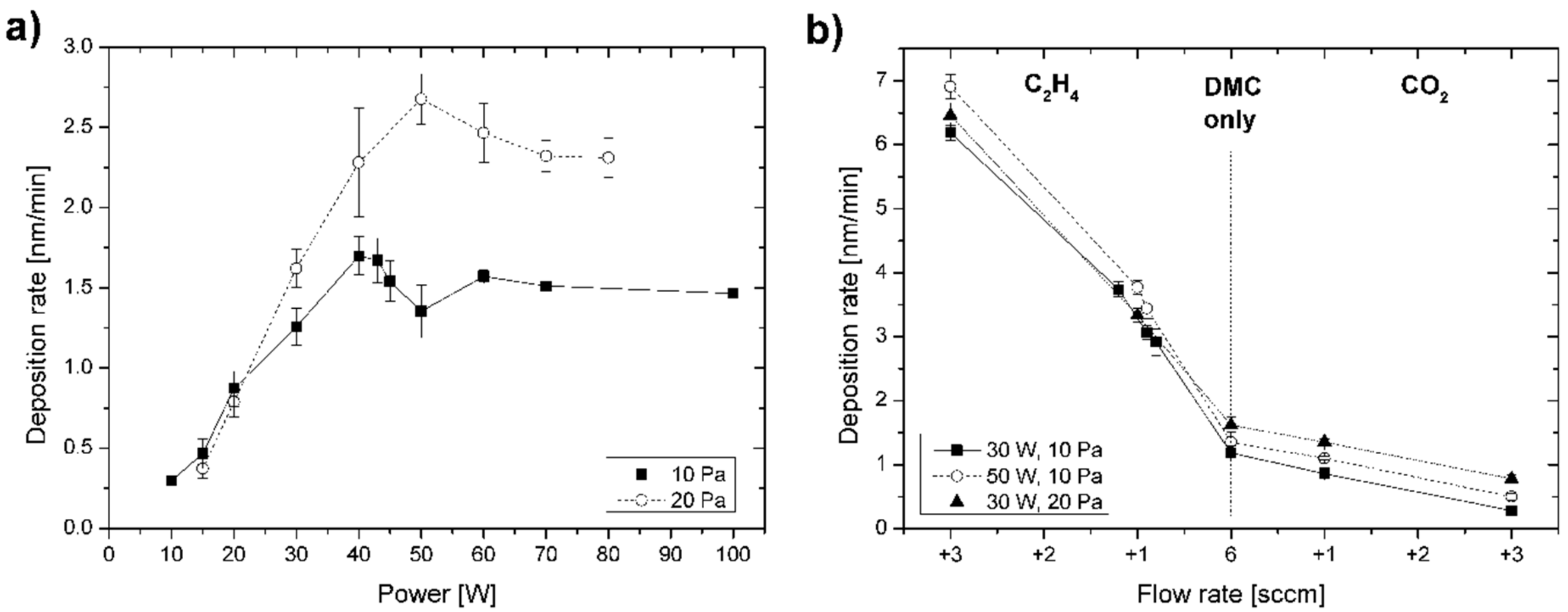
Figure 1a, the deposition rates observed were rather low owing to the high [O]/[C] ratio of 1 for DMC and reached ~1 nm·min
−1 only above a power input of 20 W. Note that no film deposition was observed below 10 W for the continuous plasma discharge mode, while pulsed power conditions enabled deposition at lower effective powers which is, however, not discussed in more detail here. The deposition rate increased with discharge power to attain maximum (1.7 nm·min
−1 at 40 W for the pressure of 10 Pa and 2.7 nm·min
−1 at 50 W for 20 Pa) before decreasing slightly and saturating above 60 W (1.5 nm·min
−1 for 10 Pa and 2.3 nm·min
−1 for 20 Pa). Higher rates were thus observed for higher pressure, yet following the same trend with power (or power per monomer flow rate,
W/
F). The energy invested per monomer molecule within the active plasma zone is enhanced with power at constant gas flow rate resulting in more film-forming species in the plasma phase and thus an increased deposition rate [
30].
The drop in the deposition rate above 40–50 W thus indicates a change in the plasma chemical reaction pathway, which was observed for an energy uptake per DMC molecule of 41 ± 6 eV (considering absorbed power and plasma length). Such energies are expected to fully destruct the monomer structure, that is, the carbonate group, as this is well known from similar monomers [
8,
31]. Interestingly, CO
2/C
2H
4 plasmas with the same [O]/[C] ratio of 1 in the gas phase (i.e., for CO
2/C
2H
4 ratios of 2:1) were found to show a similar trend in deposition rate with a transition in plasma chemistry at 37 ± 3 eV, however, at higher overall deposition rates [
18,
19,
32]. On the other hand, the increasing power simultaneously increased the energy transferred to the growing film resulting in etching processes, densification and cross-linking of the structure of the plasma polymer film [
28,
32]. The etching products are typically volatile CO and H
2O species which are pumped from the system without being incorporated into the plasma polymer film. Given that gas phase processes yielding the flux of film-forming species depend on
W/
F (and not on pressure), the observed differences in absolute deposition rates at 10 and 20 Pa are related to surface processes, that is, a different surface loss probability (involving sticking and etching processes). While the energy per monomer molecule in the plasma phase is pressure-invariant, the higher pressure enhances the residence time close to the surface and affects ion-assisted processes (due to more collisions). Additional effects related to the ion energy delivered to the surface are discussed in the literature including the contribution of hyperthermal intact molecular ions which becomes important at enhanced pressures [
33,
34]. Such ions (as formed in the gas phase at moderate energy input) arrive at low energies (several eV) at the surface resulting in their “soft landing”. Their incorporation and reduced destruction at higher pressure conditions can thus contribute to the structural retention of monomer properties in the growing film [
34,
35].
To further evaluate the influence of oxygen on the deposition of DMC-derived C:H:O PPFs, deposition rates were recorded for processes taking place at variable working gas compositions with fixed power input below and around the observed transition in the plasma phase (30 W for 10 and 20 Pa vs. 50 W for 10 Pa) as displayed in
Figure 1b. Particularly, either CO
2 or C
2H
4 reactive gases were added at flow rates of 1–3 sccm to the DMC monomer vapour (with fixed flow rate of 6 sccm) in order to investigate co-polymerization conditions regarding increasing and decreasing relative amounts of O-species in the discharge, respectively. As could be expected, introduction of more film forming species by ethylene (decreasing the O/C ratio) strongly increased the deposition rate reaching 6–7 nm·min
−1 when 3 sccm of ethylene was added. Admixture of additional O-species by CO
2 (further increasing the [O]/[C] ratio), on the other hand, led to a decrease in the deposition rate down to 0.3–0.8 nm·min
−1 when 3 sccm of CO
2 was added to the discharge. Low deposition rates indicate highly energetic conditions during film growth and rivaling deposition/etching processes: incorporation of oxygen is limited by etching and cross-linking reactions. The growth/ablation regime of the DMC discharge can therefore be supported by co-polymerization with a proper reactive gas. Again, the dependence of deposition rates on the working gas composition was qualitatively the same for different combinations of powers and pressures examined, with higher deposition rates for the higher pressure. However, it is interesting to note that discharge power has a stronger effect on the deposition rate of the films in the growth regime indicating distinct energy uptake by C
2H
4, while working gas pressure dominates in the ablation regime highlighting the importance of surface processes. While showing the same trend as CO
2/C
2H
4 and CO
2/C
3H
6O plasmas with increasing CO
2 admixture at comparable conditions [
19], the growth (+C
2H
4) and ablation (+CO
2) regimes appear to be more pronounced for DMC co-polymerization conditions (see
Appendix A,
Figure A1).
3.2. Wettability of C:H:O Plasma Polymer Films
Wettability of the deposited C:H:O PPFs is one of the most important parameters with respect to the potential application of the coatings in the biomedical field. Wettability, as the key factor for the controlled degradation of the films, was studied in terms of static water contact angle (WCA) measured by the sessile droplet method. The WCA of the as-prepared C:H:O PPFs from pure DMC vapours were largely independent of deposition power at both 10 Pa and 20 Pa showing values of 58 ± 2°. The WCA value can thus be considered as constant within the measurement error and was therefore not displayed here. Note that the WCA obtained for the films deposited at 30 W (59°) was comparable to CO
2/C
2H
4 2:1 plasmas [
36] but lower than the WCA of C:H:O coatings deposited at the same plasma conditions from acetone (C
3H
6O) vapours (67°) owing to their lower [O]/[C] ratio [
19]. The wettability of the as-prepared C:H:O PPFs depending on the gas composition (as well as pressure at various powers) are shown in
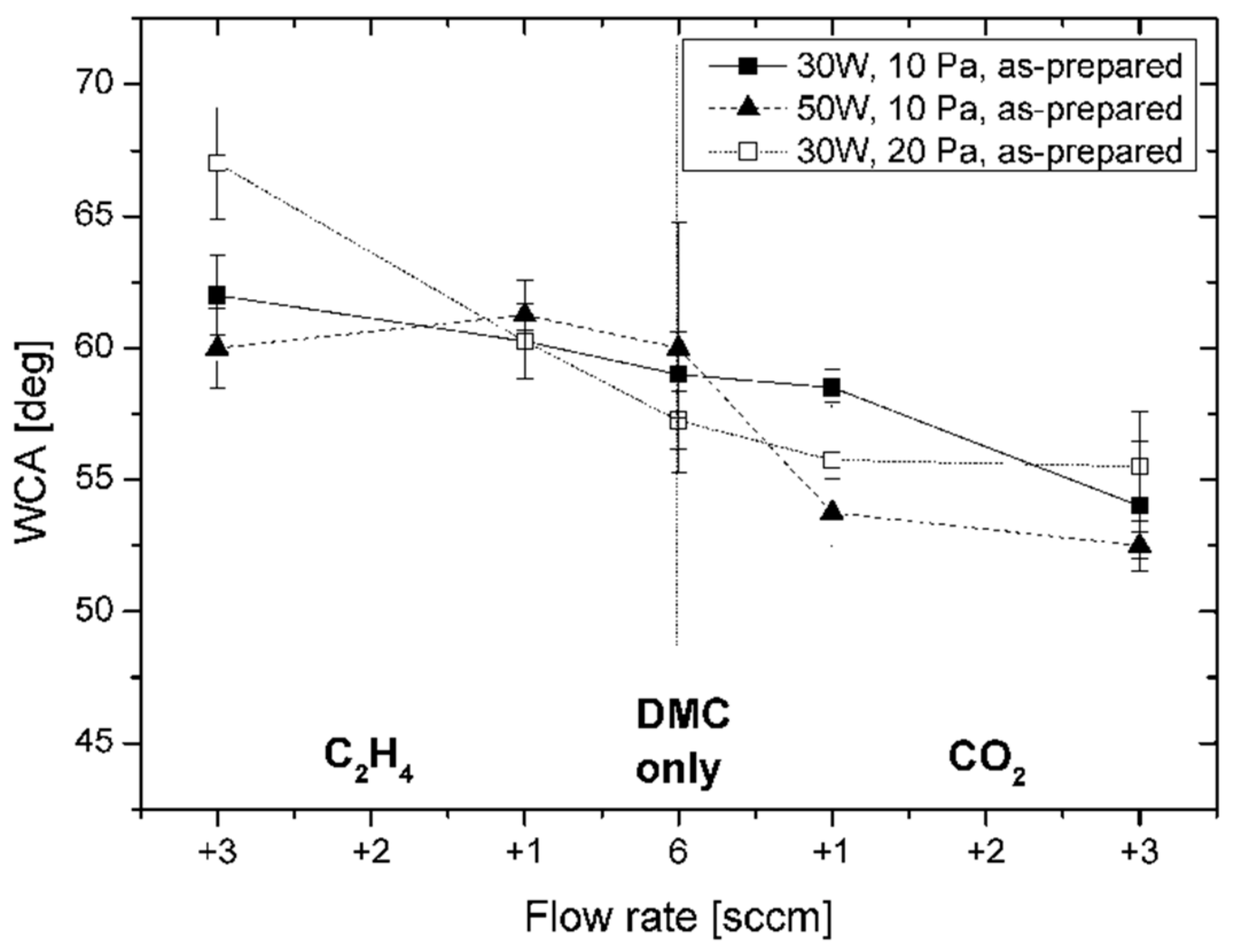
Figure 2.
The obtained values varied in the range from 53° to 67° influenced by the working gas composition (expressed in terms of flow rates for the individual working gases). The addition of CO
2 to DMC slightly decreased the contact angle with water reaching a value of 54° with 3 sccm of CO
2 in the plasma (30 W, 10 Pa), that is, at a CO
2/DMC ratio of 1:2. Again, C:H:O films deposited with the corresponding CO
2/acetone ratio of 1:2 showed a higher WCA value of 62° and ratios of 4:1 were required to reach comparable low WCAs (see
Appendix A,
Figure A2a) [
19]. The addition of C
2H
4 gas, on the other hand, increased the WCA values yielding 62° with 3 sccm of C
2H
4 (30 W, 10 Pa), that is, at a DMC:C
2H
4 ratio of 2:1. The effect of power (30 or 50 W) and pressure (10 or 20 Pa) variation was found to be small (within ±5°). By changing the working gas composition, the amount of oxygen (i.e., the [O]/[C] ratio) present in the discharge was influenced which can be expected to alter the chemical reactions taking place in the plasma volume as well as on the surface of the growing film. Related effects will be discussed in more detail below.
The aging of the deposited C:H:O PPFs was studied by following the evolution of the water contact angle values over time. As can be seen in
Figure 3, the WCA values of C:H:O films changed during their storage in ambient air for 1 or 2 months. This difference was expressed in terms of a relative WCA, that is, the ratio of the WCA value measured after aging to the initial value. The most pronounced changes were observed in the case of C:H:O films deposited from pure DMC vapours at different RF discharge powers (
Figure 3a). While all films revealed almost the same wetting properties observed right after deposition (58 ± 2°), their WCA values shifted in time depending on the deposition conditions, particularly RF power showing different behaviour for low and high powers. The highest stability (almost constant wettability) was observed for DMC-derived C:H:O films deposited at a moderate power input slightly below the plasma chemical transition (30 W at 10 Pa and 40 W at 20 Pa). For low power input of 10 and 15 W, on the other hand, the WCA dropped by up to 20°, whereas increased power resulted in enhanced WCAs up to 68°. With time the differences became even larger—the aging continued after the first month, albeit at a slower pace (
Figure 3a—black squares vs. white circles). Comparing the PPFs deposited from pure DMC at the higher pressure of 20 Pa with 10 Pa, the curve for the relative WCA was shifted towards higher powers and also the differences between the WCA values of the as-prepared and the aged C:H:O films were larger, likely owing to the nominally higher power needed to reach the transition in deposition rate and related changes in the plasma chemical reaction pathway as well as modified surface processes. It is interesting to note that the WCA aging behaviour corresponds to that described in the case of C:H:O films deposited from acetone vapours with addition of CO
2 (1:2, i.e., with the same [O]/[C] ratio of 1): the most stable films were those deposited at 30 W and revealed water contact angles of about 55° [
19]. The films deposited at lower powers revealed a decrease in the WCA values and it was concluded that these films initially contained more oxygen and were less crosslinked (allowing hydration). On the other hand, those deposited at higher powers revealed an increase in WCA upon aging (indicating hydrophobic recovery).
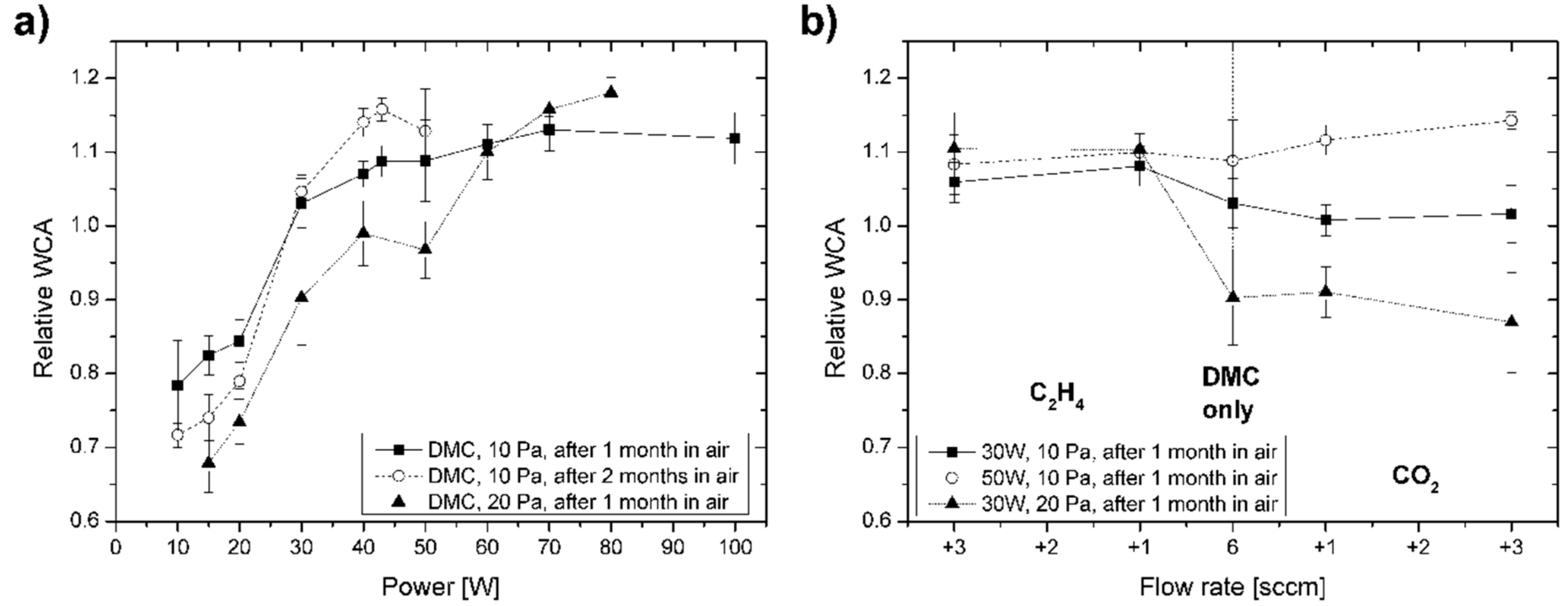
The aging of the C:H:O PPFs deposited from various mixtures of DMC with CO
2 and C
2H
4 is displayed in
Figure 3b. Obviously, also the working gas composition had a noticeable impact on the change of WCA during aging of the films stored in ambient air. Similar to the power dependence discussed before, the coatings deposited at 30 W and 10 Pa appeared to be the most stable (black squares in
Figure 3b). Especially, this holds for the case when 1 or 3 sccm of CO
2 were added to the DMC flow rate of 6 sccm during deposition where the WCA value was almost constant within 1 month of storage. On the other hand, the C:H:O PPFs prepared with higher power input of 50 W showed hydrophobic recovery, while the reduced WCAs for coatings deposited at higher pressure (30 W, 20 Pa; black triangles in
Figure 3b) indicate hydration effects. Addition of C
2H
4 resulted in a roughly 10% increase in WCAs reaching typical values for hydrocarbon-rich PPFs around 70° for all compositions. In general, the aging behaviour was again found to be very similar to the aging observed for the C:H:O coatings deposited from vapours of acetone and CO
2 (see
Appendix A,
Figure A2b) as reported in [
19].
The PPFs deposited at 30 W and 10 Pa which appeared to be the most stable in air were selected for an extended analysis of the aging effects including storage in water (
Figure 4). The observed relative changes in the WCA values (compared to the initial WCAs) upon aging in air are depicted in
Figure 4a, while the relative changes upon aging in water are given in
Figure 4b. As discussed before, the most stable coatings in air are those deposited from pure DMC vapours or with addition of low amounts of CO
2. Most of all, it can be noted that aging largely took place during the first day of storage in air after deposition.
Steadily dropping water contact angles, on the other hand, were observed in the case of storage in water. Such effects were also observed for (oxygen-rich) CO
2/C
2H
4 derived PPFs and were related to water penetration and binding of water molecules on polar groups that remain adsorbed in the films [
37]. Addition of carbon dioxide to the DMC-derived plasma polymer films resulting in the most hydrophilic C:H:O films revealed the fastest changes (within one-day storage in water), while addition of ethylene yielding more hydrophobic films delayed the interaction with water molecules during storage in water. Note that the measurement of WCA values after water storage was subject to larger deviations compared to storage in ambient air as indicated by the error bars.
Plasma polymer films deposited with the same conditions (co-polymerized DMC using 30 W power input) but at the enhanced pressure of 20 Pa generally showed an even stronger decrease in water contact angles with storage time in air and water albeit showing larger deviations indicating limited stability. Possible effects such as hydration and degradation will be further discussed below. Since wettability (described in terms of the static water contact angle) is dependent both on the structure and chemical composition of the coatings as well as the surface topography, a thorough analysis of the structure of the coatings is required. The films deposited at 30 W and 10 Pa which tend to be the most stable were chosen for further analysis of their aging effects compared to the films deposited at 30 W and 20 Pa that revealed a lower level of stability.
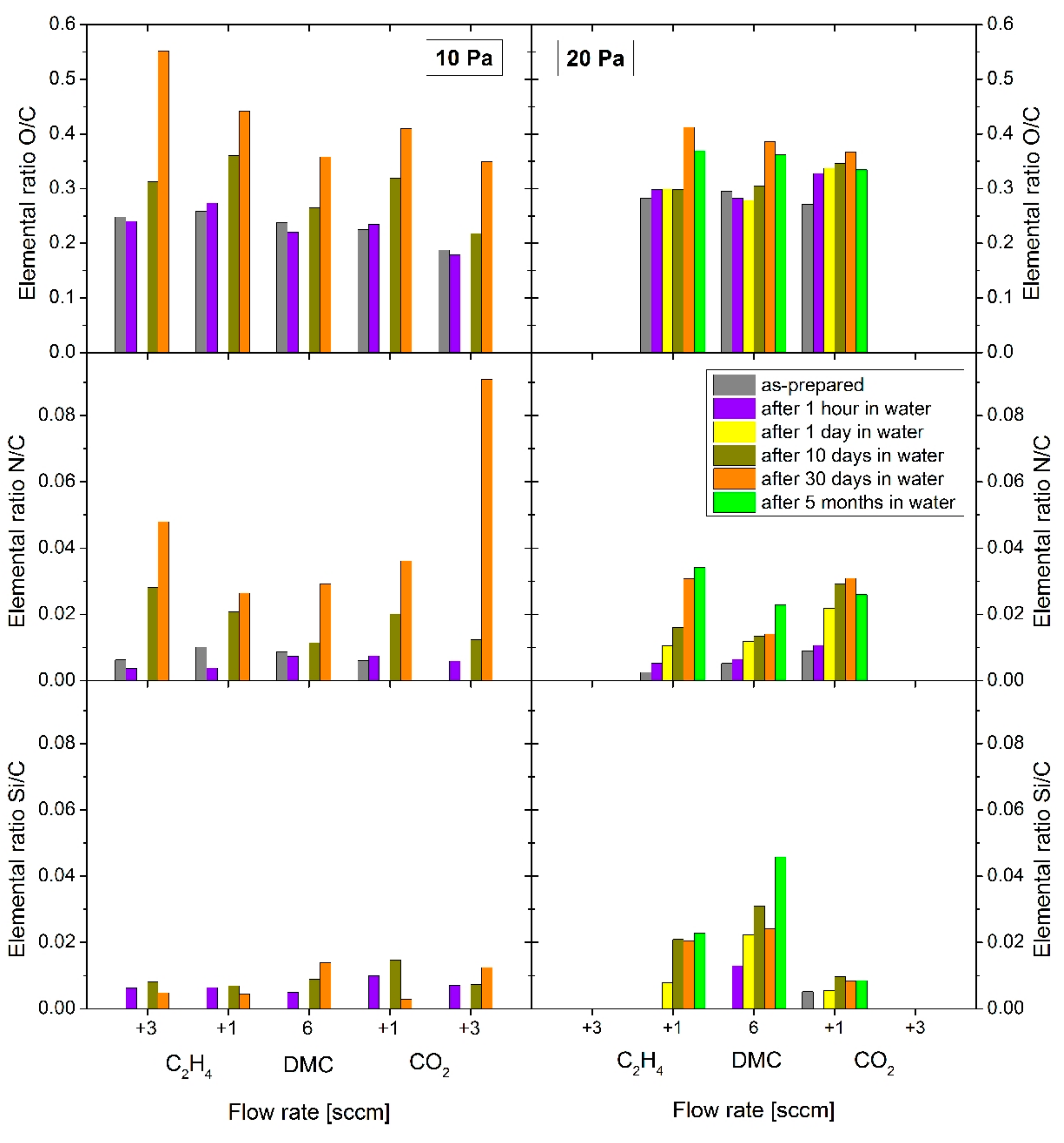
3.3. Characterization of Surface Elemental Composition of C:H:O PPFs by XPS
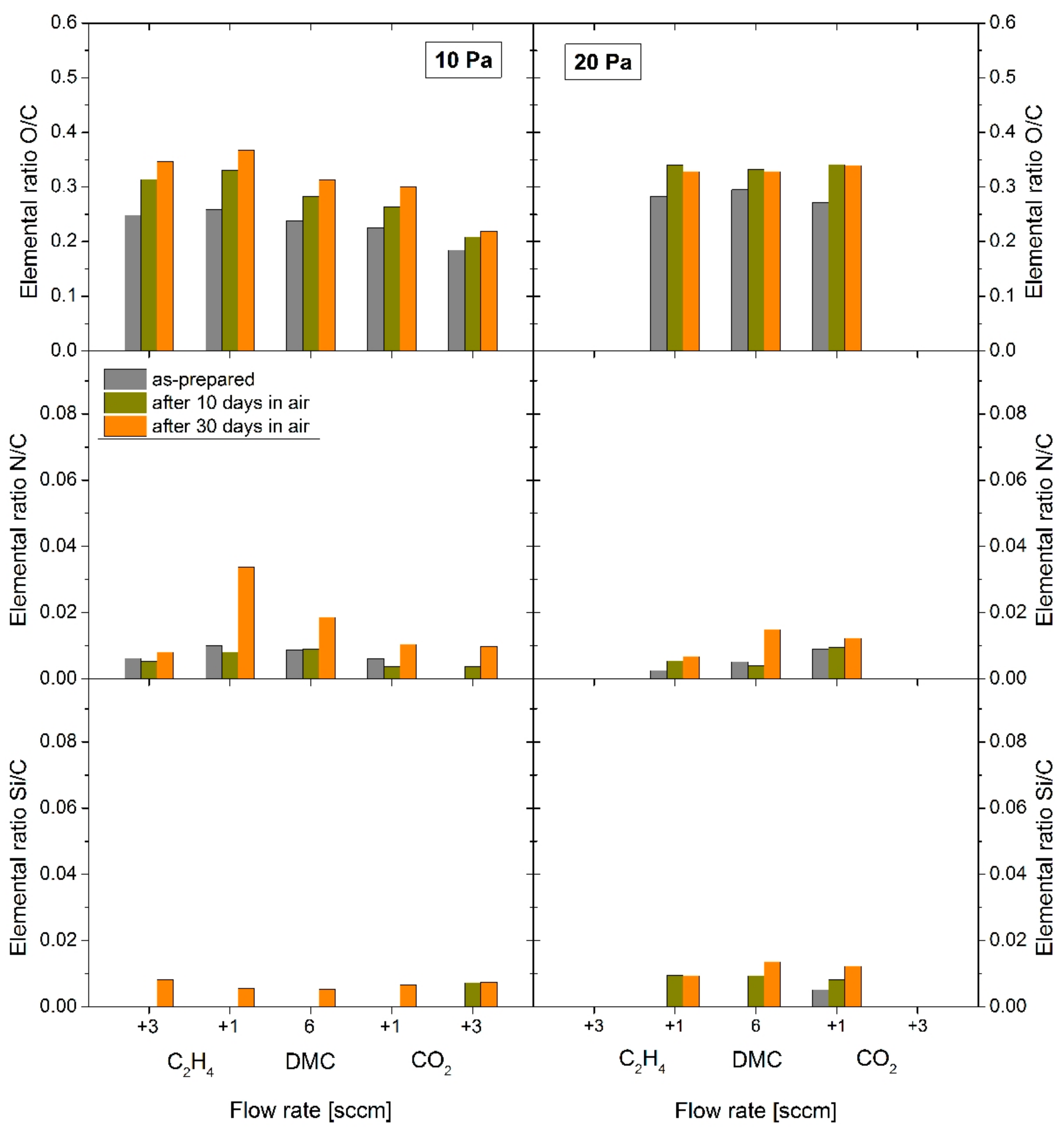
The chemical structure of the C:H:O PPFs deposited from dimethyl carbonate (DMC) vapours at RF power of 30 W and total pressure of 10 Pa or 20 Pa depending on the working gas composition was studied by XPS. The elemental composition of the films is depicted in
Figure 5 (as derived from XPS survey scans). The as-deposited films were mostly composed of carbon and oxygen which were the intrinsic constituents of the monomer gases used for plasma polymerization (except of hydrogen which cannot be analysed by XPS technique). However, also traces of nitrogen (up to 0.8 at%) could be detected in most of the film samples right after deposition. Nitrogen could be incorporated into the growing films either from residual gases during the deposition process or adsorbed on the surface after the deposition. Anyway, more important for the C:H:O film structure analysis is the [O]/[C] ratio. Compared with the monomer structure of DMC ([O]/[C] ratio of 1) a remarkable decrease in the oxygen content was observed for all plasma polymerized DMC films ([O]/[C] ratio ≤0.25) as measured shortly after deposition.
Furthermore, it was observed that the elemental composition was highly dependent on the composition of the working gas mixture, that is, addition of either ethylene or carbon dioxide. It is interesting to note that more oxygen in the plasma discharge (i.e., addition of CO2) led to less oxygen incorporation into the growing plasma polymer film. Particularly, the [O]/[C] ratio decreases from 0.24 (19 at % of oxygen) for the coating deposited from pure DMC vapours down to 0.18 (15 at % of oxygen) when 3 sccm of CO2 was added to the DMC monomer (while it was only slightly decreased for 1 sccm CO2 admixture). With addition of C2H4 (growth regime), on the other hand, the [O]/[C] ratio was slightly increased (0.25–0.26) (21 at % of oxygen). The enhanced oxygen present in the discharge indicative for the ablation regime (with low deposition rates) thus yielded an enhanced etching of the growing film by oxygen species rather than the incorporation of the oxygen into the film. This might suggest the existence of an upper limit for the [O]/[C] ratio in the plasma composition which can be transformed into an oxygenated hydrocarbon plasma polymer film for the used energetic conditions supporting cross-linking. Nevertheless, the XPS technique itself is not sufficient to assess the intrinsic amount of oxygen in the as-deposited films due to its surface sensitivity. The intrinsic amount of oxygen can be altered (increased) by post-deposition oxidation mainly as the result of the presence of the radicals trapped in the plasma polymer matrix.
The increase of the working gas pressure from 10 Pa to 20 Pa was accompanied by an overall increase in the oxygen content within the deposited PPFs. Particularly, a [O]/[C] ratio of 0.30 was reached in the case of pure DMC (23 at % of oxygen), while it decreased down to 0.27 (21 at % of oxygen) when 1 sccm of CO
2 was added to the monomer and to 0.28 (22 at % of oxygen) with 1 sccm of C
2H
4. Increased working pressure (at constant flow rate and plasma power) is known to affect the ion-assisted etching conditions at the growing film surface (while gas phase processes are maintained) and often leads to coatings better retaining the monomer structure and composition [
38]. In particular, mean ion energies at 20 Pa are roughly half compared to the values at 10 Pa due to collisions in the plasma sheath [
28,
39,
40]. Regarding the enhanced deposition rate and comparable ion flux at 20 Pa, the energy delivered to the growing film (i.e., ion flux times their energy per deposition rate) was strongly reduced [
28,
40]. Thus, a better structural retention can mainly be expected for the pure DMC discharge at elevated pressures, also allowing molecular ions of the monomer to participate in film growth [
34]. Nevertheless, the oxygen content was still far from the intrinsic DMC monomer. Hence, it can be inferred that the carbonate group (C–O–C(=O)–O–C) was not retained in the plasma polymer, forming rather ester (C–O–C(=O)–C–C), carbonyl (C–C(=O)–C–C) and ether (C–C–O–C–C) groups. Nitrogen (up to 0.7 at %) was also found in the coatings deposited at 20 Pa. In addition, a signal from the silicon substrate (0.4 at % of silicon) was recorded for the ~40 nm thick films deposited from the mixture of DMC with 1 sccm of CO
2 gas (30 W and 20 Pa). This suggests that the films might be porous and less compact (cross-linked) as the result of altered film growth conditions at the surface (more active oxygen species arriving from the plasma discharge). In cases when silicon was detected in the spectra of the studied films, the elemental content of oxygen was corrected for the signal originating from the native layer of silicon dioxide (~2.5 nm thick) grown on the surface of the silicon substrate to separate it from the oxygen contained in the C:H:O PPFs.
Furthermore, the PPFs were characterized the same day after their deposition as well as after 10 days and 30 days of storage in ambient air to assess their chemical composition with aging. As could be expected, the amount of oxygen in the coatings increased as the result of reaction of atmospheric oxygen with trapped radicals within the plasma polymer matrix. Typically, coatings contain more trapped radicals with higher C
2H
4 admixture yielding stronger post-oxidation [
41]. This can indeed be observed in the case of coatings deposited at 10 Pa at various gas compositions. Most of the oxidation occurred within the first days of storage in air, while the oxidation rate decreased with ongoing aging time. It is interesting to note that the increase in [O]/[C] ratio almost stopped after the first days in the case of the C:H:O coatings deposited at 20 Pa, which will be further discussed in the following section. Apart of oxidation of the coatings, another important change can be observed in the composition of the PPFs: presence of silicon was detected in all of the C:H:O coatings after 30 days of storage in air. Silicon signal can only origin from the substrate indicating that some decomposition can be expected within the PPFs as the result of oxidation. Silicon even appeared in the XPS signal within the first 10 days of air aging for all coatings deposited at 20 Pa and also for the coating deposited at 10 Pa with higher amount of carbon dioxide. It can thus be concluded that the stability of the plasma polymer films deposited at 20 Pa is lower compared to 10 Pa. As discussed before, the higher process pressure helps to partly retain the monomer structure and composition of the deposited films but also leads to decreased stability due to reduced ion-assisted cross-linking reactions [
38].
Similar yet more pronounced effects were observed in the case of aging in water (
Figure 6). Since higher rates of changes in the chemical composition of the PPFs were expected, the coatings were characterized after 1 h immersion in distilled water and compared with the as-prepared coatings. Furthermore, the composition of the PPFs was studied after 1, 10 and 30 days in water and extended up to 5 months. For a better comparison, all chemical composition data recorded in the PPFs stored in water (
Figure 6) are marked with the same colour for corresponding measurements on films stored in air (
Figure 5) and also the axis scales were kept the same.
Different to expectations, there was no substantial change in the amount of oxygen nor nitrogen in the PPFs after storage in water within the first hour. Nevertheless, detection of the silicon signal (up to 0.8 at %) originating from the substrate suggests that the structure of all studied C:H:O coatings deposited at 10 Pa was disturbed, possibly leading to pore opening, swelling and/or local delamination of the coating revealing the substrate material. The progressive oxidation of the coatings within the next 10 days of storage in water resemble that observed in air: the [O]/[C] ratio increased by about 20 to 25% and was higher for the coatings deposited with added ethylene reaching about 0.33. Overall, DMC-derived PPFs (30 W, 10 Pa) were found to show stronger oxidation than comparable acetone-derived films regarding admixture of CO
2 (see
Appendix A,
Figure A3), probably due to a more open (nanoporous) structure. The amount of nitrogen measured in the films was substantially higher in the case of aging in water as compared to aging in air. Since we assume that the nitrogen was incorporated into the growing coatings during the deposition process, it can be expected that the degradation of the surface structure of coatings is more significant in water. On the other hand, the increase in the intensity of the silicon signal was only minor. Different to the films stored in air, further aging of the coatings in water over 30 days resulted in substantial changes in their composition, particularly with respect to the amount of oxygen and nitrogen in the coatings. The [O]/[C] ratio gradually increased and reached much higher values: up to 0.55 in the case of the coatings deposited with 3 sccm of ethylene in the working gas mixture. A similar increase in the amount of nitrogen in the coatings occurred. Higher amount of nitrogen was observed in the case of the PPFs deposited with excess of CO
2 where it reached 2.5 at %, that is, a N/C ratio of 0.09. The amount of silicon, on the other hand, remained almost constant after the first hour in water in the case of the plasma polymer films deposited at 10 Pa.
The situation, however, was different in the case of the PPFs deposited at 20 Pa. These C:H:O coatings originally containing more oxygen oxidized at a slower rate and reached the maximum amount of oxygen after about 1 month from their immersion into the water. The highest [O]/[C] ratio of about 0.42 was attained for the C:H:O coatings deposited from vapours of DMC with admixture of C
2H
4. While the amount of incorporated nitrogen is almost the same as in the respective PPFs deposited at 10 Pa, substantially higher amounts of silicon were detected in the coatings deposited from pure DMC and also with the admixture of ethylene. This observation suggests an increased degradability of the PPFs when deposited at enhanced pressure. Furthermore, while the degradation reached a steady state after about 10 days in water for the coatings deposited from mixtures of DMC with either C
2H
4 or CO
2, the decomposition of the plasma polymer matrix continued for the C:H:O coatings deposited from pure dimethyl carbonate discharges even after 5 months of storage in water. Hence, such DMC-derived PPFs are interesting for their slow and steady degradability, which can be related to hydrolysis reactions [
16].
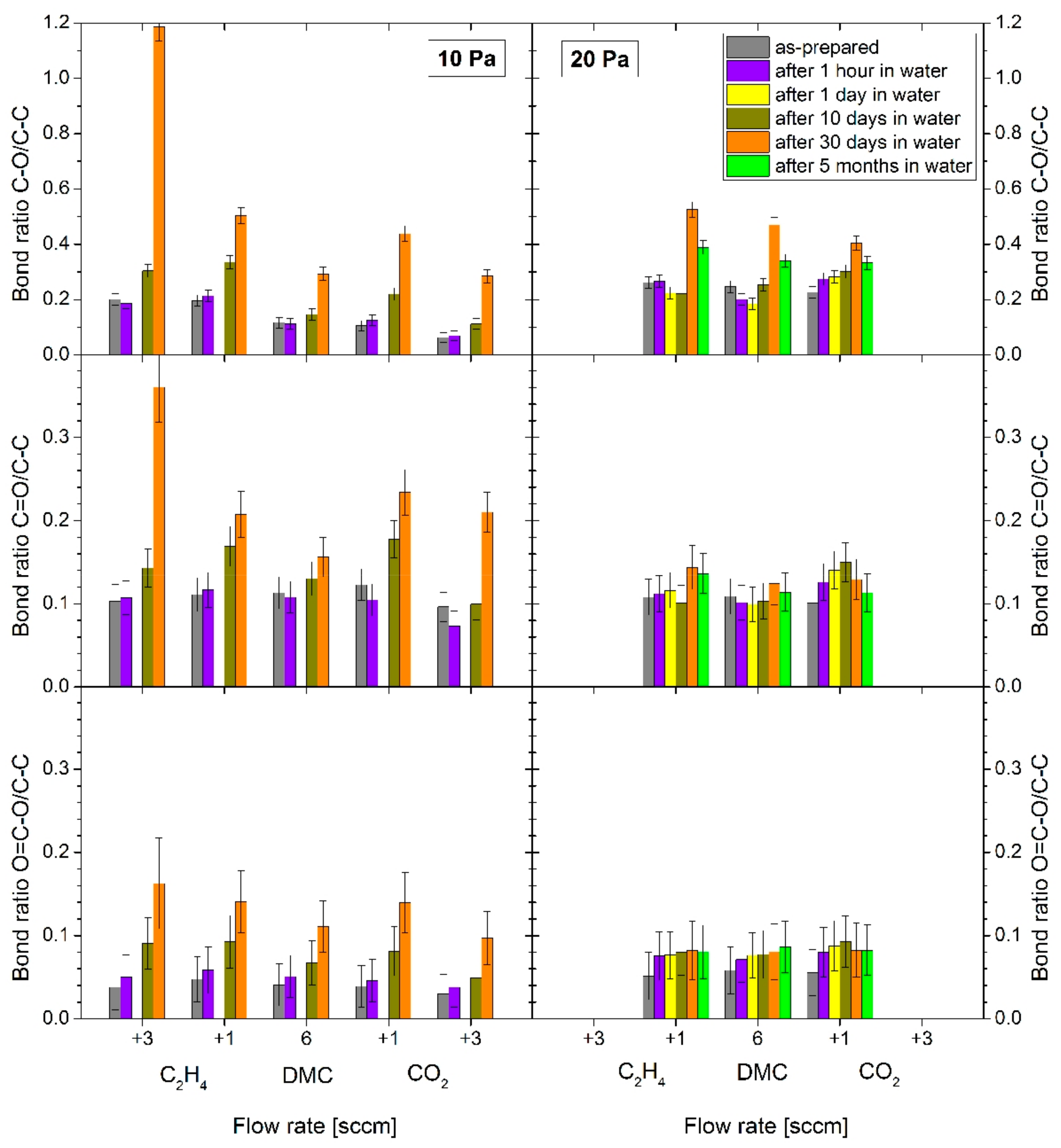
The measurement by XPS also allowed for a detailed analysis of the particular chemical bonds that were present in the surface of the studied coatings (
Figure 7). However, there are several restrictions that need to be taken into account when analysing the obtained data. The structure of a typical plasma polymer is far from being regular as is the case of traditional polymers. Statistical occurrence of chemical functional groups, random branching and crosslinking of plasma polymers complicated the chemical analysis of surfaces by XPS. The information on respective bonds and their ratios are collected from numerical fitting of measured data. The positions of specific bond energies are affected by surrounding species and thus often shifted due to the statistical nature of plasma polymers. This makes the analysis of XPS spectra (here C1s peak) rather difficult, especially if chemical groups with close values of bond energies are discussed. Therefore, in the presented case of plasma polymer films from DMC vapours, it was not possible to distinguish, for example, C–N from C–O. Nevertheless, the total amounts of nitrogen in the coatings were rather low in most of the cases and thus contribution of C–N bonds to the detected signal of the C1s peak could be neglected. Also, the error of the estimation of the respective bond ratios was rather high. Nevertheless, the trends in the aging of the coatings deposited at different conditions can be tracked.
First of all, it can be seen from
Figure 7 that pure DMC-derived PPFs contain a rather high amount of C=O groups when compared to films derived from CO
2/C
2H
4 and acetone at comparable plasma conditions (C=O/C–C of 0.12 ± 0.02 vs. 0.04 ± 0.02 for CO
2/C
2H
4 (2:1) and 0.06 ± 0.02 for acetone at 30 W and 10 Pa) demonstrating the influence of the monomer selection [
17,
19]. Some structural information from the starting monomer was thus retained, which is even more pronounced at enhanced pressure as indicated by the higher amount of O=C–O groups. Such groups are known to induce degradability in PPFs [
16]. This retention was only diminished for high CO
2 admixture (
Figure 8).
The changes in the ratios of various oxygen-containing chemical groups with respect to the amount of C–C bonds as analysed from the C1s core peak are summarized in
Figure 8. A general increase in the amount of oxygen-containing chemical groups with respect to the C–C bonds can be observed in all studied samples as a result of oxidation, which is in accordance with previously discussed changes in the elemental composition. Furthermore, it can be seen that while changes in amounts of C–O groups were rather low (the change in the C–O/C–C bond ratio was below 10%), the increase in the amount of the carbonyl (–C=O) and carboxyl/ester (O–C=O) groups was relatively high. Similar to the previous observations, it can be seen that the more ethylene was in the working gas mixture, that is, less oxygen in the plasma yielding more trapped radicals in the deposited PPFs [
40], the more readily the film became oxidized. The addition of CO
2, on the other hand, stabilized the coating against post-deposition oxidation. At the same time, it can be seen that the C:H:O coatings deposited at the low pressure of 10 Pa were more prone to oxidation than the coatings deposited at 20 Pa. The aging (oxidation) processes were still ongoing even 30 days from the deposition in the case of coatings deposited at 10 Pa, while changes were limited (mostly below the measurement error) in the case of coatings deposited at 20 Pa, which, however, showed more signs of decomposition. Again, an increased number of trapped radicals can be expected for PPFs deposited at 10 Pa with enhanced ion bombardment.
A similar analysis was also performed with coatings aged in distilled water for a duration of up to 5 months (
Figure 9). The situation was quite different when compared to aging in air and the recorded changes were rather dramatic. Similar to aging in air, the C:H:O coatings deposited at the pressure of 10 Pa strongly underwent oxidation during aging in water in comparison with those deposited at 20 Pa. Different to aging in air, however, the coatings were not stabilized, even not after 30 days from deposition. At the same time, it is interesting to note that the onset of the oxidation was rather slow and happened delayed—no changes in composition were observed within the first hours of aging in water. The increase in the amount of C–O chemical groups in comparison with carbonyl (–C=O) and carboxyl/ester (O–C=O) groups was considerably higher upon aging in water, especially in the case of PPFs deposited with excess of ethylene in the working gas mixture. The C–O/C–C bond ratio for these coatings increased 6-fold after 30 days compared to the as-prepared coatings.
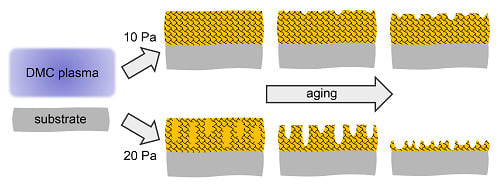
Overall, the analysis of the chemical composition of the C:H:O plasma polymer films deposited from DMC vapours or their mixtures with either C2H4 or CO2 showed different aging behaviour with respect to working gas composition (i.e., the amount of oxygen species in plasma) as well as the working gas pressure during the deposition. PPFs deposited at 20 Pa contained intrinsically more oxygen and were thus more resistant to subsequent oxidation during aging both in air and in water. On the other hand, the structure of these coatings degraded faster as it was evidenced by the detectable signal from the silicon substrate. The C:H:O PPFs deposited from pure DMC vapours at 10 Pa were overall the most stable films and revealed the lowest changes in chemistry, while their pure DMC counterpart deposited at 20 Pa revealed slow and steady degradation. In this case, co-polymerization was thus not found to be beneficial, likely due to increased fragmentation and less retention of parts of the monomer structure.
3.4. Topography of C:H:O PPFs by AFM
Atomic force microscopy (AFM) is a very effective method to analyse the film surface topography. The values of RMS roughness of the studied plasma polymer films are summarized in
Figure 10. Film surfaces were analysed as-prepared as well as during aging for up to 30 days. The as-prepared PPFs (~40 nm thick) were extremely smooth closely resembling the surface of the polished silicon substrate. RMS roughness values were measured from 1 nm up to 3 nm, where the higher values corresponded to films deposited with admixture of CO
2. Higher etching of the growing film during the deposition by active oxygen species can be expected for this ablation regime. No changes in the intrinsic surface roughness of the PPFs were observed during 30 days of aging in air (not shown here). However, the situation was different when films were immersed in the water bath. Gradual increase in the values of the RMS roughness of the coatings with the duration of storage was observed for all studied coatings.
However, the extent of swelling of the coatings did not fully correlate with the [O]/[C] elemental content of the as-prepared coatings. Different to the chemical changes, it can be seen that the coatings deposited with higher admixture of CO2 in the working gas exhibited substantial changes in their topography during aging in water. Overall, the morphology of the pure DMC-derived films deposited at 30 W and 10 Pa underwent the least changes agreeing with the finding of their highest stability as discussed before. The RMS roughness increased up to about 3 nm in average over 30 days storage in water. On the other hand, PPFs prepared from DMC with high admixture of ethylene (3 sccm) as well as all PPFs deposited at the higher pressure of 20 Pa, revealed the highest changes in the surface morphology indicating degradation processes—again strongest for pure DMC-derived PPFs at 20 Pa. The amount of oxygen was always higher than 20 at % in all of these coatings when measured right after the deposition.
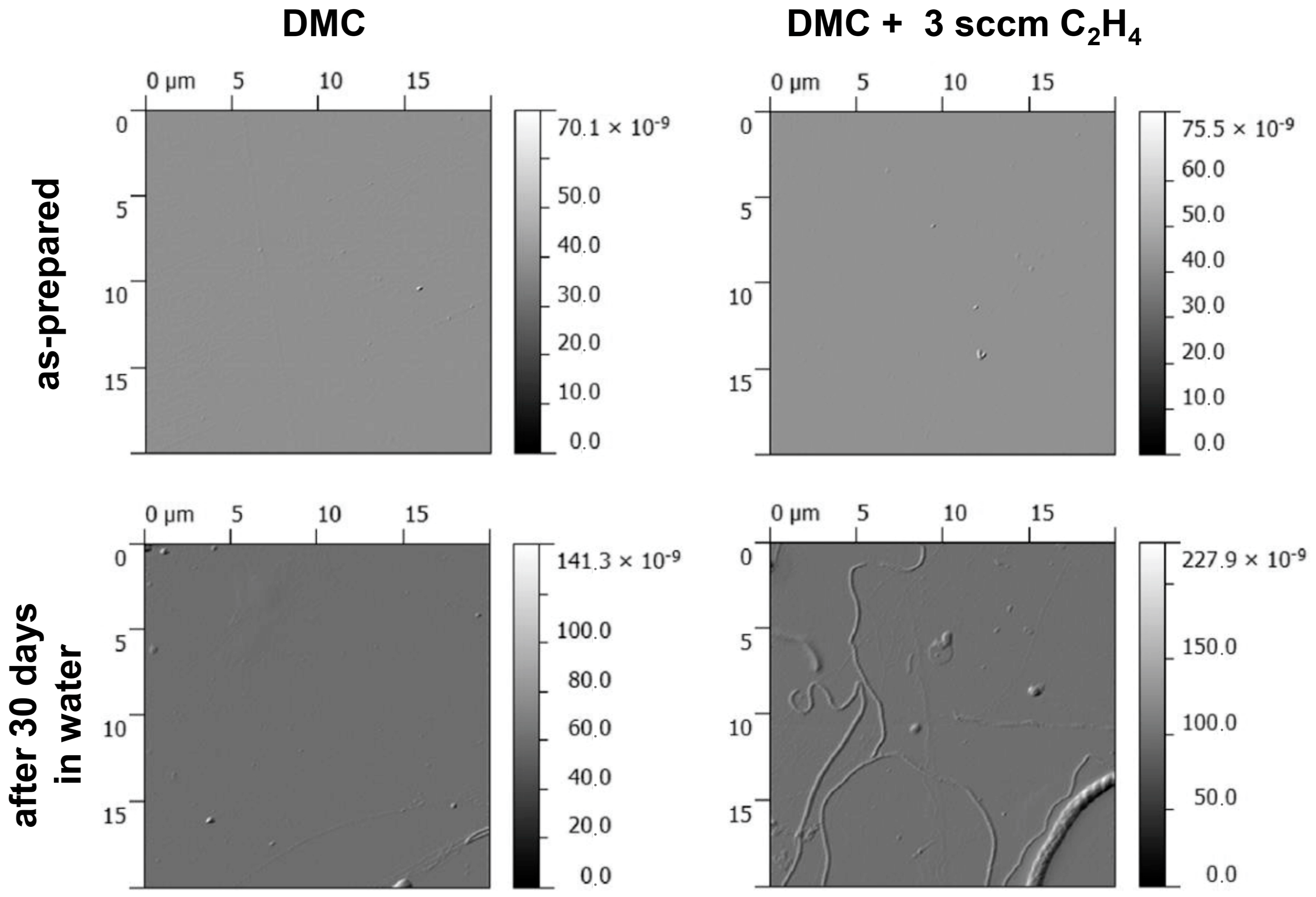
The changes in surface morphology of selected coatings deposited at a pressure of 10 Pa are displayed in
Figure 11. The surface of the rather stable DMC-derived PPFs remained smooth even after 30 days of storage in water, even though first localized defects can be seen at several spots. On the other hand, obvious swelling and formation of point and linear defects can be observed on the surface of the coating deposited from a mixture of 6 sccm of DMC and 3 sccm of ethylene (2:1). At the same time, coatings deposited at these plasma conditions revealed the highest rate of oxidation of the plasma polymer matrix during aging in water (
Figure 6). These and similar structures were observed to be not homogeneously spread on the whole surface of the analysed coatings but only on localized areas suggesting that degradation starts and proceeds from local defects. However, it has to be noted that the observed changes in the morphology of the PPFs cannot fully explain the Si signal which was detected by XPS in all studied samples already after 1 h of storage in water (
Figure 6). The amounts of Si observed were the highest in the case of coatings deposited at 20 Pa. It was also significant in the case of coatings deposited from vapours of pure DMC, while the changes in the morphology observed on these coatings were only minor. On the other hand, the coating deposited from a mixture of DMC and ethylene (2:1) underwent substantial morphological changes but revealed much lower Si signal from the substrate. These facts suggest that nanosized pore openings might have occurred in the films rather than microscopic cracking as the result of slight swelling but also removal of low molecular weight polymeric material from the plasma polymer films [
42].