Polymerization of D-Ribose in Dielectric Barrier Discharge Plasma
Abstract
:1. Introduction
2. Materials and Methods
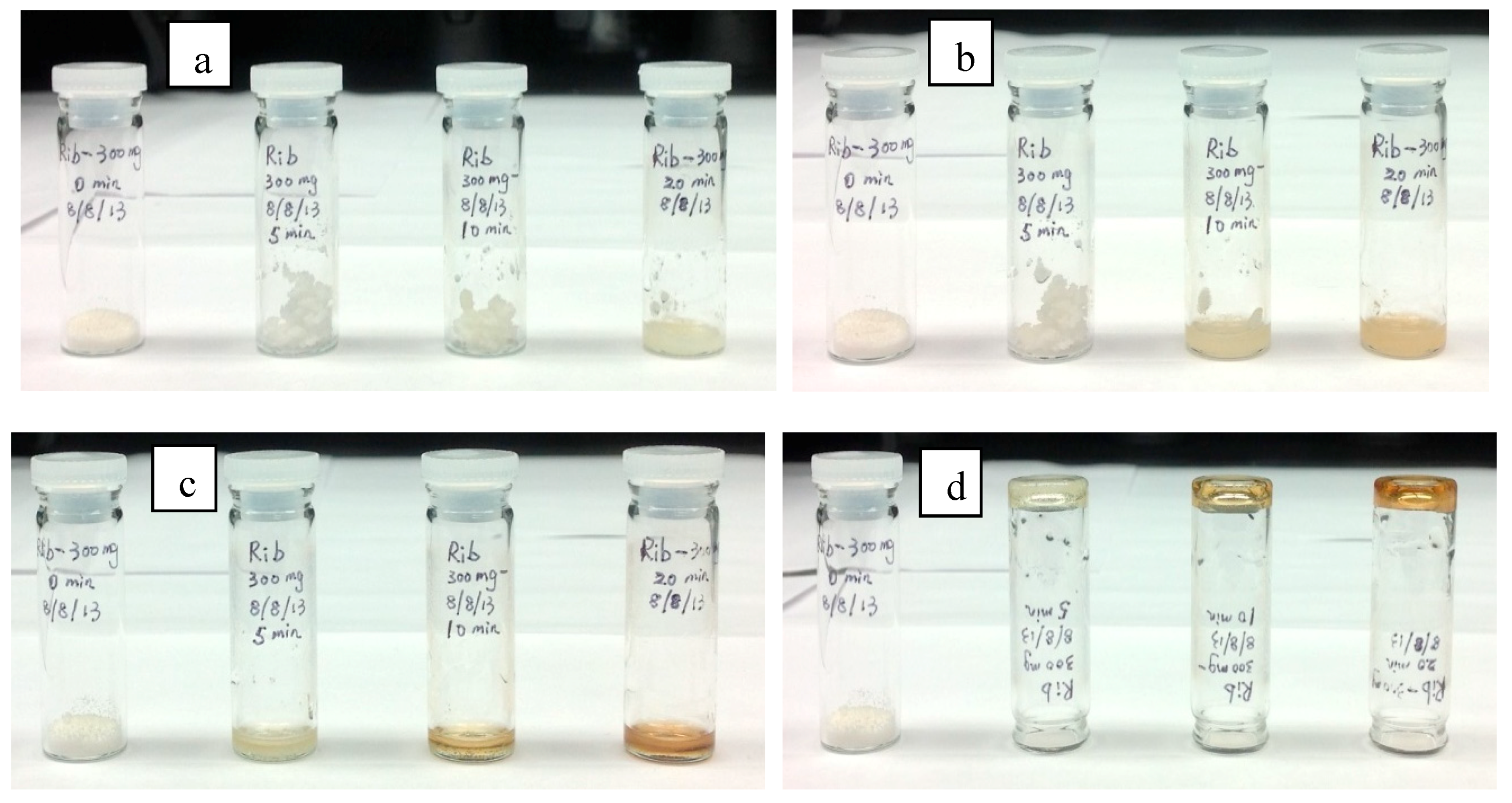
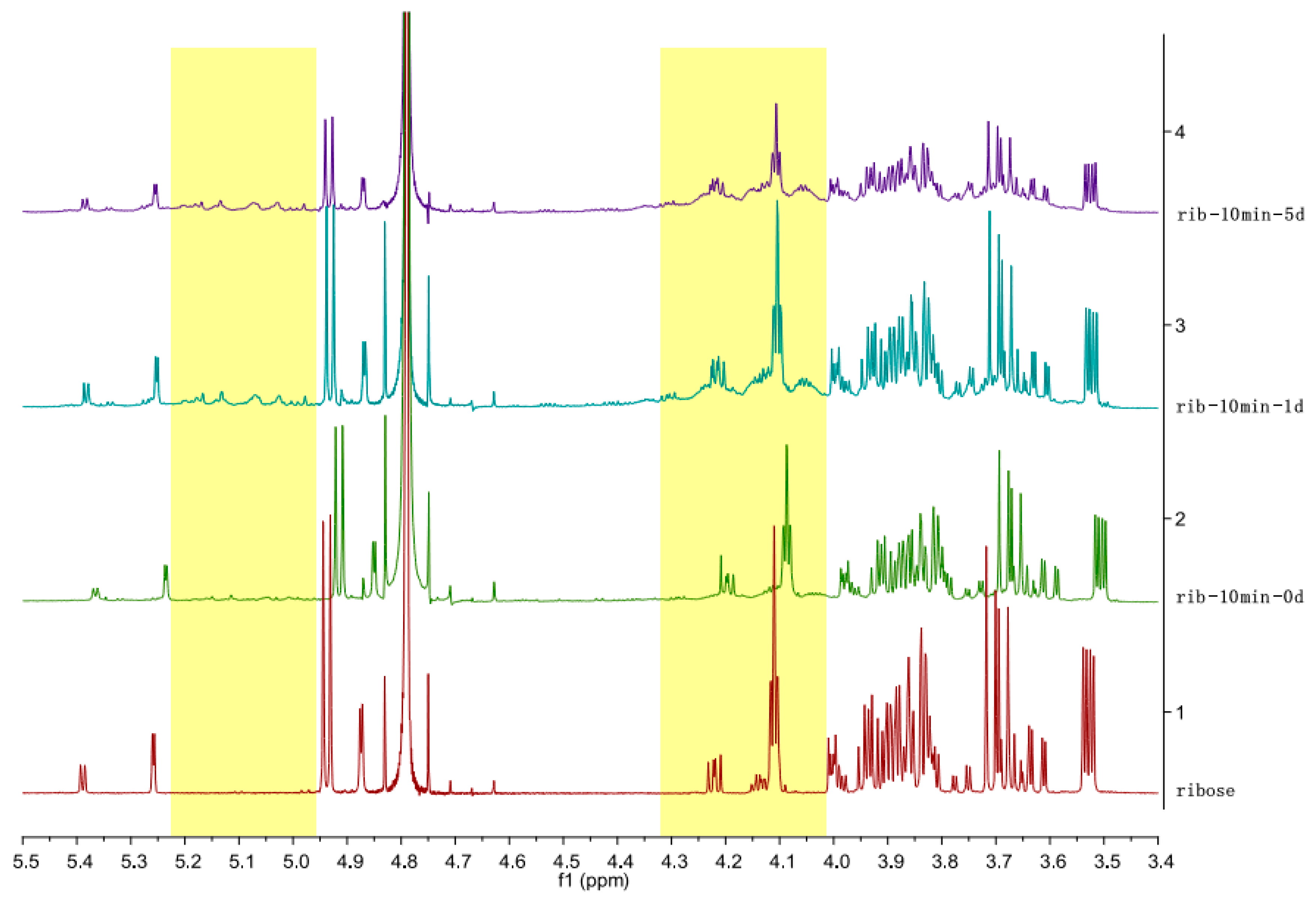
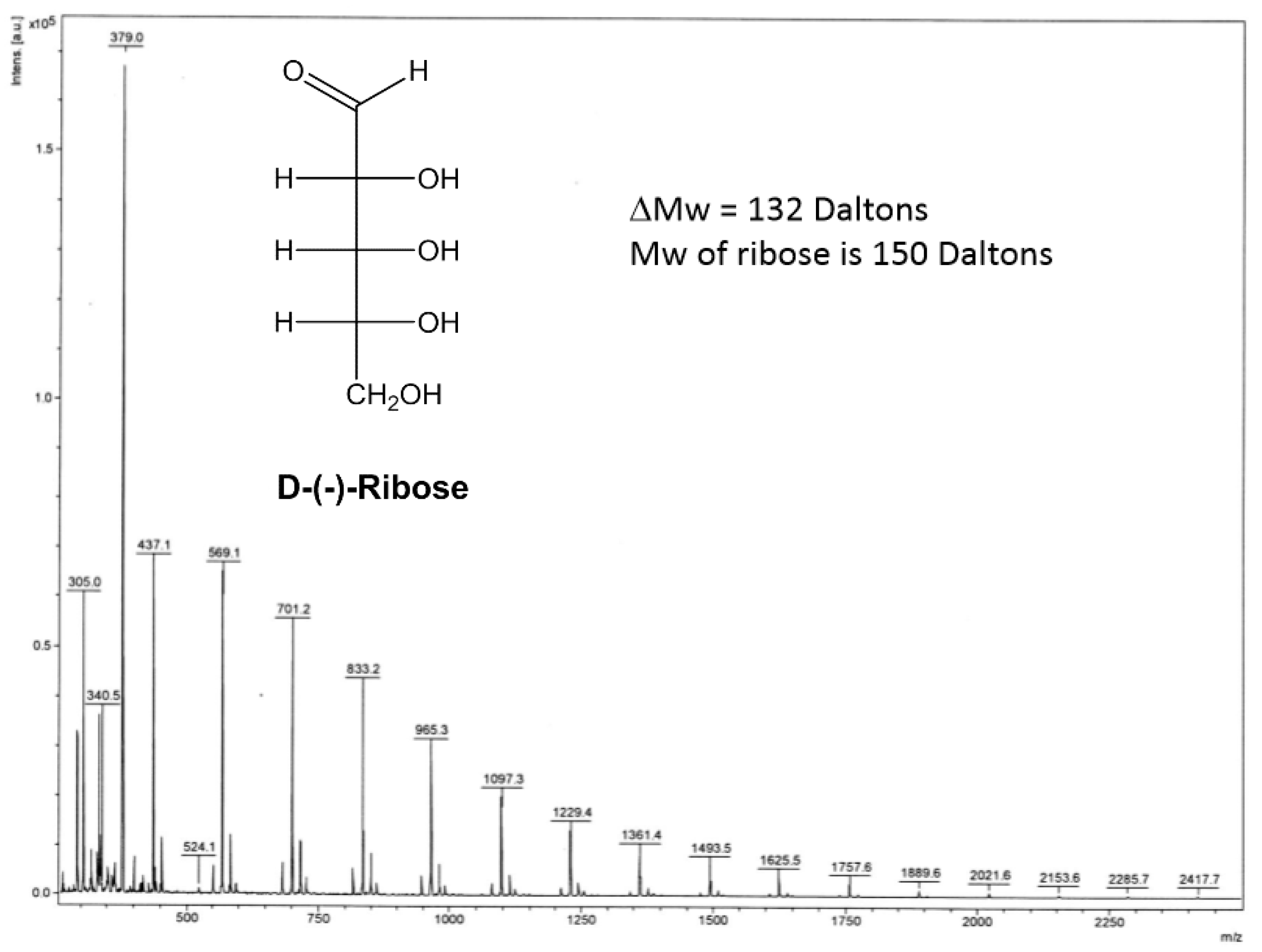
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, V.; Jolivalt, C.; Pulpytel, J.; Jafari, R.; Arefi-Khonsari, F. Development of silver nanoparticle loaded antibacterial polymer mesh using plasma polymerization process. J. Biomed. Mater. Res. A 2013, 101, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedikt, J.; Mokhtar Hefny, M.; Shaw, A.; Buckley, B.R.; Iza, F.; Schäkermann, S.; Bandow, J.E. The fate of plasma-generated oxygen atoms in aqueous solutions: Non-equilibrium atmospheric pressure plasmas as an efficient source of atomic O (aq). Phys. Chem. Chem. Phys. 2018, 20, 12037–12042. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.H.; Park, G.; Choi, E.H.; Uhm, H.S. Effects of the physical parameters of a microwave plasma jet on the inactivation of fungal spores. Thin Solid Films 2013, 547, 125–131. [Google Scholar] [CrossRef]
- Laurita, R.; Miserocchi, A.; Ghetti, M.; Gherardi, M.; Stancampiano, A.; Purpura, V.; Melandri, D.; Minghetti, P.; Bondioli, E.; Colombo, V. Cold Atmospheric Plasma Treatment of Infected Skin Tissue: Evaluation of Sterility, Viability, and Integrity. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 275–279. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977. [Google Scholar] [CrossRef] [PubMed]
- Ya, E.; Krasik, A.; Grinenko, A.; Sayapin, S.; Efimov, A.; Fedotov, V.; Gurovich, V.Z.; Oreshkin, V.I. Underwater electrical wire explosion and its applications. IEEE Trans. Plasma Sci. 2008, 36, 423–434. [Google Scholar]
- Laroussi, M.; Kong, M.G.; Morfill, G.; Stolz, W. Plasma Medicine: Applications of Low-Temperature Gas Plasmas in Medicine and Biology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Rittersdorf, I.M.; Ottinger, P.F.; Allen, R.J.; Schumer, J.W. Current density scaling expressions for a bipolar space-charge-limited cylindrical diode. IEEE Trans. Plasma Sci. 2015, 43, 3626–3636. [Google Scholar] [CrossRef]
- Ercan, U.K.; Joshi, S.S.; Yost, A.; Gogotsi, N.; O’Toole, S.; Paff, M.; Melchior, E.; Joshi, S.G. Inhibition of biofilms by non-thermal plasma treated novel solutions. Adv. Microbiol. 2014, 4, 1188–1196. [Google Scholar] [CrossRef]
- Eliasson, B.; Egli, W.; Kogelschatz, U. Modelling of dielectric barrier discharge chemistry. Pure Appl. Chem. 1994, 66, 1275–1286. [Google Scholar] [CrossRef] [Green Version]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Balasubramanian, M.; Ayan, H.; Fridman, A.; Gutsol, A.; Brooks, A. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, S.; Léveillé, V.; Yonson, S.; Leask, R.L. Miniature atmospheric pressure glow discharge torch (APGD-t) for local biomedical applications. Pure Appl. Chem. 2006, 78, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Leduc, M.; Guay, D.; Leask, R.L.; Coulombe, S. Cell permeabilization using a non-thermal plasma. New J. Phys. 2009, 11, 115021. [Google Scholar] [CrossRef]
- Jiang, C.; Vernier, P.T.; Chen, M.T.; Wu, Y.H.; Wang, L.L.; Gundersen, M.A. Low energy nanosecond pulsed plasma sterilization for endodontic applications. In Proceedings of the IEEE International Power Modulators and High-Voltage Conference, Las Vegas, NV, USA, 27–31 May 2008; pp. 77–79. [Google Scholar]
- Lu, X.; Cao, Y.; Yang, P.; Xiong, Q.; Xiong, Z.; Xian, Y.; Pan, Y. An RC plasma device for sterilization of root canal of teeth. IEEE Trans. Plasma Sci. 2009, 37, 668–673. [Google Scholar]
- Sladek, R.E.J.; Stoffels, E.; Walraven, R.; Tielbeek, P.J.A.; Koolhoven, R.A.; Koolhoven, R.A. Plasma treatment of dental cavities: A feasibility study. IEEE Trans. Plasma Sci. 2004, 32, 1540–1543. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Serezhenkov, V.A.; Rudenko, T.G.; Pekshev, A.V.; Vanin, A.F. Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric Oxide Biol. Chem. 2005, 12, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of non-thermal plasma on mammalian cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Fridman, A.; Azizkhan-Clifford, J.; Friedman, G. DNA Damage in Mammalian Cells by Non-thermal Atmospheric Pressure Microsecond Pulsed Dielectric Barrier Discharge Plasma is not Mediated by Ozone. Plasma Process. Polym. 2012, 9, 726–732. [Google Scholar] [CrossRef]
- Li, Y.; Kojtari, A.; Friedman, G.; Brooks, A.; Fridman, A.; Ji, H.-F. Decomposition of l-valine under nonthermal dielectric barrier discharge plasma. J. Phys. Chem. B 2014, 118, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.B.; Adams, I.; Ji, H.-F. Mechanism of Ampicillin Degradation by Non-Thermal Plasma Treatment with FE-DBD. Plasma 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Smith, J.B.; Adams, I.; Ji, H.-F. Biomolecule Response to Nonthermal Plasma. J. Plasma Med. 2017, 7, 427–443. [Google Scholar] [CrossRef]
- Li, Y.; Kojtari, A.; Friedman, G.; Brooks, A.; Fridman, A.; Joshi, S.G.; Ji, H.-F. Oxidation of N-Acetylcysteine (NAC) under Nanosecond-Pulsed Nonthermal Dielectric Barrier Discharge Plasma. Plasma Med. 2016, 6, 265–272. [Google Scholar] [CrossRef]
- Li, Y.; Friedman, G.; Brooks, A.; Fridman, A.; Ji, H.-F. Decomposition of sugars under non-thermal dielectric barrier discharge plasma. Clin. Plasma Med. 2014, 2, 56–63. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Atif, R.; Chen, K.; Cheng, J.; Chen, Q.; Qiao, Z.; Fridman, G.; Fridman, A.; Ji, H.-F. Polymerization of D-Ribose in Dielectric Barrier Discharge Plasma. Plasma 2018, 1, 144-149. https://doi.org/10.3390/plasma1010013
Li Y, Atif R, Chen K, Cheng J, Chen Q, Qiao Z, Fridman G, Fridman A, Ji H-F. Polymerization of D-Ribose in Dielectric Barrier Discharge Plasma. Plasma. 2018; 1(1):144-149. https://doi.org/10.3390/plasma1010013
Chicago/Turabian StyleLi, Yingying, Rida Atif, Ketao Chen, Jiushan Cheng, Qiang Chen, Zhen Qiao, Gregory Fridman, Alex Fridman, and Hai-Feng Ji. 2018. "Polymerization of D-Ribose in Dielectric Barrier Discharge Plasma" Plasma 1, no. 1: 144-149. https://doi.org/10.3390/plasma1010013





