Mechanical and Microstructural Performance of Cement Mortars with Internal Carbonation and Sustainable Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design
3. Methods
4. Results
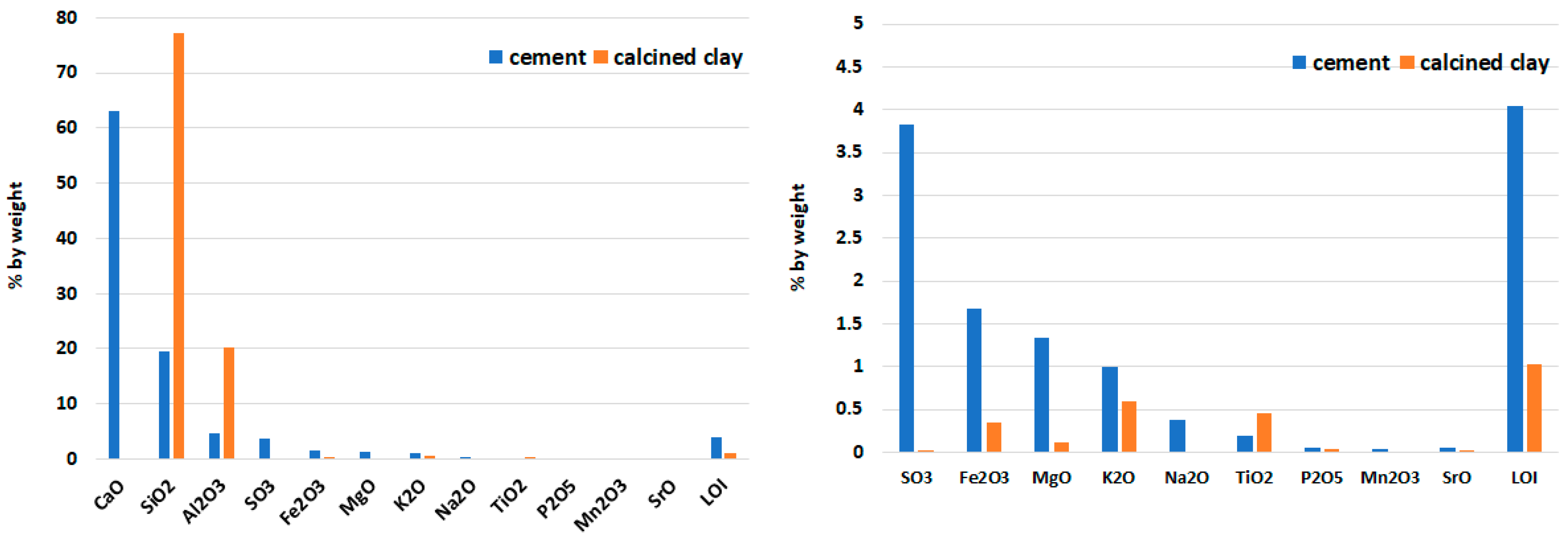
4.1. Characterization of Raw Materials
4.2. Mechanical Properties
4.3. Thermogravimetric Analysis
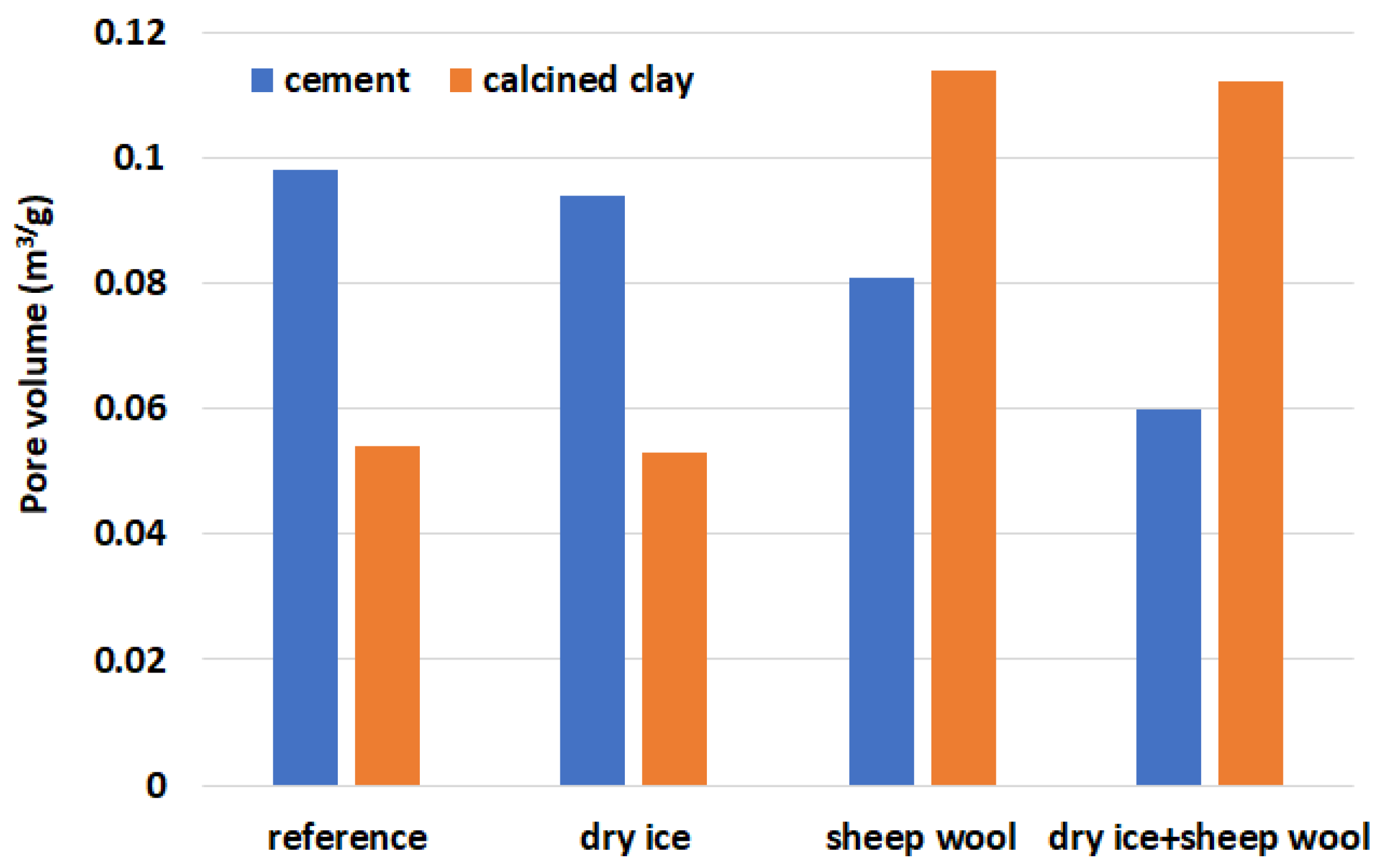
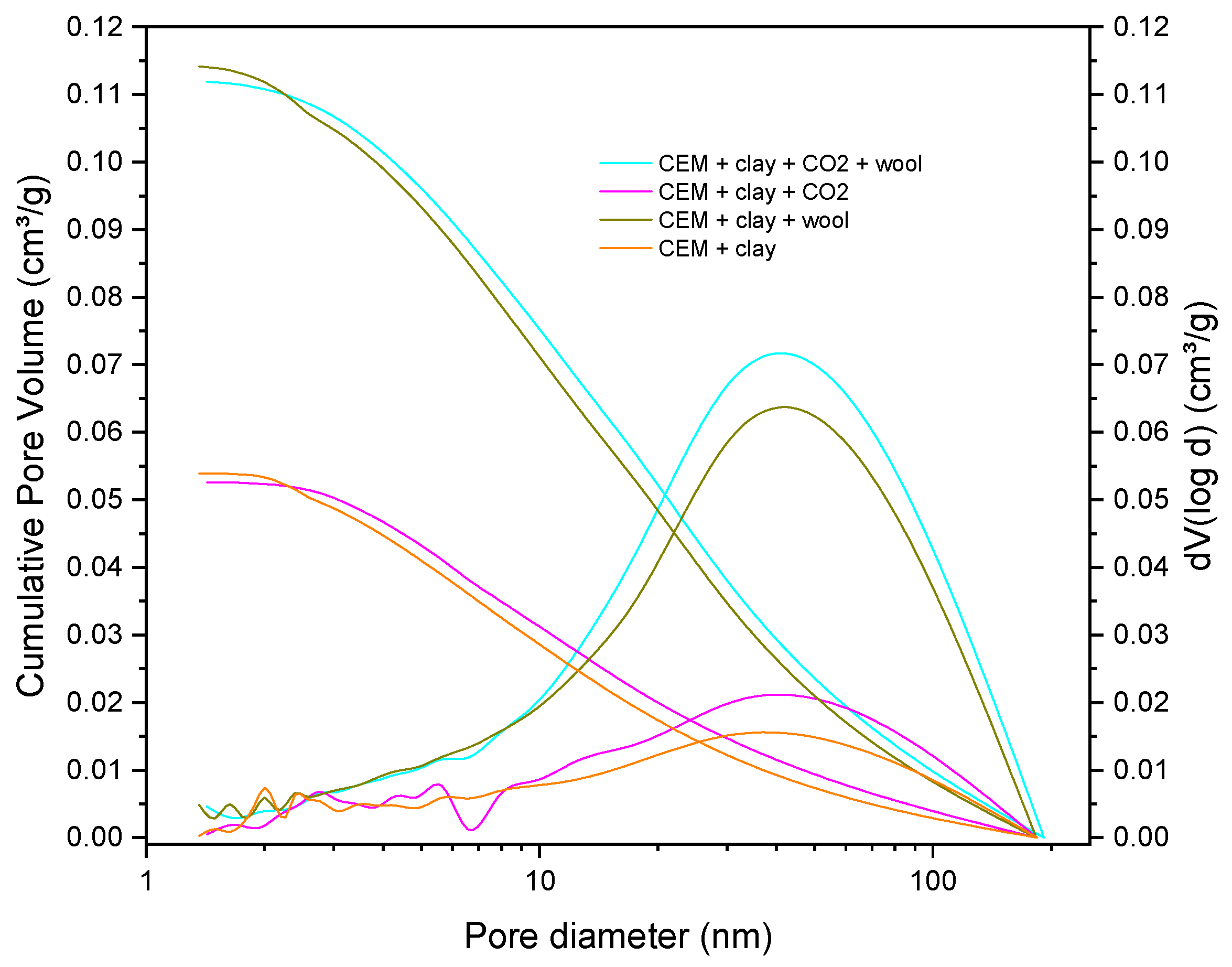
4.4. Pore Structure and BET Surface Area Analysis
4.5. Alkalinity of Mortars
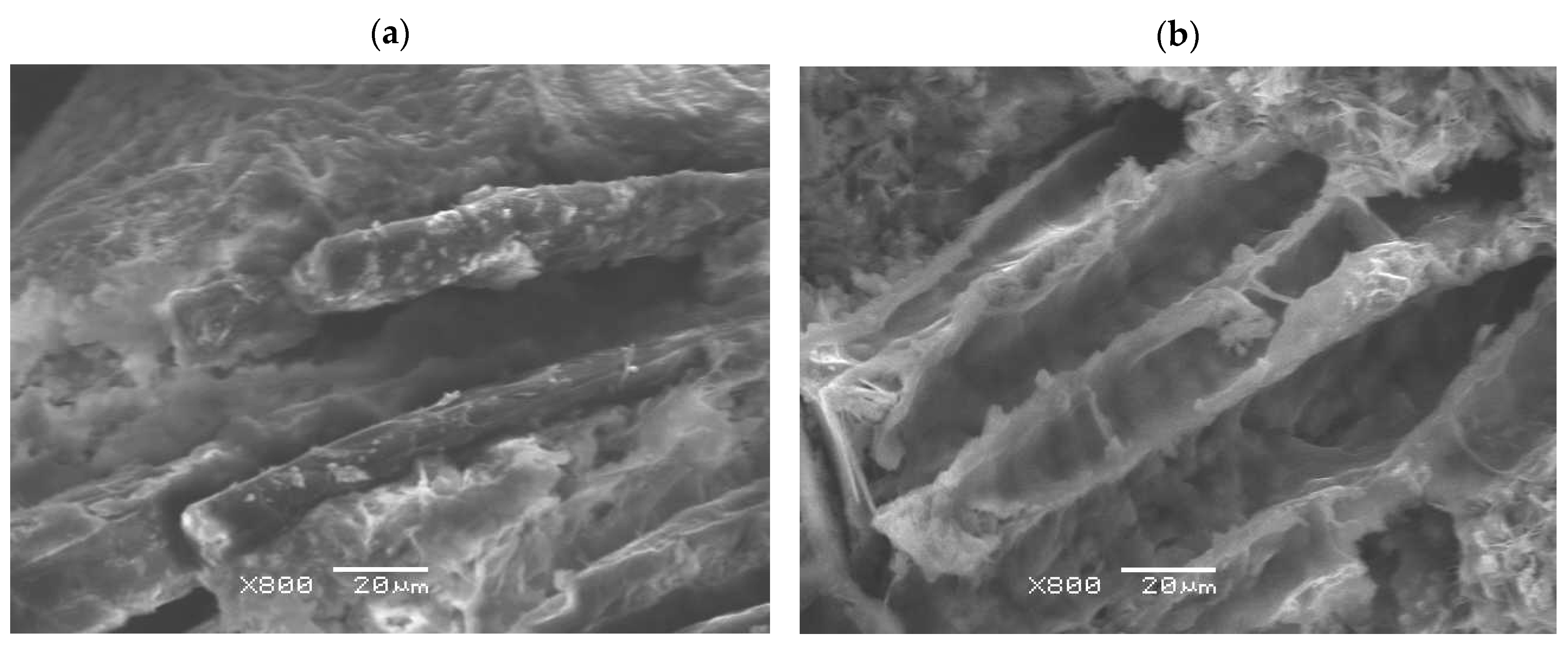
4.6. Microstructure
5. Discussion
6. Conclusions
- Internal carbonation via dry ice increased compressive strength by approximately 8% compared to the reference mix and contributed to pore structure refinement, confirming its viability for developing low-carbon cementitious systems.
- Calcined clay, used as a 15–30% cement replacement, enhanced durability by stabilizing hydration products and refining the pore network, leading to a ~12% reduction in total pore volume and a higher gel-pore fraction (<10 nm).
- Sheep wool fibers improved flexural toughness and post-cracking performance by more than 40%, validating their crack-bridging effect. However, SEM observations revealed degradation in high-pH or carbonated environments, which reduced long-term mechanical integrity. Additionally, fiber-induced porosity partially counteracted matrix densification.
- Microstructural analysis confirmed the complex interactions between carbonation, hydration, and fiber integrity, emphasizing the need to control chemical compatibility when designing multicomponent eco-mortars.
- To maximize strength and durability, fiber inclusion should be avoided when both dry ice and calcined clay are used, as their simultaneous application (M8) led to the lowest compressive strength (~30 MPa) and increased porosity.
- pH control and portlandite content were identified as key parameters influencing performance. While moderate pH reduction supports fiber stability, excessive portlandite depletion can hinder the formation of hydration products such as ettringite, whereas overly high alkalinity accelerates fiber degradation.
- The outcomes of this study indicate that internally carbonated and calcined clay-modified mortars have strong potential for real-world applications in prefabricated panels, eco-efficient repair mortars, and non-structural building components, where early strength development, durability, and reduced carbon footprint are critical. When combined with properly surface-treated natural fibers, such systems could contribute to the advancement of biogenic and circular construction materials, aligning with the goals of sustainable and low-emission building technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCM | Supplementary cementitious materials |
| ft | Flexural strength |
| fc | Compressive strength |
| AF | Flexural toughness |
| C-S-H | Calcium silicate hydrate |
| C–A–S–H | Calcium-alumino-silicate hydrate |
| LC3 | Limestone-calcined clay |
| C3A | Tricalcium aluminate |
| C4AF | Tetracalcium aluminoferrite |
| XRD | X-ray Diffraction |
| LOI | Loss on ignition |
| SEM | Scanning Electron Microscopy |
| AFt | Ettringite |
| AFm | Monosulfate |
References
- Andrew, R.M. Global CO2 Emissions from Cement Production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on Carbonation Curing of Cement-Based Materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Xuan, M.-Y.; Lee, S.; Hu, H.; Wang, X.-Y. Adding Dry Ice into Ultra-High-Performance Concrete to Enhance Engineering Performances and Lower CO2 Emissions. Constr. Build. Mater. 2023, 392, 131858. [Google Scholar] [CrossRef]
- Jaskulski, R.; Jóźwiak-Niedźwiedzka, D.; Yakymechko, Y. Calcined Clay as Supplementary Cementitious Material. Materials 2020, 13, 4734. [Google Scholar] [CrossRef] [PubMed]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement Substitution by a Combination of Metakaolin and Limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Cheah, C.B.; Liew, J.J.; Khaw, K.L.P.; bin Md Akil, H.; Alengaram, U.J. Calcined Clay as a Low-Carbon Cementitious Material: Comprehensive Review of Treatment Method, Properties, and Performance in Concrete. Clean. Waste Syst. 2025, 11, 100323. [Google Scholar] [CrossRef]
- Bhagath Singh, G.V.P.; Scrivener, K.L. Investigation of Phase Formation, Microstructure and Mechanical Properties of LC3 Based Autoclaved Aerated Blocks. Constr. Build. Mater. 2022, 344, 128198. [Google Scholar] [CrossRef]
- Maia Pederneiras, C.; Veiga, R.; de Brito, J. Rendering Mortars Reinforced with Natural Sheep’s Wool Fibers. Materials 2019, 12, 3648. [Google Scholar] [CrossRef]
- Fantilli, A.P.; Jóźwiak-Niedźwiedzka, D. Influence of Portland Cement Alkalinity on Wool Reinforced Mortar. Proc. Inst. Civil. Eng.-Constr. Mater. 2020, 174, 172–181. [Google Scholar] [CrossRef]
- Ardanuy, M.; Claramunt, J.; Toledo Filho, R.D. Cellulosic Fiber Reinforced Cement-Based Composites: A Review of Recent Research. Constr. Build. Mater. 2015, 79, 115–128. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Cho, H.-K.; Wang, X.-Y. Mixture Optimization of Sustainable Concrete with Silica Fume Considering CO2 Emissions and Cost. Buildings 2022, 12, 1580. [Google Scholar] [CrossRef]
- Tipu, R.K.; Rathi, P.; Pandya, K.S.; Panchal, V.R. Optimizing Sustainable Blended Concrete Mixes Using Deep Learning and Multi-Objective Optimization. Sci. Rep. 2025, 15, 16356. [Google Scholar] [CrossRef]
- Marandi, N.; Shirzad, S. Sustainable Cement and Concrete Technologies: A Review of Materials and Processes for Carbon Reduction. Innov. Infrastruct. Solut. 2025, 10, 408. [Google Scholar] [CrossRef]
- Wang, Y.; He, F.; Yang, L. Influence of Dry Ice on the Performance of Portland Cement and Its Mechanism. Constr. Build. Mater. 2018, 188, 898–904. [Google Scholar] [CrossRef]
- Yang, F.; Lai, C.; Liu, L.; Chen, Z.; Jia, H.; Zhu, J.; Jiang, Z.; Shi, C.; Liu, J. A New Method for Improving the Resistance of Cement-Based Materials to Carbonic Acid Water Corrosion: Carbonation Curing and Further Water Curing. Constr. Build. Mater. 2024, 422, 135733. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y.; Ghouleh, Z. Reaction Products in Carbonation-Cured Lightweight Concrete. J. Mater. Civil Eng. 2013, 25, 799–809. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, X.; Shao, Y. Carbonation Curing of Precast Fly Ash Concrete. J. Mater. Civ. Eng. 2016, 28, 04016127. [Google Scholar] [CrossRef]
- Fantilli, A.P.; Calvi, R.; Quieti, E.; Radavelli, P.L. Carbon Dioxide: A Raw Material for Cementitious Mortar. Mater. Proc. 2021, 5, 2. [Google Scholar]
- Hu, C.; Shen, K.; Qin, Y.; Qian, X.; Wang, F. Sustainable MgO-Calcined Clay Cementitious Material: Reaction Mechanism, Strength Development and Performance Enhancement via Initial CO2 Stirring. Sustain. Mater. Technol. 2024, 40, e00911. [Google Scholar] [CrossRef]
- Mastali, M.; Abdollahnejad, Z.; Pacheco-Torgal, F. Carbon Dioxide Sequestration of Fly Ash Alkaline-Based Mortars Containing Recycled Aggregates and Reinforced by Hemp Fibres. Constr. Build. Mater. 2018, 160, 48–56. [Google Scholar] [CrossRef]
- Ravikumar, D.; Zhang, D.; Keoleian, G.; Miller, S.; Sick, V.; Li, V. Carbon Dioxide Utilization in Concrete Curing or Mixing Might Not Produce a Net Climate Benefit. Nat. Commun. 2021, 12, 855. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Nepal, J.; Das, B.B. Properties, Compatibility, Environmental Benefits and Future Directions of Limestone Calcined Clay Cement (LC3) Concrete: A Review. J. Build. Eng. 2023, 79, 107794. [Google Scholar] [CrossRef]
- Usanga, I.N.; Ikeagwuani, C.C.; Okafor, F.O.; Ambrose, E.E. Exploration of Calcined Clays and Nanoclays as a Potential Construction Material for Sustainable Development: A Comprehensive and State-of-the-Art Review. Road Mater. Pavement Des. 2024, 26, 887–920. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A Review of Alternative Approaches to the Reduction of CO2 Emissions Associated with the Manufacture of the Binder Phase in Concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef]
- Abbas, Y.M.; Khan, M.I.; Abellan-Garcia, J.; Castro-Cabeza, A. Synergizing Metakaolin and Waste-Glass for Sustainable and Functional UHPCC– a Central Composite Design and Ecological Footprint Assessment. J. Build. Eng. 2024, 95, 110249. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández, D.C.; Fernández, J.M.; Jiménez, J.R. The Combined Effect of CO2 and Calcined Hydrotalcite on One-Coat Limestone Mortar Properties. Constr. Build. Mater. 2021, 280, 122532. [Google Scholar] [CrossRef]
- Alyousef, R.; Mohammadhosseini, H.; Ebid, A.A.K.; Alabduljabbar, H. An Integrated Approach to Using Sheep Wool as a Fibrous Material for Enhancing Strength and Transport Properties of Concrete Composites. Materials 2022, 15, 1638. [Google Scholar] [CrossRef]
- Jóźwiak-Niedźwiedzka, D.; Fantilli, A.P. Wool-Reinforced Cement Based Composites. Materials 2020, 13, 3590. [Google Scholar] [CrossRef] [PubMed]
- Fantilli, A.P.; Jóźwiak-Niedźwiedzka, D. The effect of hydraulic cements on the flexural behavior of wool reinforced mortars. Acad. J. Civ. Eng. 2019, 37, 287–292. [Google Scholar]
- Tonoli, G.H.D.; Santos, S.F.; Joaquim, A.P.; Savastano, H. Effect of Accelerated Carbonation on Cementitious Roofing Tiles Reinforced with Lignocellulosic Fibre. Constr. Build. Mater. 2010, 24, 193–201. [Google Scholar] [CrossRef]
- Pizzol, V.D.; Mendes, L.M.; Frezzatti, L.; Savastano, H., Jr.; Tonoli, G.H.D. Effect of Accelerated Carbonation on the Microstructure and Physical Properties of Hybrid Fiber-Cement Composites. Miner. Eng. 2014, 59, 101–106. [Google Scholar] [CrossRef]
- El-Hassan, H. Accelerated Carbonation Curing as a Means of Reducing Carbon Dioxide Emissions. In Cement Industry—Optimization, Characterization and Sustainable Application; IntechOpen: London, UK, 2021. [Google Scholar]
- García, G.; Cabrera, R.; Rolón, J.; Pichardo, R.; Thomas, C. Natural Fibers as Reinforcement of Mortar and Concrete: A Systematic Review from Central and South American Regions. J. Build. Eng. 2024, 98, 111267. [Google Scholar] [CrossRef]
- Ahmad, J.; Zhou, Z. Mechanical Properties of Natural as Well as Synthetic Fiber Reinforced Concrete: A Review. Constr. Build. Mater. 2022, 333, 127353. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Wang, H.; Lyu, H.; Zou, D.; Liu, T. Carbonation Curing of Mortars Produced with Reactivated Cementitious Materials for CO2 Sequestration. J. Clean. Prod. 2023, 383, 135501. [Google Scholar] [CrossRef]
- Haq, I.U.; Bibi, T.; Nawaz, A.; Khalid, H.R.; Elahi, A. Calcined Clay–Substituted Sustainable Cement Binders: A Holistic Review. Environ. Sci. Pollut. Res. 2025, 32, 11317–11349. [Google Scholar] [CrossRef] [PubMed]
- PN-EN 196-1; Methods of Testing Cement—Determination of Strength. Polish Committee for Standardization: Warsaw, Poland, 2016; pp. 1–33.
- Papadakis, V.G. Effect of Supplementary Cementing Materials on Concrete Resistance against Carbonation and Chloride Ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the Carbonation of Concrete with Supplementary Cementitious Materials: A Critical Review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- Saeki, N.; Kurihara, R.; Ohkubo, T.; Teramoto, A.; Suda, Y.; Kitagaki, R.; Maruyama, I. Semi-Dry Natural Carbonation at Different Relative Humidities: Degree of Carbonation and Reaction Kinetics of Calcium Hydrates in Cement Paste. Cem. Concr. Res. 2025, 189, 107777. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P.; Baza, D.; Andrade, C. Assessment of the Protective Effect of Carbonation on Portlandite Crystals. Cem. Concr. Res. 2015, 74, 68–77. [Google Scholar] [CrossRef]
- da Silva, P.M.; Mendes, J.P.; Coelho, L.C.C.; de Almeida, J.M.M.M. Real-Time Monitoring of Cement Paste Carbonation with In Situ Optical Fiber Sensors. Chemosensors 2023, 11, 449. [Google Scholar] [CrossRef]
- Kuzielová, E.; Slaný, M.; Žemlička, M.; Másilko, J. Accelerated Carbonation of Oil-Well Cement Blended with Pozzolans and Latent Hydraulic Materials. J. Therm. Anal. Calorim. 2023, 148, 9963–9977. [Google Scholar] [CrossRef]
- Missana, T.; Almendros-Ginestà, O.; Colmenero, F.; Fernández, A.M.; García-Gutiérrez, M. Investigation of the Surface Charge Behaviour of Ettringite: Influence of PH, Calcium, and Sulphate Ions. Heliyon 2024, 10, e36117. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Chang, J.; Yang, H.; Ding, S.; Liu, X.; Wu, K.; Zhao, Q. Modeling and Experimental Validation of Carbonation Mechanism of Ye’elimite-Gypsum-Water System. Mater. Struct. 2025, 58, 146. [Google Scholar] [CrossRef]
- Dousti, A.; Beyki, H.; Shekarchi, M. Strength and Durability Performance of Mortars Incorporating Calcined Clay as Pozzolan in Comparison with Silica Fume. J. Civil. Eng. Mater. Appl. 2022, 6, 159–174. [Google Scholar]
- Nguyen, Q.D.; Afroz, S.; Castel, A. Influence of Calcined Clay Reactivity on the Mechanical Properties and Chloride Diffusion Resistance of Limestone Calcined Clay Cement (LC3) Concrete. J. Mar. Sci. Eng. 2020, 8, 301. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Brice, D.G.; Kilcullen, A.; Duxson, P.; van Deventer, J.S.J. Accelerated Carbonation Testing of Alkali-Activated Binders Significantly Underestimates Service Life: The Role of Pore Solution Chemistry. Cem. Concr. Res. 2012, 42, 1317–1326. [Google Scholar] [CrossRef]
- Balestra, C.E.T.; Savaris, G.; Nakano, A.Y.; Schneider, R. Carbonation of Concretes Containing LC3 Cements with Different Supplementary Materials. Semin. Ciênc. Exatas Tecnol. 2022, 43, 161–170. [Google Scholar] [CrossRef]
- Wei, J.; Meyer, C. Degradation Rate of Natural Fiber in Cement Composites Exposed to Various Accelerated Aging Environment Conditions. Corros. Sci. 2014, 88, 118–132. [Google Scholar] [CrossRef]
- Lilargem Rocha, D.; Tambara Júnior, L.; Marvila, M.; Pereira, E.; Souza, D.; de Azevedo, A. A Review of the Use of Natural Fibers in Cement Composites: Concepts, Applications and Brazilian History. Polymers 2022, 14, 2043. [Google Scholar] [CrossRef]
- Amsalu Fode, T.; Chande Jande, Y.A.; Kivevele, T.; Rahbar, N. A Review on Degradation Improvement of Sisal Fiber by Alkali and Pozzolana for Cement Composite Materials. J. Nat. Fibers 2024, 21, 2335327. [Google Scholar] [CrossRef]
- Li, Y.; Miao, L.; Dong, Z.; Jin, Y.; Liu, W.; Gao, F.; Li, Y. Carbonation and Chloride Resistance Characteristics of Self-Developed Limestone Calcined Clay Cement (LC3) Derived from Excavated Spoil. Materials 2025, 18, 2546. [Google Scholar] [CrossRef] [PubMed]
- Rakhsh Mahpour, A.; Ventura, H.; Ardanuy, M.; Rosell, J.R.; Claramunt, J. The Effect of Fibres and Carbonation Conditions on the Mechanical Properties and Microstructure of Lime/Flax Composites. Cem. Concr. Compos. 2023, 138, 104981. [Google Scholar] [CrossRef]
- Manso, S.; Aguado, A. A Review of Sample Preparation and Its Influence on Ph Determination in Concrete Samples. Mater. Constr. 2017, 67, e108. [Google Scholar] [CrossRef]











| Mortar | CEM I | Calcined Clay | Sand | Water | Dry Ice | Wool Fibers |
|---|---|---|---|---|---|---|
| M1 | 450 | – | 1350 | 225 | – | – |
| M2 | 450 | – | 1350 | 225 | 6.75 | – |
| M3 | 450 | – | 1350 | 225 | – | 10 |
| M4 | 450 | – | 1350 | 225 | 6.75 | 10 |
| M5 | 315 | 135 | 1350 | 225 | – | – |
| M6 | 315 | 135 | 1350 | 225 | 6.75 | – |
| M7 | 315 | 135 | 1350 | 225 | – | 10 |
| M8 | 315 | 135 | 1350 | 225 | 6.75 | 10 |
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 |
|---|---|---|---|---|---|---|---|
| 12.5 | 12.4 | 12.5 | 12.1 | 12.3 | 12.2 | 12.1 | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak-Niedźwiedzka, D.; Lisowski, P.; Osial, M.; Brachaczek, A.; Alterman, D.; Fantilli, A.P. Mechanical and Microstructural Performance of Cement Mortars with Internal Carbonation and Sustainable Additives. Ceramics 2025, 8, 140. https://doi.org/10.3390/ceramics8040140
Jóźwiak-Niedźwiedzka D, Lisowski P, Osial M, Brachaczek A, Alterman D, Fantilli AP. Mechanical and Microstructural Performance of Cement Mortars with Internal Carbonation and Sustainable Additives. Ceramics. 2025; 8(4):140. https://doi.org/10.3390/ceramics8040140
Chicago/Turabian StyleJóźwiak-Niedźwiedzka, Daria, Paweł Lisowski, Magdalena Osial, Aneta Brachaczek, Dariusz Alterman, and Alessandro P. Fantilli. 2025. "Mechanical and Microstructural Performance of Cement Mortars with Internal Carbonation and Sustainable Additives" Ceramics 8, no. 4: 140. https://doi.org/10.3390/ceramics8040140
APA StyleJóźwiak-Niedźwiedzka, D., Lisowski, P., Osial, M., Brachaczek, A., Alterman, D., & Fantilli, A. P. (2025). Mechanical and Microstructural Performance of Cement Mortars with Internal Carbonation and Sustainable Additives. Ceramics, 8(4), 140. https://doi.org/10.3390/ceramics8040140









