Effects of Photobiomodulation in Association with Biomaterials on the Process of Guided Bone Regeneration: An Integrative Review
Abstract
1. Introduction
2. Methods
3. Results
3.1. Selected and Reviewed Studies
| Authors | Type of Laser (Manufacturer) | Wavelength (nm)/Spot Beam | Output Power (mW) | Energy Density (J.cm−2) | Total Delivered Energy (J, per Session) | Quantity of Radiation | Therapeutic Variables | Irradiation Site | Evaluation Time | Principal Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Alves et al., 2020 [10] | GaAlAs (PhotonLase III, DMC Equipment, São Carlos, SP, Brazil) | 808 nm | 40 mW | 4 J.cm−2 for group PBM-1 and 14 J.cm−2 for group PBM-2 | 0.48 J (PBM-1) and 1.6 J (PBM-2) | 4 points for both groups, 3 s per point for the PBM-1 group and 10 s per point for the PBM-2 group | BioGide® (Geistlich Pharma AG, Wolhusen, Switzerland) | Rat calvaria | Immediately after the procedure, 48 h, and 96 h after the surgical procedure | The PBM-treated groups, especially PBM-1, had a significantly higher bone volume fraction and number of trabeculae compared to the control group. In addition, the thickness and separation values of the trabeculae and the structural model index were significantly lower in the PBM-treated groups. Connectivity density was also significantly higher in the membrane and PBM-treated groups compared to the control group. |
| AboElsaad et al., 2009 [9] | GaAlAs (Velopex Diode Laser, MeDivance Instruments Ltd., London, UK) | 830 nm | 40 mW | 16 J.cm−2 | 2.4 J | 60 s directly on the defect area | PerioGlas® (NovaBone Products LLC, Alachua, FL, USA) | Rat jaw | At the beginning of the study and on days 3, 5, and 7 after surgery | At 3 months, there was a statistically significant difference between the sites with and without photobiomodulation. However, at 6 months, no difference was observed. According to the authors, it was possible to confirm the positive effect of the photobiomodulation in accelerating the healing of periodontal wounds. |
| Della Coletta et al., 2021 [3] | GaAIAs (Ibramed Laserpulse®, Amparo, São Paulo, Brazil) | 830 nm | 30 mW | 6.2 J.cm−2 | 2.88 J | Four spots around the surgical area, 24 s per spot | GenPhos XP® (Baumer S.A., Mogi Mirim, São Paulo, Brazil) and fibrin biopolymer developed by the Center for the Study of Venoms and Venomous Animals (CEVAP), São Paulo State University “Júlio de Mesquita Filho” (UNESP), Brazil | Rat calvaria | Immediately after surgery and three times a week until euthanasia | Bone growth was more evident in the BFMLG group at 42 days, limited to the edges of the defect and the permanence of the particles. Histomorphology tests showed an inflammatory infiltrate, with regression accompanied by the formation of mineralized bone tissue. All groups showed a progressive increase in new bone tissue, with BFMLG showing the greatest bone formation in both periods. Picrosirius-red staining revealed greater yellow-green birefringence of the collagen fibers in the BFMLG group, suggesting more advanced bone maturation. |
| de Oliveira et al., 2018 [35] | GaAlAs (Therapy XT, CW, DMC Equipment, São Carlos, SP, Brazil) | 808 nm | 100 mW | 354 J.cm−2 | 4.0 J | Photobiomodulation was conducted transcutaneously, with the laser tip in contact with the skin for 10 s per point, totaling 40 s per session. There were 7 sessions, applied every 48 h for 13 days, starting immediately after the suture. | Deproteinized bovine bone (DBB; Bio-Oss®, Geistlich AG, Wolhusen, Switzerland) and biphasic ceramic comprising hydroxyapatite and β-tricalcium phosphate (HA/βTCP; Straumann® Bone Ceramic, Straumann AG, Basel, Switzerland) | Lateral region of the rat’s mandible | There were 7 sessions, applied every 48 h for 13 days, starting immediately after the suture. | The author could see an increase in the formation of mineralized tissue and bone, especially after 90 days, an increase in the expression of BMP2, OCN, and ALP proteins, greater expression of ALP, BMP2, and Jagged1 mRNA. There was also an improvement in the osteoconductive potential of deproteinized bovine bone and HA/βTCP grafts and bone formation in areas without grafts. |
| Freitas et al., 2023 [12] | GaAlAs (TheraLase DMC®, São Carlos, São Paulo, Brazil) | 730 nm | 100 mW | 210 J.cm−2 | 24.0 J | 60 s, at four points on the edges of the surgical defect created (12 h, 3 h, 6 h and 9 h), as well as a central point on the bone graft | Bio-Oss® (Geistlich Pharma AG, Wolhusen, Switzerland) and BioGide® (Geistlich Pharma AG, Wolhusen, Switzerland) | Rat calvaria | A single application during the transoperative period | The group that received photobiomodulation in conjunction with deproteinized bovine bone showed statistically significant differences in all the variables analyzed compared to the group treated only with deproteinized bovine bone. The application of photobiomodulation in guided bone regeneration resulted in a reduction in the residual particle area compared to the group treated only with guided bone regeneration, and this difference was statistically significant. However, no significant results were observed in relation to the area of newly formed bone and the linear extension of the bone. |
| Freitas et al., 2018 [13] | GaAlAs (TheraLase DMC®, São Carlos, São Paulo, Brazil) | 808 nm | 100 mW | 30 J.cm−2 | 30.0 J | 60 s per point, applied at five points: four on the surface of the surgically created defect, according to clockwise positions (12 h, 3 h, 6 h, 9 h), plus a central point | BioGide® (Geistlich Pharma AG, Wolhusen, Switzerland) and TheraLase DMC® (DMC Equipamentos Ltd.a., São Carlos, SP, Brazil) | Rat calvaria | Only one application was conducted during the trans operative period | All the groups showed a greater area of newly formed bone compared to the control group. The PBMT + M group achieved the greatest amount of new bone, followed by the PBMT, M, AB + PBMT and AB + PBMT + M groups. The groups treated with PBMT (PBMT and PBMT + M) showed a greater amount of new bone compared to the groups treated with autogenous material (AB and AB + M). There was no statistically significant difference in the area of remaining particles between the AB + M and AB + PBMT + M groups. |
| Luca et al., 2020 [14] | GaAlAs (IRRADIA Mid-Laser®, Stockholm, Sweden) | 808 nm | 450 mW | 24.075 J.cm−2 | 30.6 J | Four opposite peripheral points and a central point of the defect using a plastic surgical guide for 17 s each point | NuOss® (natural cancellous and cortical bone matrix, ACE Surgical Supply, Brockton, MA, USA) and ACE RCM6® (resorbable collagen membrane, ACE Surgical Supply, Brockton, MA, USA) | Rat calvaria | Day of the surgery and every 48 h, for 14 days, 21 days and 30 days, respectively | The results obtained by the authors indicate that photobiomodulation had a superior healing effect compared to the support provided by the biomaterial alone, especially during the first 14 days after surgery. |
| Pinheiro et al., 2009 [15] | GaAlAs (Thera Lase®, DMC Equipamentos, São Carlos, SP, Brazil) | 830 nm | 40 mW | 4 J.cm−2 | - | Transcutaneous application at four points around the surgical site | Gen-Phos® and Gen-Derm® (Baumer S.A., Mogi Mirim, São Paulo, Brazil) | Rat femur | After placing the sutures and repeated every other day for 15 days | According to the author, the data may suggest that photobiomodulation can have a positive effect on the early healing of bone defects treated with a combination of biomaterial and guided bone regeneration. |
| Rufato et al., 2022 [36] | GaAlAs (Twin Laser, Mm Optics, São Carlos, SP, Brazil) | 780 nm | 40 mW | 30 J.cm−2 | 4.8 J | The laser was applied in contact with and perpendicular to the edges of the bone defect. Irradiation took place at four points (positions 3 h, 6 h, 9 h and 12 h), with 30 s per point, totaling 120 s per session. The protocol consisted of 12 sessions in total. | Textured membranes of poly (vinylidene fluoride–trifluoroethylene)/barium titanate [P(VDF-TrFE)/BT] (developed by UNIFEI, Itajubá, Brazil) and Surgitime PTFE (Bionnovation®, Bauru, São Paulo, Brazil) | Calvaria of ovariectomized rats | The first application was conducted 24 h after surgery and repeated every 2 days, totaling 12 sessions. | The P(VDF-TrFE)/BT membrane favored bone repair, regardless of photobiomodulation. The combination of PBM and polytetrafluoroethylene (PTFE) increased the expression of Runx2, Alp, Bsp, Bglap, Sp7 and Rankl genes. |
| Valiati et al., 2012 [37] | GaAlAs (Thera Lase®, DMC Equipamentos, São Carlos, SP, Brazil) | 830 nm | - | 16 J.cm−2 | - | The laser was applied at four points on the calvaria, with eight sessions in total | Allograft blocks harvested from two rabbits and processed by deep freezing | Rabbit calvaria | The laser was applied immediately after the surgical procedure and repeated every 48 h, totaling eight sessions | Photobiomodulation improved graft incorporation, reduced initial inflammation and increased collagen deposition. Microscopy confirmed that the allograft treated with deep freezing and PBM is a viable alternative for bone repair. |
| Authors | Type of Laser (Manufacturer) | Wavelength (nm)/Spot Beam | Output Power (mW) | Energy Density (J.cm−2) | Total Delivered Energy (J, per Session) | Quantity of Radiation | Therapeutic Variables | Irradiation Site | Evaluation Time | Principal Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Emrem Doğan et al., 2014 [11] | Nd:YAG (Smarty A10; DEKA, Firenze, Italy) | 1064 nm | 100 mW | 4 J.cm−2 | - | The beam was emitted through a phototherapeutic probe at a distance of 1 cm from the soft tissue target area covering the bone defect. The exposure time was 300 s per tooth, i.e., 60 s for each application to a defect | Bio-gen® (BGM-O5; Bioteck S.p.A., Riva Presso Chieri, Italy) and Biocollagen® (BCG-O1; Bioteck S.p.A., Riva Presso Chieri, Italy) | Bilateral intraosseous periodontal defects in humans | It was applied for 5 min at the time of surgery and on postoperative days 1, 3, 5 and 7 | The study demonstrated that RTG is an effective treatment for periodontal regeneration and that TLBP can improve the effects of RTG in the treatment of periodontal defects. |
3.2. Results of the Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO2 | Carbon dioxide |
| Er:YAG | Erbium-doped yttrium aluminum garnet |
| PBMT | Photobiomodulation therapy |
| PBM | Photobiomodulation |
| NC | Negative control |
| PC | Positive control |
| M | Collagen membrane |
| Laser | Light amplification by stimulated emission of radiation |
| LLT | Low-intensity lasers |
| HLLT | High-power lasers |
| HA/βTCP | Biphasic ceramic comprising hydroxyapatite and β-tricalcium phosphate |
| GaAIAs | Aluminum gallium arsenide diode |
| PLA | Polylactic acid |
| PGA | Polyglycolic acid |
| PTFE | Polytetrafluoroethylene |
| BMP2 | Bone morphogenetic protein 2 |
| OCN | Osteocalcin |
| Runx2 | Runt-related transcription factor 2 |
| Bsp | Bone sialoprotein |
| Bglap | Bone gamma-carboxyglutamate protein |
| Sp7 | Osterix |
| Rankl | Receptor activator of nuclear factor kappa-B ligand |
| Alp | Alkaline phosphatase |
| P(VDF-TrFE)/BT | Poly (vinylidene fluoride-trifluoroethylene)/barium titanate |
| NIR | Near infrared |
| Nd:YAG | Neodymium-doped yttrium aluminum garnet |
References
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The Role of Barrier Membranes for Guided Bone Regeneration and Restoration of Large Bone Defects: Current Experimental and Clinical Evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef]
- Della Coletta, B.B.; Jacob, T.B.; Moreira, L.A.d.C.; Pomini, K.T.; Buchaim, D.V.; Eleutério, R.G.; Pereira, E.d.S.B.M.; Roque, D.D.; Rosso, M.P.d.O.; Shindo, J.V.T.C.; et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules 2021, 26, 847. [Google Scholar] [CrossRef]
- Bosco, A.F.; Faleiros, P.L.; Carmona, L.R.; Garcia, V.G.; Theodoro, L.H.; de Araujo, N.J.; Nagata, M.J.H.; de Almeida, J.M. Effects of Low-Level Laser Therapy on Bone Healing of Critical-Size Defects Treated with Bovine Bone Graft. J. Photochem. Photobiol. B 2016, 163, 303–310. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, J.B.; Buchaim, D.V.; de Souza Bueno, C.R.; Pomini, K.T.; Barraviera, B.; Júnior, R.S.F.; Andreo, J.C.; de Castro Rodrigues, A.; Cestari, T.M.; Buchaim, R.L. Effects of Low-Level Laser Therapy on Autogenous Bone Graft Stabilized with a New Heterologous Fibrin Sealant. J. Photochem. Photobiol. B 2016, 162, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Andreo, J.C.; Ferreira Junior, R.S.; Barraviera, B.; Rodrigues, A.d.C.; Macedo, M.d.C.; Rosa Junior, G.M.; Shinohara, A.L.; Santos German, I.J.; Pomini, K.T.; et al. Efficacy of Laser Photobiomodulation on Morphological and Functional Repair of the Facial Nerve. Photomed. Laser Surg. 2017, 35, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Tissue Engineering. Mol. Ther. 2000, 1, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Janicki, P.; Schmidmaier, G. What Should Be the Characteristics of the Ideal Bone Graft Substitute? Combining Scaffolds with Growth Factors and/or Stem Cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef]
- AboElsaad, N.S.; Soory, M.; Gadalla, L.M.A.; Ragab, L.I.; Dunne, S.; Zalata, K.R.; Louca, C. Effect of Soft Laser and Bioactive Glass on Bone Regeneration in the Treatment of Bone Defects (an Experimental Study). Lasers Med. Sci. 2009, 24, 527–533. [Google Scholar] [CrossRef]
- Alves, F.A.M.; Marques, M.M.; Cavalcanti, S.C.S.X.B.; Pedroni, A.C.F.; Ferraz, E.P.; Miniello, T.G.; Moreira, M.S.; Jerônimo, T.; Deboni, M.C.Z.; Lascala, C.A. Photobiomodulation as Adjunctive Therapy for Guided Bone Regeneration. A MicroCT Study in Osteoporotic Rat Model. J. Photochem. Photobiol. B 2020, 213, 112053. [Google Scholar] [CrossRef]
- Emrem Doğan, G.; Demir, T.; Orbak, R. Effect of Low-Level Laser on Guided Tissue Regeneration Performed with Equine Bone and Membrane in the Treatment of İntrabony Defects: A Clinical Study. Photomed. Laser Surg. 2014, 32, 226–231. [Google Scholar] [CrossRef]
- Freitas, N.R.d.; Guerrini, L.B.; Esper, L.A.; Sbrana, M.C.; Santos, C.C.V.d.; Almeida, A.L.P.F.d. Photobiomodulation and Inorganic Bovine Bone in Guided Bone Regeneration: Histomorphometric Analysis in Rats. J. Funct. Biomater. 2023, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Freitas, N.R.d; Guerrini, L.B.; Esper, L.A.; Sbrana, M.C.; Dalben, G.d.S.; Soares, S.; de Almeida, A.L.P.F. Evaluation of Photobiomodulation Therapy Associated with Guided Bone Regeneration in Critical Size Defects. In Vivo Study. J. Appl. Oral Sci. 2018, 26, e20170244. [Google Scholar] [CrossRef] [PubMed]
- Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.-F.; Todea, C.D. Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. Int. J. Mol. Sci. 2020, 21, 778. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.L.B.; Martinez Gerbi, M.E.; de Assis Limeira, F.; Carneiro Ponzi, E.A.; Marques, A.M.C.; Carvalho, C.M.; de Carneiro Santos, R.; Oliveira, P.C.; Nóia, M.; Ramalho, L.M.P. Bone Repair Following Bone Grafting Hydroxyapatite Guided Bone Regeneration and Infra-Red Laser Photobiomodulation: A Histological Study in a Rodent Model. Lasers Med. Sci. 2009, 24, 234–240. [Google Scholar] [CrossRef]
- Torres, C.S.; dos Santos, J.N.; Monteiro, J.S.C.; Amorim, P.G.M.; Pinheiro, A.L.B. Does the Use of Laser Photobiomodulation, Bone Morphogenetic Proteins, and Guided Bone Regeneration Improve the Outcome of Autologous Bone Grafts? An in Vivo Study in a Rodent Model. Photomed. Laser Surg. 2008, 26, 371–377. [Google Scholar] [CrossRef]
- Cypher, T.J.; Grossman, J.P. Biological Principles of Bone Graft Healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Ferraz, M.P. An Overview on the Big Players in Bone Tissue Engineering: Biomaterials, Scaffolds and Cells. Int. J. Mol. Sci. 2024, 25, 3836. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Yang, C.; Pan, Y.; Ji, S.; Han, N.; Sun, G. Biomaterials for Bone Defect Repair: Types, Mechanisms and Effects. Int. J. Artif. Organs 2024, 47, 75–84. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B.; Aciole, G.T.S.; Ramos, T.A.; Gonzalez, T.A.; da Silva, L.N.; Soares, L.G.P.; Aciole, J.M.S.; dos Santos, J.N. The Efficacy of the Use of IR Laser Phototherapy Associated to Biphasic Ceramic Graft and Guided Bone Regeneration on Surgical Fractures Treated with Miniplates: A Histological and Histomorphometric Study on Rabbits. Lasers Med. Sci. 2014, 29, 279–288. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B.; Aciole, G.T.S.; Cangussú, M.C.T.; Pacheco, M.T.T.; Silveira, L. Effects of Laser Photherapy on Bone Defects Grafted with Mineral Trioxide Aggregate, Bone Morphogenetic Proteins, and Guided Bone Regeneration: A Raman Spectroscopic Study. J. Biomed. Mater. Res. A 2010, 95A, 1041–1047. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B.; Soares, L.G.P.; Barbosa, A.F.S.; Ramalho, L.M.P.; dos Santos, J.N. Does LED Phototherapy Influence the Repair of Bone Defects Grafted with MTA, Bone Morphogenetic Proteins, and Guided Bone Regeneration? A Description of the Repair Process on Rodents. Lasers Med. Sci. 2012, 27, 1013–1024. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B.; Santos, N.R.S.; Oliveira, P.C.; Aciole, G.T.S.; Ramos, T.A.; Gonzalez, T.A.; da Silva, L.N.; Barbosa, A.F.S.; Silveira, L. The Efficacy of the Use of IR Laser Phototherapy Associated to Biphasic Ceramic Graft and Guided Bone Regeneration on Surgical Fractures Treated with Wire Osteosynthesis: A Comparative Laser Fluorescence and Raman Spectral Study on Rabbits. Lasers Med. Sci. 2013, 28, 815–822. [Google Scholar] [CrossRef]
- Deana, A.M.; de Souza, A.M.; Teixeira, V.P.; Mesquita-Ferrari, R.A.; Bussadori, S.K.; Fernandes, K.P.S. The Impact of Photobiomodulation on Osteoblast-like Cell: A Review. Lasers Med. Sci. 2018, 33, 1147–1158. [Google Scholar] [CrossRef]
- Furtado, G.S.; Martin, V.; Araújo, R.; Gomes, P.S.; Lago, A.D.N. Osteoinductive Activity of Photobiomodulation in an Organotypic Bone Model. Photodiagn. Photodyn. Ther. 2024, 45, 103936. [Google Scholar] [CrossRef]
- Greben, A.I.; Eremin, P.S.; Kostromina, E.Y.; Markov, P.A.; Greben, T.N.; Gilmutdinova, I.R.; Konchugova, T.V. Low Level Laser Therapy: Molecular Mechanisms of Anti-Inflammatory and Regenerative Effects. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2023, 100, 61–68. [Google Scholar] [CrossRef]
- Lu, P.; Peng, J.; Liu, J.; Chen, L. The Role of Photobiomodulation in Accelerating Bone Repair. Prog. Biophys. Mol. Biol. 2024, 188, 55–67. [Google Scholar] [CrossRef]
- Shokri, A.; Moradhaseli, H.; Fekrazad, R.; Jazaeri, M.; Farhadian, M. Effect of Photobiomodulation Therapy with Different Wavelengths on Bone Mineral Density in Osteoporotic Rats. Lasers Med. Sci. 2023, 38, 59. [Google Scholar] [CrossRef] [PubMed]
- Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G.; et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, Q. Current Advances of Photobiomodulation Therapy in Treating Knee Osteoarthritis. Front. Cell Dev. Biol. 2023, 11, 1286025. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.F.; Bobinski, F.; Martins, D.F.; Palandi, J.; Folmer, V.; da Silva, M.D. Photobiomodulation Therapy in Knee Osteoarthritis Reduces Oxidative Stress and Inflammatory Cytokines in Rats. J. Biophotonics 2020, 13, e201900204. [Google Scholar] [CrossRef] [PubMed]
- Chiari, S. Photobiomodulation and Lasers. Front. Oral Biol. 2016, 18, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Escudero, J.S.B.; Perez, M.G.B.; de Oliveira Rosso, M.P.; Buchaim, D.V.; Pomini, K.T.; Campos, L.M.G.; Audi, M.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Bone Repair: A Systematic Review. Injury 2019, 50, 1853–1867. [Google Scholar] [CrossRef]
- Lopes, C.d.C.A.; Limirio, J.P.J.O.; Zanatta, L.S.A.; Simamoto, V.R.N.; Dechichi, P.; Limirio, P.H.J.O. Effectiveness of Photobiomodulation Therapy on Human Bone Healing in Dentistry: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2022, 40, 440–453. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.J.P.L.; Aroni, M.A.T.; Medeiros, M.C.; Marcantonio, E.; Marcantonio, R.A.C. Effect of Low-level Laser Therapy on the Healing of Sites Grafted with Coagulum, Deproteinized Bovine Bone, and Biphasic Ceramic Made of Hydroxyapatite and Β-tricalcium Phosphate. In Vivo Study in Rats. Lasers Surg. Med. 2018, 50, 651–660. [Google Scholar] [CrossRef]
- Rufato, F.C.T.; de Sousa, L.G.; Scalize, P.H.; Gimenes, R.; Regalo, I.H.; Rosa, A.L.; Beloti, M.M.; de Oliveira, F.S.; Bombonato-Prado, K.F.; Regalo, S.C.H.; et al. Texturized P(VDF-TrFE)/BT Membrane Enhances Bone Neoformation in Calvaria Defects Regardless of the Association with Photobiomodulation Therapy in Ovariectomized Rats. Clin. Oral Investig. 2022, 26, 1053–1065. [Google Scholar] [CrossRef]
- Valiati, R.; Paes, J.V.; de Moraes, A.N.; Gava, A.; Agostini, M.; Masiero, A.V.; de Oliveira, M.G.; Pagnoncelli, R.M. Effect of Low-Level Laser Therapy on Incorporation of Block Allografts. Int. J. Med. Sci. 2012, 9, 853–861. [Google Scholar] [CrossRef]
- Rando, R.G.; Buchaim, D.V.; Cola, P.C.; Buchaim, R.L. Effects of Photobiomodulation Using Low-Level Laser Therapy on Alveolar Bone Repair. Photonics 2023, 10, 734. [Google Scholar] [CrossRef]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological Effects of Low Level Laser Therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar]
- Crous, A.; Abrahamse, H. The Signalling Effects of Photobiomodulation on Osteoblast Proliferation, Maturation and Differentiation: A Review. Stem Cell Rev. Rep. 2021, 17, 1570–1589. [Google Scholar] [CrossRef]
- Chong, W.L.; Chu, S.A.; Dam, J.G.; Ong, K.S. Oral Rehabilitation Using Dental Implants and Guided Bone Regeneration. Ann. Acad. Med. Singap. 1999, 28, 697–703. [Google Scholar]
- Drăgan, E.; Nemţoi, A. Review of the Long-Term Outcomes of Guided Bone Regeneration and Autologous Bone Block Augmentation for Vertical Dental Restoration of Dental Implants. Med. Sci. Monit. 2022, 28, e937433-1–e937433-9. [Google Scholar] [CrossRef]
- Johnson, T.B.; Siderits, B.; Nye, S.; Jeong, Y.-H.; Han, S.-H.; Rhyu, I.-C.; Han, J.-S.; Deguchi, T.; Beck, F.M.; Kim, D.-G. Effect of Guided Bone Regeneration on Bone Quality Surrounding Dental Implants. J. Biomech. 2018, 80, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Tim, C.R.; Pinto, K.N.Z.; Rossi, B.R.O.; Fernandes, K.; Matsumoto, M.A.; Parizotto, N.A.; Rennó, A.C.M. Low-Level Laser Therapy Enhances the Expression of Osteogenic Factors during Bone Repair in Rats. Lasers Med. Sci. 2014, 29, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Nissan, J.; Assif, D.; Gross, M.D.; Yaffe, A.; Binderman, I. Effect of Low Intensity Laser Irradiation on Surgically Created Bony Defects in Rats. J. Oral Rehabil. 2006, 33, 619–924. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.; Ridgway, T.D.; Higbee, R.G.; Howard, E.W.; Lucroy, M.D. Effect of Wavelength on Low-Intensity Laser Irradiation-Stimulated Cell Proliferation in Vitro. Lasers Surg. Med. 2005, 36, 8–12. [Google Scholar] [CrossRef]
- Khoo, N.K.; Shokrgozar, M.A.; Kashani, I.R.; Amanzadeh, A.; Mostafavi, E.; Sanati, H.; Habibi, L.; Talebi, S.; Abouzaripour, M.; Akrami, S.M. In Vitro Therapeutic Effects of Low Level Laser at MRNA Level on the Release of Skin Growth Factors from Fibroblasts in Diabetic Mice. Avicenna J. Med. Biotechnol. 2014, 6, 113–118. [Google Scholar]
- Hernández-Bule, M.L.; Naharro-Rodríguez, J.; Bacci, S.; Fernández-Guarino, M. Unlocking the Power of Light on the Skin: A Comprehensive Review on Photobiomodulation. Int. J. Mol. Sci. 2024, 25, 4483. [Google Scholar] [CrossRef]
- Pulicari, F.; Pellegrini, M.; Pascadopoli, M.; Porrini, M.; Kuhn, E.; Scribante, A.; Spadari, F. Plasma Cell Gingivitis Treated with Photobiomodulation, with No Recurrence for a Five-Year Follow-Up. Case Rep. Dent. 2022, 2022, 2992656. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Machado, C.d.S.M.; De Marchi, T.; Casalechi, H.L.; Bjordal, J.M.; de Carvalho, P.d.T.C.; Leal-Junior, E.C.P. Infrared Low-Level Laser Therapy (Photobiomodulation Therapy) before Intense Progressive Running Test of High-Level Soccer Players: Effects on Functional, Muscle Damage, Inflammatory, and Oxidative Stress Markers—A Randomized Controlled Trial. Oxid. Med. Cell Longev. 2019, 2019, 6239058. [Google Scholar] [CrossRef]
- Balbinot, G.; Schuch, C.P.; Nascimento, P.S.d.; Lanferdini, F.J.; Casanova, M.; Baroni, B.M.; Vaz, M.A. Photobiomodulation Therapy Partially Restores Cartilage Integrity and Reduces Chronic Pain Behavior in a Rat Model of Osteoarthritis: Involvement of Spinal Glial Modulation. Cartilage 2021, 13, 1309S–1321S. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B. Advances and Perspectives on Tissue Repair and Healing. Photomed. Laser Surg. 2009, 27, 833–836. [Google Scholar] [CrossRef]
- Selestin Raja, I.; Kim, C.; Oh, N.; Park, J.-H.; Hong, S.W.; Kang, M.S.; Mao, C.; Han, D.-W. Tailoring Photobiomodulation to Enhance Tissue Regeneration. Biomaterials 2024, 309, 122623. [Google Scholar] [CrossRef]
- Coelho, R.C.P.; Zerbinati, L.P.S.; de Oliveira, M.G.; Weber, J.B.B. Systemic Effects of LLLT on Bone Repair around PLLA–PGA Screws in the Rabbit Tibia. Lasers Med. Sci. 2014, 29, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Freddo, A.L.; Rodrigo, S.M.; Massotti, F.P.; Etges, A.; de Oliveira, M.G. Effect of Low-Level Laser Therapy after Implantation of Poly-L-Lactic/Polyglycolic Acid in the Femurs of Rats. Lasers Med. Sci. 2009, 24, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Vigliar, M.F.R.; Marega, L.F.; Duarte, M.A.H.; Alcalde, M.P.; Rosso, M.P.d.O.; Ferreira Junior, R.S.; Barraviera, B.; Reis, C.H.B.; Buchaim, D.V.; Buchaim, R.L. Photobiomodulation Therapy Improves Repair of Bone Defects Filled by Inorganic Bone Matrix and Fibrin Heterologous Biopolymer. Bioengineering 2024, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.L.B.; Soares, L.G.P.; Marques, A.M.C.; Cangussú, M.C.T.; Pacheco, M.T.T.; Silveira, L. Biochemical Changes on the Repair of Surgical Bone Defects Grafted with Biphasic Synthetic Micro-Granular HA + β-Tricalcium Phosphate Induced by Laser and LED Phototherapies and Assessed by Raman Spectroscopy. Lasers Med. Sci. 2017, 32, 663–672. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Rocha, G.S.; Ribeiro Junior, V.L.; Sakakura, C.E.; de Mello Neto, J.M.; Garcia, V.G.; Ervolino, E.; Marcantonio Junior, E. Bone Formed After Maxillary Sinus Floor Augmentation by Bone Autografting with Hydroxyapatite and Low-Level Laser Therapy. Implant. Dent. 2018, 27, 547–554. [Google Scholar] [CrossRef]
- Reis, C.H.B.; Buchaim, R.L.; Pomini, K.T.; Hamzé, A.L.; Zattiti, I.V.; Duarte, M.A.H.; Alcalde, M.P.; Barraviera, B.; Ferreira Júnior, R.S.; Pontes, F.M.L.; et al. Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair. Polymers 2022, 14, 2075. [Google Scholar] [CrossRef]
- Ivandic, T. Low-Level Laser Therapy. Dtsch. Arztebl. Int. 2021, 118, 69. [Google Scholar] [CrossRef]
- Nadhreen, A.; Alamoudi, N.; Elkhodary, H. Low-Level Laser Therapy in Dentistry: Extra-Oral Applications. Niger. J. Clin. Pract. 2019, 22, 1313–1318. [Google Scholar] [CrossRef]
- Biala, M. Low-Level Laser Therapy: A Literature Review of the Prevention and Reduction of Oral Mucositis in Patients Undergoing Stem Cell Transplantation. Clin. J. Oncol. Nurs. 2022, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-W.; Hong, C.-H.; Shih, M.-C.; Tam, K.-W.; Huang, Y.-H.; Kuan, Y.-C. Low-Level Laser Therapy for Fibromyalgia: A Systematic Review and Meta-Analysis. Pain Physician 2019, 22, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Glazov, G.; Yelland, M.; Emery, J. Low-Level Laser Therapy for Chronic Non-Specific Low Back Pain: A Meta-Analysis of Randomised Controlled Trials. Acupunct. Med. 2016, 34, 328–341. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Hamid, M.S.A.; Yusof, A. Effects of Low-Level and High-Intensity Laser Therapy as Adjunctive to Rehabilitation Exercise on Pain, Stiffness and Function in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Physiotherapy 2022, 114, 85–95. [Google Scholar] [CrossRef]
- Ezzati, K.; Laakso, E.-L.; Saberi, A.; Yousefzadeh Chabok, S.; Nasiri, E.; Bakhshayesh Eghbali, B. A Comparative Study of the Dose-Dependent Effects of Low Level and High Intensity Photobiomodulation (Laser) Therapy on Pain and Electrophysiological Parameters in Patients with Carpal Tunnel Syndrome. Eur. J. Phys. Rehabil. Med. 2021, 56, 733–740. [Google Scholar] [CrossRef]
- Sant’Anna, E.F.; Araújo, M.T.d.S.; Nojima, L.I.; da Cunha, A.C.; da Silveira, B.L.; Marquezan, M. High-Intensity Laser Application in Orthodontics. Dental Press J. Orthod. 2017, 22, 99–109. [Google Scholar] [CrossRef]
- de la Barra Ortiz, H.A.; Parizotto, N.; Arias, M.; Liebano, R. Effectiveness of High-Intensity Laser Therapy in the Treatment of Patients with Frozen Shoulder: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2023, 38, 266. [Google Scholar] [CrossRef]
- de la Barra Ortiz, H.A.; Avila, M.A.; Miranda, L.G.; Liebano, R.E. Effect of High-Intensity Laser Therapy in Patients with Non-Specific Chronic Neck Pain: Study Protocol for a Randomized Controlled Trial. Trials 2023, 24, 563. [Google Scholar] [CrossRef]
- Lopes, C.B.; Pacheco, M.T.T.; Silveira, L.; Duarte, J.; Cangussú, M.C.T.; Pinheiro, A.L.B. The Effect of the Association of NIR Laser Therapy BMPs, and Guided Bone Regeneration on Tibial Fractures Treated with Wire Osteosynthesis: Raman Spectroscopy Study. J. Photochem. Photobiol. B 2007, 89, 125–130. [Google Scholar] [CrossRef]
- Tong, L.; Liao, Q.; Zhao, Y.; Huang, H.; Gao, A.; Zhang, W.; Gao, X.; Wei, W.; Guan, M.; Chu, P.K.; et al. Near-Infrared Light Control of Bone Regeneration with Biodegradable Photothermal Osteoimplant. Biomaterials 2019, 193, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, P.; Lv, L.; Zhou, Y. NIR Light-Assisted Phototherapies for Bone-Related Diseases and Bone Tissue Regeneration: A Systematic Review. Theranostics 2020, 10, 11837–11861. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Di Cesare Mannelli, L.; Lucarini, E.; Cialdai, F.; Vignali, L.; Ghelardini, C.; Monici, M. Photobiomodulation Therapy by NIR Laser in Persistent Pain: An Analytical Study in the Rat. Lasers Med. Sci. 2017, 32, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P. Photobiomodulation in Promoting Wound Healing: A Review. Regener. Med. 2016, 11, 107–122. [Google Scholar] [CrossRef]
- Mosca, R.C.; Ong, A.A.; Albasha, O.; Bass, K.; Arany, P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv. Skin Wound Care 2019, 32, 157–167. [Google Scholar] [CrossRef]
- Incerti Parenti, S.; Tschon, M.; Sartori, M.; Visani, A.; Aroni, E.; Fini, M.; Alessandri-Bonetti, G. Evidence from Systematic Reviews on Photobiomodulation of Human Bone and Stromal Cells: Where Do We Stand? Arch. Biochem. Biophys. 2020, 685, 108333. [Google Scholar] [CrossRef]
- Varela, P.J.R.; Barros, P.A.G.; Montagner, P.G.; Provout, M.B.; Martinez, E.F.; Suzuki, S.S.; Garcez, A.S. Can Collagen Membrane on Bone Graft Interfere with Light Transmission and Influence Tissue Neoformation During Photobiomodulation? A Preliminary Study. Photobiomodul. Photomed. Laser Surg. 2023, 41, 167–174. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Amaroli, A.; De Angelis, N.; Benedicenti, S. Effects of Photobiomodulation on Bone Defects Grafted with Bone Substitutes: A Systematic Review of in Vivo Animal Studies. J. Biophotonics 2021, 14, e202000267. [Google Scholar] [CrossRef]
- Brassolatti, P.; de Andrade, A.L.M.; Bossini, P.S.; Orth, D.L.; Duarte, F.O.; dos Anjos Souza, A.B.; Parizotto, N.A.; de Freitas Anibal, F. Photobiomodulation on Critical Bone Defects of Rat Calvaria: A Systematic Review. Lasers Med. Sci. 2018, 33, 1841–1848. [Google Scholar] [CrossRef]

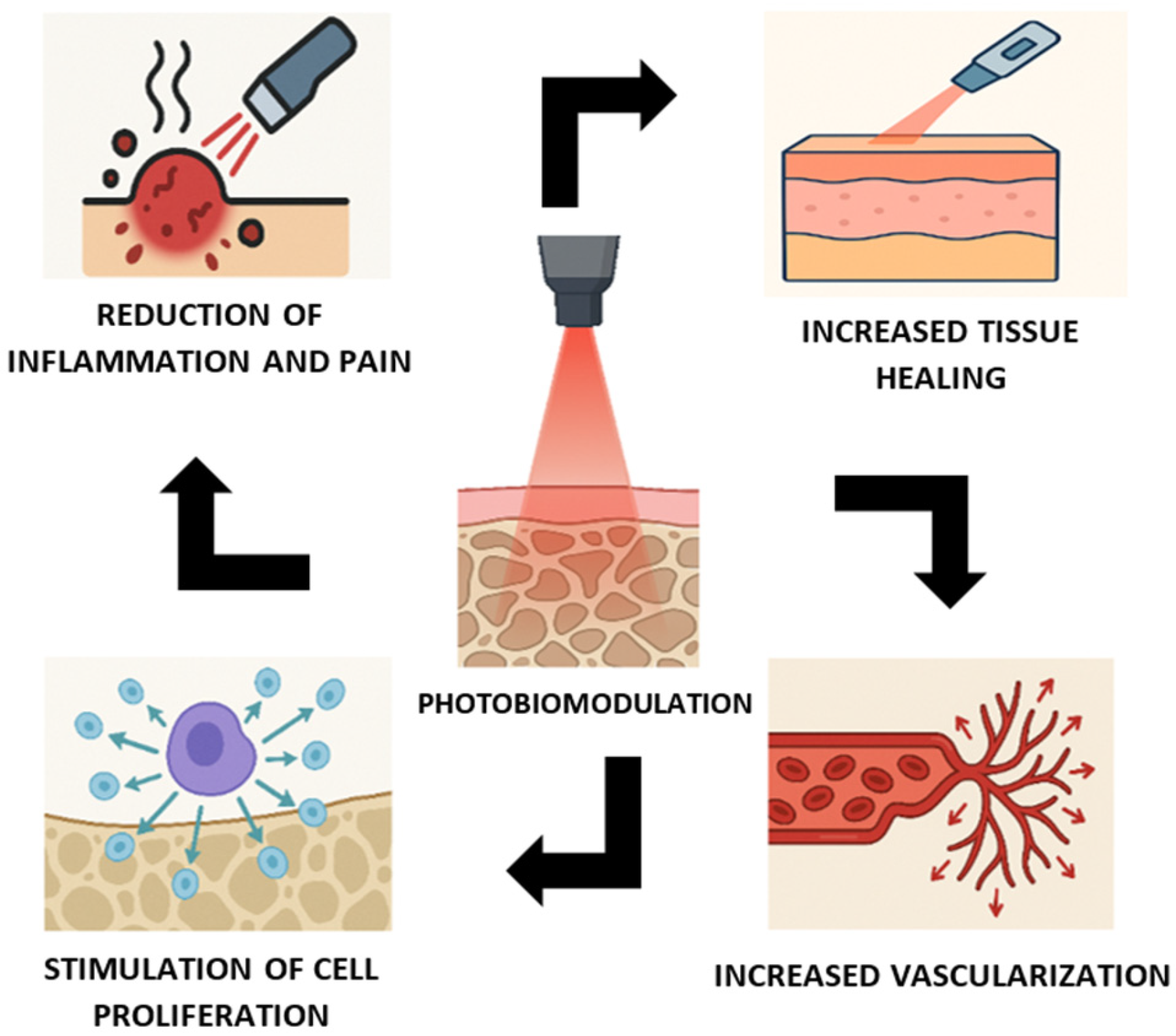
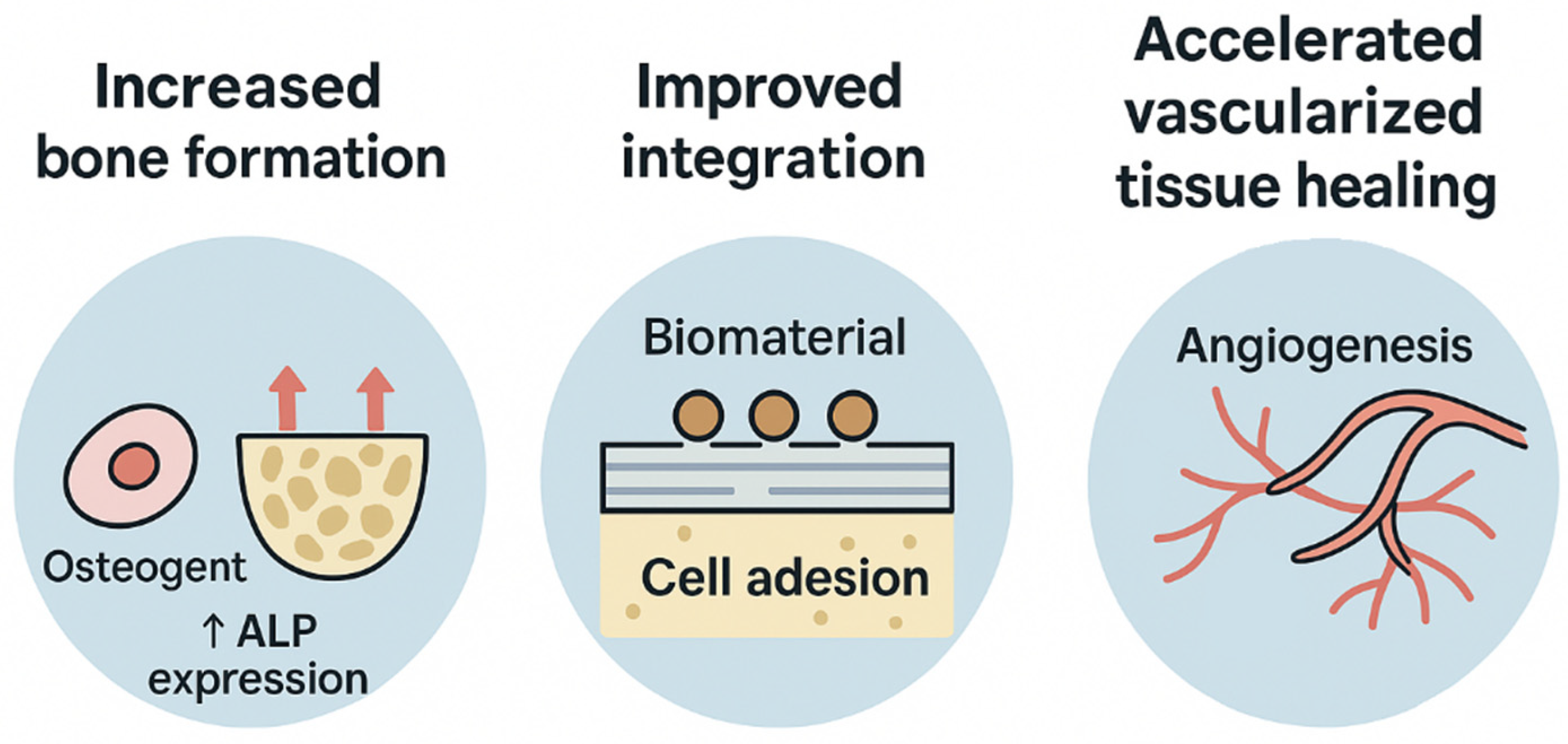



| Study | Laser (λ) | Outcome Type | Direction | Interpretation |
|---|---|---|---|---|
| Della Coletta et al., 2021 [3] | GaAlAs (830 nm) | Histology | ↑ | Greater bone formation and maturation in PBM group. |
| AboElsaad et al., 2009 [9] | GaAlAs (830 nm) | Clinical/Histologic | ↑ | Accelerated healing at 3 months; no difference at 6 months. |
| Alves et al., 2020 [10] | GaAlAs (808 nm) | Micro-CT | ↑ | Increased bone volume fraction, trabecular number, and connectivity; reduced trabecular separation. |
| Freitas et al., 2023 [12] | GaAlAs (730 nm) | Histomorphometry | = | Significant reduction in residual graft particles; no difference in new bone area. |
| Freitas et al., 2018 [13] | GaAlAs (808 nm) | Histomorphometry | ↑ | More new bone in PBM groups compared to autogenous graft groups. |
| Luca et al., 2020 [14] | GaAlAs (808 nm) | Histology | ↑ | Superior healing during early postoperative period (first 14 days). |
| Pinheiro et al., 2009 [15] | GaAlAs (830 nm) | Histology | ↑ | Suggests early positive effect of PBM in GBR context. |
| de Oliveira et al., 2018 [35] | GaAlAs (808 nm) | Histology/Molecular | ↑ | Increased mineralized tissue and gene expression (BMP2, ALP, OCN). |
| Rufato et al., 2022 [36] | GaAlAs (780 nm) | Molecular | ↑ | Upregulation of osteogenic genes with PBM + PTFE. |
| Valiati et al., 2012 [37] | GaAlAs (830 nm) | Histology | ↑ | Improved graft incorporation, reduced inflammation, enhanced collagen. |
| Study | Laser (λ) | Outcome Type | Direction | Interpretation |
|---|---|---|---|---|
| Emrem Doğan et al., 2014 [11] | Nd:YAG (1064 nm) | Clinical | ↑ | PBM improved clinical outcomes in periodontal defects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscatel, M.B.M.; Pagani, B.T.; Trazzi, B.F.d.M.; Reis, C.H.B.; Ribeiro, C.A.; Buchaim, D.V.; Buchaim, R.L. Effects of Photobiomodulation in Association with Biomaterials on the Process of Guided Bone Regeneration: An Integrative Review. Ceramics 2025, 8, 94. https://doi.org/10.3390/ceramics8030094
Moscatel MBM, Pagani BT, Trazzi BFdM, Reis CHB, Ribeiro CA, Buchaim DV, Buchaim RL. Effects of Photobiomodulation in Association with Biomaterials on the Process of Guided Bone Regeneration: An Integrative Review. Ceramics. 2025; 8(3):94. https://doi.org/10.3390/ceramics8030094
Chicago/Turabian StyleMoscatel, Matheus Bento Medeiros, Bruna Trazzi Pagani, Beatriz Flávia de Moraes Trazzi, Carlos Henrique Bertoni Reis, Camila Aparecida Ribeiro, Daniela Vieira Buchaim, and Rogerio Leone Buchaim. 2025. "Effects of Photobiomodulation in Association with Biomaterials on the Process of Guided Bone Regeneration: An Integrative Review" Ceramics 8, no. 3: 94. https://doi.org/10.3390/ceramics8030094
APA StyleMoscatel, M. B. M., Pagani, B. T., Trazzi, B. F. d. M., Reis, C. H. B., Ribeiro, C. A., Buchaim, D. V., & Buchaim, R. L. (2025). Effects of Photobiomodulation in Association with Biomaterials on the Process of Guided Bone Regeneration: An Integrative Review. Ceramics, 8(3), 94. https://doi.org/10.3390/ceramics8030094








