1. Introduction
Partially stabilized zirconium dioxide (PSZ) is one of the promising ceramic materials for operation in extreme conditions, such as nuclear power engineering, space technology, gas turbine engines, and plasma reactors. Its high mechanical strength, chemical resistance, heat resistance, and resistance to radiation exposure make it a key component for structural elements operating at high temperatures and in aggressive environments [
1,
2,
3]. The introduction of yttrium oxide stabilizes the high-temperature tetragonal and cubic phases of ZrO
2, preventing phase transitions to the monoclinic form, which is accompanied by volume change (~3–5%) and a decrease in mechanical strength, which is especially critical during cyclic heating and cooling [
4,
5]. Due to these properties, partially stabilized zirconium dioxide is widely used in components operating under severe radiation and thermal loads, such as in the following: in nuclear power engineering (fuel rod claddings, core elements, and structural components of gas-cooled and fast reactors); in space technology (thermal protection coatings and engine components of spacecraft); in gas turbine and aircraft engines (thermal barrier coatings for blades and combustion chambers); as well as in plasma systems and fusion reactors (linings, insulating, and barrier layers) [
6,
7]. However, exposure to high-energy particles such as neutrons and heavy ions can lead to changes in the structure and properties of the material, which requires a detailed study of the mechanisms of radiation damage and phase transformations [
8].
One of the most effective methods for modeling radiation effects is ion irradiation, which allows the study of the evolution of the defect structure and phase transformations without significantly heating the material, which is unlike neutron irradiation, where the accumulation of defects occurs over a long period of time [
9]. The use of ion irradiation allows for controlled exposure with a certain penetration depth, which is important for studying surface effects and subsequent structural analysis [
10]. Heavy inert elements such as krypton (Kr) and xenon (Xe) are usually used as irradiating ions, since their high mass and energy allow for reproducing conditions typical of the operating environments of nuclear reactors and spacecraft [
11]. The impact of Kr
15+ ions with an energy of 1–5 MeV leads to the formation of collision cascades, which are accompanied by the formation of point defects (vacancies and interstitial atoms), a change in the parameters of the crystal lattice, the accumulation of dislocations, and possible phase transitions [
12,
13].
When heavy ions act on stabilized zirconium dioxide, several key effects are observed. First, the formation of radiation-induced defects leads to an expansion of the lattice parameters and, in some cases, to partial amorphization of the material, especially at high irradiation fluences (>10
15 ions/cm
2) [
14]. Second, phase transformations are observed, in particular the transition of the metastable monoclinic phase to the stabilized tetragonal phase, which are accompanied by a change in the mechanical properties of the material [
15]. Third, the mechanical characteristics change depending on the fluence and energy of ions: at the initial stages (at fluences of 10
13–10
14 ions/cm
2), the material can be strengthened due to the formation of dislocation structures that prevent plastic deformation, whereas at higher fluences (>1016 ions/cm
2), defects accumulate, leading to a decrease in strength and increased brittleness [
16,
17]. Fourth, radiation defects can affect the thermal conductivity of the material, which is especially critical for thermal barrier coatings used in the aviation and energy industries [
18].
In the present study, a composition of ZrO
2 + 3 mol% Y
2O
3 with partial stabilization by yttria was selected in order to investigate the transformation of the crystal structure from monoclinic to tetragonal and cubic phases under radiation exposure. The radiation-induced structural changes in partially stabilized ZrO
2–Y
2O
3 may depend on the irradiation temperature. At low temperatures (<300 K), defects have limited mobility, which promotes their accumulation and the formation of amorphized regions. At elevated temperatures (>800 K), partial restoration of the lattice occurs due to defect recombination, which can reduce the degree of radiation damage [
19]. Thus, the study of the radiation resistance of stabilized ZrO
2 is a key task for materials science aimed at developing strategies for improving its resistance to radiation exposure and increasing its service life under extreme operating conditions.
The aim of this study is to investigate the effect of Kr15+ ion irradiation on the structure and properties of Y2O3-ZrO2 ceramics.
The practical significance of the present study lies in the potential application of PSZ ceramics in nuclear technology due to a number of unique properties that are particularly important under conditions of radiation, high temperatures, and aggressive environments. PSZ composition remains stable at temperatures exceeding 2000 °C, which is critical in nuclear accident scenarios. The structure of partially stabilized ceramics transforms from the monoclinic phase to the cubic or tetragonal phases, which are more resistant to radiation-induced defects. Furthermore, the low thermal conductivity of PSZ makes it an effective thermal barrier material for both fuel cells and protective coatings. The composition does not react with nuclear fuel, cladding materials, or coolant media. Due to its stabilized crystal lattice, the material maintains its strength and fracture toughness during thermal cycling, which is especially important for components subjected to rapid temperature gradients (e.g., fuel cladding under loss-of-coolant conditions), as PSZ is chemically inert with respect to uranium, plutonium, and thorium oxides as well as structural alloys.
2. Materials and Methods
Ceramic samples were obtained by solid-phase synthesis from commercial powders: ZrO
2 (CAS No. 1314-23-4, ≥99.9%, Shandong Xuke Chemical Technology Co., Ltd., Dongying, China) and Y
2O
3 (CAS No. 1314-36-9, 99.99%, Sigma Aldrich, Darmstadt, Germany). The ratio of the components of the finished mixture was ZrO
2 + 3 mol. % Y
2O
3.
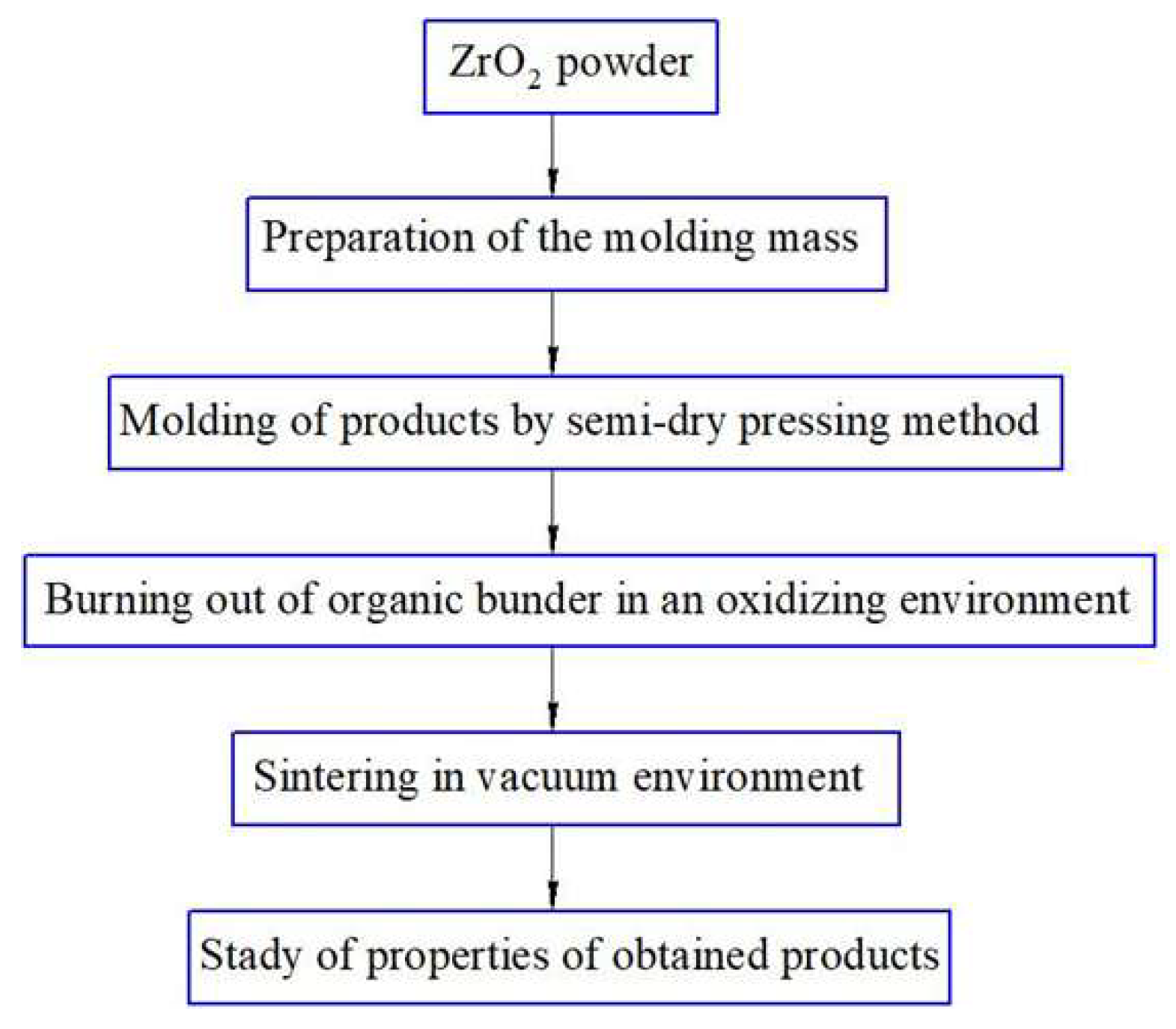
Figure 1 shows a flow chart for obtaining ZrO
2 + 3 mol. % Y
2O
3 using the mechanochemical synthesis method with subsequent sintering of the samples in a vacuum.
Primary combined milling (mixing) was performed in a ball mill in an ethyl alcohol environment for 5 h. After mixing, the powders were dried in a chamber furnace at a temperature of 300 °C until complete evaporation of moisture, then the mixture was calcined (heat treated) at a temperature of 1100 °C for 4 h.
After calcination, the mixture was re-grinded in a planetary mill to obtain a fine powder. The zirconium oxide and yttrium oxide mixture were ground in a ball planetary mill with the addition of oleic acid to prevent particle agglomeration and improve dispersion. The oleic acid concentration was 3.0% of the powder weight. Grinding cups and balls made of zirconium oxide were used to prevent contamination of the material with foreign impurities. Long-term grinding was performed for 10 h to ensure a solid-phase reaction between ZrO2 and Y2O3 to form stabilized zirconium oxide. The ratio of grinding media to the mineral composition ZrO2 + Y2O3 was 10:1, and the ratio of the speeds of the planetary disk and cup was 1:−2.5.
The samples were formed from the obtained mixture using the semi-dry pressing method according to the diagram shown in
Figure 2.
Ceramic samples with a diameter of 20.0 mm and a thickness of 5 mm were obtained.
Primary grinding (mixing) was carried out in a ball mill IBMU-100-2 (Ht Machinery Co., Ltd., Wuxi, China). Drying for moisture evaporation and the burning out of the organic binder was carried out in an oxidizing environment at Tmax = 1200 °C using an electric furnace SNOL 400/12-VP (VNIIETO, Istra, Russia); the accuracy of temperature maintaining was ±5 °C.
Co-milling (mechanochemical activation) was carried out in a planetary ball mill XQM-8A (Changsha Tianchuang Powder Technology Co., Ltd., Changsha, China).
The press mass was prepared with the addition of an organic binder based on a 10% solution of polyvinyl alcohol mixed with a plasticizer (glycerin), in a ratio of 7:3. The amount of the introduced organic binder according to the formation of granules of the press powder was 10 mol. %. The molding of blanks from the obtained press mass was carried out by the method of uniaxial semi-dry pressing on a hydraulic press ES0501 (EQFS, Reutov, Russia); the pressing force was 1000–1200 kg/cm2, holding at maximum pressure for 30 s.
The final sintering of the samples was carried out in an electric vacuum chamber furnace SNVE-1.3.1/20 (Prizma LLC, Iskitim, Russia), and the vacuum depth was 1.1·10−2 Pa. Ceramics were sintered according to the following regime: heating to 1600 °C → holding for 2 h → cooling together with the furnace to 70 °C.
Determination of apparent density, porosity, and water absorption was carried out according to ASTM C20-00.
Measurements of the mass of the powders, binder components, and other materials during the experiment and analysis were carried out on an AUM210-E electronic analytical scale (Azzota Scientific, Claymont, DE, USA), with an accuracy of 0.0001 g. Drying of the powders was carried out in a drying cabinet ShS-80-01-SPU (Smolensk SKTB SPU, Smolensk, Russia), with an accuracy of temperature maintaining ± 1°C.
Microhardness of the synthesized ceramics was determined by the Vickers method using a DuraScan-20 microhardness tester (EMCO TEST, Kuchl, Austria), test load 0.2; 0.5 and 1.0 kN, holding at maximum load—5 s.
The crystal structure of powders and sintered samples was studied using a X PertPRO diffractometer (Malvern Panalytical Empyrean, Almelo, Netherlands) using X-ray diffraction with monochromated copper (Sica) radiation, with a scanning step of 0.03° (K-Alpha1 [Å] 0.1542). The measurement angle was 20–100°, the X-ray tube voltage was 40 kV, the current was 30 mA, the measurement time at each step was 10 s, and an aluminum rectangular multipurpose sample holder (PW1172/01, Malvern Panalytical Empyrean, Almelo, Netherlands) was used.
Quantitative analysis of the phase composition and structure of the samples was performed using additional analysis software: HighScore Plus 5.1 and Powder Cell 2.4. Raman spectra were obtained on an EnSpectr R 532 Raman express analyzer (Spectr-M LLC, Yoshkar-Ola, Russia), laser wavelength 532 nm, spectral resolution 4–6 cm−1, and range 160–4000 cm−1.
The specific surface area was determined using a BSD-660 analyzer (BSD Instrument Technology Co., Ltd., Beijing, China), which has a surface area range starting from 0.0005 m2/g, a pore diameter range from 0.35 to 500 nm, and a pore volume range starting from 0.0001 cm3/g.
The size of the initial powders and the powder mixture was assessed using laser diffraction on a Bettersizer S3 particle size and shape analyzer (Bettersize Instruments Ltd., Dandong, China); particle size: 0.01–3500 μm; particle shape: 2–3500 μm.
Microstructure analysis of the samples was carried out using a Phenom ™ XL G2 Desktop scanning electron microscope (ThermoFisher Scientific, Waltham, MA, USA). Maximum magnification of ×100,000; resolution of 14 nm; accelerating voltage of 5, 10, 15 kV, backscattered electron detector, EMF attachment.
Irradiation of zirconia-based ceramic samples with krypton ions Kr15+ (atomic mass 84, charge 15, A/Z = 5.6) at an energy of 1.75 MeV per nucleon was carried out using the DC-60 cyclotron. The irradiation process was conducted on water-cooled targets to prevent overheating of the samples under the ion beam. The selected fluences—1 × 1011, 1 × 1012, 1 × 1013, and 1 × 1014 ions/cm2—cover a range from the initial stages of radiation damage, where isolated point defects and dislocation nuclei form, through the phase of intense defect accumulation and the onset of phase transitions, to quasi-extreme conditions with defect saturation, clustering, and local amorphization. This range enables the study of the material’s evolution up to its radiation resistance limit. Higher fluences on the employed cyclotron would lead to local surface overheating and distortion of results; therefore, the chosen values represent a compromise between achieving significant radiation effects and preserving the original morphology of the samples.
The calculation of energy loss of the incident ions, as well as the maximum ion penetration depth in the ceramic, was performed based on model data on ceramic density using the SRIM-2008 Pro software. According to SRIM simulations for irradiation with Kr15+ ions at 1.75 MeV per nucleon (corresponding to a total ion energy of approximately 147 MeV) of ZrO2—3 wt.% Y2O3 ceramic targets with a density of 5.7 g/cm3, the average ion range in the material is approximately 5.2 µm. The maximum structural damage occurs at a depth of about 2.9 µm from the surface, and the number of atomic displacements per ion reaches approximately 8275.
The recalculated dpa (displacements per atom) values for the selected fluences are presented in
Table 1.
3. Results and Discussion
Obtaining stabilized zirconium oxide by solid-phase synthesis is a common method for producing ceramic materials.
Figure 3 shows electron microscopic images of the microstructure of the initial ZrO
2 and Y
2O
3 powders.
Zirconium oxide particles are predominantly rounded in shape, including agglomerates and particles with a fragment morphology. The particle size varies from 20 to 100 μm. Yttrium oxide particles are represented by fragments of an exclusively fragmentary shape, 20–100 μm in size.
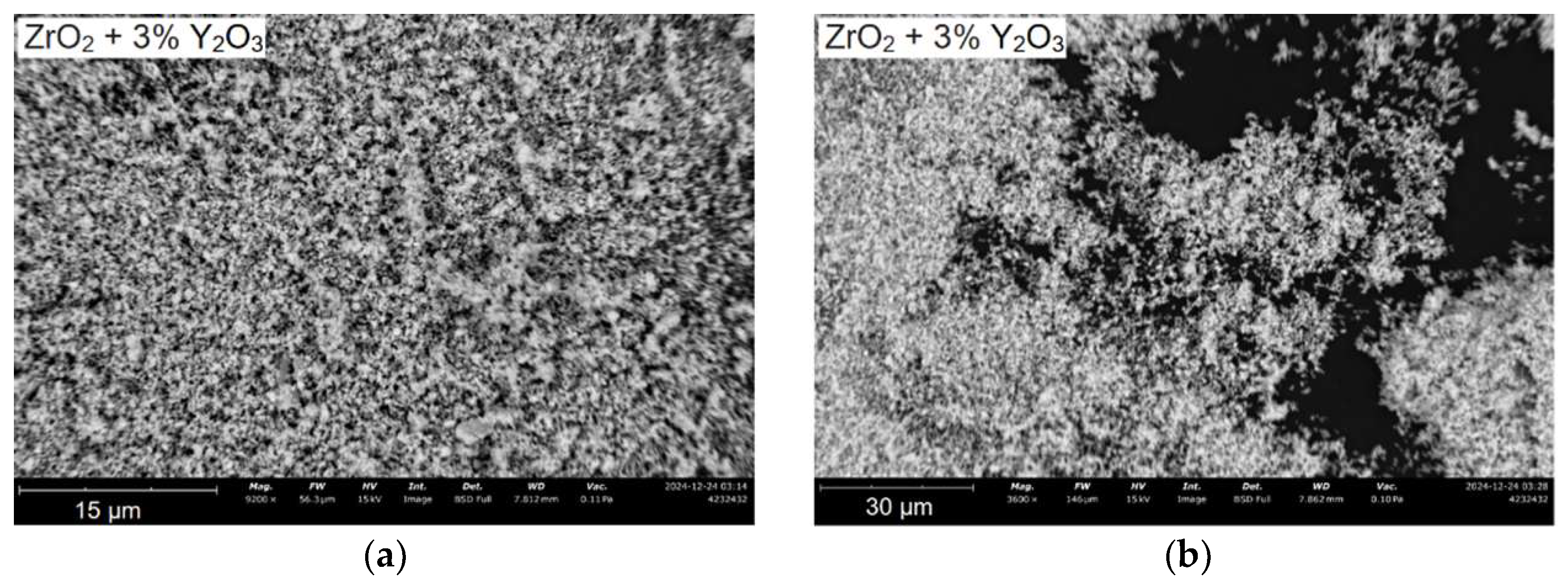
SEM images of particles after calcination and combined milling are shown in
Figure 4.
Particles of ZrO2 + 3 mol. % Y2O3 mixture after calcination and combined milling have a finely dispersed size distribution about 1–3 µm. The shape of the particles is predominantly fragmentary. Since the ZrO2 + Y2O3 mixture is a dielectric material, a charge accumulates on the surface of the particles, which distorts the trajectory of secondary electrons, preventing the obtaining of high-resolution photographs.
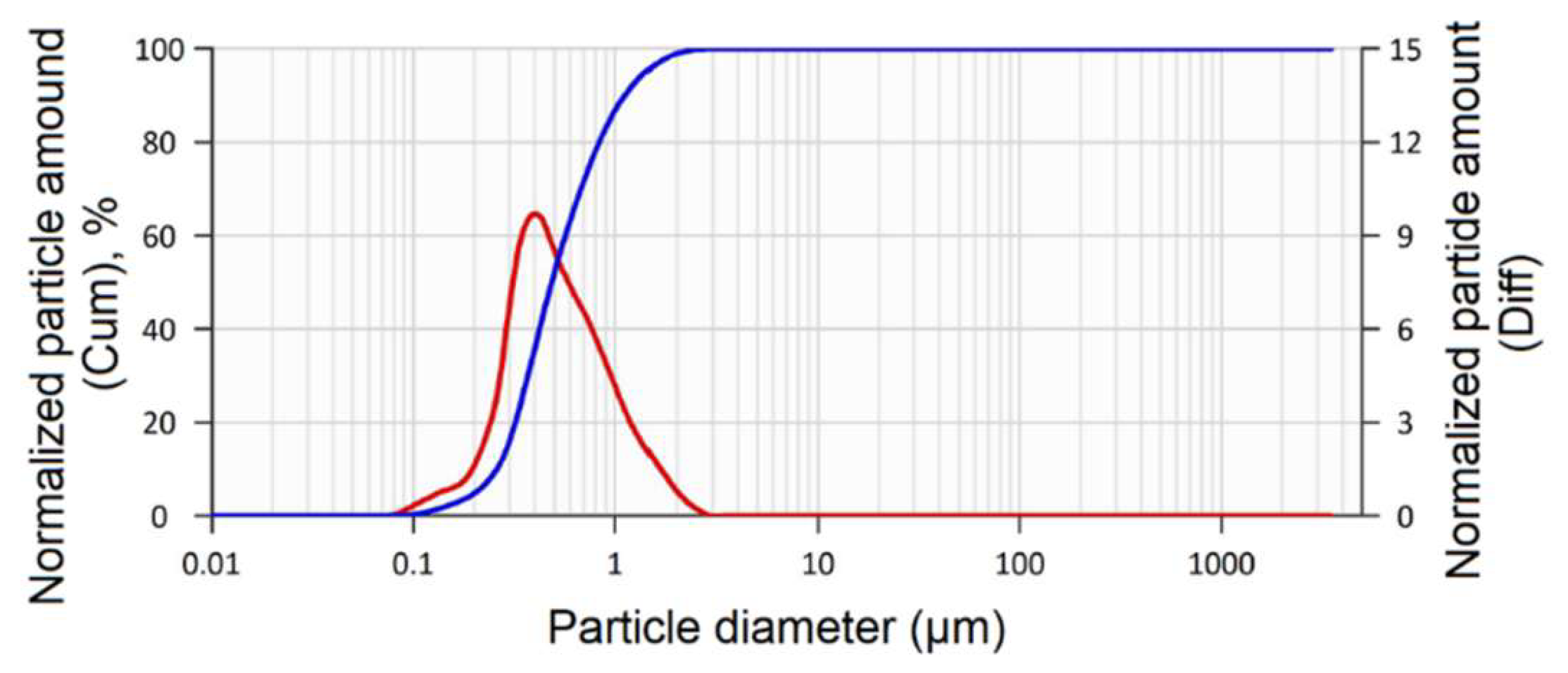
In order to establish a more detailed particle size distribution in the powder mixture after calcination and milling, a particle size analysis was carried out using laser diffraction (
Figure 5).
The particle size distribution in the ZrO2 + 3 mol. % Y2O3 mixture is represented by a linear dependence, ascending from the nanoscale range of ~80 nm, and then the peak occurs at a particle size of ~0.6 μm.
The specific surface area of the ZrO
2 and Y
2O
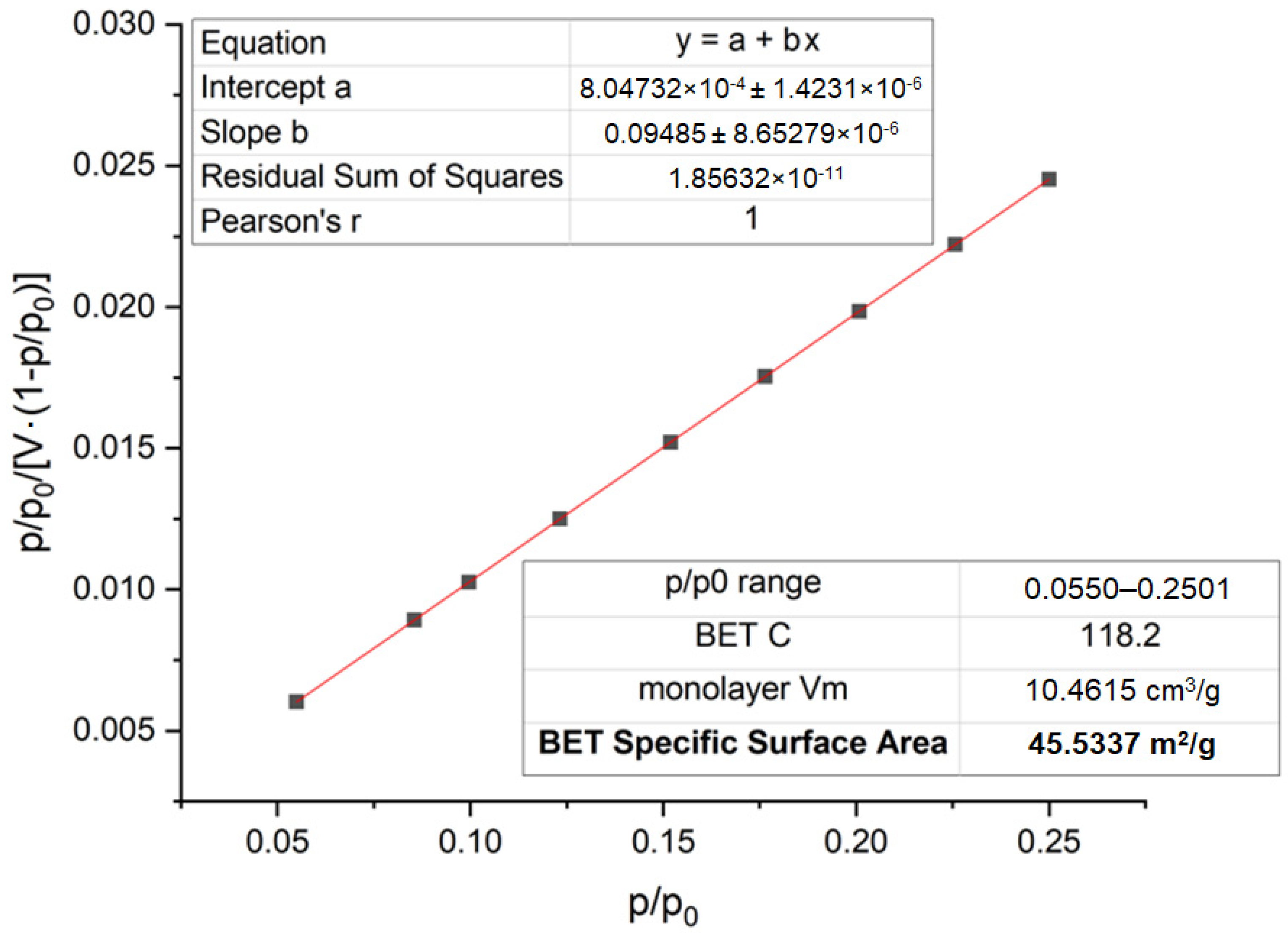
3 powder mixture after secondary milling was also studied using the BET method (
Figure 6).
Figure 6 shows the nitrogen adsorption isotherm obtained using the BET method for the ZrO
2 + 3 mol% Y
2O
3 powder mixture after repeated mechanochemical milling. The graph corresponds to an isotherm characteristic of mesoporous materials, with a clearly defined capillary condensation region at a relative pressure of P/P
0 > 0.8, indicating the formation of a well-developed porous structure after activation. According to the obtained data, the specific surface area of the powder after milling is 45.53 m
2/g (compared to 10.1 m
2/g before milling), which confirms the effectiveness of the applied milling process. The secondary grinding resulted in a significant reduction in particle size (down to 0.25–1.1 μm), which contributed to the increase in the external surface area and the formation of pore channels within the mesoporous range. The change in morphology positively affects the reactivity of the system and promotes a more complete progression of the solid-state reaction during the synthesis of stabilized zirconia upon subsequent calcination. The BET analysis data after re-milling indicate the formation of a highly active powder system with a developed specific surface area suitable for producing ceramic materials with a dense and homogeneous structure.
Based on the results of the experiment, about 30 samples of the composition ZrO
2 + 3 mol. % Y
2O
3 were synthesized using the semi-dry pressing technology. The average value of the results of measuring the physical and mechanical properties of the samples is given in
Table 2.
Next, one sample with the values of physical and mechanical properties corresponding to the average indicators was selected (
Table 2). In order to evaluate the effect of the intensity of krypton ion irradiation with four fluences of 1 × 10
11, 1 × 10
12, 1 × 10
13, and 1 × 10
14 ions/cm
2, the sample, after its characterization, was divided into four equal parts for irradiation.
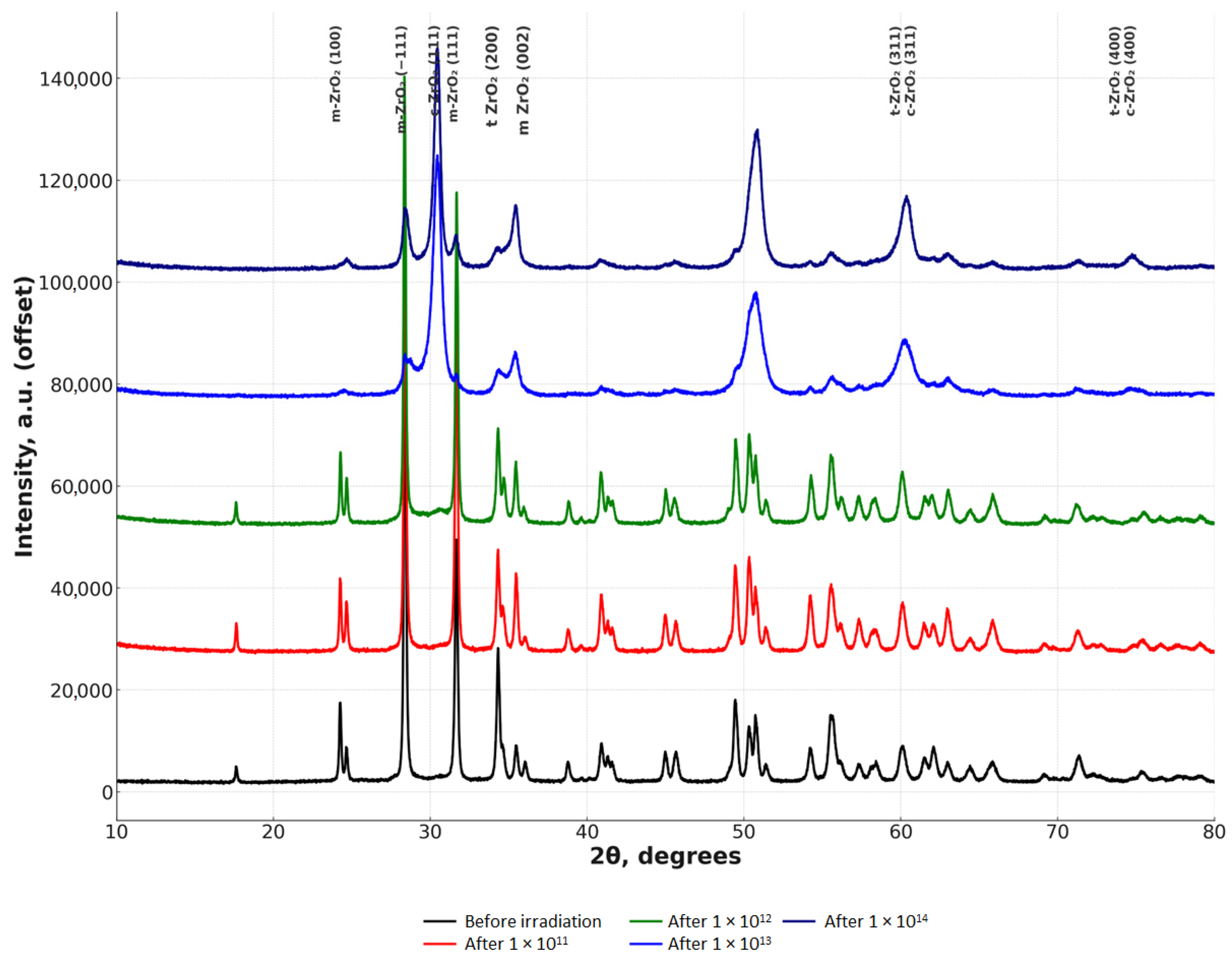
The results of the study of the X-ray phase composition of the samples are presented in
Figure 7.
Analysis of the crystallite size data shows (
Table 3) that with an increase in the irradiation fluence, there is a systematic decrease in the crystallite sizes in all phases (m-ZrO
2, t-ZrO
2, c-ZrO
2). Before irradiation, the crystallite size of the monoclinic phase (m-ZrO
2) was 13 nm, but at a fluence of 1 × 10
14 it decreased to 8 nm, which indicates significant deformation of the structure and accumulation of radiation defects. A similar trend is observed for the tetragonal phase (t-ZrO
2), where the crystallite size decreases from 12 nm to 7 nm, as well as for the cubic phase (c-ZrO
2), where a decrease from 10 nm to 6 nm is observed. These changes indicate the processes of radiation-induced crystallite refinement caused by the intense formation of defects such as vacancies, interstitial atoms and dislocations. A particularly pronounced reduction in size is observed at fluences of 1 × 10
13–1 × 10
14, which indicates the saturation of defects in the lattice and a possible approach to the state of a nanostructured material. These results confirm that irradiation not only causes phase transformations, but also significantly changes the morphology of the crystal structure of stabilized zirconium dioxide.
Irradiation of sintered zirconia ceramics with krypton ions with an energy of 1.75 MeV/nucleon resulted in significant changes in the phase composition of the material (
Table 4). Before irradiation, the sample consisted predominantly of the monoclinic (m-ZrO
2) phase (66.1%), while the content of the tetragonal (t-ZrO
2) and cubic (c-ZrO
2) phases were 30.0% and 3.9%, respectively. Already at 1 × 10
11, the content of the monoclinic phase decreased by almost 1.5 times (to 43.8%), and the amount of the cubic phase sharply increased to 27.5%, which indicates a radiation-stimulated phase transition. A further increase in the irradiation fluence to 1 × 10
12 did not result in significant changes compared to 1 × 10
11, which may indicate saturation of the transformation processes at this stage. However, at 1 × 10
13–1 × 10
14 a further decrease in the proportion of the monoclinic phase (to 34.9% and 29.6%, respectively) and an increase in the proportion of the tetragonal and cubic phases (t-ZrO
2: 32.5% → 35.2%, c-ZrO
2: 32.5% → 35.2%) are observed. These changes indicate the accumulation of radiation defects and the transition of the material to more symmetrical structures. As a result, at 1 × 10
14, an almost equal ratio of the tetragonal and cubic phases (~35.2%) is observed, which may indicate the ultimate stabilization of the material under the influence of irradiation.
Under the influence of ion irradiation, various types of defects are formed and accumulated in the crystal lattice of zirconium dioxide (ZrO2), which leads to a change in its phase composition, structural characteristics, and morphology of the material. Radiation-induced stabilization of the tetragonal and cubic phases is due to the formation of interstitial zirconium atoms and oxygen vacancies, which reduces the energy barrier between the monoclinic (m-ZrO2) and tetragonal (t-ZrO2) phases. As a result of such transformations, the proportion of tetragonal and cubic structures (c-ZrO2) increases, which is a characteristic transition in the presence of stabilizing impurities such as Y2O3.
With increasing radiation fluence, the concentration of point defects increases, which facilitates the transition of less stable phases to more symmetrical ones. Lattice disorder and radiation-stimulated defects have a key effect on phase transformations. Penetration of krypton ions deep into the material causes a cascade of atomic collisions, which leads to an intensive accumulation of radiation defects and a destabilization of the crystal lattice. During this process, a redistribution of structural elements occurs, and the parameters of the unit cell change, which increases the probability of a phase transition.
When irradiated with fluences of about 1 × 1013–1 × 1014 ions/cm2, the phase transition m-ZrO2 → t-ZrO2 → c-ZrO2 occurs, since the high content of point defects and local inclusions makes the highly symmetric phases energetically more favorable. These changes are accompanied by an increase in the dislocation density, which leads to a refinement of the crystallites and an increase in mechanical stresses inside the material.
An additional factor contributing to phase transformations is local radiation pressure, which occurs due to the uneven distribution of interstitial atoms and vacancies. These defects lead to a local change in the lattice parameters and an increase in internal stresses, which accelerates the process of phase transitions. A gradual decrease in the proportion of the monoclinic structure with an increase in the radiation fluence is a direct consequence of this phenomenon. These changes are confirmed by X-ray phase analysis, where a redistribution of the phase composition and a decrease in the size of crystallites are observed.
At extremely high irradiation fluences (>1 × 1014 ions/cm2) the development of amorphization of the structure is possible. This process is associated with a critical accumulation of radiation defects, leading to the destruction of the long-range order in the crystal lattice. As a result, it becomes impossible to maintain a regular periodic structure. Although at such fluences amorphous peaks may not be observed in X-ray patterns, a further increase in the irradiation fluence can lead to partial amorphization of the material, accompanied by an increase in the degree of disorder in the structure.
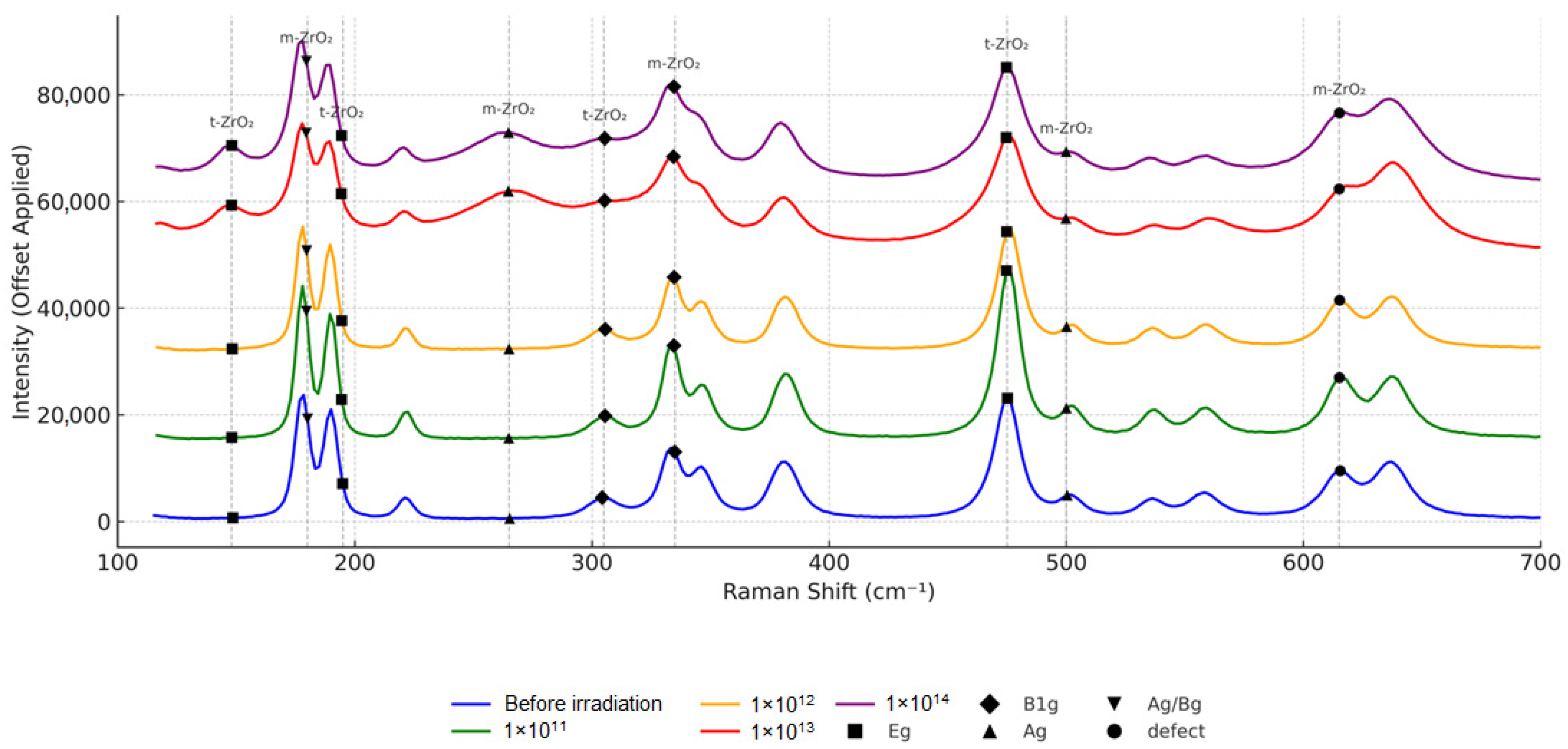
Figure 8 shows Raman spectra of zirconium dioxide ceramic samples. The studies confirm the XRD data.
The radiation effect on stabilized zirconium dioxide leads to significant changes in its crystal structure, which is clearly manifested in changes in the Raman spectra. As the radiation fluence increases, a consistent decrease in the intensity of characteristic peaks, their broadening and shifting, are observed, which indicates an increase in the defectiveness of the material and a change in its phase composition. Particularly pronounced changes are observed at fluences of 1 × 1013–1 × 1014, when the spectrum loses clarity and the peaks become blurred and less pronounced, indicating the possible amorphization of the structure. At the early stages of irradiation (1 × 1011–1 × 1012), a gradual destruction of the monoclinic phase (m-ZrO2) occurs with an increase in the proportion of the tetragonal (t-ZrO2). At higher fluences (1 × 1013), the transition to the cubic phase (c-ZrO2) is enhanced, and at the maximum fluence (1 × 1014), partial or complete lattice disordering is possible, leading to the formation of amorphous regions.
The main mechanisms of these changes are the accumulation of radiation defects (vacancies, interstitial atoms, defect complexes), phase transitions between ZrO2 modifications, and the occurrence of internal stresses that change the position of atoms in the lattice. Radiation defects, formed during the interaction of high-energy krypton ions with the material, destroy the original order in the crystal lattice, leading to the disorganization of the structure and changes in the spectral characteristics. This is manifested in the shift of peaks, which may indicate local mechanical stresses in the crystal caused by radiation damage.
Phase transitions during irradiation occur sequentially: first (low fluences), the monoclinic phase is destroyed. Then, the tetragonal phase stabilizes; at high fluences, a cubic phase appears, characteristic of highly defective crystalline structures. At critical fluences (1 × 1014), the process goes so far that zirconium dioxide begins to lose its crystalline order, and its structure acquires signs of an amorphous state.
The decrease in peak intensity observed in the spectra may also be due to an increase in the defect density. The broadening of the peaks indicates an increased dislocation density, as well as the probable occurrence of defect clusters, which further contribute to the disordering of the structure.
The analysis of the geometry of the indentations and the nature of the microcracks allows us to evaluate the mechanical stability of the material, its ability to resist plastic deformation, and the propagation of defects under local external influence. The shape and size of the indentations directly depend on the microstructure of the samples, the presence of internal stresses, and the distribution of defects in the material, which allows us to conduct a comprehensive assessment of its mechanical properties. The general appearance of the indentation marks indicates the high strength of the samples and their significant resistance to crack formation, since with increasing load there is no catastrophic failure, and the process of crack formation is controlled.
The cracks observed with increasing load are predominantly semi-disk-shaped and are localized near the indentation vertices, which is typical for brittle materials experiencing significant mechanical stresses in the area of contact with the indenter. Such a mechanism of destruction indicates that the material is capable of withstanding significant loads without a sharp decrease in strength, and the resulting microcracks do not lead to the instantaneous destruction of the sample. It is important to note that the shape of the indenter imprints in most cases remains close to isotropic, which indicates a uniform distribution of the resulting stresses and plastic deformations in the near-surface layer of the material. This indicates a high homogeneity of the structure of the studied samples, which is important for predicting their behavior under operating conditions, especially when exposed to cyclic loads.
At the same time, in the areas adjacent to the spherical inclusions in the structure of the material, a significant decrease in the intensity of crack formation is observed, which indicates their strengthening effect. Such inclusions perform a reinforcing function, similar to how this occurs in composite materials and amorphous glasses, where dispersed phases prevent the propagation of cracks by redistributing stresses and absorbing the energy of destruction. This confirms that the presence of spherical inclusions in the structure of the material helps to increase its resistance to mechanical damage, reducing the likelihood of catastrophic destruction and increasing the overall service life.
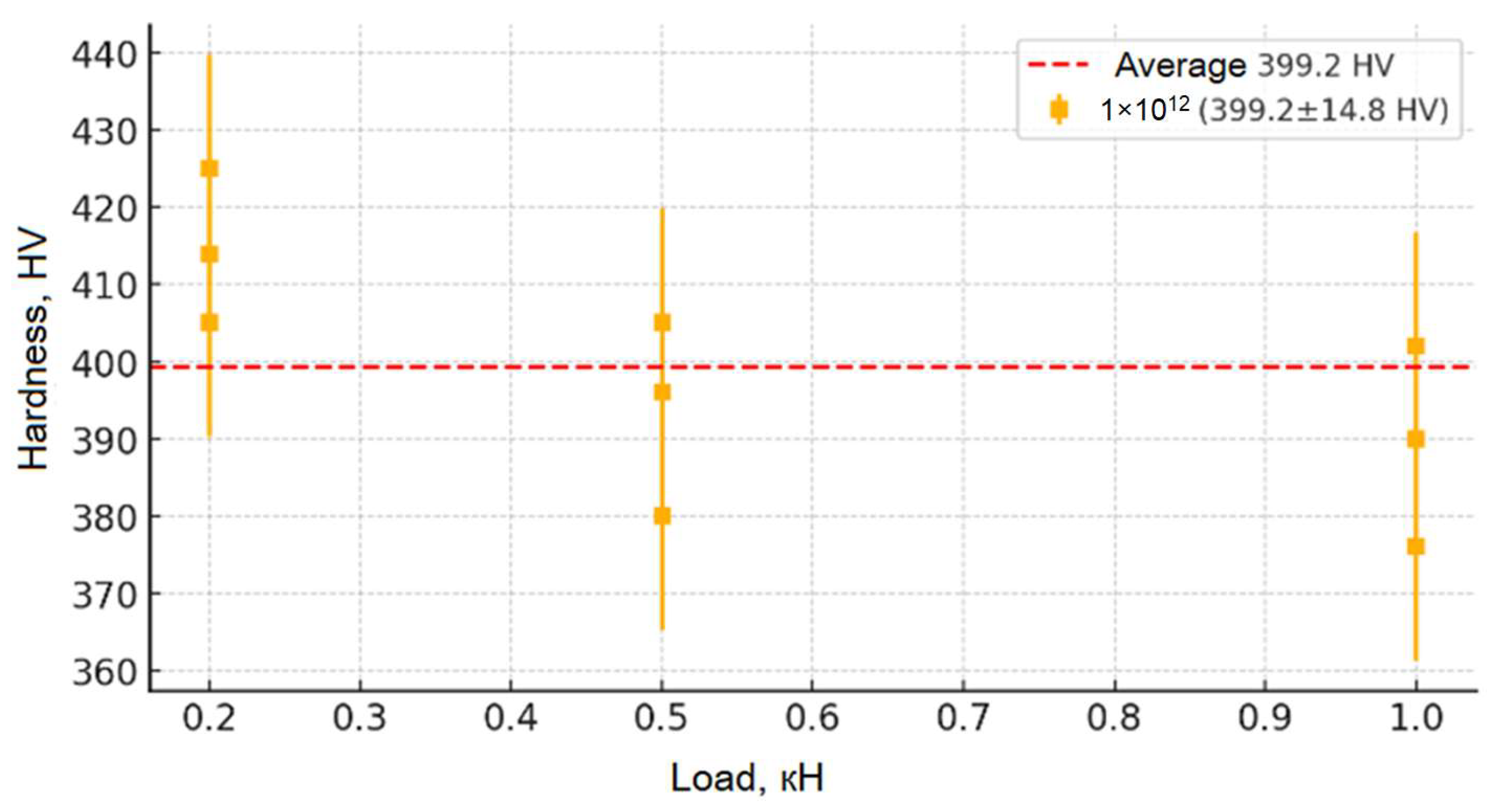
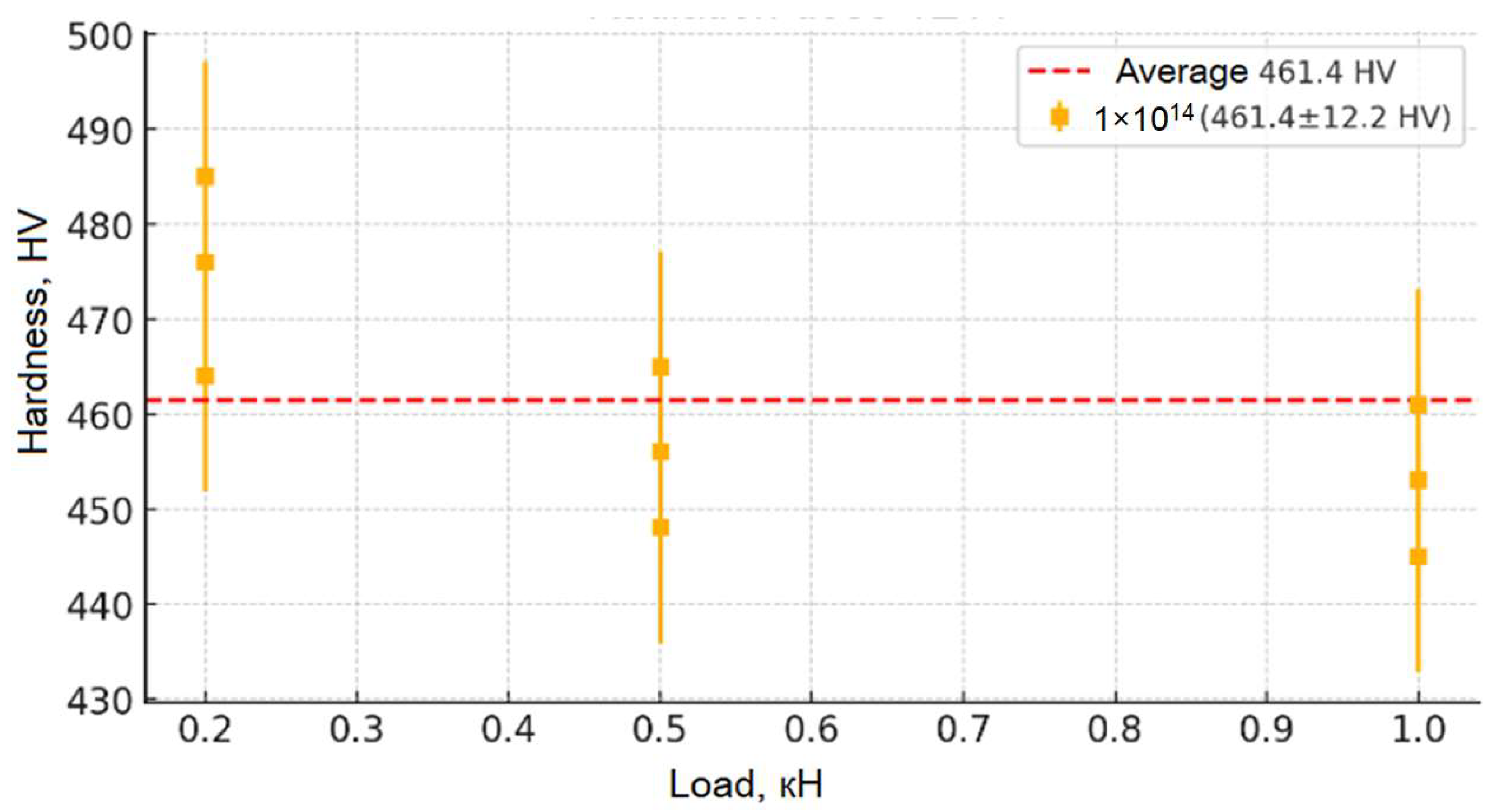
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
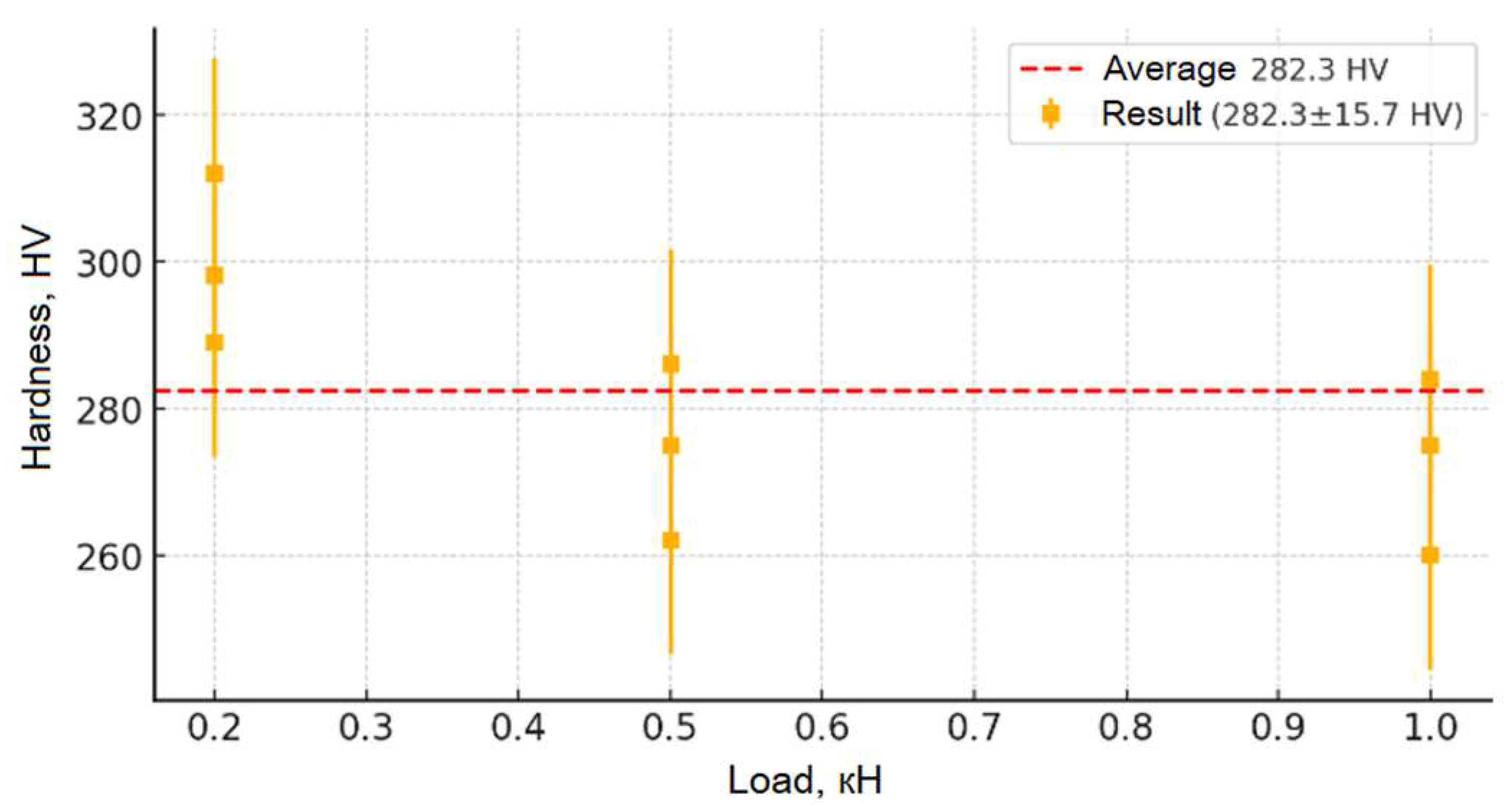
Figure 13 show the results of the study of the microhardness of the synthesized ceramic samples. Analysis of the presented graphs allows us to trace in detail the changes in the microhardness mechanisms of the samples depending on the applied load and the irradiation fluence. The initial (non-irradiated) sample of moderate hardness (~280 HV) remains relatively stable in the average value (from 0.2 to 0.5 kN), which indicates the originality of its structure and the absence of significant concentrations of defects and internal stresses. However, at low loads (less than 0.05 kN), slightly increased hardness values are observed, which may be due to the presence of a surface layer with increased structural stresses causing local deformation. With an increase in the penetration depth of the indenter (loads up to 0.5 kN), the hardness values stabilize, confirming the depth of the inner layer of the material and its resistance to moderate mechanical impacts. At higher loads (above 0.5 kN), a slight decrease in hardness is observed, which allows us to explain the change in depth in the indenter recesses, involving deeper layers with other structures in the measurement, as well as the accumulation of local defects and the formation of microcracks, leading to partial destruction of the structure.
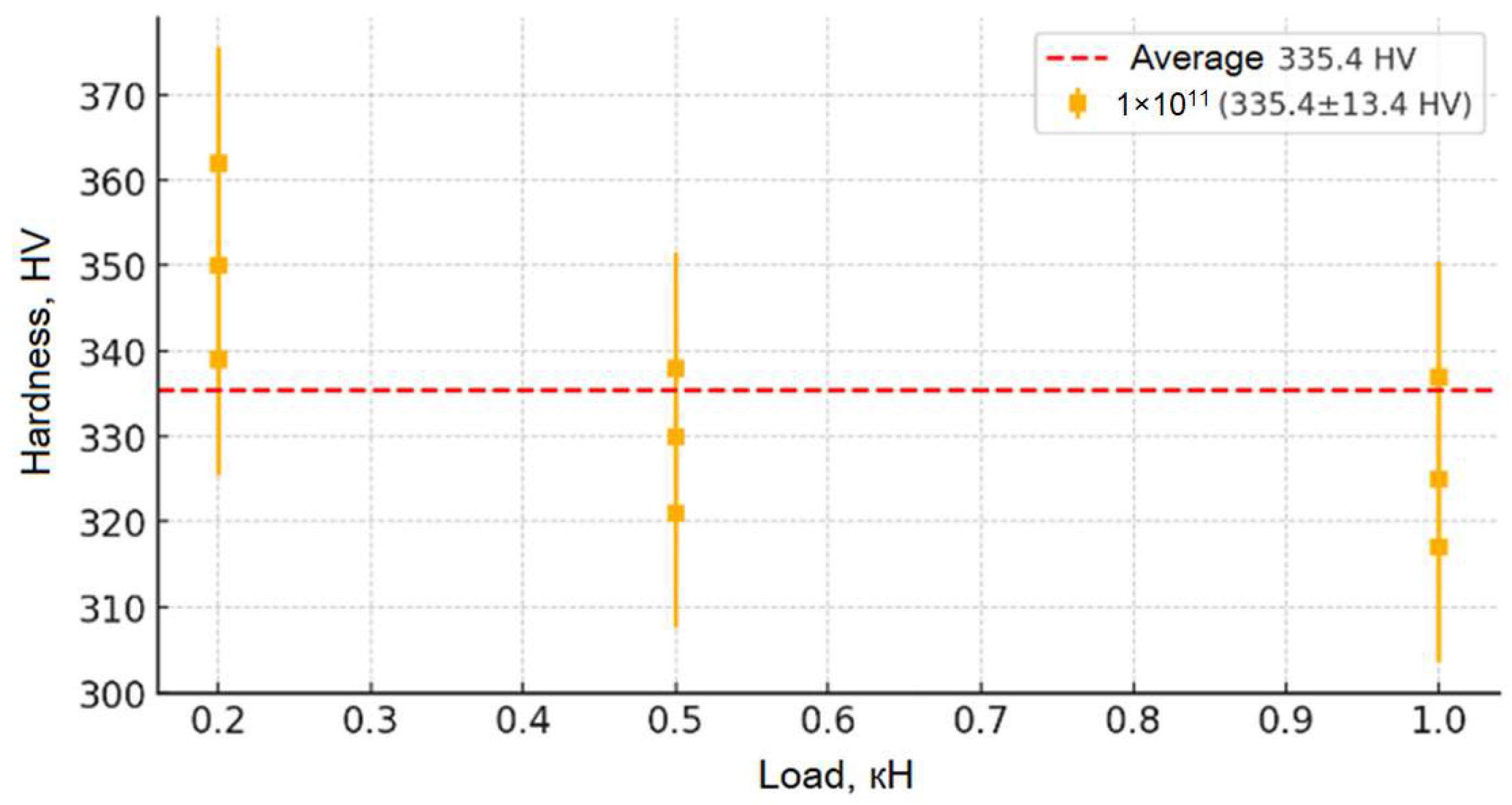
From the moment of receiving the irradiation fluence (from 1 × 1011 to 1 × 1013), the microhardness of the samples increases significantly (up to ~480 HV for the fluence of 1 × 1013), which indicates radiation-induced changes in their crystalline stage. First, this is due to the formation of point defect centers (vacancies, interstitial atoms) and dislocation networks that cause dislocation movement and hinder plastic deformation, which leads to the strengthening of the material. However, a broader study of the irradiation fluence (1 × 1014) reveals a slight decrease in hardness compared to the fluence of 1 × 1013, which may be due to the onset of stress relaxation processes, partial recrystallization, or local amorphization of the structure, leading to a weakening of the physical strength. Despite this, the general picture of the change in hardness depending on the load is evident: at low loads, its increase is observed due to surface stresses; on average, it stabilizes; and at high loads, a slight decrease is recorded, which leads to an increase in microdamage. This set of changes allows for an impact on the mechanical properties of the material and allows us to draw a conclusion about its resistance to moderate loads, as well as about possible structural changes at maximum radiation fluences.