Control of the SiC Polytypes in SiC Bonded Diamond Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results
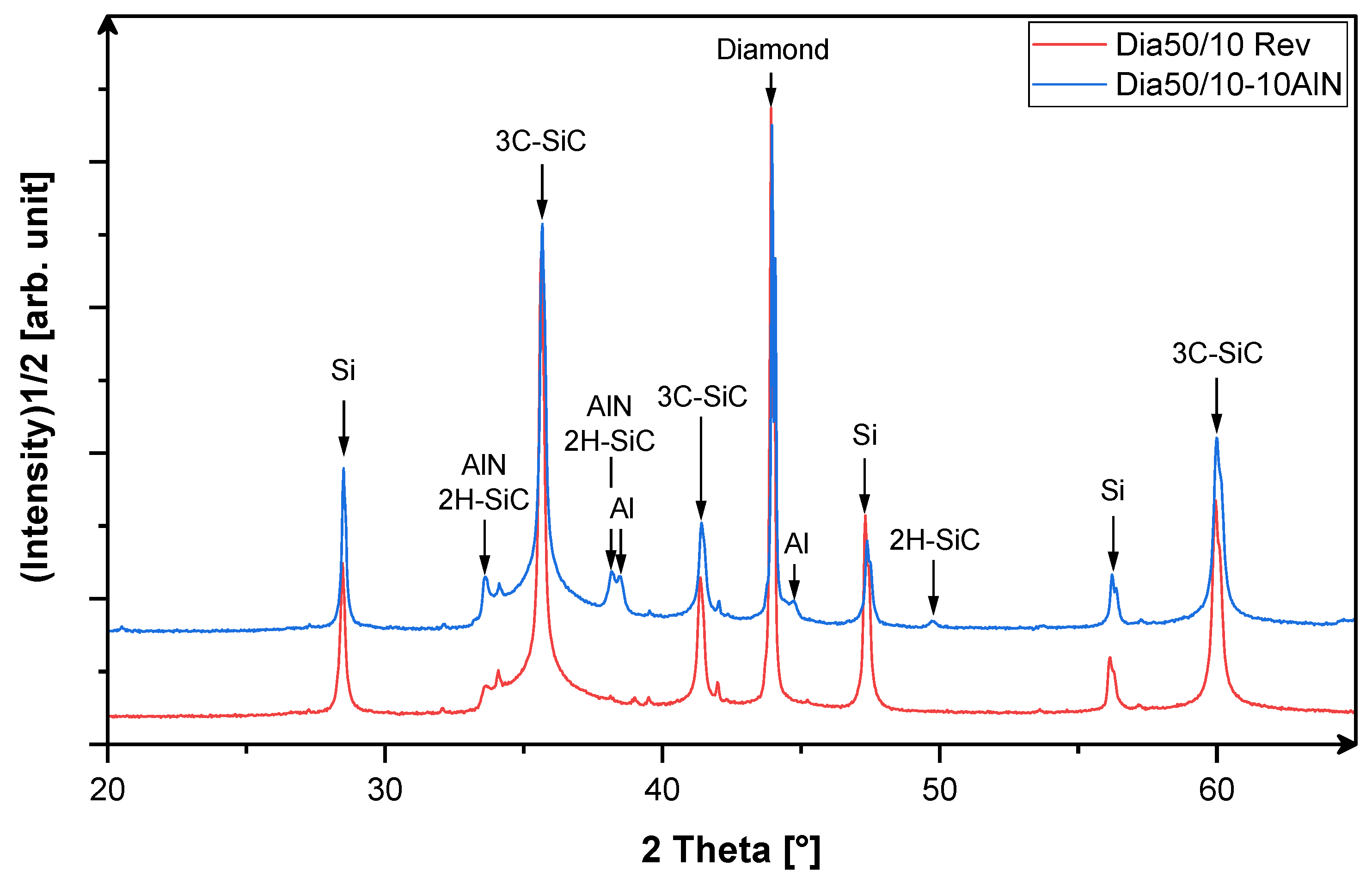
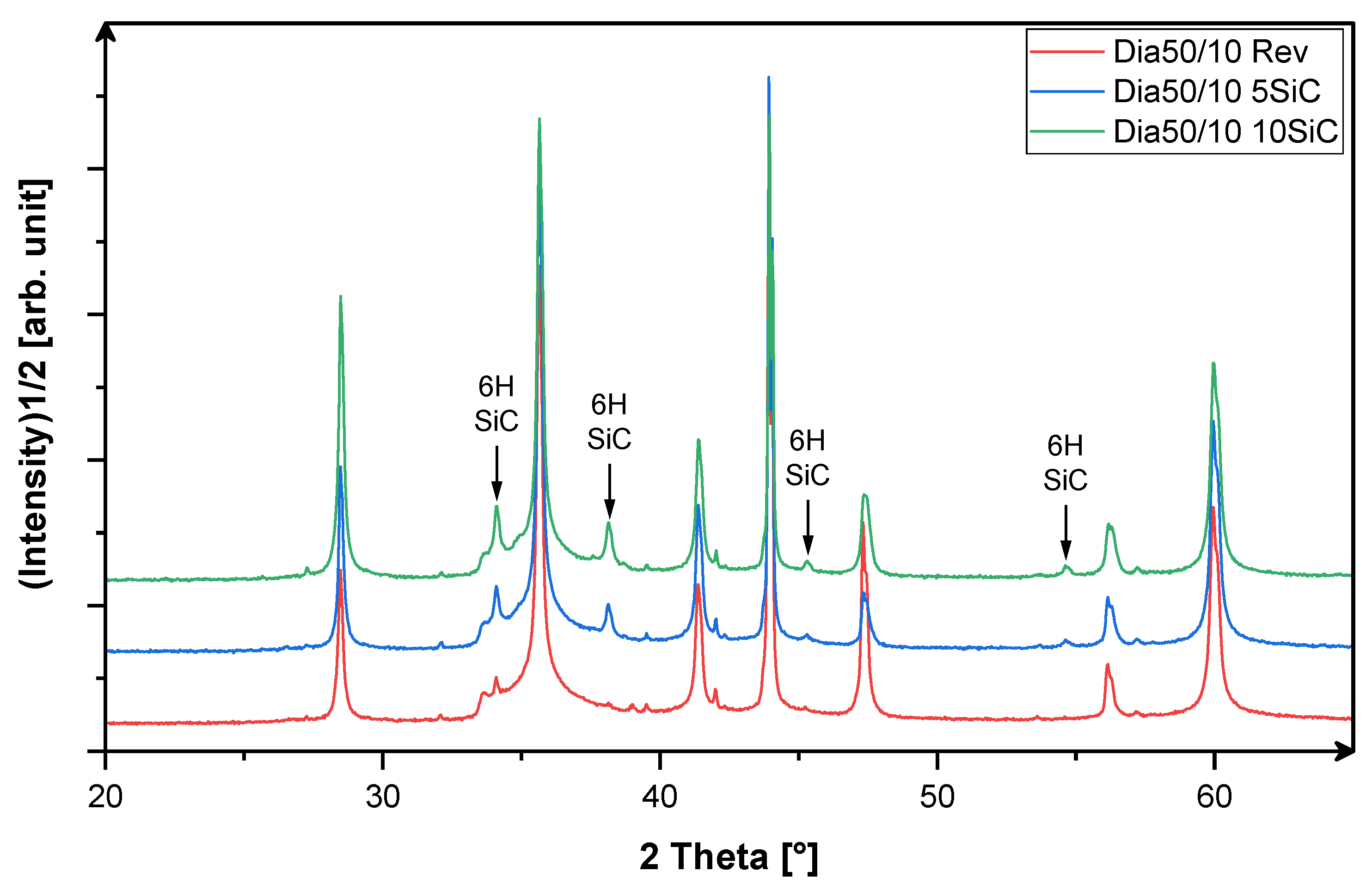
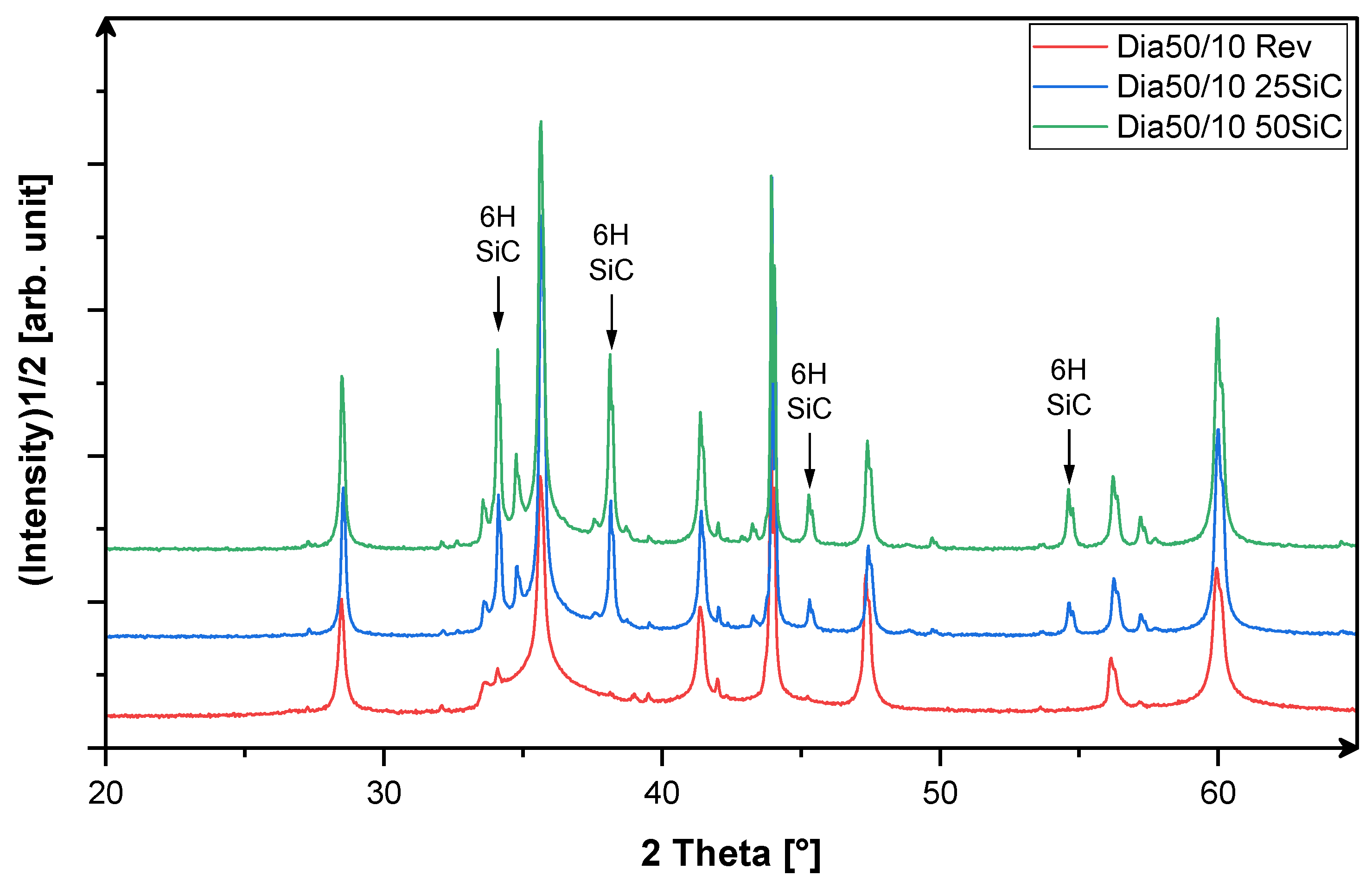
3.1. X-Ray-Phase Analysis
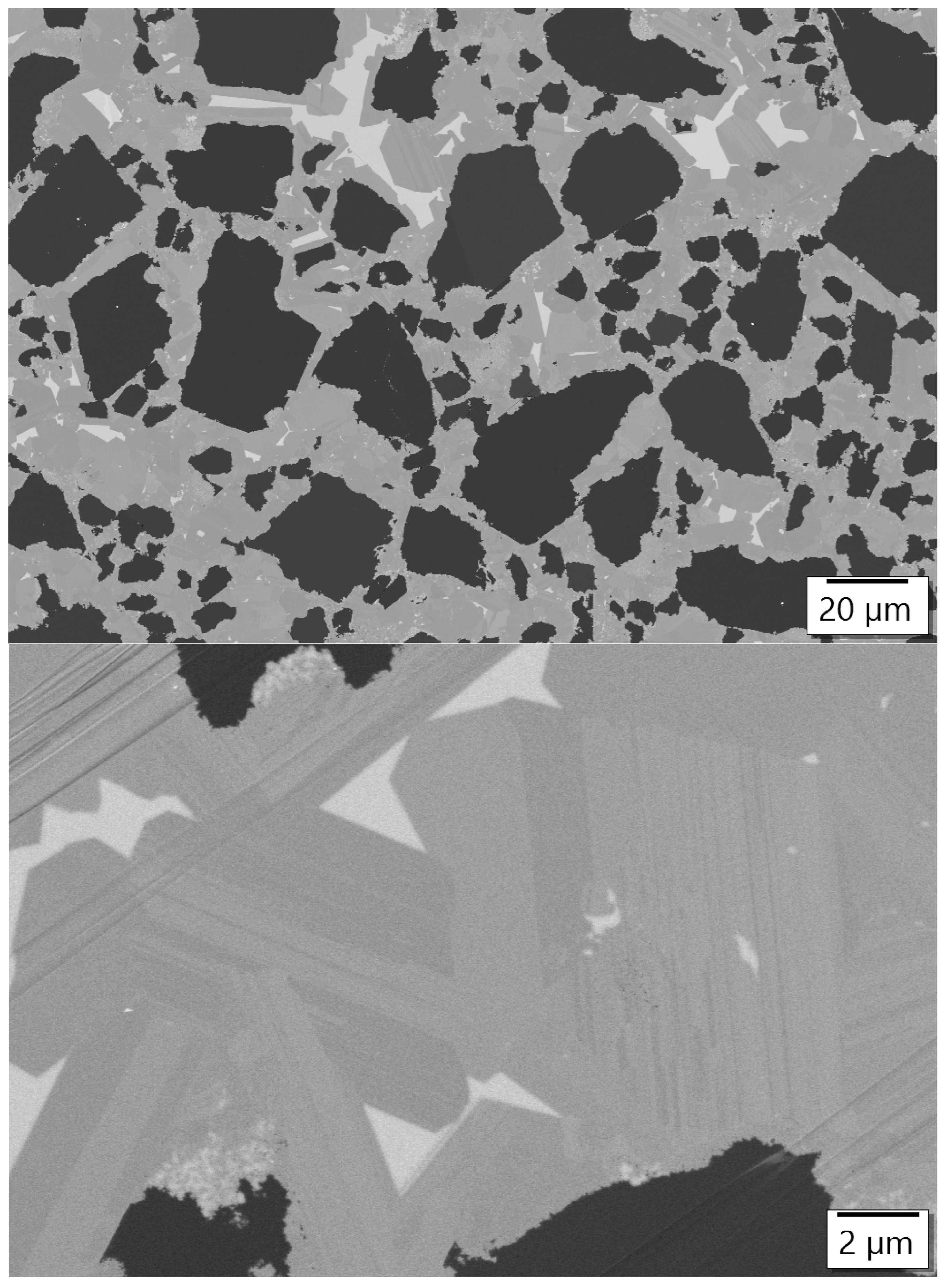
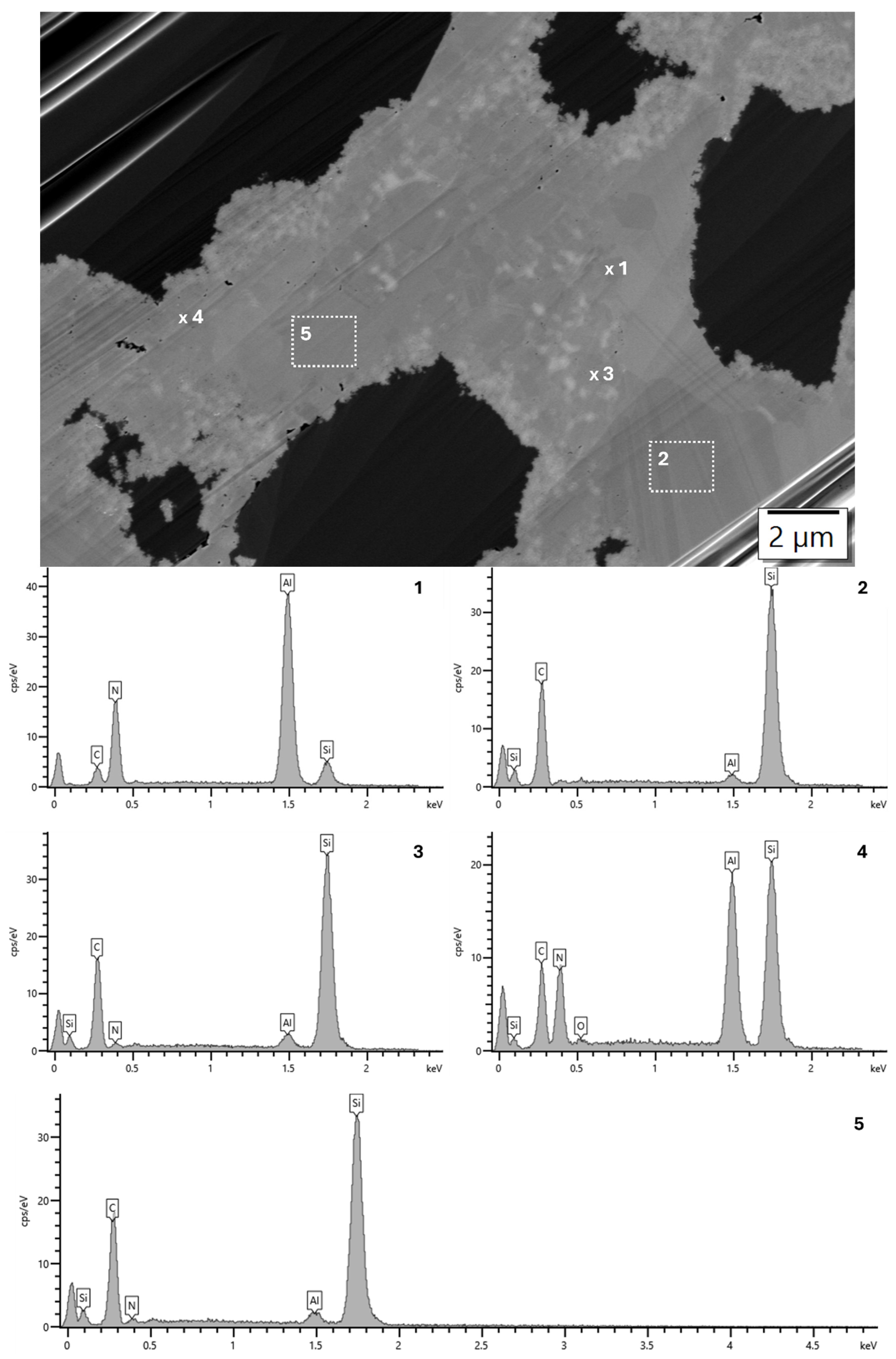
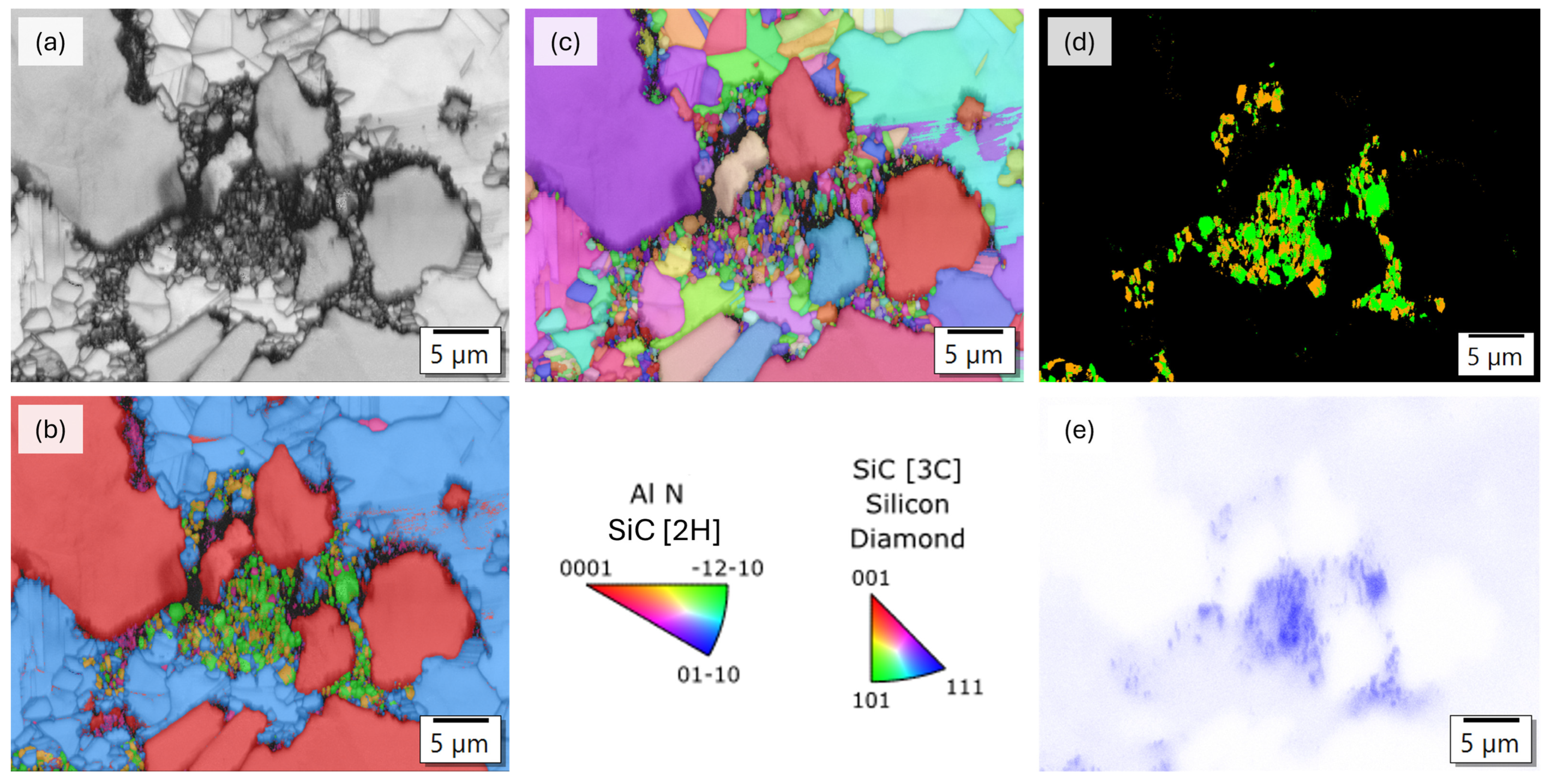
3.2. Analysis of the Microstructure of the AlN Containing Material Dia50/10 10AlN
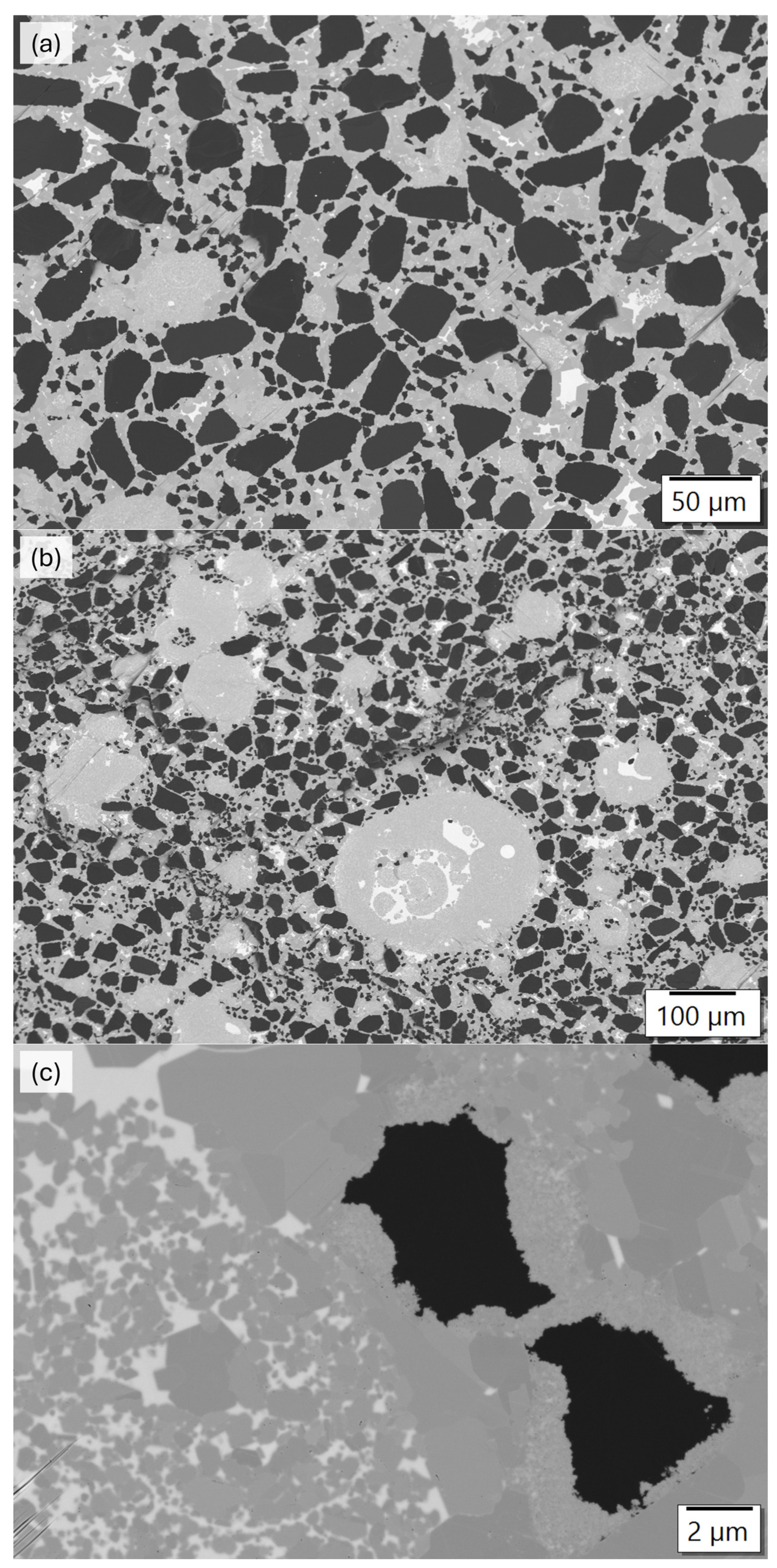
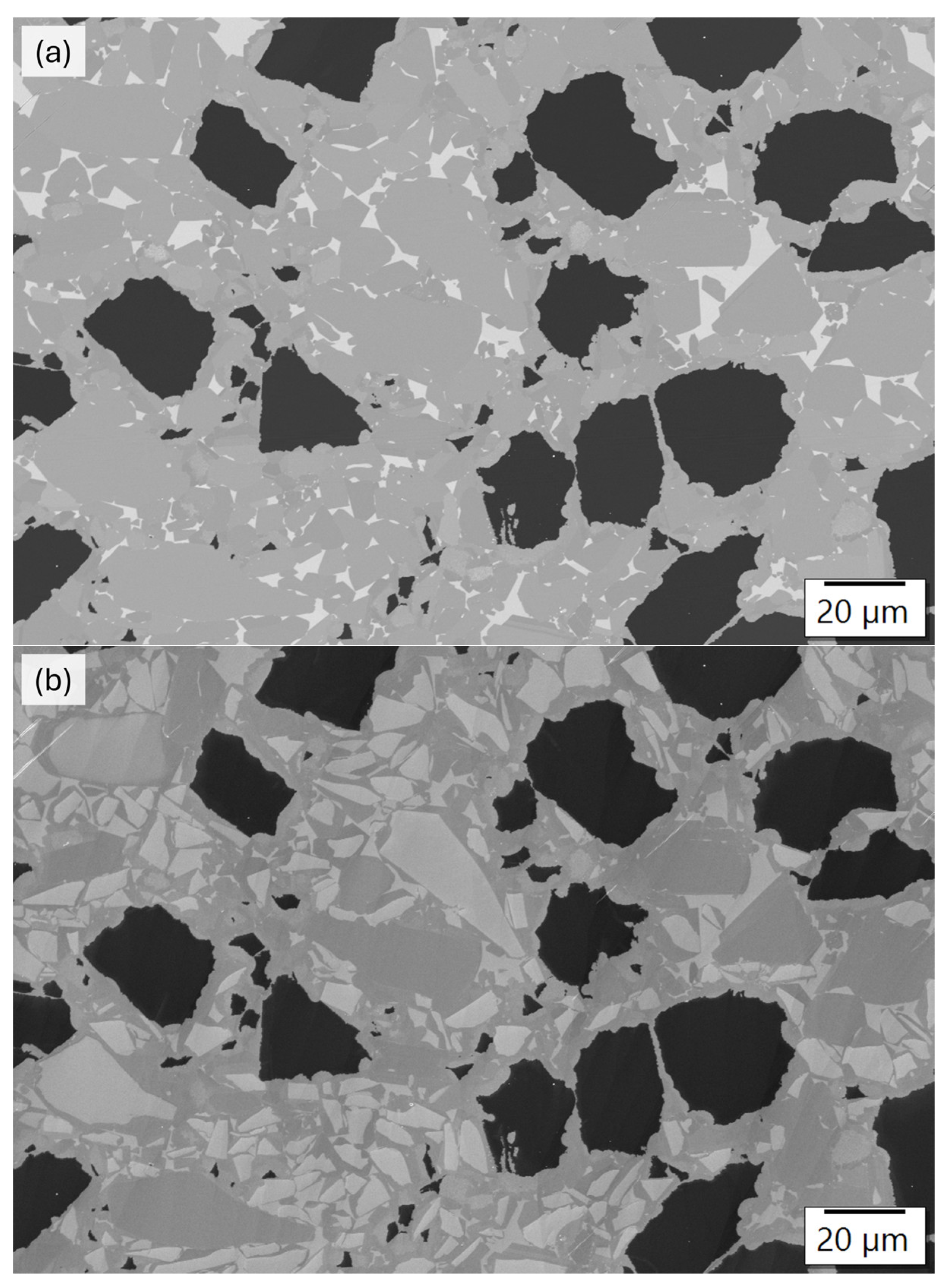
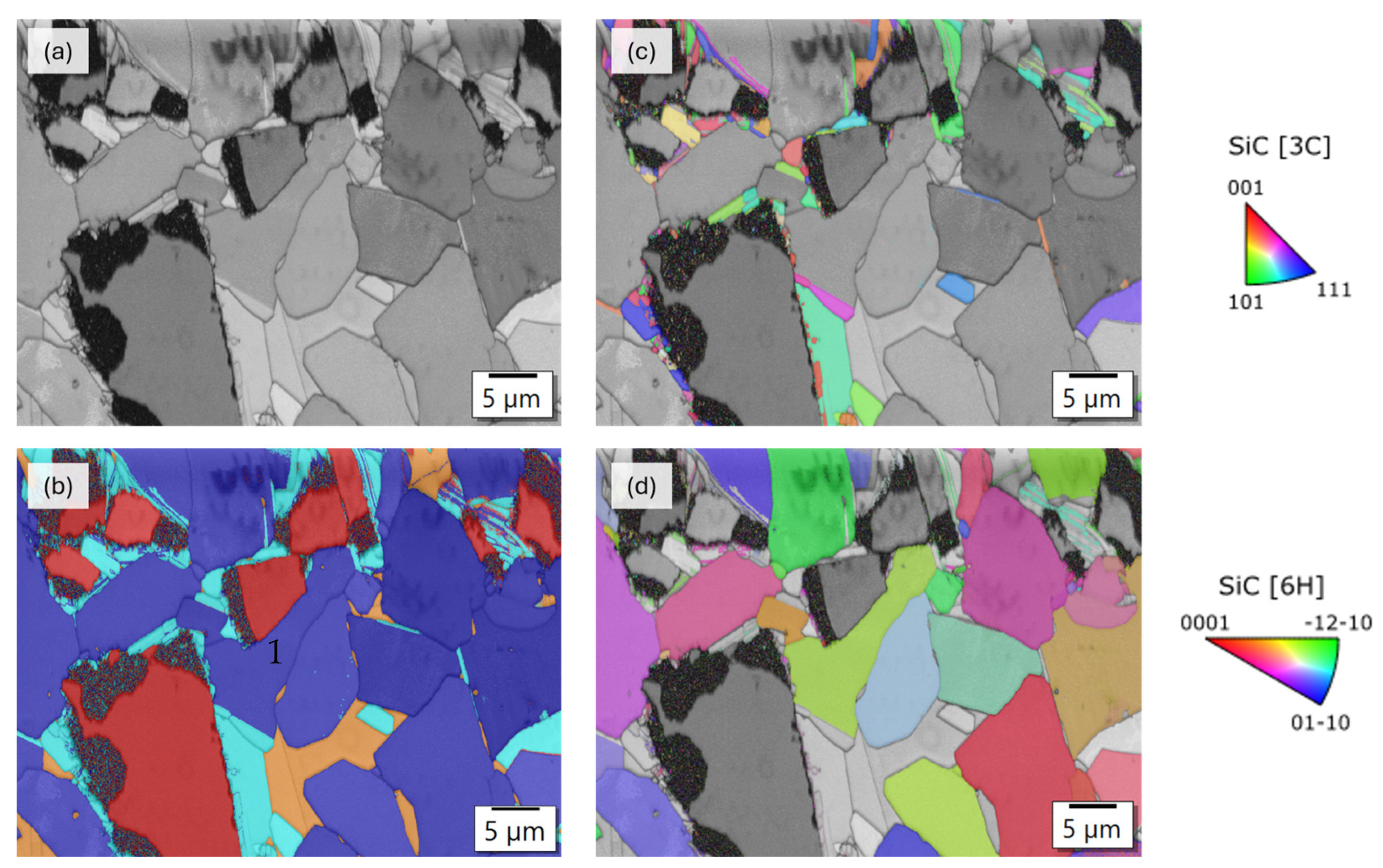
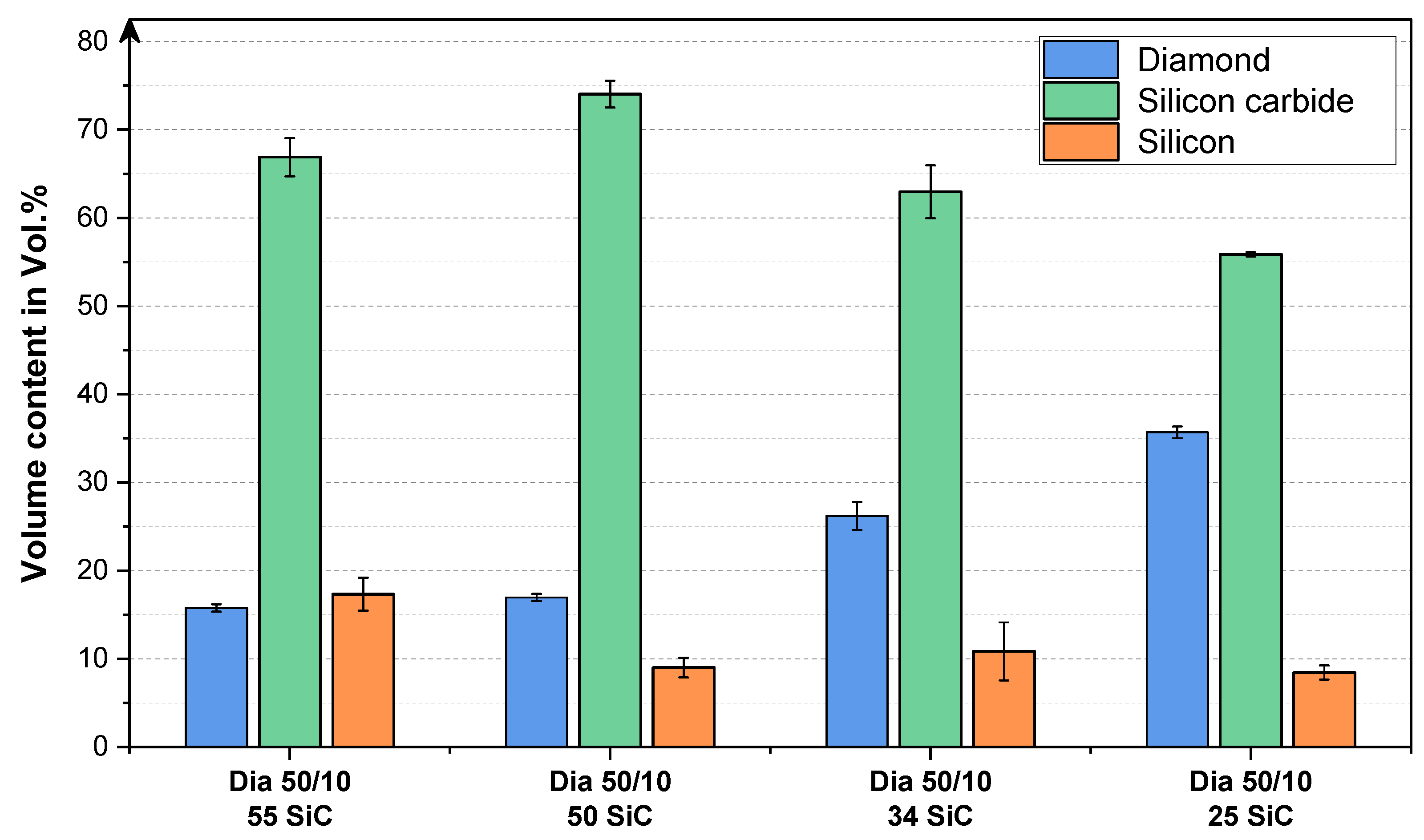
3.3. Analysis of the Microstructure of the SiC Containing Materials
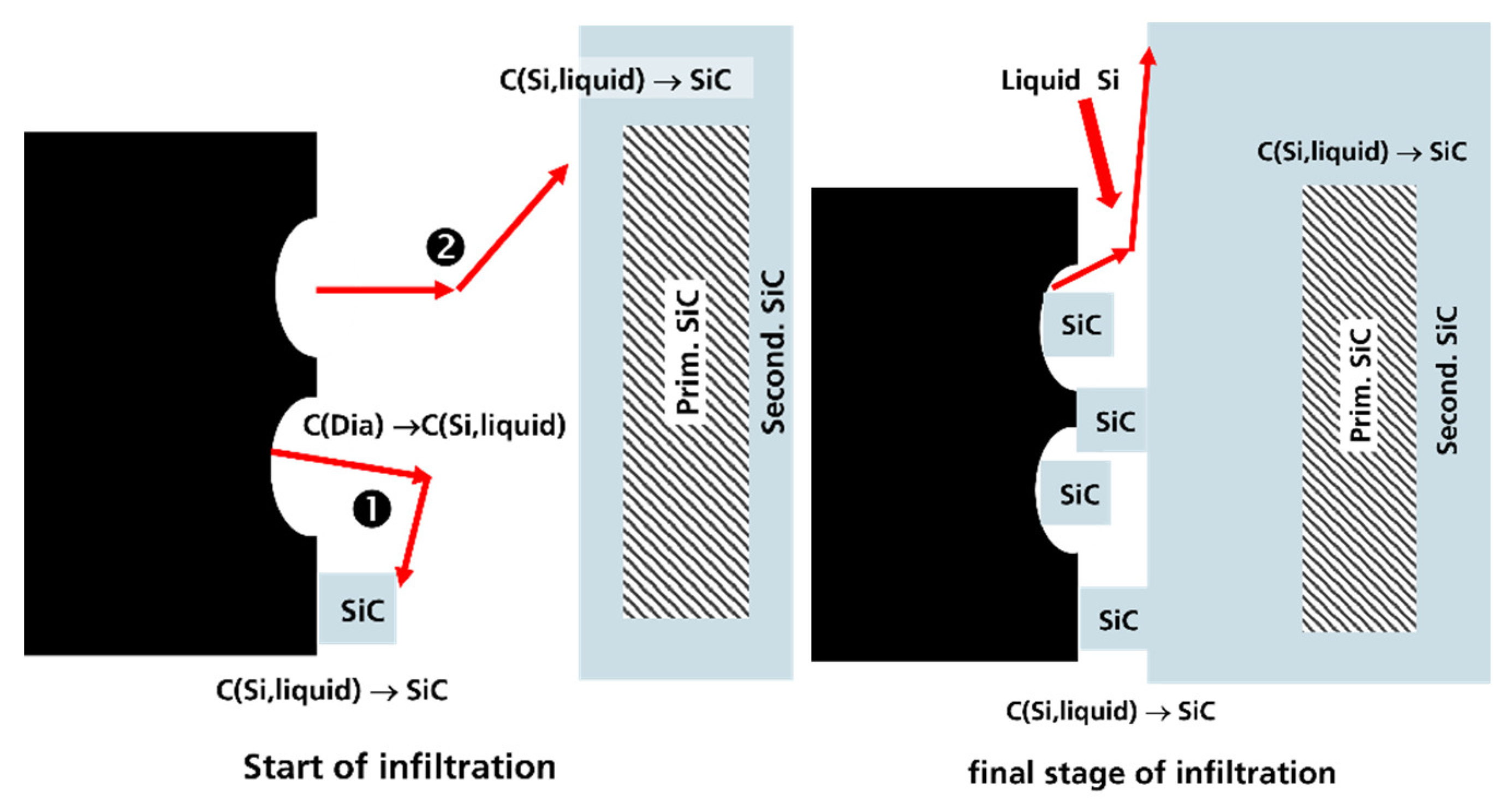
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthey, B. Siliciumcarbid Gebundene Diamantwerkstoffe—Herstellung. Mikrostruktur und Eigenschaften. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 2018. [Google Scholar]
- Gordeev, S.; Zhukov, S.; Danchukova, L.; Ekstrom, T. Low-Pressure Fabrication of Diamond–SiC–Si Composites. Inorg. Mater. 2001, 6, 579–583. [Google Scholar] [CrossRef]
- Matthey, B.; Kunze, S.; Hörner, M.; Blug, B.; van Geldern, M.; Michaelis, A.; Herrmann, M. SiC-bonded diamond materials produced by pressureless silicon infiltration. J. Mater. Res. 2017, 17, 3362–3371. [Google Scholar] [CrossRef]
- Yang, Z.; He, X.; Wu, M.; Zhang, L.; Ma, A.; Liu, R.; Hu, H.; Zhang, Y.; Qu, X. Infiltration mechanism of diamond/SiC composites fabricated by Si-vapor vacuum reactive infiltration process. J. Eur. Ceram. Soc. 2013, 4, 869–878. [Google Scholar] [CrossRef]
- Yang, Z.; He, X.; Wu, M.; Zhang, L.; Ma, A.; Liu, R.; Hu, H.; Zhang, Y.; Qu, X. Fabrication of diamond/SiC composites by Si-vapor vacuum reactive infiltration. Ceram. Int. 2013, 3, 3399–3403. [Google Scholar] [CrossRef]
- Shevchenko, V.Y.; Dolgin, A.; Sychov, M.M.A.; Perevislov, S. Ideal: A promising diamond-silicon carbide composite for enhanced amor. Ceram. Int. 2024, 50, 4264–4273. [Google Scholar] [CrossRef]
- Shevchenko, V.; Perevislov, S. Reaction–Diffusion Mechanism of Synthesis in the Diamond–Silicon Carbide System. Russ. J. Inorg. Chem. 2021, 66, 1107–1114. [Google Scholar] [CrossRef]
- Bogdanov, S.P.; Dolgin, A.S.; Perevislov, S.N.; Khristyuk, N.A.; Sychov, M.M. Effect of Diamond Phase Dispersion on the Properties of Diamond-SiC-Si Composites. Ceramics 2023, 6, 1632–1645. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Zhang, T.; Liu, P.; Qu, X. Preparation of bimodal-diamond/SiC composite with high thermal conductivity. J. Eur. Ceram. Soc. 2024, 44, 643–650. [Google Scholar] [CrossRef]
- Schmalzried, C.; Schwetz, K.A. Silicon-Carbide-and Boron Carbide-Based Hard Materials. In Ceramics Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 131–227. [Google Scholar]
- Madelung, O.; Rössler, U.; Schulz, M. (Eds.) Landolt-Börnstein—Group III Condensed Matter: Numerical Data and Functional Relationships in Science and Technology; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar] [CrossRef]
- Gmelin Handbook of Inorganic and Organometallic Chemistry, 8th ed.; Springer: Berlin/Heidelberg, Germany, 1984; Silicon Volume B2.
- Ruys, A.J. Silicon Carbide Ceramics: Structure, Properties and Manufacturing; Elsevier: Amsterdam, The Netherlands, 2023; p. 89. [Google Scholar]
- Grinchuk, P.S.; Abuhimd, H.M.; Kiyashko, M.V. Advanced reaction-bonded SiC ceramics for space mirror blanks. J. Manuf. Process. 2024, 113, 275–290. [Google Scholar] [CrossRef]
- Oleinik, G.S.; Danilenko, N.V. Polytype formation in nonmetallic substances. Russ. Chem. Rev. 1997, 66, 553. [Google Scholar] [CrossRef]
- Xu, W.-W.; Xia, F.; Chen, L.; Wu, M.; Gang, T.; Huang, Y. High-temperature mechanical and thermodynamic properties of silicon carbide polytypes. J. Alloys Compd. 2018, 768, 722–732. [Google Scholar] [CrossRef]
- Luo, X.; Goel, S.; Reuben, R.L. A quantitative assessment of nanometric machinability of major polytypes of single crystal silicon carbide. J. Eur. Ceram. Soc. 2012, 32, 3423–3434. [Google Scholar] [CrossRef]
- Prasad, K.E.; Ramesh, K.T. Hardness and mechanical anisotropy of hexagonal SiC single crystal polytypes. J. Alloys Compd. 2019, 770, 158–165. [Google Scholar] [CrossRef]
- Eddy, C.R.; Wu, P.; Zwieback, I.; VanMil, B.L.; Myers-Ward, R.L.; Tedesco, J.L. Microhardness of 6H-and 4H-SiC Substrates. Mater. Sci. Forum. 2009, 615–617, 323–326. [Google Scholar] [CrossRef]
- Sugiyama, S.; Togaya, M. Phase Relationship between 3C- and 6H-Silicon Carbide at High Pressure and High Temperature. J. Am. Ceram. Soc. 2001, 84, 3013–3016. [Google Scholar] [CrossRef]
- Atkinson, C.M.; Guziewski, M.C.; Coleman, S.P.; Nayak, S.K.; Alpay, S.P. First principles analysis of impurities in silicon carbide grain boundaries. Acta Mater. 2021, 221, 117421. [Google Scholar] [CrossRef]
- Kuang, J.; Cao, W. Elder Synthesis of α-SiC particles at 1200 °C by microwave heating. Powder Technol. 2013, 247, 106–111. [Google Scholar] [CrossRef]
- Wei, S.; Guan, L.; Song, B.; Fan, B.; Zhao, B.; Zhang, R. Seeds-induced synthesis of SiC by microwave heating. Ceram. Int. 2019, 45, 9771–9775. [Google Scholar] [CrossRef]
- Turan, S.; Knowles, K.M. Effect of Boron Nitride on the Phase Stability and Phase Transformations in Silicon Carbide. J. Am. Ceram. Soc. 1996, 79, 3325–3328. [Google Scholar] [CrossRef]
- Mainzer, B. How to Tame the Aggressiveness of Liquid Silicon in the LSI Process. Key Eng. Mater. 2017, 742, 238–245. [Google Scholar] [CrossRef]
- Quintana Freire, J.A.; Matthey, B.; Höhn, S.; Kunze, S.; Herrmann, M. Boron Containing SiC Bonded Diamond Composites. Fraunhofer Institute for Ceramic Technologies and Systems, Dresden, Germany. 2025; manuscript in preparation. [Google Scholar]
- Herrmann, M.; Sempf, K.; Kremmer, K.; Schneider, M.; Michaelis, A. Electrochemical corrosion of silicon-infiltrated silicon carbide ceramics in aqueous solutions. Ceram. Int. 2015, 41, 4422–4429. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, C.-Y.; Karandikar, P.; Ni, C. Interfacial zone surrounding the diamond in reaction bonded diamond/SiC composites: Interphase structure and formation mechanism. J. Eur. Ceram. Soc. 2019, 39, 5190–5196. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.J.; Kim, S.W.; Youm, M.R.; Nahm, S.; Park, S.W. Effects of diamond as a main carbon source on the fabrication and mechanical properties of reaction-bonded SiC. Ceram. Int. 2024, 50, 35169–35177. [Google Scholar] [CrossRef]
- Mlungwane, K. A Study on the Sintering of fine-Grained Diamond-SiC Composites. Ph.D. Thesis, University of Witwatersrand, Johannesburg, South Africa, 2014. [Google Scholar]
- Kunze, S.; Matthey, B.; Herrmann, M. SiC-bonded diamond material with excellent abrasive wear resistance. Open Ceram. 2024, 19, 100627. [Google Scholar] [CrossRef]
- Matthey, B.; Kunze, S.; Kaiser, A.; Herrmann, M. Thermal properties of SiC-bonded diamond materials produced by liquid silicon infiltration. Open Ceram. 2023, 15, 100386. [Google Scholar] [CrossRef]
- DIN EN ISO 18754:2022-06; Hochleistungskeramik- Bestimmung der Dichte und der scheinbaren Porosität. Deutsches Institut für Normung (German Institute for Standardization): Berlin, Germany, 2022.
- Pujar, V.V.; Cawley, J.D. Computer Simulations of Diffraction Effects due to Stacking Faults in β-SiC: I, Simulation Results. J. Am. Ceram. Soc. 1997, 80, 1653–1662. [Google Scholar] [CrossRef]
- Pujar, V.V.; Cawley, J.D. Computer Simulations of Diffraction Effects due to stacking faults in β-SiC: II, Experimental Verification. J. Am. Ceram. Soc. 2001, 84, 2645–2651. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, C.; Wang, T.; Karandikar, P.; Ni, C. Mechanical and thermal characteristics of interfaces and defects in diamond/SiC composites (Invited). In Proceedings of the 44th ICACC, Daytona Beach, FL, USA, 29 January 2020. ICACC-S4-030-2020. [Google Scholar]
- Yousefi, P.; Matthey, B.; Fontanot, T.; Herre, P.; Höhn, S.; Christiansen, S.H.; Herrmann, M. Strength of diamond-silicon carbide interfaces in silicon carbide bonded diamond materials containing graphitic interlayers. Open Ceram. 2022, 11, 100296. [Google Scholar] [CrossRef]
- Drevet, B.; Eustathopoulos, N. Wetting of ceramics by molten silicon and silicon alloys: A review. J. Mater. Sci. 2012, 47, 8247–8260. [Google Scholar] [CrossRef]
- Mlungwane, K.; Sigalas, I.; Herrmann, M.; Rodrıguez, M. The wetting behaviour and reaction kinetics in diamond–silicon carbide systems. Ceram. Int. 2009, 35, 2435–2441. [Google Scholar] [CrossRef]


| Polytype (Ramsdell Notation) | Layer Sequency | Space Group | Z | Lattice Parameter | Band Gap, eV | E, GPa | H (100) Plane, GPa | |
|---|---|---|---|---|---|---|---|---|
| a, nm | c, nm | |||||||
| 3C | ABC | F43m | 4 | 0.43590 | 2.3 | 314 | 25–30 | |
| 2H | AB | P63mc | 2 | 0.30790 | 0.5055 | 3.3 | ||
| 4H | ABCB | P63mc | 4 | 0.30800 | 1.0080 | 3.2 | 347 | 26 |
| 6H | ABCACB | P63mc | 6 | 0.30806 | 1.5117 | 3.0 | 347 | 20–26 |
| Additives | Density, g/cm3 | Open Porosity, % | Phase Content | Temperature Conductivity, mm2/s | Thermal Conductivity, W/m∗K | ||
|---|---|---|---|---|---|---|---|
| Dia 50/10 Rev | - | 1575 °C 20 min | 3.29 | 0 | Diamond, Si, 3C-SiC | 218.4 | 439 |
| Dia 50/10 10AlN | 10 wt. % AlN | 1575 °C 20 min | 3.12 | 4.5 | Diamond, 3C-SiC, 2H-SiC, AlN, Si, Al; Al(OH)3 | 96.0 | approx. 180 |
| Dia 50/10 5SiC | 5 wt. % SiC UF15 | 1575 °C 20 min | 3.32 | 0 | Diamond, Si3C-SiC, 6HSiC | 213.3 | 433 |
| Dia 50/10 10SiC | 10 wt. % SiC UF15 | 1575 °C 20 min | 3.30 | 0 | Diamond, Si, 3C-SiC 6HSiC | 184.0 | 370 |
| Dia 50/10 55 SiC | 55 SiC F900/F360 | 1600 °C 60 min | 3.19 | 0 | Diamond, Si, 3C-SiC, 6H-SiC | ||
| Dia 50/10 50 SiC | 50 SiC F900/F360 | 1600 °C 60 min | 3.19 | 0 | Diamond, Si 3C-SiC, 6H-SiC | ||
| Dia 50/10 34SiC | 34 SiC F900/F360 | 1600 °C 60 min | 3.26 | 0 | Diamond, Si 3C-SiC, 6H-SiC | ||
| Dia 50/10 25-SiC | 25 SiC F900/F360 | 1600 °C 60 min | 3.26 | 0 | Diamond, Si 3C-SiC, 6H-SiC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrmann, M.; Quintana Freire, J.A.; Matthey, B.; Kunze, S.; Höhn, S. Control of the SiC Polytypes in SiC Bonded Diamond Materials. Ceramics 2025, 8, 90. https://doi.org/10.3390/ceramics8030090
Herrmann M, Quintana Freire JA, Matthey B, Kunze S, Höhn S. Control of the SiC Polytypes in SiC Bonded Diamond Materials. Ceramics. 2025; 8(3):90. https://doi.org/10.3390/ceramics8030090
Chicago/Turabian StyleHerrmann, Mathias, Jesus Andres Quintana Freire, Björn Matthey, Steffen Kunze, and Sören Höhn. 2025. "Control of the SiC Polytypes in SiC Bonded Diamond Materials" Ceramics 8, no. 3: 90. https://doi.org/10.3390/ceramics8030090
APA StyleHerrmann, M., Quintana Freire, J. A., Matthey, B., Kunze, S., & Höhn, S. (2025). Control of the SiC Polytypes in SiC Bonded Diamond Materials. Ceramics, 8(3), 90. https://doi.org/10.3390/ceramics8030090









