Sunflower Shells Biomass Fly Ash as Alternative Alkali Activator for One-Part Cement Based on Ladle Slag
Abstract
1. Introduction
2. Materials and Methods
3. Results
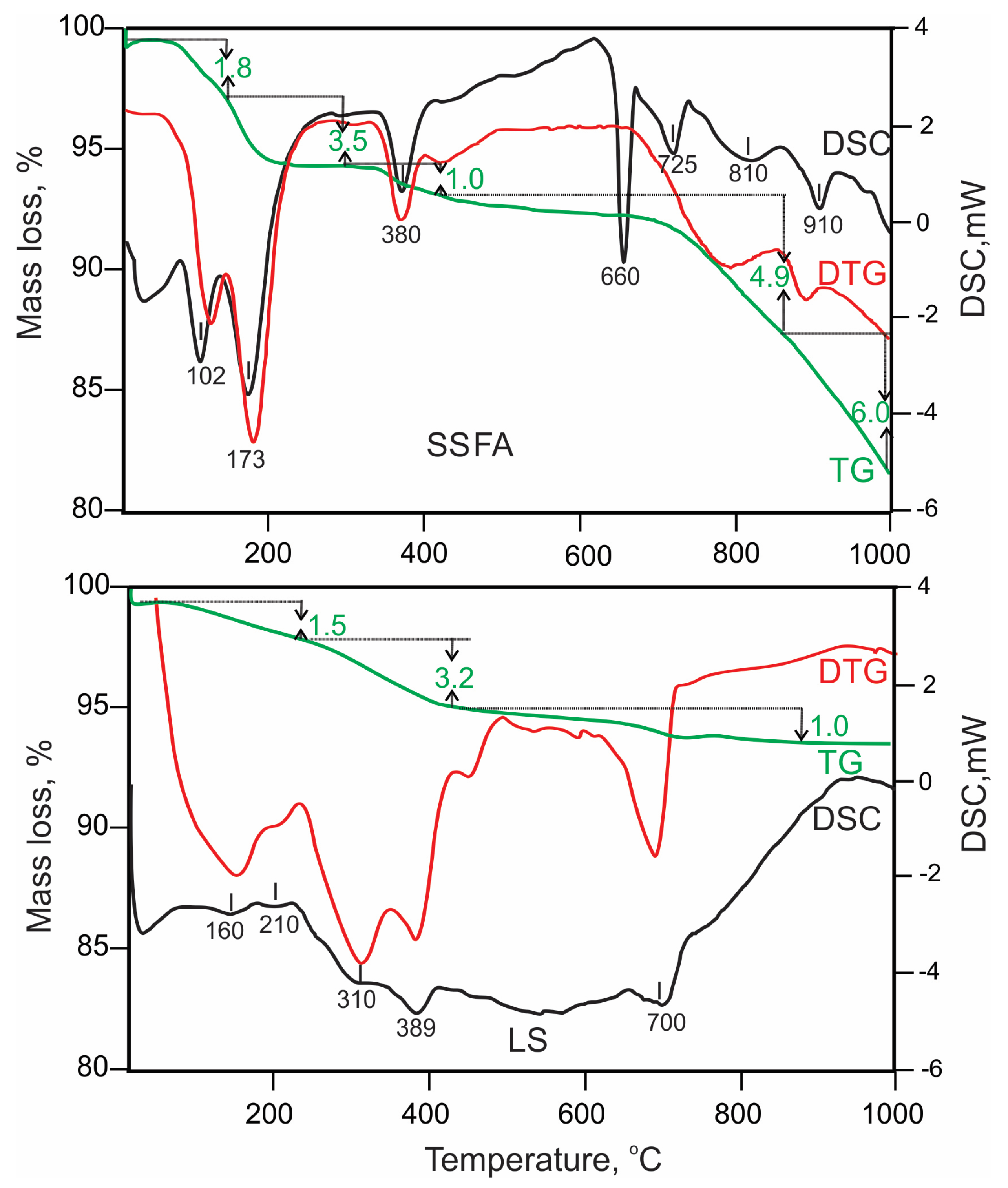
3.1. Characterization of the Precursors
3.2. Influence of Sunflower Shells Fly Ash Addition to Ladle Slag
3.2.1. Physical Properties
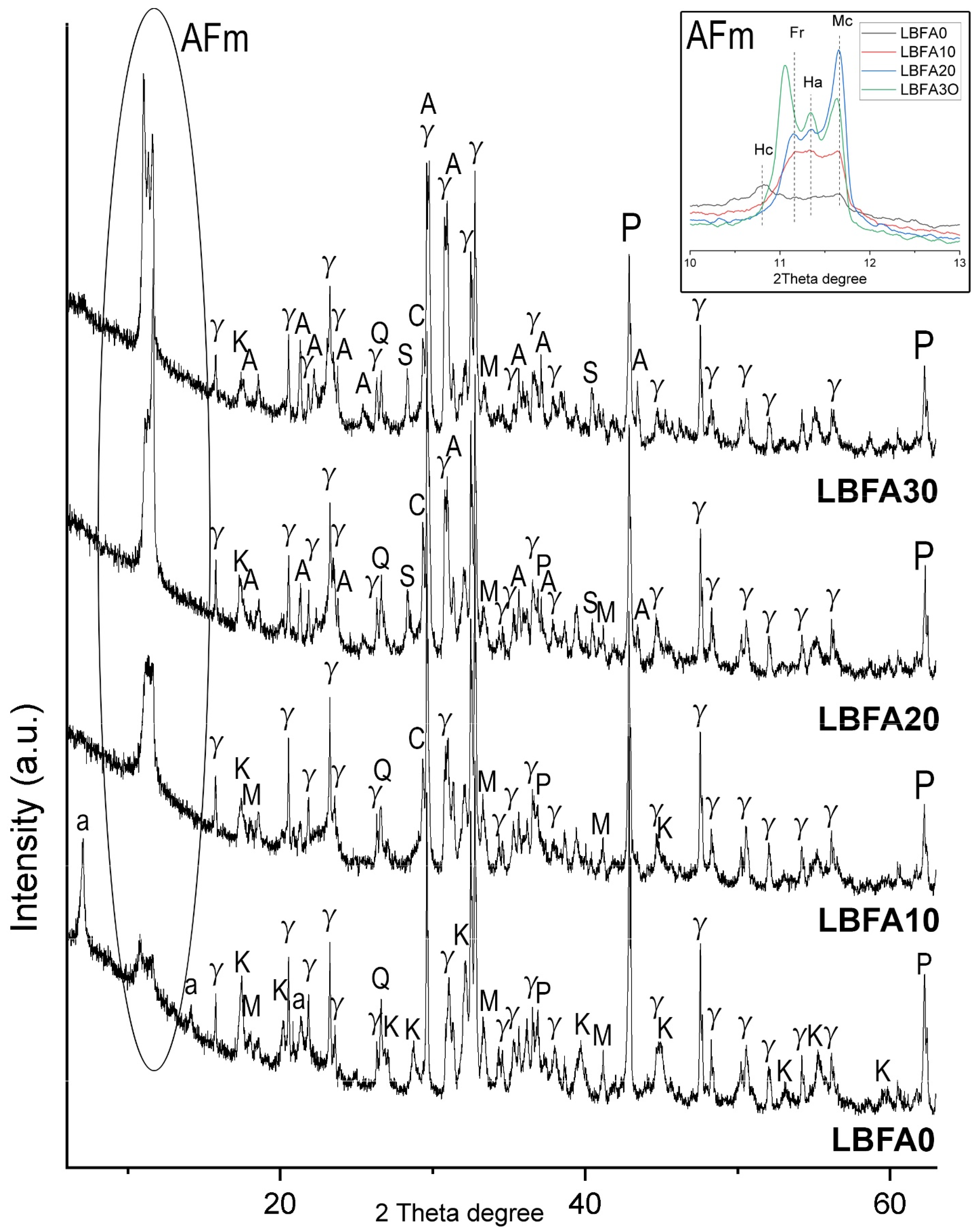
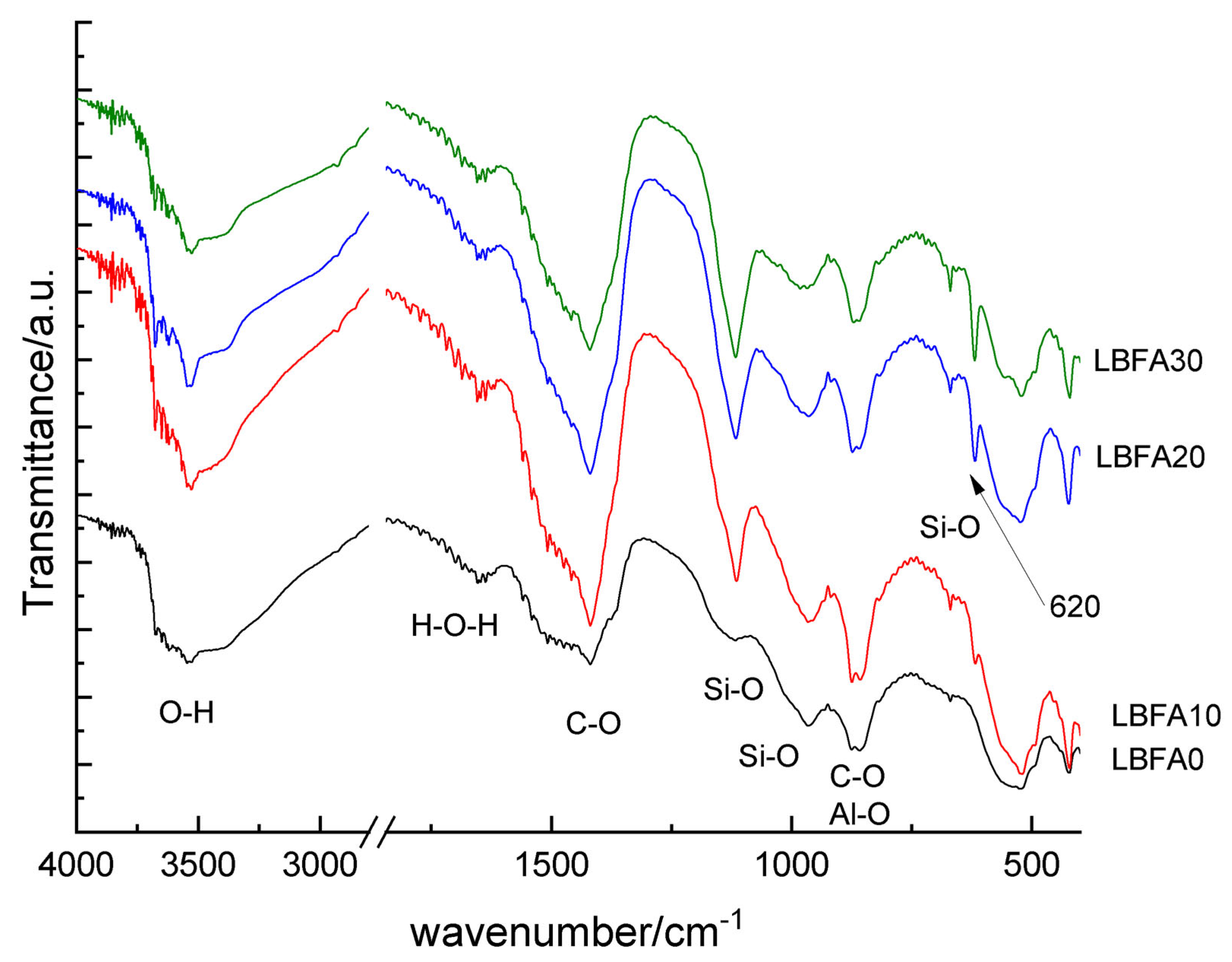
3.2.2. Microstructural Characterization
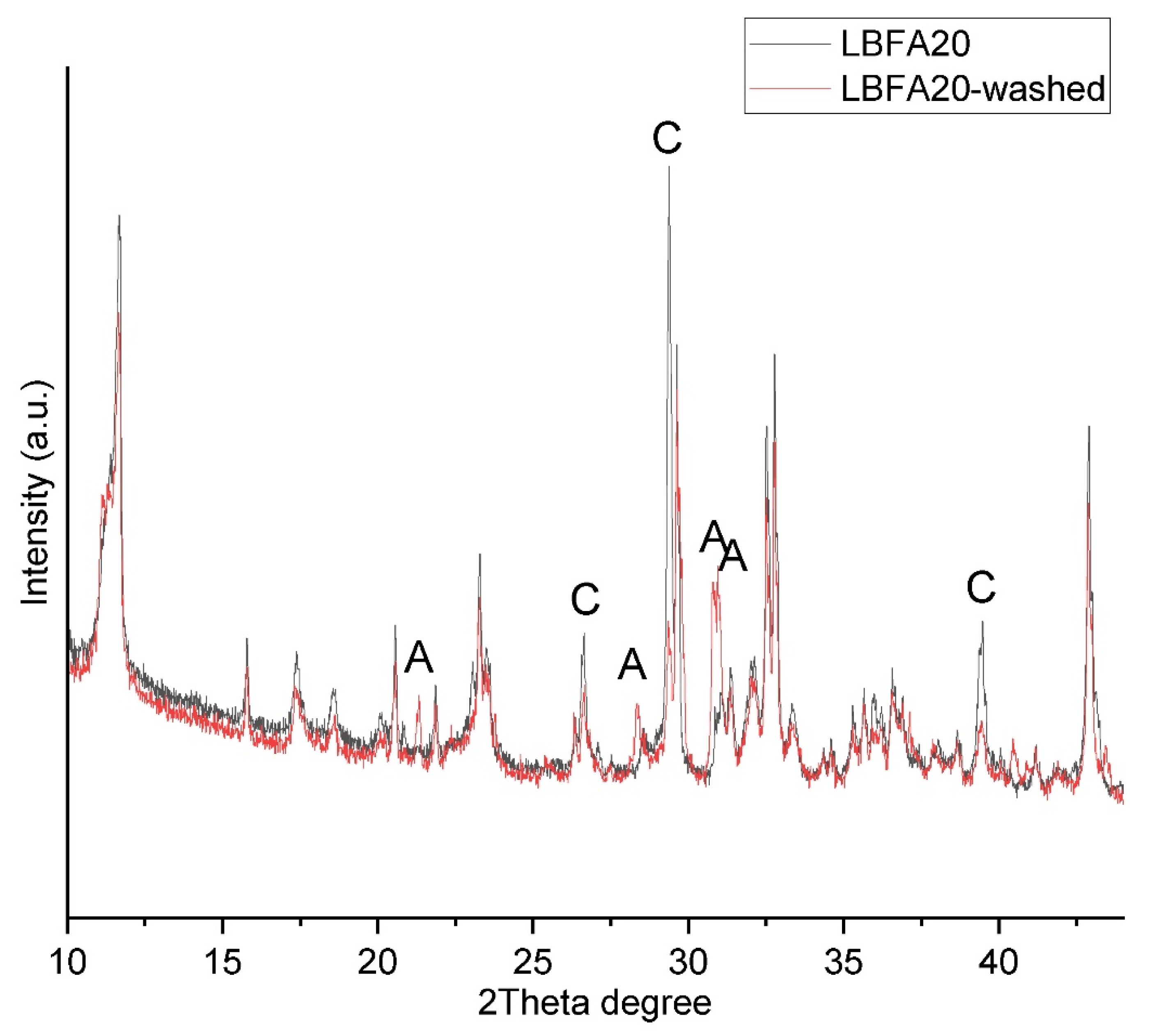
3.3. Leaching Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFm | A family of hydrated calcium aluminate phases |
| SSFA | Sunflower shells fly ash |
| LS | Ladle slag |
References
- World Steel Association. Steel Statistical Yearbook 2022: A Cross-Section of Steel Industry Statistics 2012–2021; World Steel Association: Brussels, Belgium, 2022. [Google Scholar]
- Chen, J.; Xing, Y.; Wang, Y.; Zhang, W.; Guo, Z.; Su, W. Application of iron and steel slags in mitigating greenhouse gas emissions: A review. Sci. Total Environ. 2022, 844, 157041. [Google Scholar] [CrossRef] [PubMed]
- Baalamurugan, J.; Kumar, V.G.; Chandrasekaran, S.; Balasundar, S.; Venkatraman, B.; Padmapriya, R.; Raja, V.B. Recycling of steel slag aggregates for the development of high density concrete: Alternative & environment-friendly radiation shielding composite. Compos. Part B Eng. 2021, 216, 108885. [Google Scholar] [CrossRef]
- Raut, S.R.; Saklecha, P.; Kedar, R. Review on ground granulated blast-furnace slag as a supplementary cementitious material. Int. J. Comput. Appl. 2015, 975, 8887. [Google Scholar]
- Aiban, S.A. Utilization of steel slag aggregate for road bases. J. Test. Eval. 2006, 34, 65–75. [Google Scholar] [CrossRef]
- Kumar, H.; Varma, S. A review on utilization of steel slag in hot mix asphalt. Int. J. Pavement Res. Technol. 2021, 14, 232–242. [Google Scholar] [CrossRef]
- Pinheiro, C.; Rios, S.; da Fonseca, A.V.; Fernández-Jiménez, A.; Cristelo, N. Application of the response surface method to optimize alkali activated cements based on low-reactivity ladle furnace slag. Constr. Build. Mater. 2020, 264, 120271. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.; Mei, H.; Rao, L.; Wang, H.; Lv, N. Generation, utilization, and environmental impact of ladle furnace slag: A minor review. Sci. Total Environ. 2023, 895, 165070. [Google Scholar] [CrossRef]
- Brand, A.S.; Fanijo, E.O. A review of the influence of steel furnace slag type on the properties of cementitious composites. Appl. Sci. 2020, 10, 8210. [Google Scholar] [CrossRef]
- Najm, O.; El-Hassan, H.; El-Dieb, A. Ladle slag characteristics and use in mortar and concrete: A comprehensive review. J. Clean. Prod. 2021, 288, 125584. [Google Scholar] [CrossRef]
- Shi, C. Steel slag—Its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Serjun, V.Z.; Mirti, B.; Mladenovič, A. Evaluation of ladle slag as a potential material for building and civil engineering. Mater. Technol. 2013, 47, 543–550. [Google Scholar]
- Taylor, H. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Yan, Z.; Jiang, Y.; Yin, K.; Wang, L.; Pan, T. Enhancement of Hydration Activity and Microstructure Analysis of γ-C2S. Materials 2023, 16, 6762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, J.; Yan, P. Influence of initial alkalinity on the hydration of steel slag. Sci. China Technol. Sci. 2012, 55, 3378–3387. [Google Scholar] [CrossRef]
- Shi, C. Characteristics and cementitious properties of ladle slag fines from steel production. Cem. Concr. Res. 2002, 32, 459–462. [Google Scholar] [CrossRef]
- Krivenko, P.V. Alkaline cements. In Proceedings of the 1st International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 11–14 October 1994; pp. 11–129. [Google Scholar]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Wang, W.-C.; Wang, H.-Y.; Tsai, H.-C. Study on engineering properties of alkali-activated ladle furnace slag geopolymer. Constr. Build. Mater. 2016, 123, 800–805. [Google Scholar] [CrossRef]
- Murri, A.N.; Rickard, W.; Bignozzi, M.; Van Riessen, A. High temperature behaviour of ambient cured alkali-activated materials based on ladle slag. Cem. Concr. Res. 2013, 43, 51–61. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Alkali activation of ladle slag from steel-making process. J. Sustain. Metall. 2017, 3, 300–310. [Google Scholar] [CrossRef]
- Češnovar, M.; Traven, K.; Horvat, B.; Ducman, V. The potential of ladle slag and electric arc furnace slag use in synthesizing alkali activated materials; the influence of curing on mechanical properties. Materials 2019, 12, 1173. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Properties and durability of alkali-activated ladle slag. Mater. Struct. 2017, 50, 255. [Google Scholar] [CrossRef]
- Xu, B.; Yi, Y. Use of ladle furnace slag containing heavy metals as a binding material in civil engineering. Sci. Total Environ. 2020, 705, 135854. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, I.; Ponzoni, C.; Bignozzi, M.C.; Barbieri, L.; Leonelli, C. Incinerator bottom ash and ladle slag for geopolymers preparation. Waste Biomass Valor. 2014, 5, 393–401. [Google Scholar] [CrossRef]
- Passuello, A.; Rodríguez, E.D.; Hirt, E.; Longhi, M.; Bernal, S.A.; Provis, J.L.; Kirchheim, A.P. Evaluation of the potential improvement in the environmental footprint of geopolymers using waste-derived activators. J. Clean. Prod. 2017, 166, 680–689. [Google Scholar] [CrossRef]
- Adesanya, E.; Perumal, P.; Luukkonen, T.; Yliniemi, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Opportunities to improve sustainability of alkali-activated materials: A review of side-stream based activators. J. Clean. Prod. 2021, 286, 125558. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass as a precursor in alkaline activation: Chemical process and hydration products. Constr. Build. Mater. 2017, 139, 342–354. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium waterglass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Billong, N.; Oti, J.; Kinuthia, J. Using silica fume based activator in sustainable geopolymer binder for building application. Constr. Build. Mater. 2021, 275, 122177. [Google Scholar] [CrossRef]
- Moraes, J.; Font, A.; Soriano, L.; Akasaki, J.; Tashima, M.; Monzó, J.; Borrachero, M.V.; Payá, J. New use of sugar cane straw ash in alkali-activated materials: A silica source for the preparation of the alkaline activator. Constr. Build. Mater. 2018, 171, 611–621. [Google Scholar] [CrossRef]
- Nikolov, A.; Nugteren, H.; Rostovsky, I. Optimization of geopolymers based on natural zeolite clinoptilolite by calcination and use of aluminate activators. Constr. Build. Mater. 2020, 243, 118257. [Google Scholar] [CrossRef]
- Jamieson, E.; van Riessen, A.; McLellan, B.; Penna, B.; Kealley, C.; Nikraz, H. Introducing Bayer Liquor-Derived Geopolymers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 159–189. [Google Scholar]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Peys, A.; Rahier, H.; Pontikes, Y. Potassium-rich biomass ashes as activators in metakaolin-based inorganic polymers. Appl. Clay Sci. 2016, 119, 401–409. [Google Scholar] [CrossRef]
- Font, A.; Soriano, L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. One-part eco-cellular concrete for the precast industry: Functional features and life cycle assessment. J. Clean. Prod. 2020, 269, 122203. [Google Scholar] [CrossRef]
- Soriano, L.; Font, A.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Bonifácio, T.; Payá, J. Almond-shell biomass ash (ABA): A greener alternative to the use of commercial alkaline reagents in alkali-activated cement. Constr. Build. Mater. 2021, 290, 123251. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.; Vásquez-García, S.; Israde-Alcántara, I.; Flores-Ramirez, N.; Rico, J.; Mohammed, M.S. Cleaner production of one-part white geopolymer cement using pre-treated wood biomass ash and diatomite. J. Clean. Prod. 2019, 209, 1420–1428. [Google Scholar] [CrossRef]
- Lima, F.S.; Gomes, T.C.F.; de Moraes, J.C.B. Novel one-part alkali-activated binder produced with coffee husk ash. Mater. Lett. 2022, 313, 131733. [Google Scholar] [CrossRef]
- Lokare, S.S.; Dunaway, J.D.; Moulton, D.; Rogers, D.; Tree, D.R.; Baxter, L.L. Investigation of ash deposition rates for a suite of biomass fuels and fuel blends. Energy Fuels 2006, 20, 1008–1014. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Baxter, D. Trace element concentrations and associations in some biomass ashes. Fuel 2014, 129, 292–313. [Google Scholar] [CrossRef]
- Amin, M.; Zeyad, A.M.; Agwa, I.S.; Heniegal, A.M. Effect of peanut and sunflower shell ash on properties of sustainable high-strength concrete. J. Build. Eng. 2024, 89, 109208. [Google Scholar] [CrossRef]
- Shahbazpanahi, S.; Faraj, R.H. Feasibility study on the use of shell sunflower ash and shell pumpkin ash as supplementary cementitious materials in concrete. J. Build. Eng. 2020, 30, 101271. [Google Scholar] [CrossRef]
- Nikolov, A.; Kostov-Kytin, V.; Tarassov, M.; Tsvetanova, L.; Jordanov, N.B.; Karamanova, E.; Rostovsky, I. Characterization of cement kiln dust from Bulgarian cement plants. J. Chem. Technol. Metall. 2025, 60, 455–463. [Google Scholar] [CrossRef]
- Lyu, H.; Hao, L.; Zhang, S.; Poon, C.S. High-performance belite rich eco-cement synthesized from solid wastes: Raw feed design, sintering temperature optimization, and property analysis. Resour. Conserv. Recycl. 2023, 199, 107211. [Google Scholar] [CrossRef]
- He, Z.; Li, Y. The influence of mayenite employed as a functional component on hydration properties of ordinary Portland cement. Materials 2018, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cordonnier, B.; Zhu, W.; Renard, F.; Jamtveit, B. Effects of confinement on reaction-induced fracturing during hydration of periclase. Geochem. Geophys. Geosyst. 2018, 19, 2661–2672. [Google Scholar] [CrossRef]
- Tolkacheva, A.; Shkerin, S.; Plaksin, S.; Vovkotrub, E.; Bulanin, K.; Kochedykov, V.; Ordinartsev, D.; Gyrdasova, O.; Molchanova, N. Synthesis of dense ceramics of single-phase mayenite (Ca12Al14O32)O. Russ. J. Appl. Chem. 2011, 84, 907–911. [Google Scholar] [CrossRef]
- Horgnies, M.; Chen, J.; Bouillon, C. Overview about the use of Fourier transform infrared spectroscopy to study cementitious materials. WIT Trans. Eng. Sci. 2013, 77, 251–262. [Google Scholar]
- Fernández Carrasco, L.; Torrens Martín, D.; Morales, L.; Martínez Ramírez, S. Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012; pp. 357–372. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Braterman, P.S.; Cygan, R.T. Vibrational spectroscopy of brucite: A molecular simulation investigation. Am. Mineral. 2006, 91, 1188–1196. [Google Scholar] [CrossRef]
- Chukanov, N.V. The Application of IR Spectroscopy to the Investigation of Minerals. In Infrared Spectra of Mineral Species: Extended Library; Springer: Dordrecht, The Netherlands, 2014; pp. 1–19. [Google Scholar]
- Schutte, C.; Buijs, K. The infra-red spectra of K2CO3 and its hydrates. Spectrochim. Acta 1961, 17, 921–926. [Google Scholar] [CrossRef]
- Okoronkwo, M.U.; Glasser, F.P. Stability of strätlingite in the CASH system. Mater. Struct. 2016, 49, 4305–4318. [Google Scholar] [CrossRef]
- Litwinek, E.; Madej, D. Structure, microstructure and thermal stability characterizations of C3AH6 synthesized from different precursors through hydration. J. Therm. Anal. Calorim. 2020, 139, 1693–1706. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Fang, H.; Li, S.; Yao, Y.; He, Y. A novel preparation of nano-sized hexagonal Mg(OH)2. Procedia Eng. 2015, 102, 388–394. [Google Scholar] [CrossRef]
- Sabzevari, M.; Sajjadi, S.A.; Moloodi, A. Physical and mechanical properties of porous copper nanocomposite produced by powder metallurgy. Adv. Powder Technol. 2016, 27, 105–111. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
- Broström, M.; Enestam, S.; Backman, R.; Mäkelä, K. Condensation in the KCl–NaCl system. Fuel Process. Technol. 2013, 105, 142–148. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Rai, R.K.; Serra-Maia, R.; Shi, Y.; Psarras, P.; Vojvodic, A.; Stach, E.A. Enhanced mineral carbonation on surface functionalized MgO as a Proxy for mine tailings. Environ. Sci. Nano 2025, 12, 2630–2646. [Google Scholar] [CrossRef]
- Khan, M.; Kayali, O.; Troitzsch, U. Chloride binding capacity of hydrotalcite and the competition with carbonates in ground granulated blast furnace slag concrete. Mater. Struct. 2016, 49, 4609–4619. [Google Scholar] [CrossRef]
- Damidot, D.; Glasser, F. Thermodynamic investigation of the CaO—Al2O3—CaSO4—CaCO3-H2O closed system at 25 °C and the influence of Na2O. Adv. Cem. Res. 1995, 7, 129–134. [Google Scholar] [CrossRef]
- Damidot, D.; Glasser, F. Investigation of the CaO-Al2O3-SiO2-H2O system at 25 °C by thermodynamic calculations. Cem. Concr. Res. 1995, 25, 22–28. [Google Scholar] [CrossRef]
- Landy, R.A. Magnesia refractories. Mech. Eng. 2004, 178, 109. [Google Scholar]
- Thomas, J.J.; Musso, S.; Prestini, I. Kinetics and activation energy of magnesium oxide hydration. J. Am. Ceram. Soc. 2014, 97, 275–282. [Google Scholar] [CrossRef]
- Madej, D. Hydration, carbonation and thermal stability of hydrates in Ca7−xSrxZrAl6O18 cement. J. Therm. Anal. Calorim. 2018, 131, 2411–2420. [Google Scholar] [CrossRef]
- Petkova, V.; Stoyanov, V.; Pelovski, Y. TG–DTG–DTA in studying white self-compacting cement mortars. J. Therm. Anal. Calorim. 2012, 109, 797–806. [Google Scholar] [CrossRef]
- Gartner, E.; Walenta, G.; Morin, V.; Termkhajornkit, P.; Baco, I.; Casabonne, J. Hydration of a belite-calciumsulfoaluminate-ferrite cement: Aether™. In Proceedings of the 13th International Congress on the Chemistry of Cement, Madrid, Spain, 3–8 July 2011. [Google Scholar]
- Birnin-Yauri, U.; Glasser, F. Friedel’s salt, Ca2Al(OH)6(Cl, OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, W.; Liu, H.; Jin, H.; Chen, H.; Su, K.; Tu, Y.; Wang, W. Thermochemical behavior of three sulfates (CaSO4, K2SO4 and Na2SO4) blended with cement raw materials (CaO-SiO2-Al2O3-Fe2O3) at high temperature. J. Anal. Appl. Pyrolysis 2019, 142, 104617. [Google Scholar] [CrossRef]
- Lv, Z.; Tan, H.; Liu, X.; Chen, P.; Wang, Y.; Liang, W.; Hong, J. Chloride binding of AFm in the presence of Na⁺, Ca2⁺ and Ba2⁺. Constr. Build. Mater. 2023, 364, 129804. [Google Scholar] [CrossRef]
- Jun, Y.; Yoon, S.; Oh, J.E. A comparison study for chloride-binding capacity between alkali-activated fly ash and slag in the use of seawater. Appl. Sci. 2017, 7, 971. [Google Scholar] [CrossRef]



| Na2O | MgO | Al2O3 | SiO2 | SO3 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ladle slag | - | 6.50 | 15.50 | 15.60 | 2.45 | - | 0.10 | 55.50 | 0.70 | 0.80 | 2.69 | 0.16 |
| SSFA | 0.03 | 2.33 | 0.04 | 0.22 | 14.02 | 4.78 | 61.34 | 15.43 | 0.04 | 0.03 | 0.12 | - |
| Series | LS | SSFA | Water to Solid | Compressive Strength, MPa | Density, g/cm3 |
|---|---|---|---|---|---|
| LBFA0 | 100 | 0 | 0.35 | 9.5 ± 0.5 | 1.591 ± 0.006 |
| LBFA10 | 90 | 10 | 0.35 | 29.6 ± 0.2 | 1.569 ± 0.001 |
| LBFA20 | 80 | 20 | 0.35 | 22.4 ± 0.3 | 1.560 ± 0.002 |
| LBFA30 | 70 | 30 | 0.35 | 11.6 ± 0.2 | 1.548 ± 0.004 |
| Series | (I) Dehydration–(II) Dehydroxylation, Mass %, (Process-Related New Phases) | (III) Decarbonization, Mass % (Process-Related New Phase) | Total Loss, Mass % |
|---|---|---|---|
| LBFA0 | 15.8 (strätlingite, katoite) | 3.4 (hemi-, monocarboaluminate, calcite) | 19.2 |
| LBFA10 | 13.8 (AFm) | 7.4 (hemi-, monocarboaluminate, calcite) | 21.2 |
| LBFA20 | 13.5 (AFm) | 7.5 (hemi-, monocarboaluminate, calcite) | 21.0 |
| LBFA30 | 13.4 (AFm) | 7.6 (hemi-, monocarboaluminate, calcite) | 21.0 |
| MgO | Al2O3 | SiO2 | SO3 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LBFA20 | 5.67 | 10.9 | 9.28 | 5.91 | 0.983 | 13.4 | 48.6 | 0.654 | 0.868 | 2.84 | 0.895 |
| LBFA20-washed | 6.97 | 11.0 | 11.8 | 0.692 | 0.254 | 1.93 | 60.3 | 0.912 | 1.22 | 3.78 | 1.142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolov, A.; Kostov, V.; Petrova, N.; Tsvetanova, L.; Vassilev, S.V.; Titorenkova, R. Sunflower Shells Biomass Fly Ash as Alternative Alkali Activator for One-Part Cement Based on Ladle Slag. Ceramics 2025, 8, 79. https://doi.org/10.3390/ceramics8030079
Nikolov A, Kostov V, Petrova N, Tsvetanova L, Vassilev SV, Titorenkova R. Sunflower Shells Biomass Fly Ash as Alternative Alkali Activator for One-Part Cement Based on Ladle Slag. Ceramics. 2025; 8(3):79. https://doi.org/10.3390/ceramics8030079
Chicago/Turabian StyleNikolov, Aleksandar, Vladislav Kostov, Nadia Petrova, Liliya Tsvetanova, Stanislav V. Vassilev, and Rositsa Titorenkova. 2025. "Sunflower Shells Biomass Fly Ash as Alternative Alkali Activator for One-Part Cement Based on Ladle Slag" Ceramics 8, no. 3: 79. https://doi.org/10.3390/ceramics8030079
APA StyleNikolov, A., Kostov, V., Petrova, N., Tsvetanova, L., Vassilev, S. V., & Titorenkova, R. (2025). Sunflower Shells Biomass Fly Ash as Alternative Alkali Activator for One-Part Cement Based on Ladle Slag. Ceramics, 8(3), 79. https://doi.org/10.3390/ceramics8030079











