Life Cycle Assessment of Industrial Glass Cullet Recycling Process Based on Alkaline Activation
Abstract
1. Introduction
- -
- What are the environmental impacts of the four alkaline activation processes?
- -
- How much do the environmental impacts change when using a different energy source?
2. Materials and Methods
2.1. Goal and Scope Definition
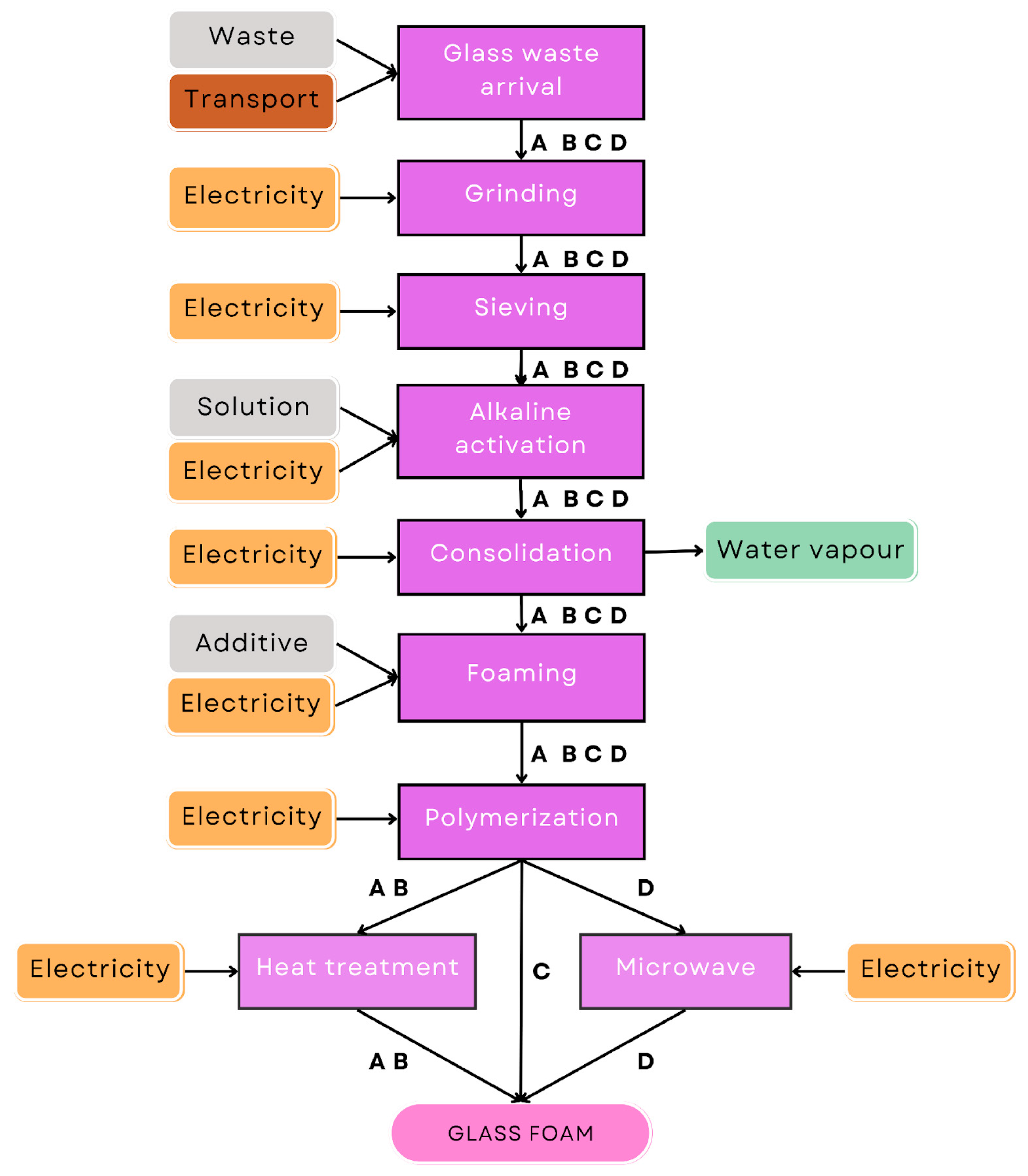
2.1.1. Description of the Alkali Activation Process
- A.
- Following the protocol outlined by Rincon et al. [23], after the AA process, the resulting slurry is transferred into closed polystyrene molds. The samples are subsequently dried in an oven at 75 °C for 2 h using a Bio-tech oven (Biomedica Elettronica, Padova, Italy) to facilitate preliminary gelation. Afterward, Triton X-100 (polyoxyethylene octyl phenyl ether—C14H22O(C2H4O)n, n = 9–10, Sigma-Aldrich, Gillingham, UK) was incorporated to the suspension at a concentration of 4 wt%. Aeration was achieved through vigorous mechanical mixing at 2000 rpm for 10 min. The resultant foams were removed from the molds after an additional drying phase at 75 °C for 24 h, after which they underwent thermal firing at 700 °C for 1 h (BSF-laboratory chamber furnace, Elite Thermal Systems Limited, Leicestershire, UK), with a controlled heating rate of 10 °C/min.
- B.
- Direct foaming experiments are carried out by introducing sodium perborate (NaBO3·H2O, Sigma Aldrich, Schnelldorf, Germany) and sodium dodecyl sulphate (SDS, CH3(CH2)11OSO3Na, Sigma Aldrich, Schnelldorf, Germany) into a mixture at a concentration of 1 wt% relative to the mass of the glass powder. Homogenization was achieved via mechanical mixing for 10 min at 2000 rpm. The drying phase was extended to 24 h at 40 °C, followed by thermal treatment at 600 °C for 1 h [13].
- C.
- The experimental conditions and methodologies are comparable to those outlined in process B. The type and quantity of foaming agent employed remained consistent. Notably, no additional sintering process was applied during this phase, as the hardening process occurs just upon drying at nearly room temperature (40 °C), for seven days [12], in a condition of ‘cold consolidation’.
- D.
- To mitigate the time-consuming nature of the hardening phase, the integration of microwave technology is explored. After the direct foaming expressed as in process B, the specimens are left for 1 day and later subjected to microwave heating (microwave oven MJ3965BPS, LG, Samsung MS23F300EEK, Samsung Electronics Italia S.p.A, Milano, Italy) for 5 min at 400 W [49].
2.1.2. System Boundaries of Processes
2.2. Life Cycle Inventory
2.3. Life Cycle Impact Assessment and Interpretation
3. Results and Discussions
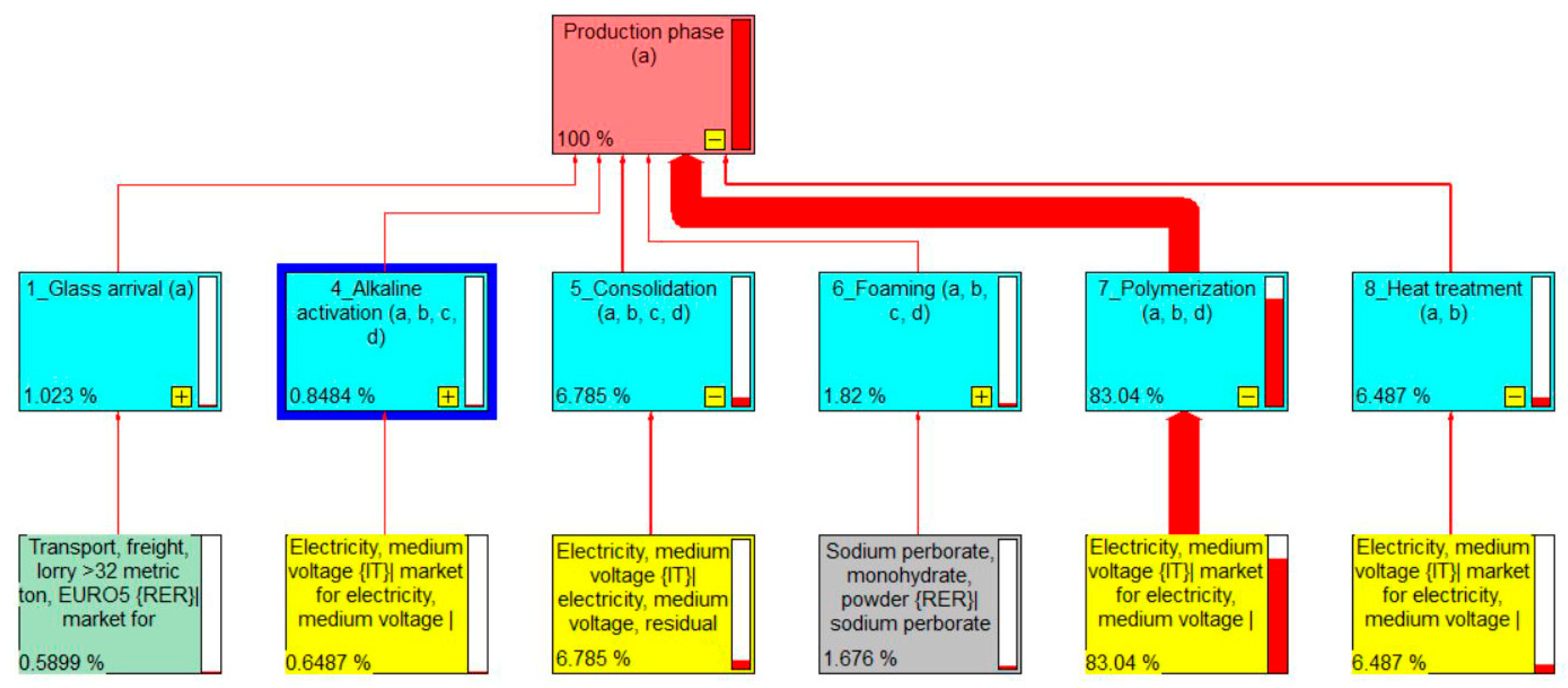
3.1. Life Cycle Inventory Results
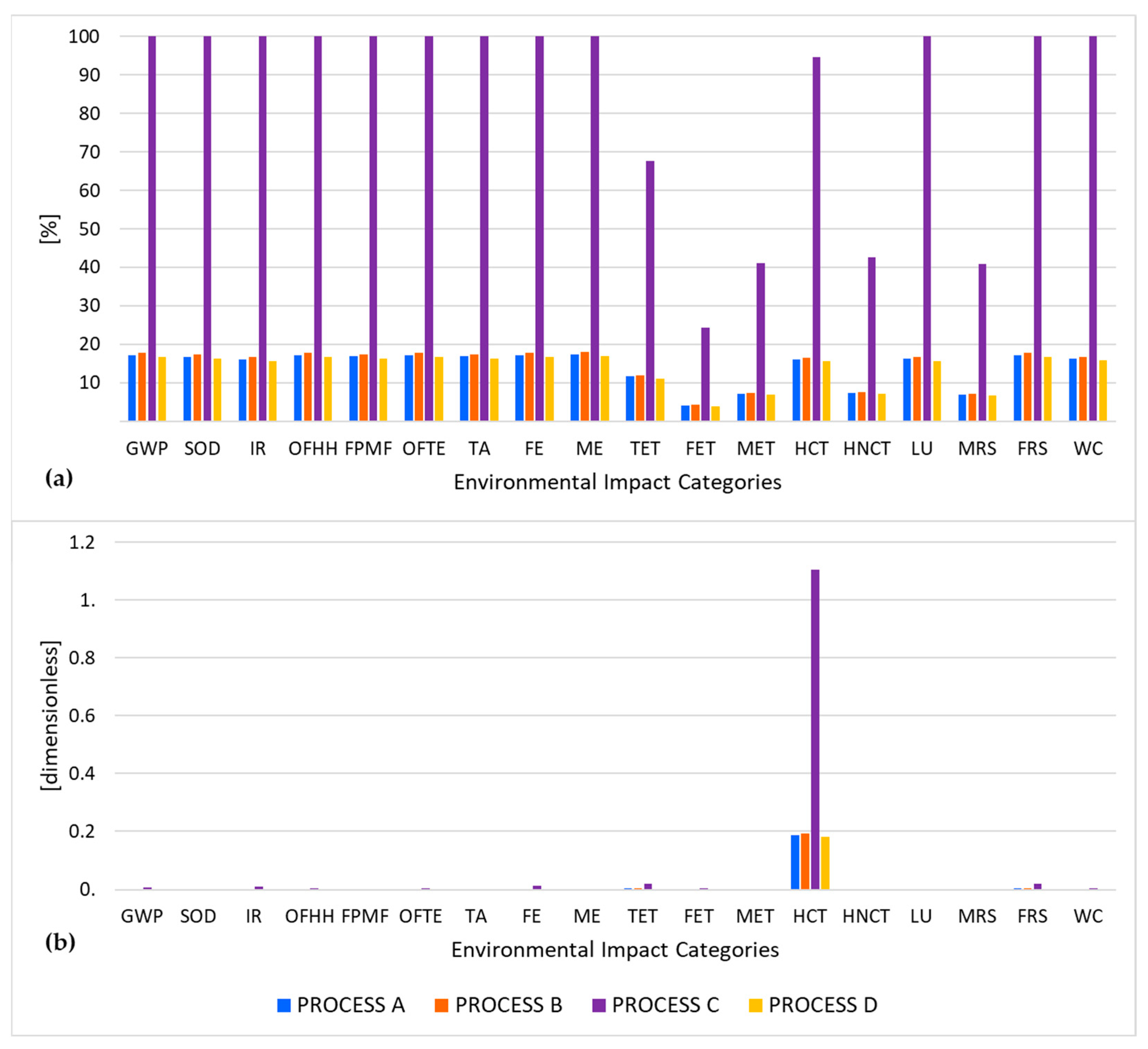
3.2. Life Cycle Impact Assessment Results
3.3. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Alkaline Activation |
| BASG | Boro-alumino-silicate glass |
| CE | Circular Economy |
| E | Egalitarian |
| EoL | End of Life |
| FPMF | Fine Particulate Matter Formation |
| FRS | Fossil Resource Scarcity |
| FET | Freshwater Eco-Toxicity |
| FE | Freshwater Eutrophication |
| GWP | Global warming potential |
| H | Hierarchist |
| HCT | Human Carcinogenic toxicity |
| HNCT | Human Non-Carcinogenic Toxicity |
| I | Individualist |
| IR | Ionizing Radiation |
| LU | Land Use |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| LCIA | Life Cycle Impact Assessment |
| MET | Marine Eco-Toxicity |
| ME | Marine Eutrophication |
| MRS | Mineral Resource Scarcity |
| OFHH | Ozone Formation, Human Health |
| OFTE | Ozone Formation, Terrestrial Ecosystems |
| PV | Photovoltaic |
| SOD | Stratospheric Ozone Depletion |
| TA | Terrestrial Acidification |
| TE | Terrestrial Eco-Toxicity |
| WC | Water Consumption |
References
- Curran, M.A. Life Cycle Impact Assessment. In Life Cycle Assessment Handbook: A Guide for Environmentally Sustainable Products; Curran, M.A., Ed.; Wiley: Hoboken, NJ, USA, 2012; Chapter 16. [Google Scholar] [CrossRef]
- Adekomaya, O.; Majozi, T. Mitigating environmental impact of waste glass materials: Review of the existing reclamation options and future outlook. Environ. Sci. Pollut. Res. 2021, 28, 10488–10502. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liang, J.; Yang, G.; Dagestani, A.A.; Liu, W.; Luo, X.; Zeng, B.; Wu, H.; Huang, M.; Lin, L.; et al. Recycling of waste glass as raw materials for the preparation of self-cleaning, light-weight and high-strength porous ceramics. J. Clean. Prod. 2021, 317, 128395. [Google Scholar] [CrossRef]
- Bonifazi, G.; Serranti, S. Imaging spectroscopy based strategies for ceramic glass contaminants removal in glass recycling. Waste Manag. 2006, 26, 627–639. [Google Scholar] [CrossRef]
- Farcomeni, A.; Serranti, S.; Bonifazi, G. Non-parametric analysis of infrared spectra for recognition of glass and glass ceramic fragments in recycling plants. Waste Manag. 2008, 28, 557–564. [Google Scholar] [CrossRef]
- Rincón, A.; Giacomello, G.; Pasetto, M.; Bernardo, E. Novel ‘inorganic gel casting’ process for the manufacturing of glass foams. J. Eur. Ceram. Soc. 2017, 37, 2227–2234. [Google Scholar] [CrossRef]
- Delbari, S.A.; Hof, L.A. Glass waste circular economy—Advancing to high-value glass sheets recovery using industry 4.0 and 5.0 technologies. J. Clean. Prod. 2024, 462, 142629. [Google Scholar] [CrossRef]
- Rincón, A.; Marangoni, M.; Cetin, S.; Bernardo, E. Recycling of inorganic waste in monolithic and cellular glass-based materials for structural and functional applications. J. Chem. Technol. Biotechnol. 2016, 91, 1946–1961. [Google Scholar] [CrossRef]
- Singh, N.; Li, J.; Zeng, X. Solutions and challenges in recycling waste cathode-ray tubes. J. Clean. Prod. 2016, 133, 188–200. [Google Scholar] [CrossRef]
- Baek, C.R.; Kim, H.D.; Jang, Y.-C. Exploring glass recycling: Trends, technologies, and future trajectories. Environ. Eng. Res. 2025, 30, 240241. [Google Scholar] [CrossRef]
- Guadagnino, E.; Guglielmi, M.; Nicoletti, F. Glass: The best material for pharmaceutical packaging. Int. J. Appl. Glass Sci. 2022, 13, 281–291. [Google Scholar] [CrossRef]
- Tameni, G.; Lago, D.; Kaňková, H.; Buňová, L.; Kraxner, J.; Galusek, D.; Dawson, D.M.; Ashbrook, S.E.; Bernardo, E. Alkaline attack of boro-alumino-silicate glass: New insights of the molecular mechanism of cold consolidation and new applications. Open Ceram. 2025, 21, 100726. [Google Scholar] [CrossRef]
- Mehta, A.; Karbouche, K.; Kraxner, J.; Elsayed, H.; Galusek, D.; Bernardo, E. Upcycling of Pharmaceutical Glass into Highly Porous Ceramics: From Foams to Membranes. Materials 2022, 15, 3784. [Google Scholar] [CrossRef] [PubMed]
- Bristogianni, T.; Oikonomopoulou, F. Glass up-casting: A review on the current challenges in glass recycling and a novel approach for recycling ‘as-is’ glass waste into volumetric glass components. Glass Struct. Eng. 2023, 8, 255–302. [Google Scholar] [CrossRef]
- Iacocca, R.G.; Allgeier, M. Corrosive attack of glass by a pharmaceutical compound. J. Mater. Sci. 2007, 42, 801–811. [Google Scholar] [CrossRef]
- Singh, P.J.; Sung, K.; Cooper, T.; West, K.; Mont, O. Challenges and opportunities for scaling up upcycling businesses—The case of textile and wood upcycling businesses in the UK. Resour. Conserv. Recycl. 2019, 150, 104439. [Google Scholar] [CrossRef]
- Shi, X.; Liao, Q.; Liu, L.; Deng, F.; Chen, F.; Wang, F.; Zhu, H.; Zhang, L.; Liu, C. Utilizing multi-solid waste to prepare and characterize foam glass ceramics. Ceram. Int. 2023, 49, 35534–35543. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhang, S. A review of glass ceramic foams prepared from solid wastes: Processing, heavy-metal solidification and volatilization, applications. Sci. Total Environ. 2021, 781, 146727. [Google Scholar] [CrossRef]
- Meylan, G.; Ami, H.; Spoerri, A. Transitions of municipal solid waste management. Part II: Hybrid life cycle assessment of Swiss glass-packaging disposal. Resour. Conserv. Recycl. 2014, 86, 16–27. [Google Scholar] [CrossRef]
- Sepúlveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Hill, R.G.; Yue, S.; Nightingale, D.; Lee, P.D.; Jones, J.R. Melt-derived bioactive glass scaffolds produced by a gel-cast foaming technique. Acta Biomater. 2011, 7, 1807–1816. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Appendini, M.; Rivoira, L.; Onida, B.; Del Bubba, M.; Jana, P.; Sorarù, G.D. Polymer-derived ceramic aerogels as sorbent materials for the removal of organic dyes from aqueous solutions. J. Am. Ceram. Soc. 2018, 101, 821–830. [Google Scholar] [CrossRef]
- Romero, A.R.; Tamburini, S.; Taveri, G.; Toušek, J.; Dlouhy, I.; Bernardo, E. Extension of the “Inorganic Gel Casting” Process to the Manufacturing of Boro-Alumino-Silicate Glass Foams. Materials 2018, 11, 2545. [Google Scholar] [CrossRef]
- Riondet, L.; Rio, M.; Perrot-Bernardet, V.; Zwolinski, P. Emerging technologies upscaling: A framework for matching LCA practices with upscaling archetypes. Sustain. Prod. Consum. 2024, 50, 347–363. [Google Scholar] [CrossRef]
- ISO 14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Bjørn, A.; Owsianiak, M.; Molin, C.; Laurent, A. Main characteristics of LCA. In Life Cycle Assessment; Hauschild, M., Rosenbaum, R., Olsen, S., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Hetherington, A.C.; Borrion, A.L.; Griffiths, O.G.; Panoutsou, N.L.; Webb, J.S.; Kendrick, A.M. Use of LCA as a development tool within early research: Challenges and issues across different sectors. Int. J. Life Cycle Assess. 2014, 19, 130–143. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, G.; Tian, Y. The historical evolution and research trends of life cycle assessment. Green Carbon 2024, 2, 425–437. [Google Scholar] [CrossRef]
- Cucurachi, S.; van der Giesen, C.; Guinée, J. Ex-ante LCA of emerging technologies. Procedia CIRP 2018, 69, 463–468. [Google Scholar] [CrossRef]
- Tan, L.; Mandley, S.J.; Peijnenburg, W.; der Loop, S.L.W.-V.; Giesen, D.; Legradi, J.B.; Shen, L. Combining ex-ante LCA and EHS screening to assist green design: A case study of cellulose nanocrystal foam. J. Clean. Prod. 2018, 178, 494–506. [Google Scholar] [CrossRef]
- Georgiades, M.; Kilcan, C.Ö.; Giels, M.; Stewart, D.I.; Zhan, A.; Muñoz, J.F.; Vandepitte, N.; Dewulf, S. Ex-ante life cycle assessment of bauxite residue vitrification technology. Int. J. Life Cycle Assess. 2025, 30, 1896–1911. [Google Scholar] [CrossRef]
- Bergerson, A.; Brandt, A.; Cresko, J.; Carbajales-Dale, M.; MacLean, H.L.; Matthews, H.S.; McCoy, S.; McManus, M.; Miller, S.A.; Morrow, W.R.; et al. Life cycle assessment of emerging technologies: Evaluation techniques at different stages of market and technical maturity. J. Ind. Ecol. 2020, 24, 11–25. [Google Scholar] [CrossRef]
- Moni, S.M.; Mahmud, R.; High, K.; Carbajales-Dale, M. Life cycle assessment of emerging technologies: A review. J. Ind. Ecol. 2020, 24, 52–63. [Google Scholar] [CrossRef]
- Subal, L.; Braunschweig, A.; Hellweg, S. The relevance of life cycle assessment to decision-making in companies and public authorities. J. Clean. Prod. 2024, 435, 140520. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Das, B.B. Life Cycle Assessment of construction materials: Methodologies, applications and future directions for sustainable decision-making. Case Stud. Constr. Mater. 2023, 19, e02326. [Google Scholar] [CrossRef]
- Pryshlakivsky, J.; Searcy, C. Life Cycle Assessment as a decision-making tool: Practitioner and managerial considerations. J. Clean. Prod. 2021, 309, 127344. [Google Scholar] [CrossRef]
- European Commission. Recommendation on the Use of Common Methods to Measure and Communicate the Life Cycle Environmental Performance of Products and Organisations. (2013/179/EU). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013H0179 (accessed on 30 July 2025).
- Directive 2009/125/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for the Setting of Eco-Design Requirements for Energy-Related Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32009L0125 (accessed on 30 July 2025).
- ISO 14025:2006; Environmental Labels and Declarations—Type III Environmental Declarations—Principles and Procedures. International Organization for Standardization: Geneva, Switzerland, 2006.
- Farahzadi, L.; Tellnes, L.G.F.; Shafei, B.; Kioumarsi, M. Life-cycle environmental assessment of ultra-high-performance concrete with sustainable materials and fiber substitutions. Clean. Eng. Technol. 2024, 23, 100846. [Google Scholar] [CrossRef]
- Mazzi, A.; Sciarrone, M.; Bernardo, E. Environmental performance of glass foam as insulation material from waste glass with the alkali activation process. Heliyon 2023, 9, 19001. [Google Scholar] [CrossRef]
- Rodrigues, C.; König, J.; Freire, F. Prospective life cycle assessment of a novel building system with improved foam glass incorporating high recycled content. Sustain. Prod. Consum. 2023, 36, 161–170. [Google Scholar] [CrossRef]
- Nodehi, M.; Aguayo, F.; Madey, N.; Zhou, L. A comparative review of polymer, bacterial-based, and alkali-activated (also geopolymer) binders: Production, mechanical, durability, and environmental impacts life cycle assessment (LCA). Constr. Build. Mater. 2024, 422, 135816. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework, 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2006.
- EN 15804:2012+A2:2019; Sustainability of Construction Works—Environmental Product Declarations—Core Rules for the Product Category of Construction Products. European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- European Commission. Joint Research Centre. Institute for Environment and Sustainability, International Reference Life Cycle Data System (ILCD) Handbook: General Guide for Life Cycle Assessment: Detailed Guidance. LU: Publications Office, 2010. Available online: https://data.europa.eu/doi/10.2788/38479 (accessed on 15 May 2025).
- Yue, Y.; Tuheen, M.I.; Du, J. Borosilicate glasses. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 519–539. [Google Scholar] [CrossRef]
- Tameni, G.; Cammelli, F.; Elsayed, H.; Stangherlin, F.; Bernardo, E. Upcycling of Boro-Alumino-Silicate Pharmaceutical Glass in Sustainable Construction Materials. Detritus 2022, 20, 17–21. [Google Scholar] [CrossRef]
- Tameni, G.; Carollo, F.; Cavazzini, A.M.; Forzan, M.; Bernardo, E. Microwave assisted cold consolidation of alkali activated suspension of glass waste powders. Mater. Lett. 2025, 389, 138354. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, C.; Xie, R.; Yu, H.; Sun, M.; Li, F. Environmental assessment of fabric wet processing from gate-to-gate perspective: Comparative study of weaving and materials. Sci. Total Environ. 2023, 857, 159495. [Google Scholar] [CrossRef]
- Pražanová, A.; Fridrich, M.; Weinzettel, J.; Knap, V. Gate-to-gate life cycle assessment of lithium-ion battery recycling pre-treatment. Clean. Environ. Syst. 2025, 16, 100263. [Google Scholar] [CrossRef]
- Karadirek, I.E.; Erkaya, O.; Ciggin, A.S. Comparative life cycle assessment of sewage sludge drying by solar and thermal drying technologies. Waste Manag. 2025, 201, 114826. [Google Scholar] [CrossRef] [PubMed]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Rybaczewska-Błażejowska, M.; Jezierski, D. Comparison of ReCiPe 2016, ILCD 2011, CML-IA baseline and IMPACT 2002+ LCIA methods: A case study based on the electricity consumption mix in Europe. Int. J. Life Cycle Assess. 2024, 29, 1799–1817. [Google Scholar] [CrossRef]
| Elements | Process | Assumptions |
|---|---|---|
| Functional unit | A, B, C, D | 1 kg of glass foam produced |
| Function | A, B, C, D | Insulating material in construction sector |
| System boundaries | A, B, C, D | Gate-to-gate approach |
| Data sources | A, B, C, D | Primary data, Technical datasheet or Ecoinvent |
| Impact assessment method | A, B, C, D | ReCiPe 2016 v1.1 midpoint Egalitarian |
| SiO2 [wt%] | B2O3 [wt%] | Al2O3 [wt%] | CaO [wt%] | Na2O [wt%] | K2O [wt%] | BaO [wt%] | |
|---|---|---|---|---|---|---|---|
| BASG | 72 | 12 | 7 | 1 | 6 | 2 | <0.1 |
| Step | Process | Input |
|---|---|---|
| 1-arrival of waste | A, B, C, D A, B, C, D | Boro-alumino-silicate glass; 1 kg Road transport; 30 km |
| 2-grinding | A, B, C, D | Italian energy mix; 0.75 kWh |
| 3-sieving | A, B, C, D | Italian energy mix; 0.10 kWh |
| 4-alkaline activation | A, B, C, D A, B, C, D | Italian energy mix; 0.15 kWh NaOH and KOH; 0.5 kg |
| 5-consolidation | A, B, C, D | Italian energy mix, 1.60 kWh |
| 6-foaming | A, B, C, D A, B, C, D | Italian energy mix, 0.008 kWh C14H22O(C2H4O)n, n = 9–10; 0.04 kg NaBO3 ·H2O and CH3(CH2)11OSO3Na; 0.01 kg |
| 7-polymerization | A, B, D C | Italian energy mix; 19.2 kWh Italian energy mix; 134.4 kWh |
| 8-heat treatment | A, B | Italian energy mix; 1.50 kWh |
| 9-microwave | D | Italian energy mix; 0.037 kWh |
| Process A | Process B | Process C | Process D | |||||
|---|---|---|---|---|---|---|---|---|
| Mix | PV | Mix | PV | Mix | PV | Mix | PV | |
| GWP | 17.2719 | 3.5211 | 17.824 | 3.5843 | 100 | 20.3544 | 16.767 | 3.3686 |
| SOD | 16.776 | 2.6563 | 17.332 | 2.6887 | 100 | 15.3279 | 16.2686 | 2.5261 |
| IR | 16.044 | 2.3608 | 16.6579 | 2.4366 | 100 | 13.8092 | 15.5858 | 2.2903 |
| OFHH | 17.2646 | 6.178 | 17.7544 | 6.2728 | 100 | 35.6697 | 16.6965 | 5.8947 |
| FPMF | 16.8444 | 9.4965 | 17.3918 | 9.7759 | 100 | 56.5377 | 16.3292 | 9.1744 |
| OFTE | 17.263 | 6.027 | 17.7529 | 6.1165 | 100 | 34.784 | 16.695 | 5.7477 |
| TA | 16.8444 | 7.6399 | 17.4114 | 7.8709 | 100 | 45.5077 | 16.349 | 7.3868 |
| FE | 17.1324 | 14.9438 | 17.7158 | 15.4624 | 100 | 89.0582 | 16.6574 | 14.5157 |
| ME | 17.4473 | 11.9418 | 18.0169 | 12.183 | 100 | 68.4856 | 16.9623 | 11.5959 |
| TET | 11.7015 | 16.837 | 11.8814 | 17.218 | 67.768 | 100 | 11.1626 | 16.1533 |
| FET | 4.1011 | 16.4889 | 4.2329 | 17.0897 | 24.4053 | 100 | 3.9735 | 16.0233 |
| MET | 7.1044 | 16.6557 | 7.2897 | 17.2074 | 41.0687 | 100 | 6.8552 | 16.1425 |
| HCT | 16.11 | 16.8113 | 16.5735 | 17.3087 | 94.7238 | 100 | 15.5683 | 16.245 |
| HNCT | 7.397 | 16.6655 | 7.5917 | 17.2169 | 42.6434 | 100 | 7.1409 | 16.1521 |
| LU | 16.3363 | 5.1039 | 16.7882 | 5.113 | 100 | 29.0885 | 15.7179 | 4.8046 |
| MRS | 6.9983 | 16.6622 | 7.2039 | 17.2343 | 40.9521 | 100 | 6.7698 | 16.1697 |
| FRS | 17.26 | 2.7176 | 17.8134 | 2.7526 | 100 | 15.5826 | 16.7562 | 2.5875 |
| WC | 16.2135 | 6.2732 | 16.828 | 6.497 | 100 | 37.3995 | 15.7582 | 6.0996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battiston, E.; Carollo, F.; Tameni, G.; Bernardo, E.; Mazzi, A. Life Cycle Assessment of Industrial Glass Cullet Recycling Process Based on Alkaline Activation. Ceramics 2025, 8, 109. https://doi.org/10.3390/ceramics8030109
Battiston E, Carollo F, Tameni G, Bernardo E, Mazzi A. Life Cycle Assessment of Industrial Glass Cullet Recycling Process Based on Alkaline Activation. Ceramics. 2025; 8(3):109. https://doi.org/10.3390/ceramics8030109
Chicago/Turabian StyleBattiston, Elena, Francesco Carollo, Giulia Tameni, Enrico Bernardo, and Anna Mazzi. 2025. "Life Cycle Assessment of Industrial Glass Cullet Recycling Process Based on Alkaline Activation" Ceramics 8, no. 3: 109. https://doi.org/10.3390/ceramics8030109
APA StyleBattiston, E., Carollo, F., Tameni, G., Bernardo, E., & Mazzi, A. (2025). Life Cycle Assessment of Industrial Glass Cullet Recycling Process Based on Alkaline Activation. Ceramics, 8(3), 109. https://doi.org/10.3390/ceramics8030109









