Synthesis of Magnesia–Hercynite-Based Refractories from Mill Scale and Secondary Aluminum Dross: Implication for Recycling Metallurgical Wastes
Abstract
1. Introduction
2. Materials and Methods
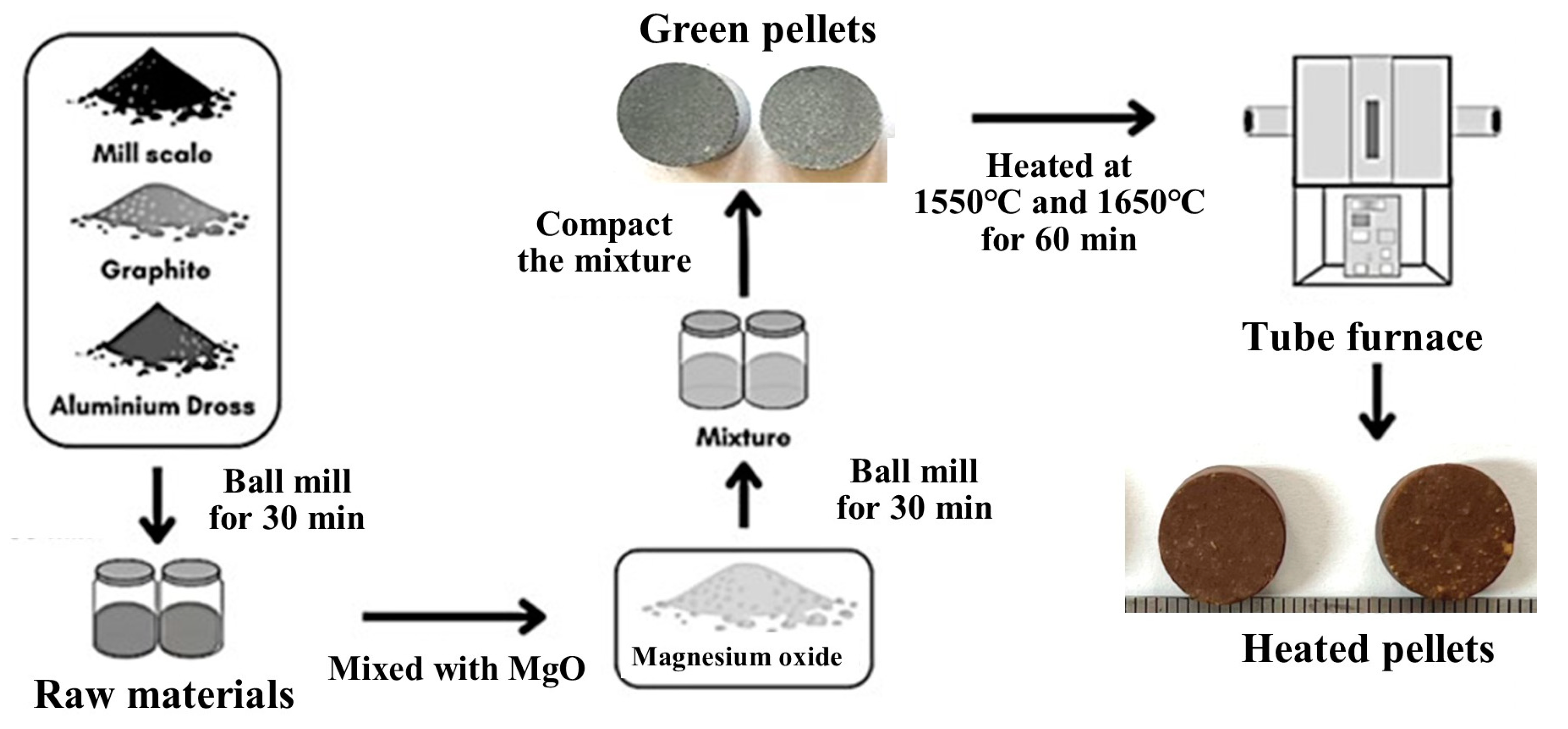
2.1. Materials Preparation
2.2. Material Synthesis
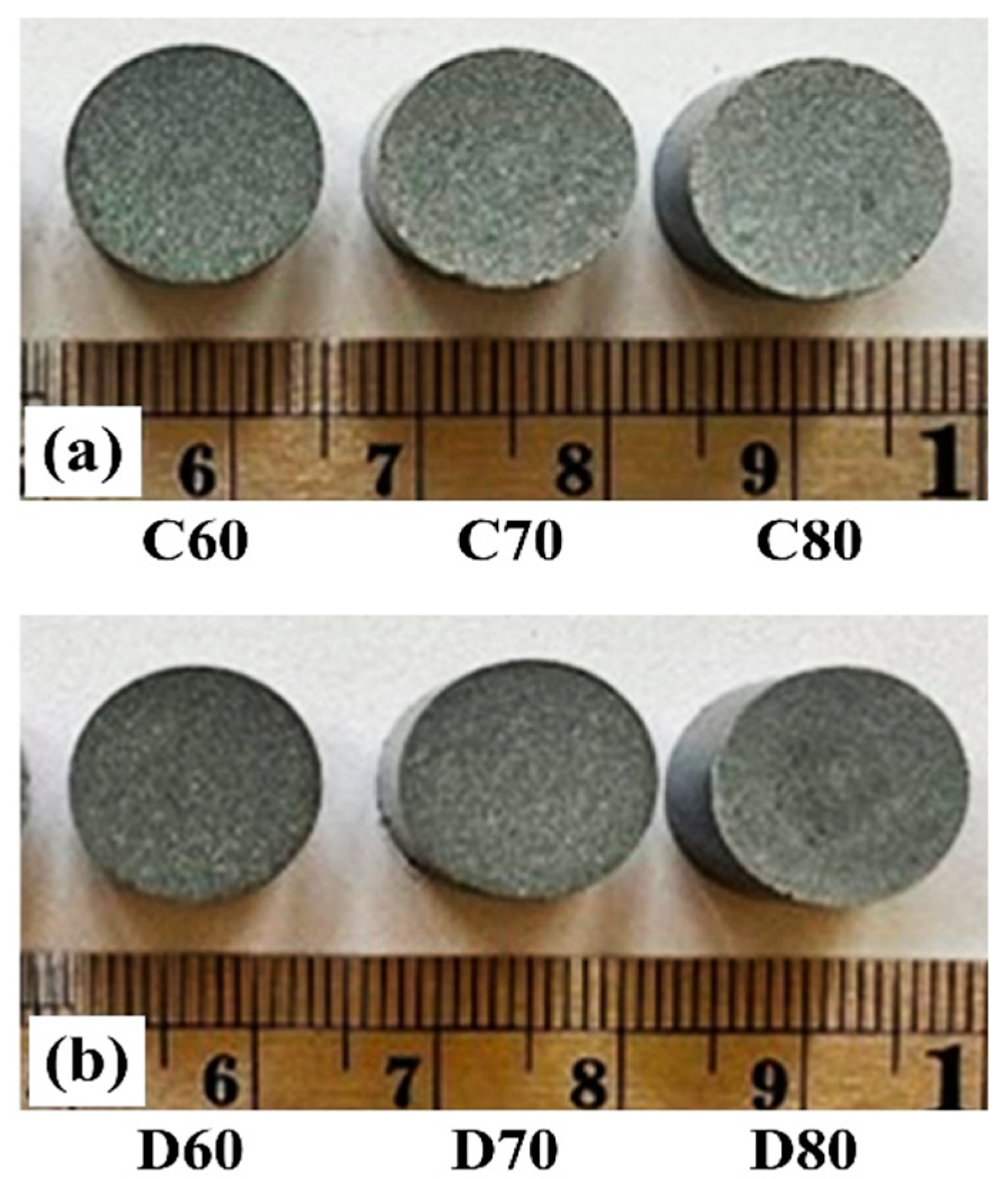
2.3. Formulation of the Reacting Raw Materials
2.4. Material Analysis
3. Results and Discussion
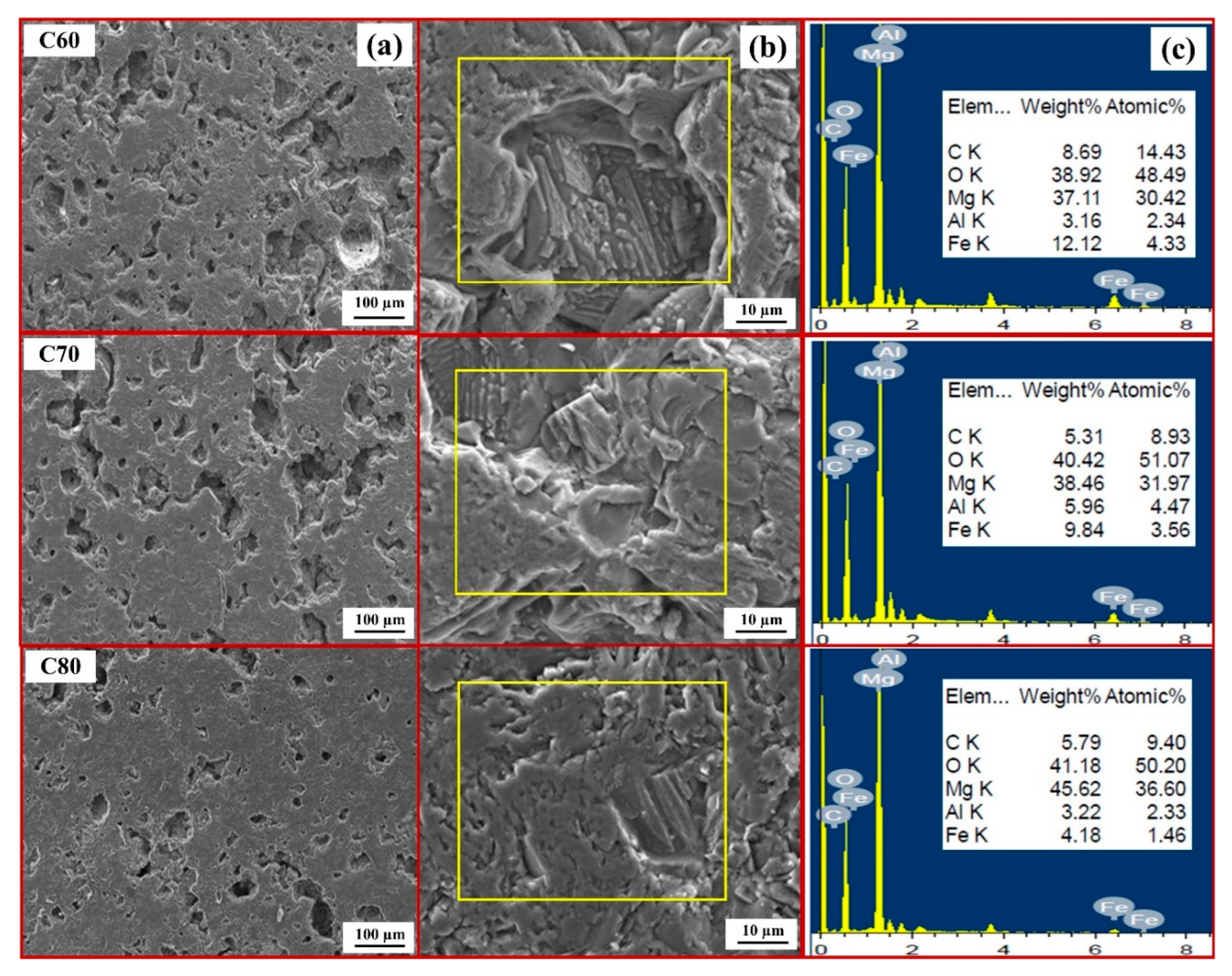
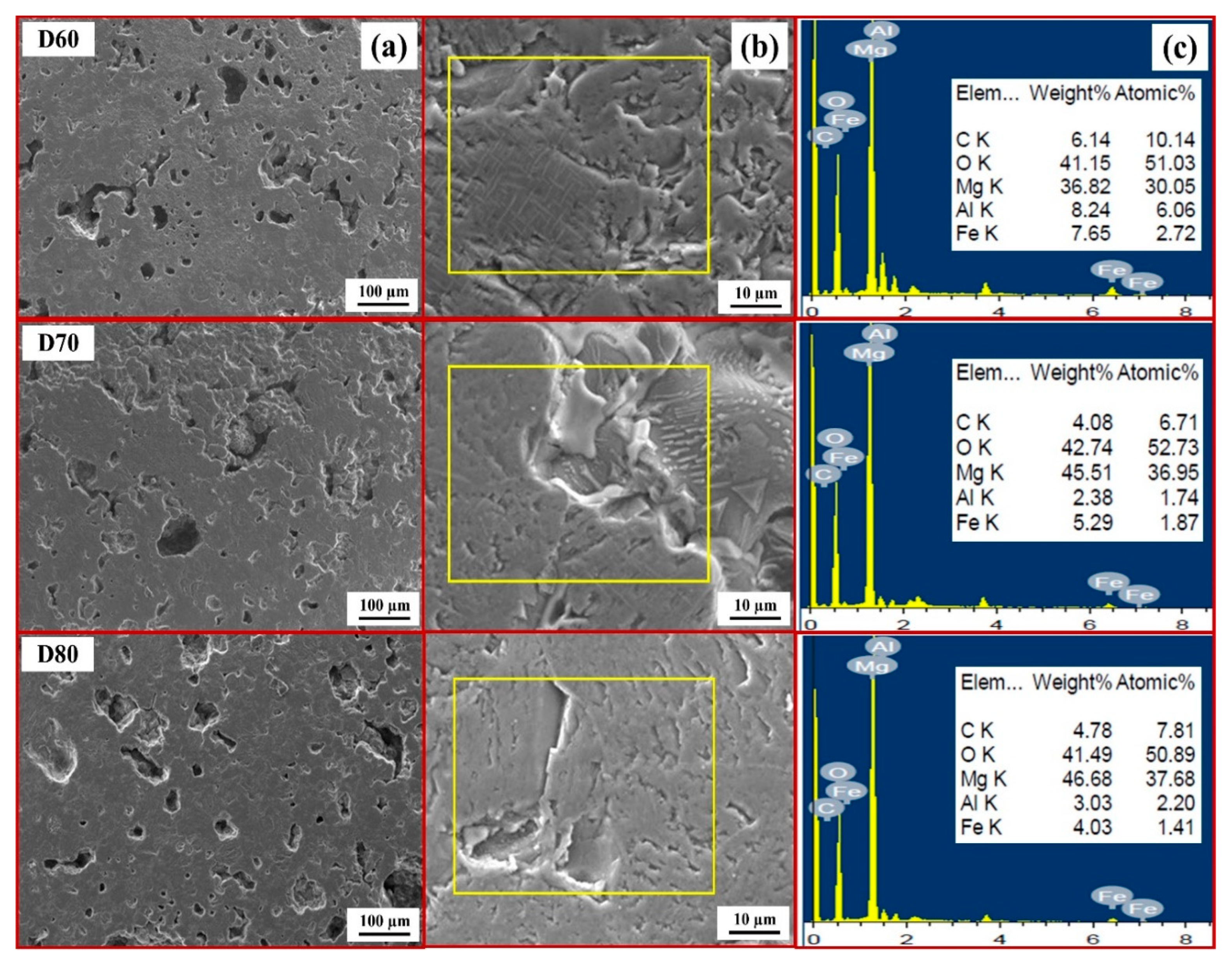
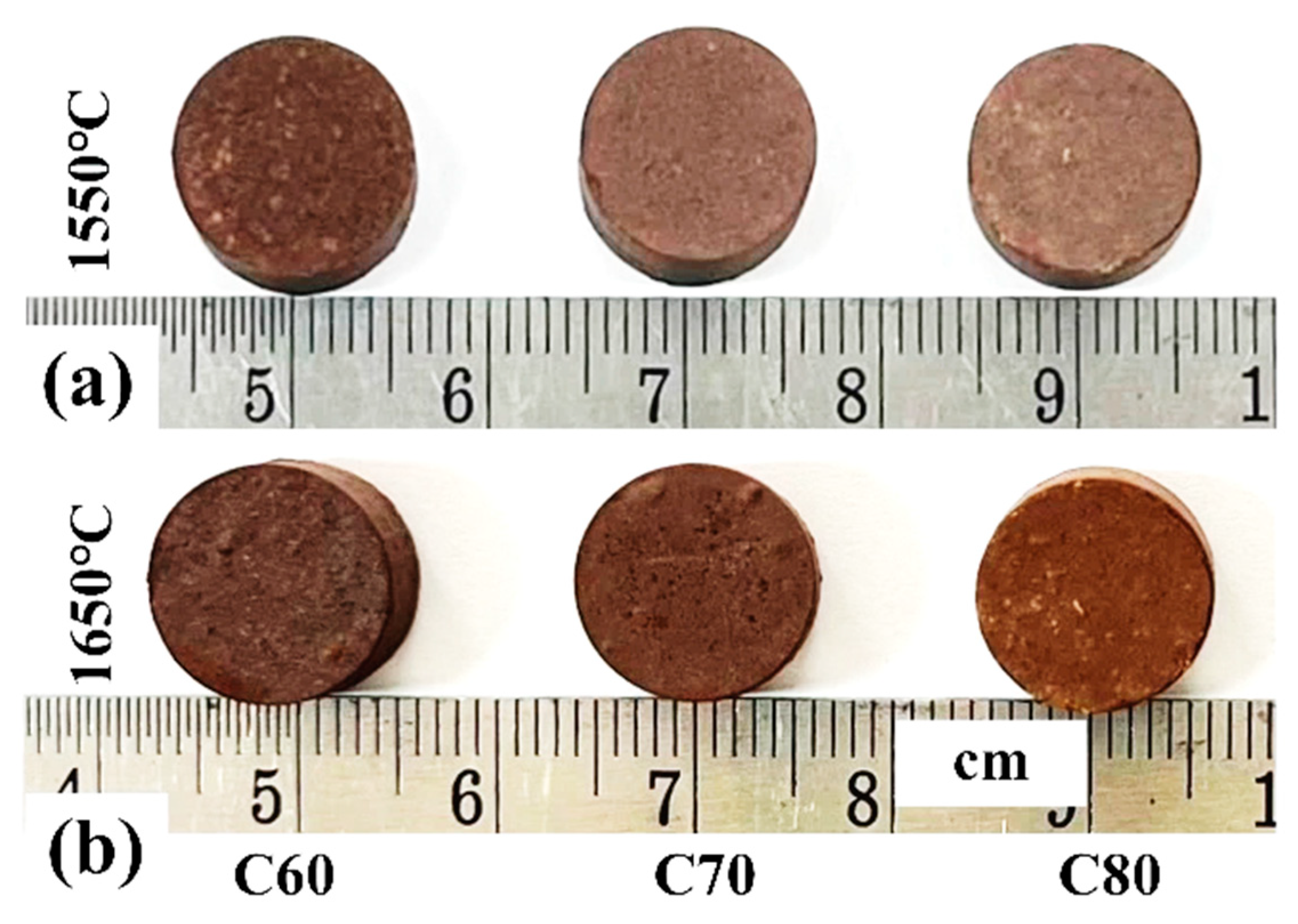
3.1. Effect of Carbon
3.2. Effect of Temperature
3.3. Evaluation of the Magnesia–Hercynite Ceramic Produced from Commercial and Waste Resources
4. Conclusions
- Magnesia–hercynite-based refractories can be successfully synthesized from MgO, MS, AD, and graphite via reactions at 1550 °C and 1650 °C for 1 h in a normal air atmosphere. The single-step blending of raw materials was employed for this process.
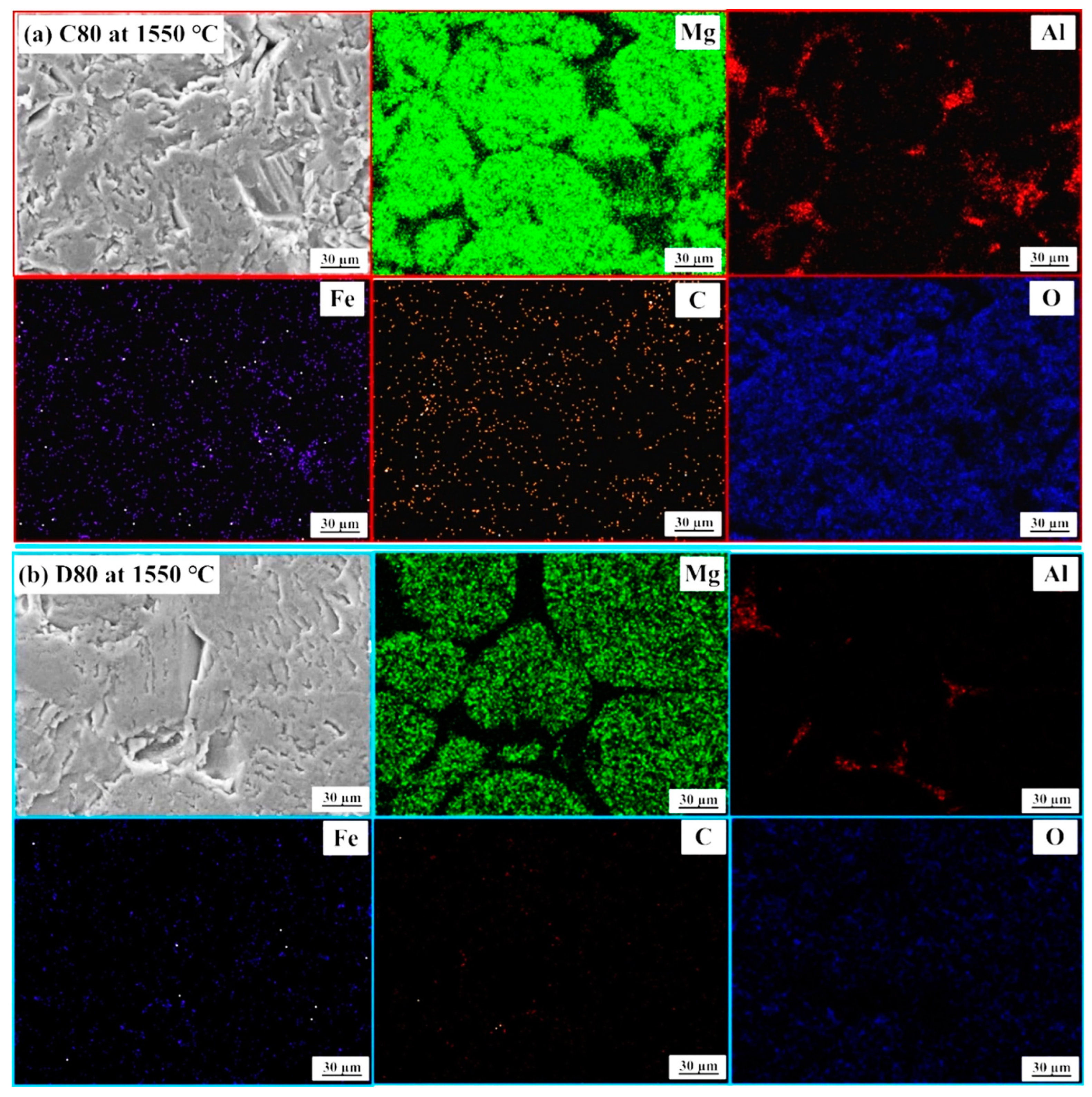
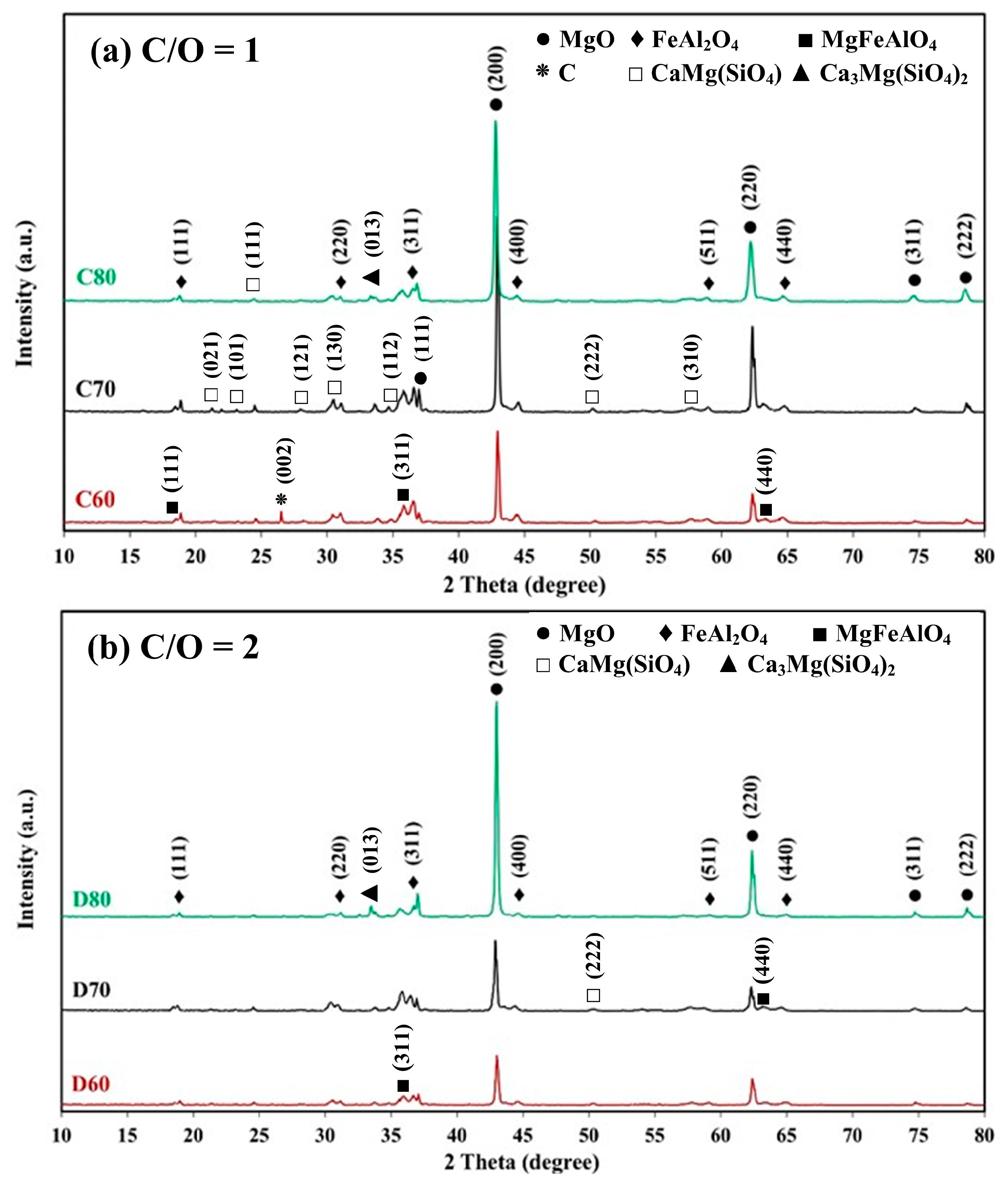
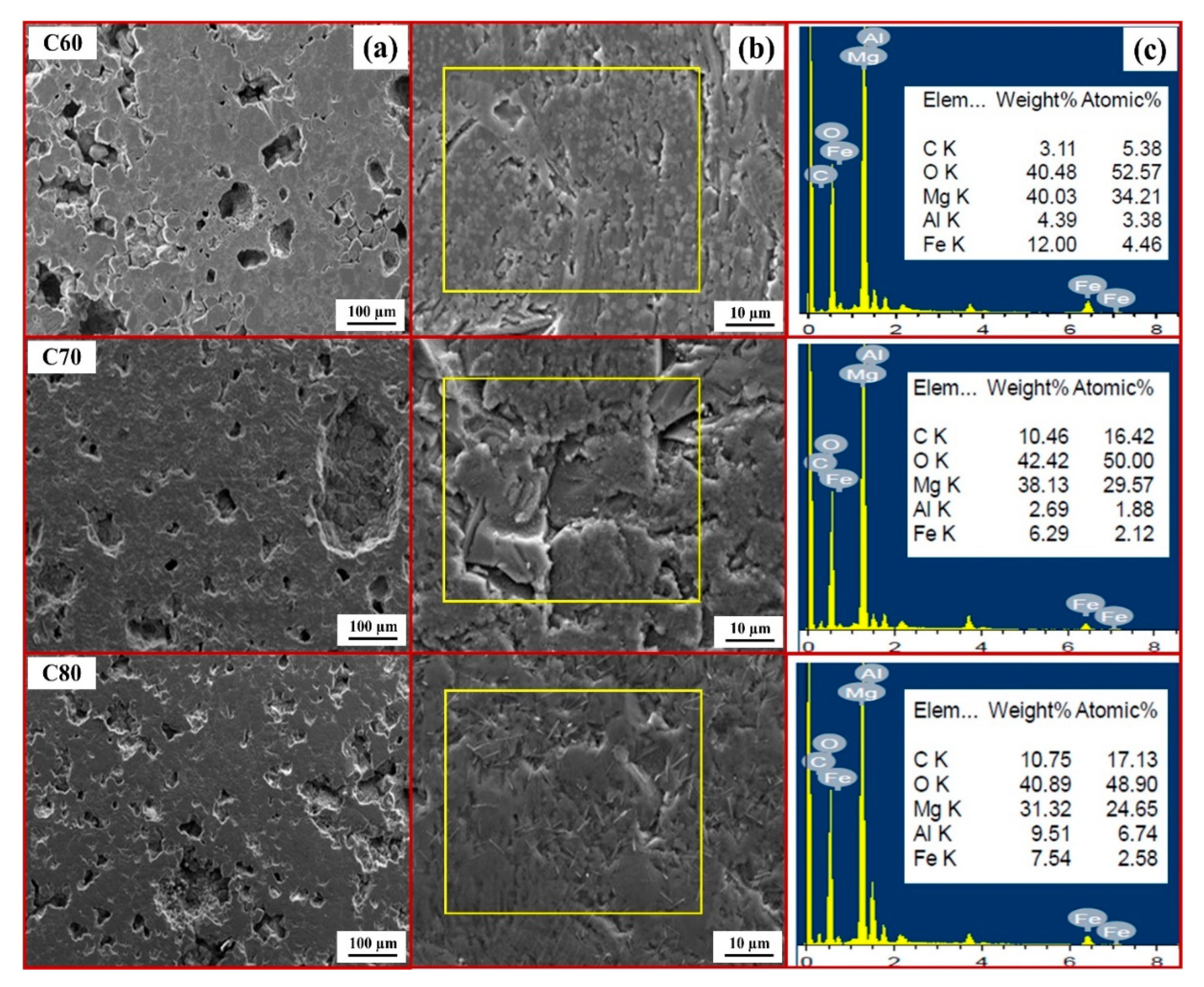
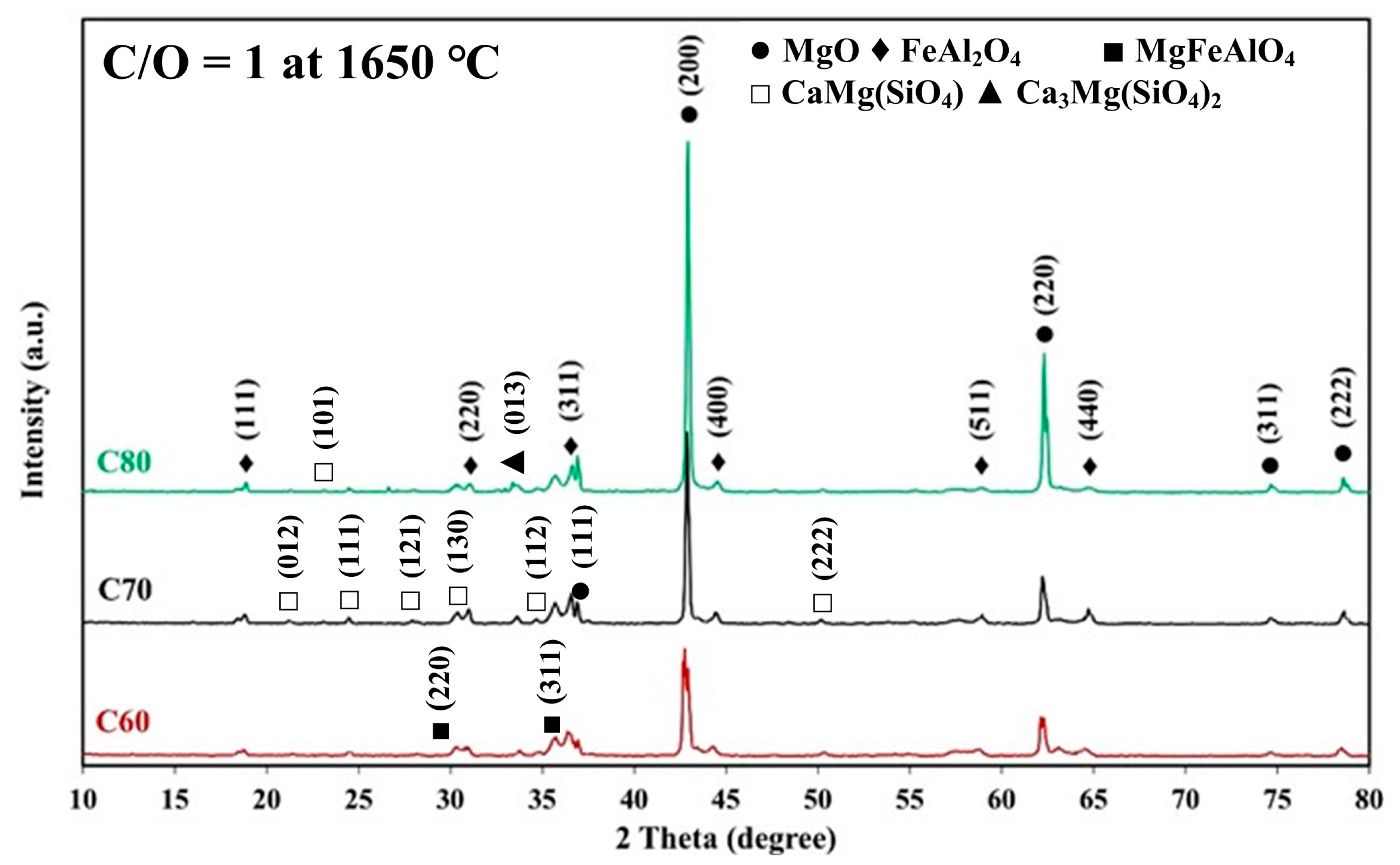
- The combination of blend samples with high magnesia content (C80 and D80) and high levels of impurity oxides (CaO and SiO2) leads to variations in the chemical composition and the formation of distinct phases in the resulting ceramic. The phases present in the resulting ceramic include MgO, FeAl2O4, MgFeAlO4, CaMg(SiO4), and Ca3Mg(SiO4)2. These phases, in turn, affect the microstructure, as well as the physical and mechanical properties of the produced magnesia–hercynite-based refractory.
- The produced magnesia–hercynite-based refractories consist of equiaxed MgO grains and an FeO-Al2O3 spinel phase at the boundaries. The formation of MgFeAlO4, CaMg(SiO4), and Ca3Mg(SiO4)2 is anticipated to occur throughout the bulk ceramic, but it is primarily concentrated at the red regions or boundaries of the MgO grains.
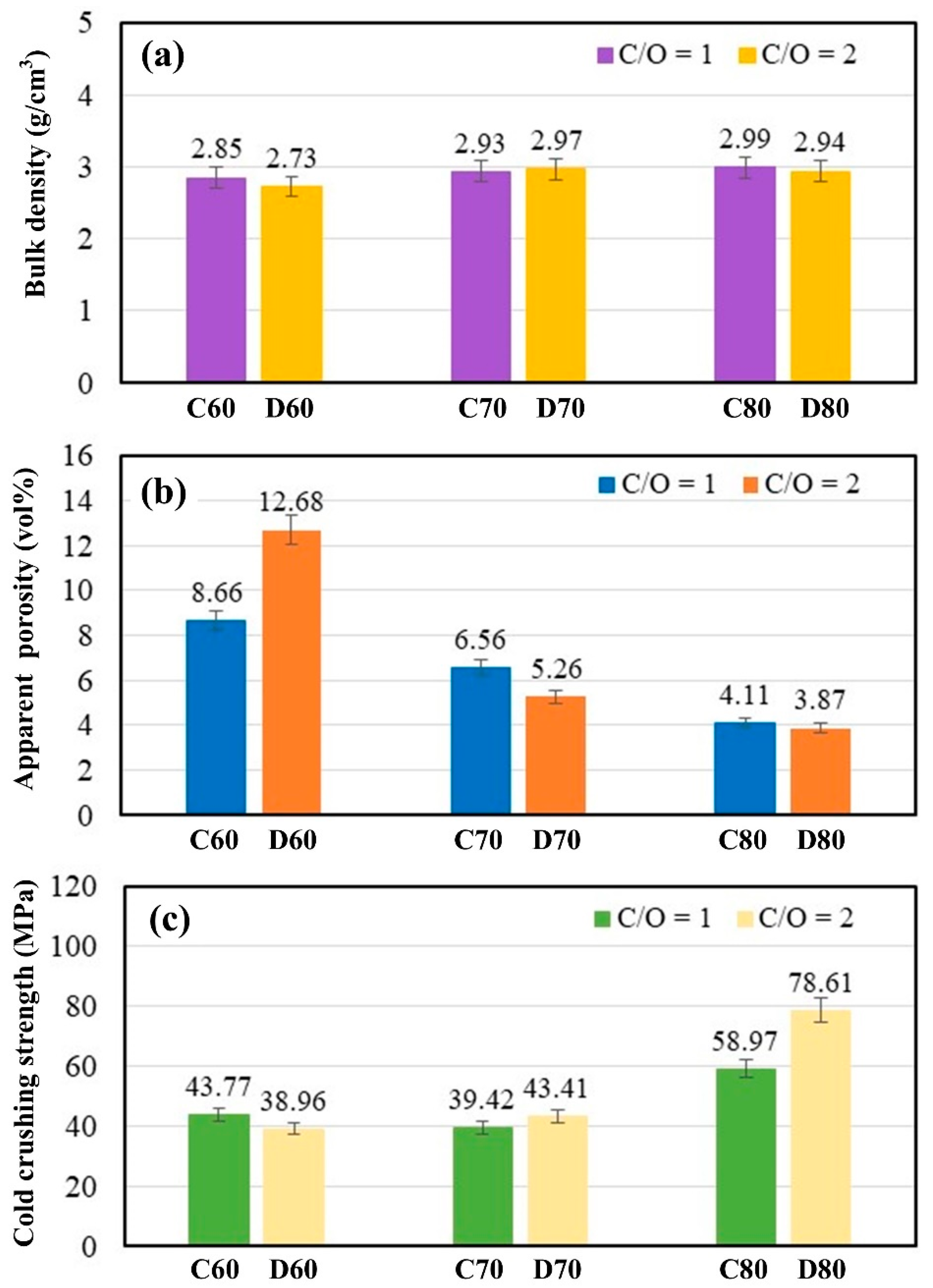
- Varying the carbon content (C/O ratios of 1 and 2) has a minimal impact on the bulk density and apparent porosity of the resulting ceramic. Increasing the magnesia powder content (60–80 wt%) does not significantly affect bulk density but tends to reduce apparent porosity. A higher MgO content combined with increased carbon concentration (C/O = 2) leads to a more notable increase in crushing strength compared with a C/O ratio of 1. At 1550 °C, the pellets with the highest MgO content (C80 and D80) exhibit the greatest cold crushing strength.
- Increasing the heating temperature from 1550 °C to 1650 °C results in a slight reduction in bulk density and a significant decrease in apparent porosity, enhancing the ceramic’s densification and compactness. This leads to a notable improvement in mechanical properties, with cold crushing strength nearly doubling from 43.77–58.97 MPa at 1550 °C to 76.79–95.67 MPa at 1650 °C.
- Blends with high magnesia content (C80 and D80) show optimal conditions for synthesizing magnesia–hercynite-based refractories from the magnesia–dross–scale–graphite system, exhibiting physical and mechanical properties comparable to commercial magnesia–hercynite bricks. However, the quantity and type of phases in the ceramic differ from those in commercial bricks, indicating that further investigation of the ceramic’s large-scale and thermal properties is necessary for potential application in industrial rotary kilns.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.Q. Technical Progress in Basic Refractories for Cement Rotary Kiln. J. Tech. Assoc. Refract. 2003, 23, 218–225. [Google Scholar]
- Guo, Z.; Nievoll, J. An Overview of Magnesia-Hercynite Refractories for Cement Rotary Kilns. China’s Refract. 2007, 16, 65–71. [Google Scholar]
- Zhang, X.; Yu, R.; Yu, X. Characteristics of hercynite and its application: In refractories. China’s Refract. 2012, 21, 17–22. [Google Scholar]
- Padhi, L.; Sahu, P.; Sahoo, N.; Singh, S.; Tripathy, J. A Novel Process for Synthesis of Iron-Alumina Spinel and Its Application in Refractory for Cement Rotary Kiln. Trans. Indian Ceram. Soc. 2017, 76, 196–201. [Google Scholar] [CrossRef]
- Chen, J.; Su, J.; Yan, M.; Yang, S. Two methods of synthesizing hercynite. Appl. Mech. Mater. 2014, 543–547, 3830–3833. [Google Scholar] [CrossRef]
- Chen, J.; Yu, L.; Sun, J.; Li, Y.; Xue, W. Synthesis of hercynite by reaction sintering. Eur. Ceram. Soc. 2011, 31, 259–263. [Google Scholar] [CrossRef]
- Perdomo-Gonzales, L.; Quintana-Puchol, R.; Alujas-Diaz, A.; Perdomo-Gomez, L.A.; Ruiz-Perez, R.; Cruz-Crespo, A. Aluminothermic synthesis of ceramics from the hercynite-alumina system, using mill scale and aluminium Chips. DYNA 2023, 90, 106–113. [Google Scholar] [CrossRef]
- Rodríguez, E.; Castillo, G.; Contreras, J.; Puente-Ornelas, R.; Aguilar-Martínez, J.; García, L.; Gómez, C. Hercynite and magnesium aluminate spinels acting as a ceramic bonding in an electro fused MgO–CaZrO3 refractory brick for the cement industry. Ceram. Int. 2012, 38, 6769–6775. [Google Scholar] [CrossRef]
- Khlifi, I.; Pop, O.; Dupré, J.; Doumalin, P.; Huger, M. Investigation of microstructure-property relationships of magnesia-hercynite refractory composites by a refined digital image correlation technique. J. Eur. Ceram. Soc. 2019, 39, 3893–3902. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Ma, S.; Xia, W.; Li, Y. Effects of Atmosphere on the Periclase-Hercynite Brick. Adv. Mater. Res. 2012, 476–478, 1523–1528. [Google Scholar] [CrossRef]
- Eissa, M.; Ahmed, A.; El-Fawkhry, M. Conversion of mill scale waste into valuable products via carbothermic reduction. J. Metall. 2015, 2015, 926028. [Google Scholar] [CrossRef][Green Version]
- Bagatini, M.C.; Zymla, V.; Osorio, E.; Vilela, A.C.F. Characterization and reduction behavior of mill scale. ISIJ Int. 2011, 51, 1072. [Google Scholar] [CrossRef]
- Sen, R.; Dehiya, S.; Pandel, U.; Banerjee, M.K. Utilization of low-grade coal for direct reduction of mill scale to obtain sponge iron: Effect of reduction time and particle size. Procedia Earth Planet. Sci. 2015, 11, 8–14. [Google Scholar] [CrossRef]
- Martin, M.I.; Lopez, F.A.; Torralba, J.M. Production of sponge iron powder by reduction of rolling mill scale. Ironmak. Steelmak. 2012, 39, 155–162. [Google Scholar] [CrossRef]
- Baganiti, M.C.; Kan, T.; Evans, T.J.; Strezov, V. Iron ore reduction by biomass volatiles. J. Sustain. Metall. 2021, 7, 215–226. [Google Scholar]
- Khaerudini, D.S.; Chanif, I.; Insiyanda, D.R.; Destyorini, F.; Alva, S.; Premono, A. Preparation and characterization of mill scale industrial waste reduced by biomass-based carbon. J. Sustain. Metall. 2019, 5, 510–518. [Google Scholar] [CrossRef]
- Sista, K.S.; Dwarapudi, S.; Nerune, V.P. Direct reduction recycling of mill scale through iron powder synthesis. ISIJ Int. 2019, 5, 787–794. [Google Scholar] [CrossRef]
- Shi, J.; Wang, D.R.; He, Y.D.; Qi, H.B.; Wei, G. Reduction of oxide scale on hot-rolled strip steels by carbon monoxide. Mater. Lett. 2018, 62, 3500–3502. [Google Scholar]
- Ye, Q.; Zhu, H.; Zhang, L.; Ma, J.; Zhou, L.; Liu, P.; Chen, J.; Chen, G.; Peng, J. Preparation of reduced iron powder using combined distribution of wood-charcoal by microwave heating. J. Alloys Compd. 2014, 613, 102–106. [Google Scholar] [CrossRef]
- Xie, H.; Guo, Z.; Xu, R.; Zhang, Y. Particle sorting to improve the removal of fluoride and aluminum nitride from secondary aluminum dross by roasting. Environ. Sci. Pollut. Res. 2023, 30, 54536–54546. [Google Scholar] [CrossRef]
- Lin, W.C.; Tsai, C.H.; Zhang, D.N.; Syu, S.S.; Kuo, Y.M. Recycling of aluminum dross for producing calcinated alumina by microwave plasma. Sustain. Environ. Res. 2022, 32, 50. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, H.; Xiang, Z.; Zhang, H.; He, Q.; Li, J. Effect of hercynite content on the properties of magnesia-spinel composite refractories sintered in different atmospheres. Ceram. Int. 2016, 42, 19058–19062. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, J.; Yan, M.; Li, B.; Su, J.; Hou, X. Morphology characterization of periclase- hercynite refractories by reaction sintering. Int. J. Miner. Metall. Mater. 2015, 22, 1219–1224. [Google Scholar] [CrossRef]
- Wongsawan, P.; Srichaisiriwech, W.; Kongkarat, S. Synthesis of Ferroalloys via Mill Scale-Dross-Graphite Interaction: Implication for Industrial Wastes Upcycling. Metals 2022, 12, 1909. [Google Scholar] [CrossRef]
- Huang, W.M.; Hillert, M.; Wang, X. Thermodynamic assessment of the CaO-MgO-SiO2 System. Metall. Mater. Trans. A 1995, 26, 2293. [Google Scholar] [CrossRef]
- Shankar, A.; Görnerup, M.; Lahiri, A.K.; Seetharaman, S. Experimental Investigation of the Viscosities in CaO-SiO2-MgO-Al2O3 and CaO-SiO2-MgO-Al2O3-TiO2 Slags. Metall. Mater. Trans. B 2007, 38, 911. [Google Scholar] [CrossRef]



| Oxides (wt%) | |||
|---|---|---|---|
| MgO | SiO2 | CaO | Fe2O3 |
| 92.87 | 3.62 | 2.56 | 0.95 |
| Oxides (wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | Fe2O3 | CaO | K2O | MgO | MnO | Na2O | SO3 | CuO | TiO2 | ZnO | Others |
| 69.94 | 5.01 | 0.54 | 1.0 | 0.76 | 4.91 | 0.15 | 10.65 | 2.46 | 0.37 | 0.17 | 0.25 | 3.79 |
| Oxides (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | Al2O3 | CaO | SO3 | TiO2 | K2O | P2O5 | other |
| 93.66 | 1.42 | 0.82 | 0.17 | 0.08 | 0.04 | 0.02 | 0.04 | 3.75 |
| Blends | AD (wt%) | MS (wt%) | Graphite (wt%) | MgO (wt%) | Total (wt%) |
|---|---|---|---|---|---|
| C60 | 21.00 | 15.68 | 3.32 | 60 | 100 |
| C70 | 15.75 | 11.76 | 2.49 | 70 | 100 |
| C80 | 10.50 | 7.84 | 1.66 | 80 | 100 |
| D60 | 19.40 | 14.48 | 6.12 | 60 | 100 |
| D70 | 14.55 | 10.86 | 4.59 | 70 | 100 |
| D80 | 9.70 | 7.24 | 3.06 | 80 | 100 |
| Pellet | Oxides (wt%) | |||||
|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | MgO | SiO2 | CaO | Other | |
| C60 | 25.52 | 12.50 | 51.16 | 5.93 | 3.44 | 1.45 |
| C70 | 20.88 | 11.57 | 55.59 | 6.14 | 4.52 | 1.30 |
| C80 | 15.29 | 9.04 | 64.60 | 5.37 | 4.88 | 0.82 |
| D60 | 33.28 | 9.35 | 43.10 | 6.58 | 6.16 | 1.53 |
| D70 | 27.81 | 11.30 | 49.85 | 6.15 | 3.71 | 1.18 |
| D80 | 10.04 | 7.70 | 69.39 | 7.32 | 4.60 | 0.95 |
| Pellet | Oxides (wt%) | |||||
|---|---|---|---|---|---|---|
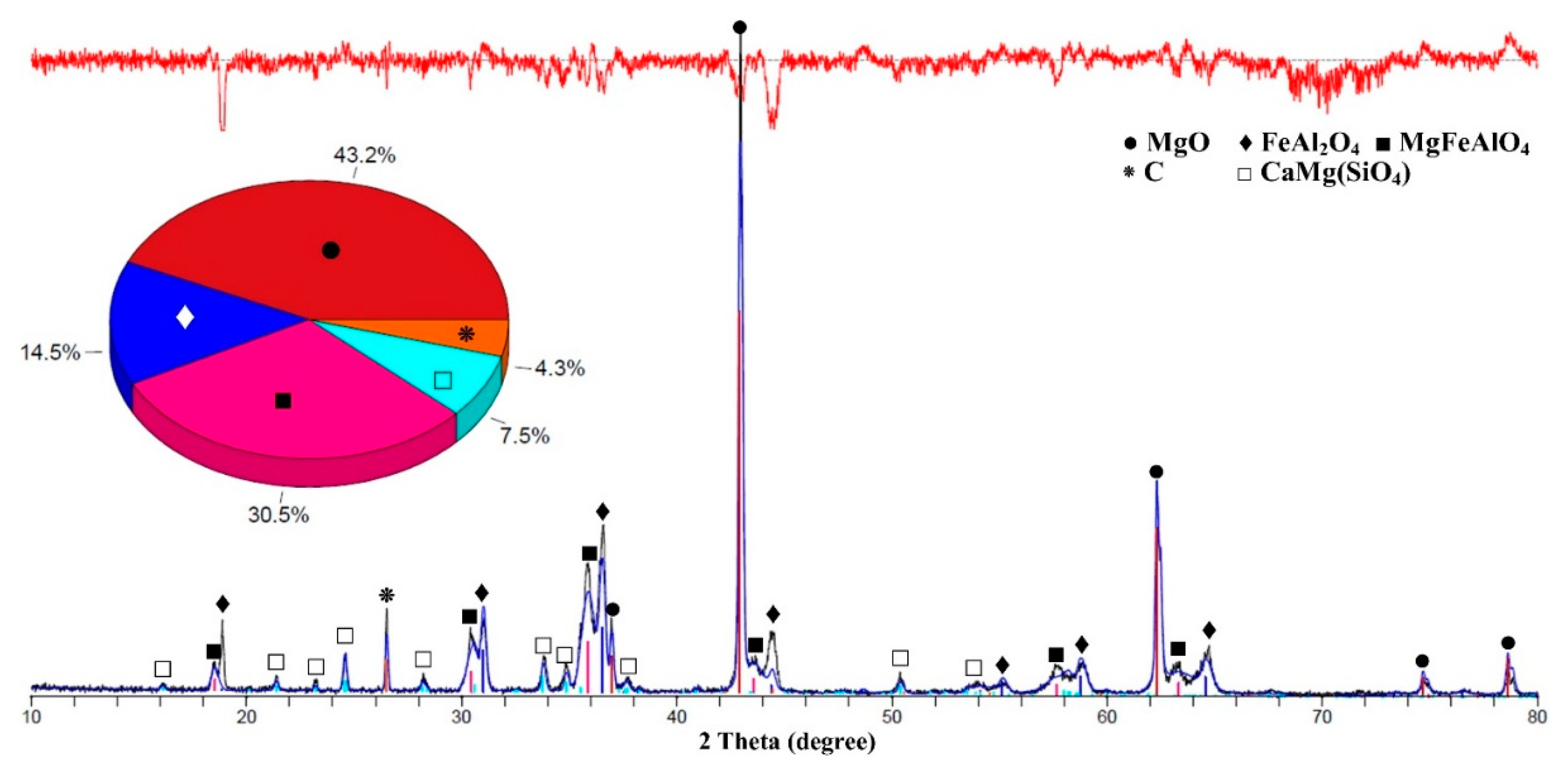
| MgO | FeAl2O4 | MgFeAlO4 | CaMg(SiO4) | Ca3Mg(SiO4)2 | C | |
| C60 | 43.2 | 14.5 | 30.5 | 7.5 | - | 4.3 |
| C70 | 60.7 | 9.0 | 24.1 | 6.2 | - | - |
| C80 | 73.3 | 5.7 | 14.4 | 2.8 | 3.8 | - |
| D60 | 54.4 | 12.0 | 25.6 | 8 | - | - |
| D70 | 47.3 | 15.2 | 28.8 | 8.2 | - | - |
| D80 | 76.2 | 5.2 | 9.6 | 2.1 | 6.9 | - |
| Pellet | Oxides (wt%) | |||||
|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | MgO | SiO2 | CaO | Other | |
| C60 | 26.33 | 13.67 | 46.21 | 8.12 | 4.12 | 1.55 |
| C70 | 20.76 | 12.82 | 51.06 | 8.58 | 5.22 | 1.56 |
| C80 | 14.54 | 10.89 | 60.32 | 7.99 | 5.33 | 0.93 |
| Pellet | Oxides (wt%) | |||||
|---|---|---|---|---|---|---|
| MgO | FeAl2O4 | MgFeAlO4 | CaMg(SiO4) | Ca3Mg(SiO4)2 | C | |
| C60 | 54.6 | 8.9 | 29.0 | 7.4 | - | - |
| C70 | 58.1 | 14.7 | 19.9 | 7.3 | - | - |
| C80 | 77.9 | 7.8 | 12.9 | 3.0 | 3.6 | 0.7 |
| Refractory Sample | Commercial Resources | Present Study | |||
|---|---|---|---|---|---|
| RFC#1 [2] | RFC#2 [4] | C80 at 1550 °C | D80 at 1550 °C | C80 at 1650 °C | |
| Raw materials | |||||
| Magnesia | China | India | Thailand magnesia | ||
| Hercynite | Commercial electro-fused | Mill scale and aluminum dross combustion | |||
| Chemical composition (wt%) | |||||
| MgO | 85.0 | 87.8 | 64.6 | 69.93 | 60.32 |
| Fe2O3 | 3.8 | 4.8 | 15.29 | 10.04 | 14.54 |
| Al2O3 | 3.4 | 4.89 | 9.04 | 7.7 | 10.89 |
| CaO | 0.7 | 1.25 | 4.88 | 4.6 | 5.33 |
| SiO2 | 0.3 | 0.6 | 5.37 | 7.32 | 7.99 |
| Basic physical properties | |||||
| Bulk density (g/cm3) | 3.06 | 2.97 | 2.99 | 2.94 | 2.81 |
| Apparent porosity (vol%) | 14.0 | 16.5 | 4.11 | 3.87 | 1.58 |
| Cold crushing strength (MPa) | 70 | 57.3 | 58.97 | 78.61 | 95.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongsawan, P.; Boonlom, N.; Vantar, M.; Kongkarat, S. Synthesis of Magnesia–Hercynite-Based Refractories from Mill Scale and Secondary Aluminum Dross: Implication for Recycling Metallurgical Wastes. Ceramics 2024, 7, 1440-1458. https://doi.org/10.3390/ceramics7040093
Wongsawan P, Boonlom N, Vantar M, Kongkarat S. Synthesis of Magnesia–Hercynite-Based Refractories from Mill Scale and Secondary Aluminum Dross: Implication for Recycling Metallurgical Wastes. Ceramics. 2024; 7(4):1440-1458. https://doi.org/10.3390/ceramics7040093
Chicago/Turabian StyleWongsawan, Praphaphan, Nantiya Boonlom, Muenfahn Vantar, and Somyote Kongkarat. 2024. "Synthesis of Magnesia–Hercynite-Based Refractories from Mill Scale and Secondary Aluminum Dross: Implication for Recycling Metallurgical Wastes" Ceramics 7, no. 4: 1440-1458. https://doi.org/10.3390/ceramics7040093
APA StyleWongsawan, P., Boonlom, N., Vantar, M., & Kongkarat, S. (2024). Synthesis of Magnesia–Hercynite-Based Refractories from Mill Scale and Secondary Aluminum Dross: Implication for Recycling Metallurgical Wastes. Ceramics, 7(4), 1440-1458. https://doi.org/10.3390/ceramics7040093










