Fabrication and Characterization of Narrow-Wavelength Phosphors of Tb-Doped Yttrium-Silicon-Aluminum Oxynitride Using Spray Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Y3.92SiAlO8N:0.08Tb3+ Phosphors Powders
2.2. Characterization of Y3.92SiAlO8N:0.08Tb3+ Phosphors Powders
3. Results
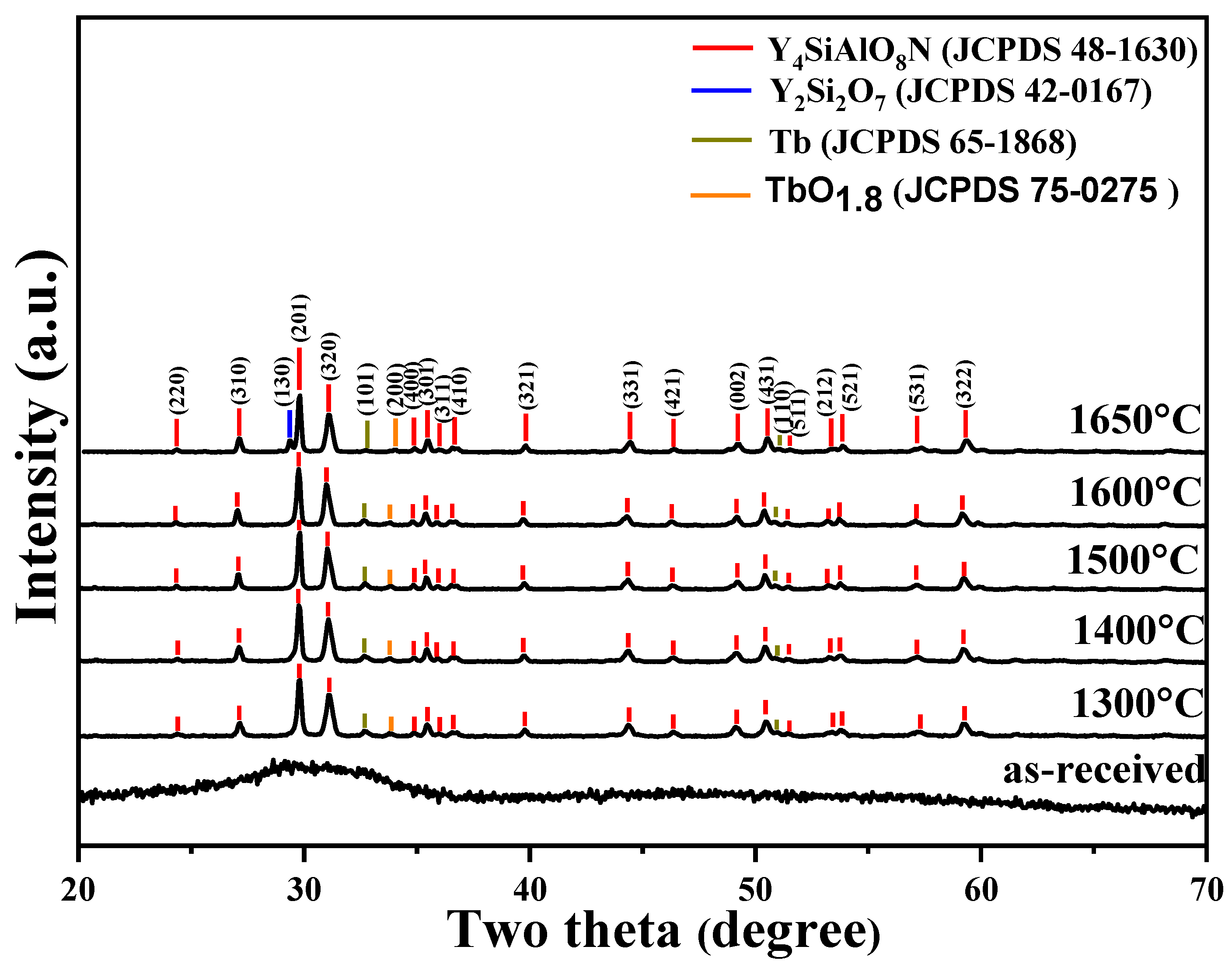
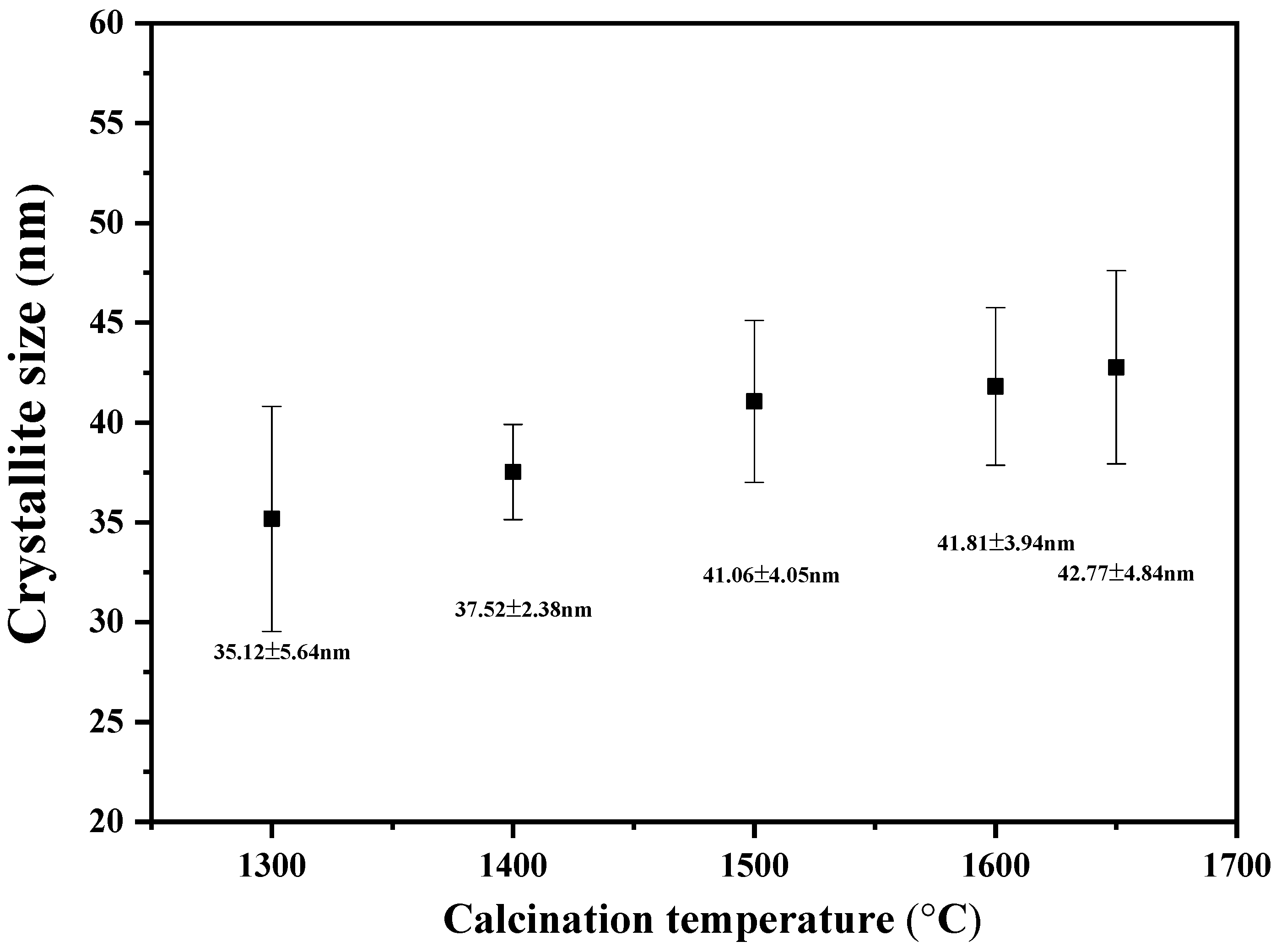
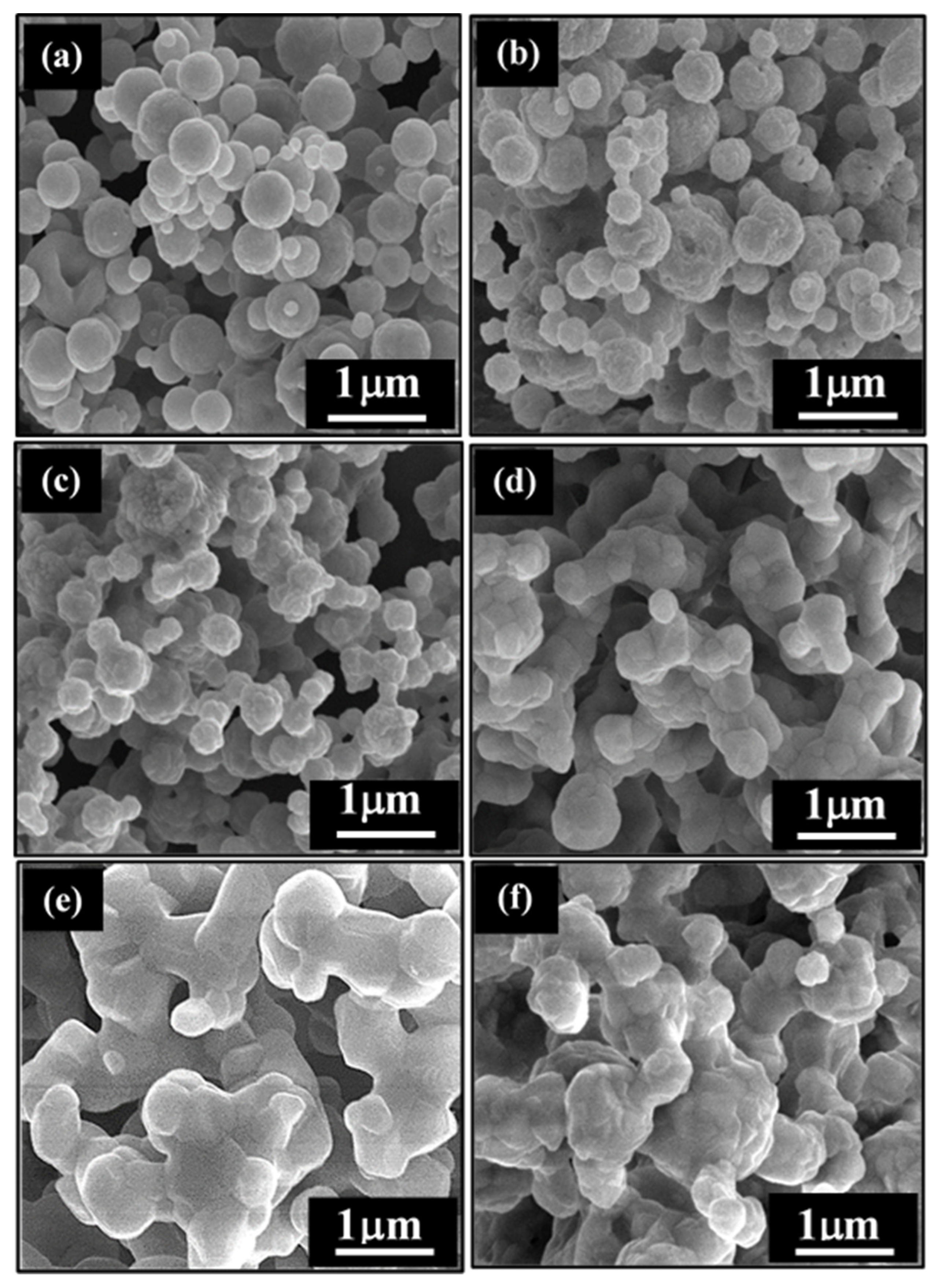
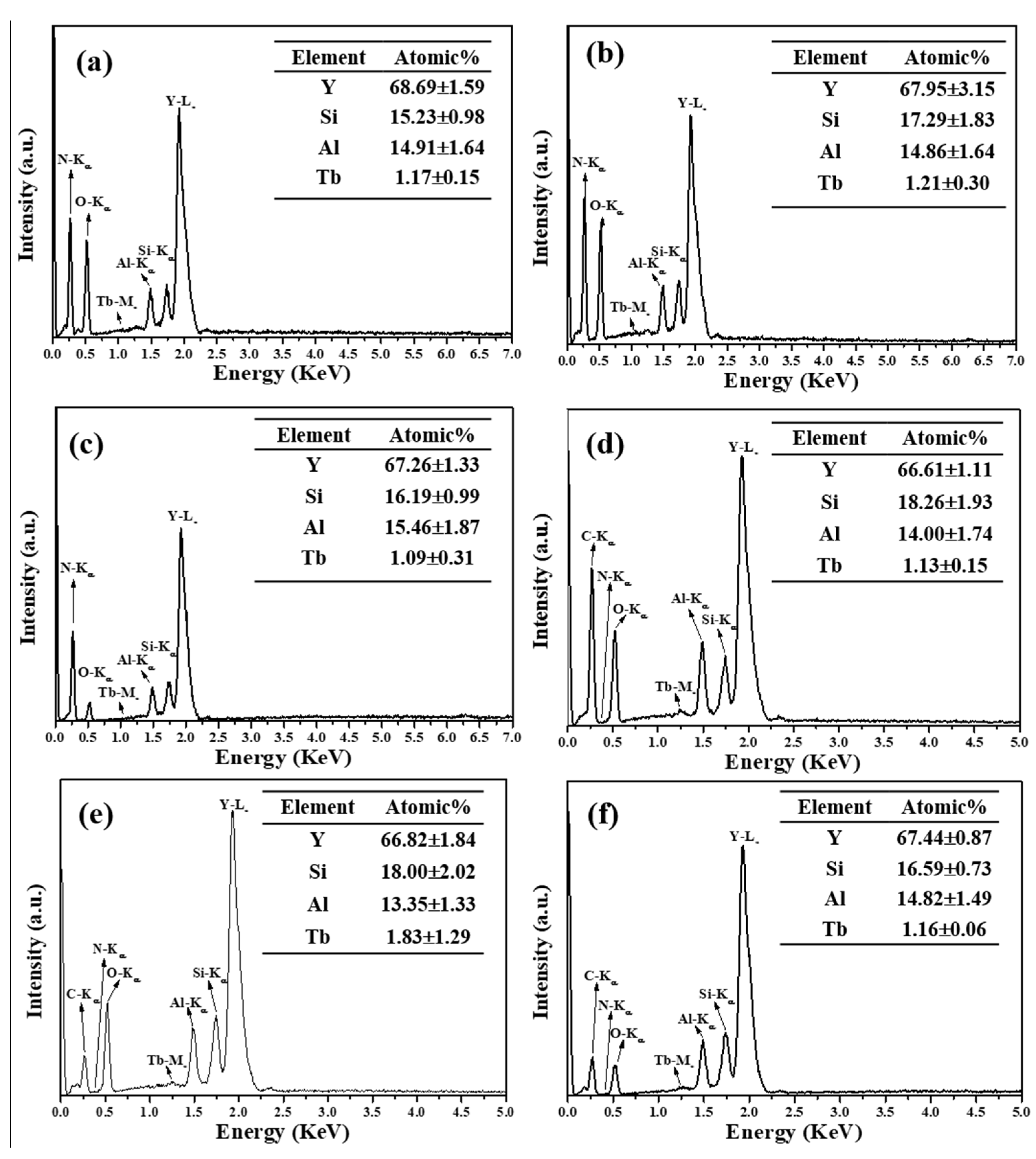
3.1. Phase Compositions and Morphology Analysis
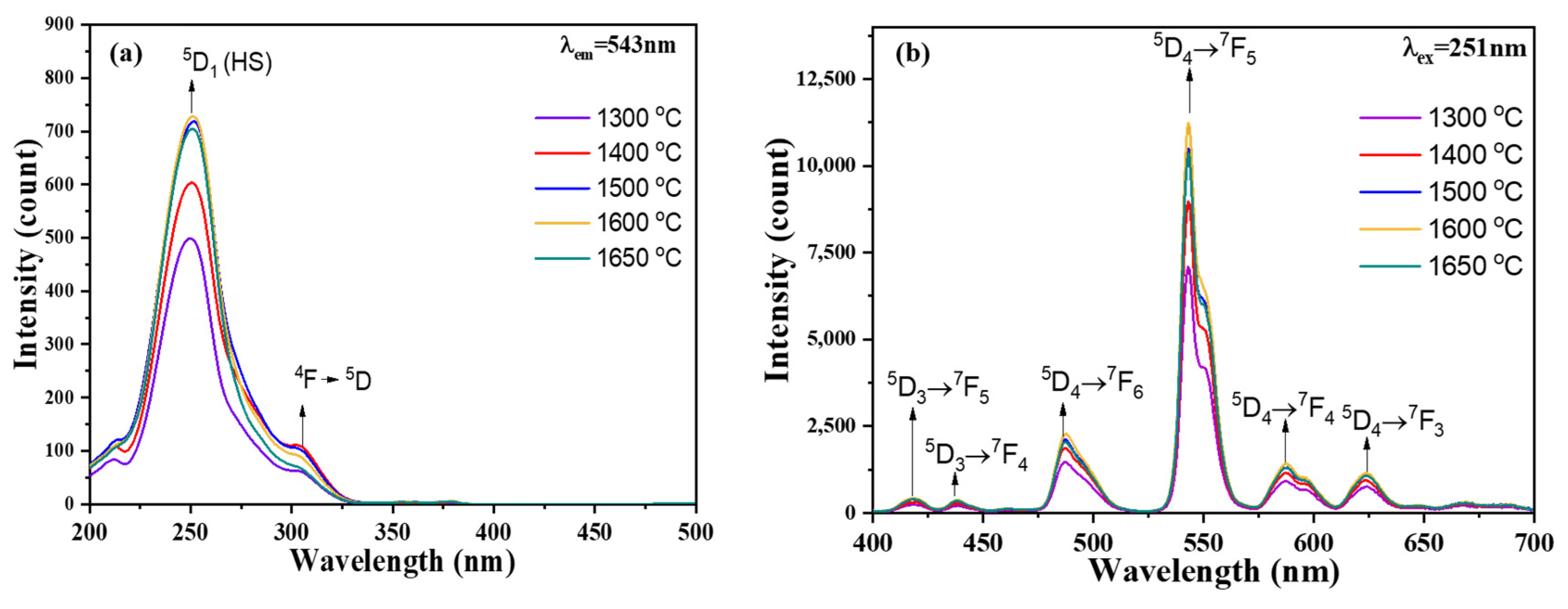
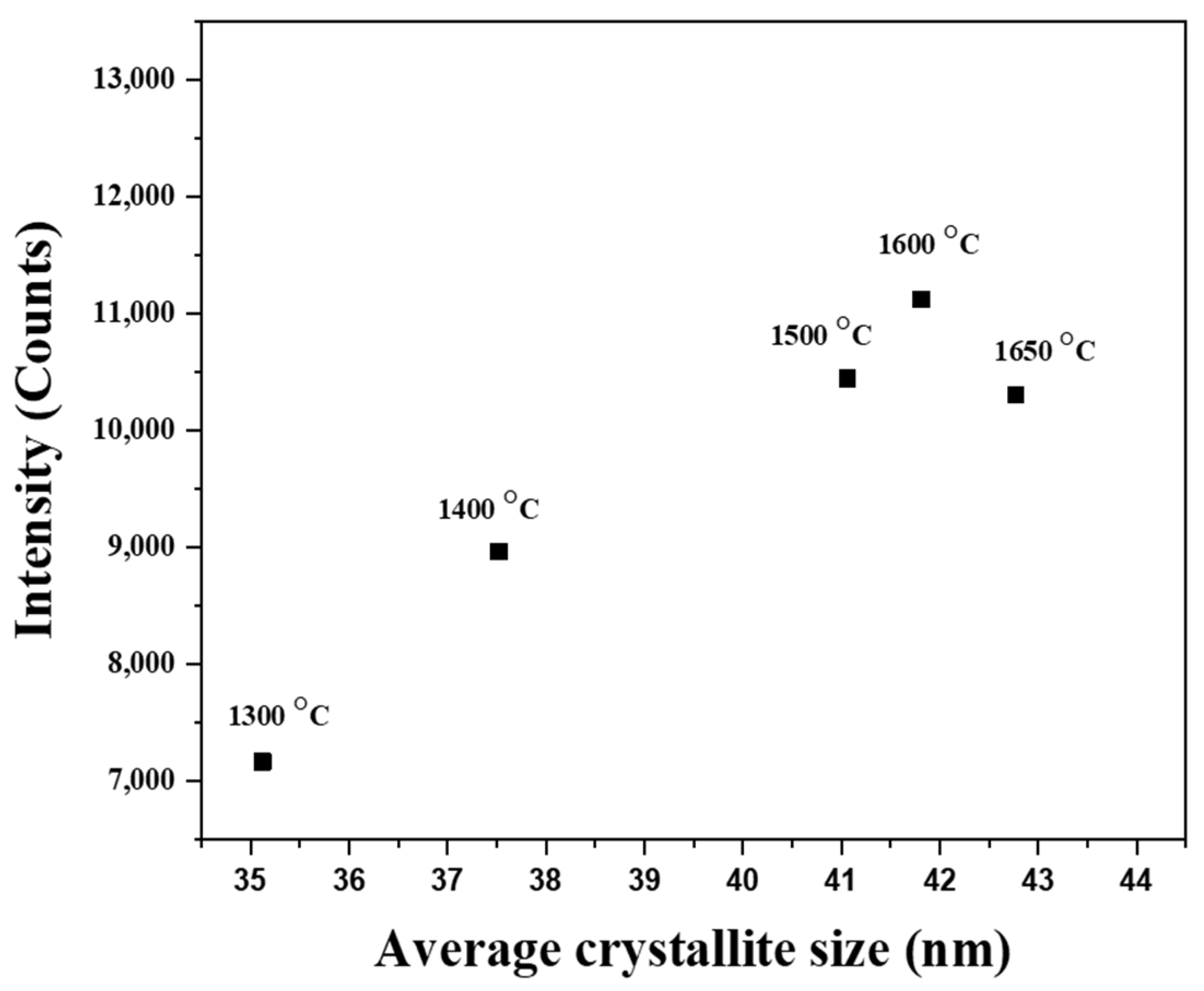
3.2. PL Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nair, G.B.; Dhoble, S.J. 1—Introduction to luminescence. In The Fundamentals and Applications of Light-Emitting Diodes; Nair, G.B., Dhoble, S.J., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 3–33. [Google Scholar]
- Satpute, N.S.; Dhoble, S.J. Chapter 14—Role of rare-earth ions for energy-saving LED lighting devices. In Energy Materials; Dhoble, S.J., Kalyani, N.T., Vengadaesvaran, B., Kariem Arof, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 407–444. [Google Scholar]
- Cho, J.; Park, J.H.; Kim, J.K.; Schubert, E.F. White light-emitting diodes: History, progress, and future. Laser Photonics Rev. 2017, 11, 1600147. [Google Scholar] [CrossRef]
- Li, Y.; Ding, J.; Wu, Q.; Long, Q.; Wang, X.; Wang, Y. Blue to green emission and energy transfer between Ce3+ ions in Ca15Si20O10N30. J. Mater. Chem. C 2015, 3, 8949–8955. [Google Scholar] [CrossRef]
- Ito, Y.; Hori, T.; Kusunoki, T.; Nomura, H.; Kondo, H. A phosphor sheet and a backlight system providing wider color gamut for LCDs. J. Soc. Inf. Disp. 2014, 22, 419–428. [Google Scholar] [CrossRef]
- Bachmann, V.; Ronda, C.R.; Meijerink, A. Temperature Quenching of Yellow Ce3+ Luminescence in YAG:Ce. Chem. Mater. 2009, 21, 2077–2084. [Google Scholar] [CrossRef]
- Tran, N.T.; You, J.P.; Shi, F.G. Effect of Phosphor Particle Size on Luminous Efficacy of Phosphor-Converted White LED. J. Light. Technol. 2009, 27, 5145–5150. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, Q. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Pust, P.; Schmidt, P.J.; Schnick, W. A revolution in lighting. Nat. Mater. 2015, 14, 454–458. [Google Scholar] [CrossRef]
- Xie, R.-J.; Hirosaki, N.; Takeda, T. Wide Color Gamut Backlight for Liquid Crystal Displays Using Three-Band Phosphor-Converted White Light-Emitting Diodes. Appl. Phys. Express 2009, 2, 022401. [Google Scholar] [CrossRef]
- Fang, M.-H.; Leaño, J.L., Jr.; Liu, R.-S. Control of Narrow-Band Emission in Phosphor Materials for Application in Light-Emitting Diodes. ACS Energy Lett. 2018, 3, 2573–2586. [Google Scholar] [CrossRef]
- Li, S.; Xie, R.-J.; Takeda, T.; Hirosaki, N. Critical Review—Narrow-Band Nitride Phosphors for Wide Color-Gamut White LED Backlighting. ECS J. Solid State Sci. Technol. 2018, 7, R3064. [Google Scholar] [CrossRef]
- Kim, Y.H.; Arunkumar, P.; Kim, B.Y.; Unithrattil, S.; Kim, E.; Moon, S.-H.; Hyun, J.Y.; Kim, K.H.; Lee, D.; Lee, J.-S.; et al. A zero-thermal-quenching phosphor. Nat. Mater. 2017, 16, 543–550. [Google Scholar] [CrossRef]
- Rao, R.P. Tb3 + Activated Green Phosphors for Plasma Display Panel Applications. J. Electrochem. Soc. 2003, 150, H165. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Y.; Wang, T.; Wu, J.; Zhang, J.; Yu, S.; Zhang, M.; Zhao, L.; Wang, W. Ultra-bright green-emitting phosphors with an internal quantum efficiency of over 90% for high-quality WLEDs. Dalton Trans. 2021, 50, 4159–4166. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Yin, S.; Wu, X.; You, H. Highly efficient green-emitting phosphors with high color rendering for WLEDs. J. Alloys Compd. 2022, 911, 165149. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, D.; Ma, H.; Deng, D.; Xu, S. Synthesis, luminescence properties and electronic structure of Tb3+-doped Y4−xSiAlO8N:xTb3+—A novel green phosphor with high thermal stability for white LEDs. RSC Adv. 2016, 6, 113249–113259. [Google Scholar] [CrossRef]
- Takeda, T.; Hirosaki, N.; Funahshi, S.; Xie, R.-J. Narrow-Band Green-Emitting Phosphor Ba2LiSi7AlN12:Eu2+ with High Thermal Stability Discovered by a Single Particle Diagnosis Approach. Chem. Mater. 2015, 27, 5892–5898. [Google Scholar] [CrossRef]
- Strobel, P.; Schmiechen, S.; Siegert, M.; Tücks, A.; Schmidt, P.J.; Schnick, W. Narrow-Band Green Emitting Nitridolithoalumosilicate Ba[Li2(Al2Si2)N6]: Eu2+ with Framework Topology whj for LED/LCD-Backlighting Applications. Chem. Mater. 2015, 27, 6109–6115. [Google Scholar] [CrossRef]
- Yoshimura, K.; Fukunaga, H.; Izumi, M.; Masuda, M.; Uemura, T.; Takahashi, K.; Xie, R.-J.; Hirosaki, N. White LEDs using the sharp β-sialon: Eu phosphor and Mn-doped red phosphor for wide-color gamut display applications. J. Soc. Inf. Disp. 2016, 24, 449–453. [Google Scholar] [CrossRef]
- Larker, R. Reaction Sintering and Properties of Silicon Oxynitride Densified by Hot Isostatic Pressing. J. Am. Ceram. Soc. 1992, 75, 62–66. [Google Scholar] [CrossRef]
- Hua, Y.; Li, X.; Zhang, D.; Ma, H.; Deng, D.; Xu, S. The crystal structure and luminescence properties of novel Ce3+ and Ce3+, Sm3+-activated Y4SiAlO8N phosphors for near-UV white LEDs. New J. Chem. 2016, 40, 5458–5466. [Google Scholar] [CrossRef]
- Segawa, H.; Yoshimizu, H.; Hirosaki, N.; Inoue, S. Fabrication of silica glass containing yellow oxynitride phosphor by the sol–gel process. Sci. Technol. Adv. Mater. 2011, 12, 034407. [Google Scholar] [CrossRef][Green Version]
- Osip, H.; Czosnek, C.; Janik, J.F.; Marchewka, J.; Sitarz, M. Amorphous Silicon Oxynitride-Based Powders Produced by Spray Pyrolysis from Liquid Organosilicon Compounds. Materials 2021, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Liddell, K.; Mandal, H.; Thompson, D.P. X-ray data for new Y-Si-Al-O-N glass ceramics. J. Eur. Ceram. Soc. 1997, 17, 781–787. [Google Scholar] [CrossRef]
- Brinker, C.J.; Haaland, D.M. Oxynitride Glass Formation from Gels. J. Am. Ceram. Soc. 1983, 66, 758–765. [Google Scholar] [CrossRef]
- Shih, S.-J.; Lin, Y.-C.; Lin, S.-H.; Veteška, P.; Galusek, D.; Tuan, W.-H. Preparation and characterization of Eu-doped gehlenite glassy particles using spray pyrolysis. Ceram. Int. 2016, 42, 11324–11329. [Google Scholar] [CrossRef]
- Messing, G.L.; Zhang, S.-C.; Jayanthi, G.V. Ceramic Powder Synthesis by Spray Pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Joffin, N.; Caillier, B.; Garcia, A.; Guillot, P.; Galy, J.; Fernandes, A.; Mauricot, R.; Dexpert-Ghys, J. Phosphor powders elaborated by spray-pyrolysis: Characterizations and possible applications. Opt. Mater. 2006, 28, 597–601. [Google Scholar] [CrossRef]
- Shih, S.-J.; Lin, Y.-C.; Lin, S.-H.; Yu, C.-Y. Correlation of morphology and photoluminescence properties of gehlenite: Eu glassy phosphors. Int. J. Appl. Ceram. Technol. 2017, 14, 56–62. [Google Scholar] [CrossRef]
- Min, B.H.; Jung, K.Y. Synthesis and luminescence characteristics of fine-sized Ba3Si6O12N2:Eu green phosphor through spray pyrolysis using TEOS/Si3N4 mixed precursors. RSC Adv. 2017, 7, 44759–44765. [Google Scholar] [CrossRef]
- Gültekin, S.; Yıldırım, S.; Yılmaz, O.; Keskin, İ.Ç.; Katı, M.İ.; Çelik, E. Structural and optical properties of SrAl2O4: Eu2+/Dy3+ phosphors synthesized by flame spray pyrolysis technique. J. Lumin. 2019, 206, 59–69. [Google Scholar] [CrossRef]
- Mazlan, M.R.; Jamadon, N.H.; Rajabi, A.; Sulong, A.B.; Mohamed, I.F.; Yusof, F.; Jamal, N.A. Necking mechanism under various sintering process parameters—A review. J. Mater. Res. Technol. 2023, 23, 2189–2201. [Google Scholar] [CrossRef]
- Jung, K.Y.; Lee, C.H.; Kang, Y.C. Effect of surface area and crystallite size on luminescent intensity of Y2O3:Eu phosphor prepared by spray pyrolysis. Mater. Lett. 2005, 59, 2451–2456. [Google Scholar] [CrossRef]
- Dinger, T.R.; Rai, R.S.; Thomas, G. Crystallization Behavior of a Glass in the Y2O3-SiO2-AlN System. J. Am. Ceram. Soc. 1988, 71, 236–244. [Google Scholar] [CrossRef]
- Liddell, K.; Thompson, D.P. Heat treatment of wollastonite-type Y–Si–Al–O–N glasses. J. Mater. Sci. 1997, 32, 887–892. [Google Scholar] [CrossRef]
- Salkind, N.J. Encyclopedia of Research Design; SAGE Publications: Thousand Oaks, CA, USA, 2010. [Google Scholar]
- Spiridonova, T.I.; Tverdokhlebov, S.I.; Anissimov, Y.G. Investigation of the Size Distribution for Diffusion-Controlled Drug Release From Drug Delivery Systems of Various Geometries. J. Pharm. Sci. 2019, 108, 2690–2697. [Google Scholar] [CrossRef]
- Back, M.; Massari, A.; Boffelli, M.; Gonella, F.; Riello, P.; Cristofori, D.; Riccò, R.; Enrichi, F. Optical investigation of Tb3+-doped Y2O3 nanocrystals prepared by Pechini-type sol–gel process. J. Nanopart. Res. 2012, 14, 792. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, B.; Xu, W.; Yang, Y.; Zhao, X.; Di, W.; Lu, S.; Wang, X.; Sun, J.; Cheng, L.; et al. Size-dependent excitation spectra and energy transfer in Tb3+-doped Y2O3 nanocrystalline. J. Appl. Phys. 2007, 102. [Google Scholar] [CrossRef]
- Park, K.; Kim, H.; Hakeem, D.A. Photoluminescence properties of Eu3+-and Tb3+-doped YAlO3 phosphors for white LED applications. Ceram. Int. 2016, 42, 10526–10530. [Google Scholar] [CrossRef]
- Schubert, E.F. Light-Emitting Diodes, 4th ed.; E. Fred Schubert: New York, NY, USA, 2023; pp. 4–14. [Google Scholar]
- Dobrovolsky, A.; Merdasa, A.; Unger, E.L.; Yartsev, A.; Scheblykin, I.G. Defect-induced local variation of crystal phase transition temperature in metal-halide perovskites. Nat. Commun. 2017, 8, 34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aji, B.B.; Huang, Y.-H.; Oishi, M.; Moriga, T.; Shih, S.-J. Fabrication and Characterization of Narrow-Wavelength Phosphors of Tb-Doped Yttrium-Silicon-Aluminum Oxynitride Using Spray Pyrolysis. Ceramics 2023, 6, 2307-2319. https://doi.org/10.3390/ceramics6040141
Aji BB, Huang Y-H, Oishi M, Moriga T, Shih S-J. Fabrication and Characterization of Narrow-Wavelength Phosphors of Tb-Doped Yttrium-Silicon-Aluminum Oxynitride Using Spray Pyrolysis. Ceramics. 2023; 6(4):2307-2319. https://doi.org/10.3390/ceramics6040141
Chicago/Turabian StyleAji, Bramantyo Bayu, Yu-Hsiuan Huang, Masatsugu Oishi, Toshihiro Moriga, and Shao-Ju Shih. 2023. "Fabrication and Characterization of Narrow-Wavelength Phosphors of Tb-Doped Yttrium-Silicon-Aluminum Oxynitride Using Spray Pyrolysis" Ceramics 6, no. 4: 2307-2319. https://doi.org/10.3390/ceramics6040141
APA StyleAji, B. B., Huang, Y.-H., Oishi, M., Moriga, T., & Shih, S.-J. (2023). Fabrication and Characterization of Narrow-Wavelength Phosphors of Tb-Doped Yttrium-Silicon-Aluminum Oxynitride Using Spray Pyrolysis. Ceramics, 6(4), 2307-2319. https://doi.org/10.3390/ceramics6040141





