3.1. Properties of Input Materials
The properties of the alloy are listed in
Table 3. They are 2.76 ± 0.20 g/cc, 83.0 ± 1.3 HRB, 241.6 ± 4.5 MPa, 60.3 ± 2.6 GPa, 255.5 ± 3.9 MPa, and 209.8 ± 2.7 MPa for density, Brinell hardness, tensile strength, tensile modulus, flexural strength, and compressive strength, respectively. From the result, EDS (elemental composition) is displayed in
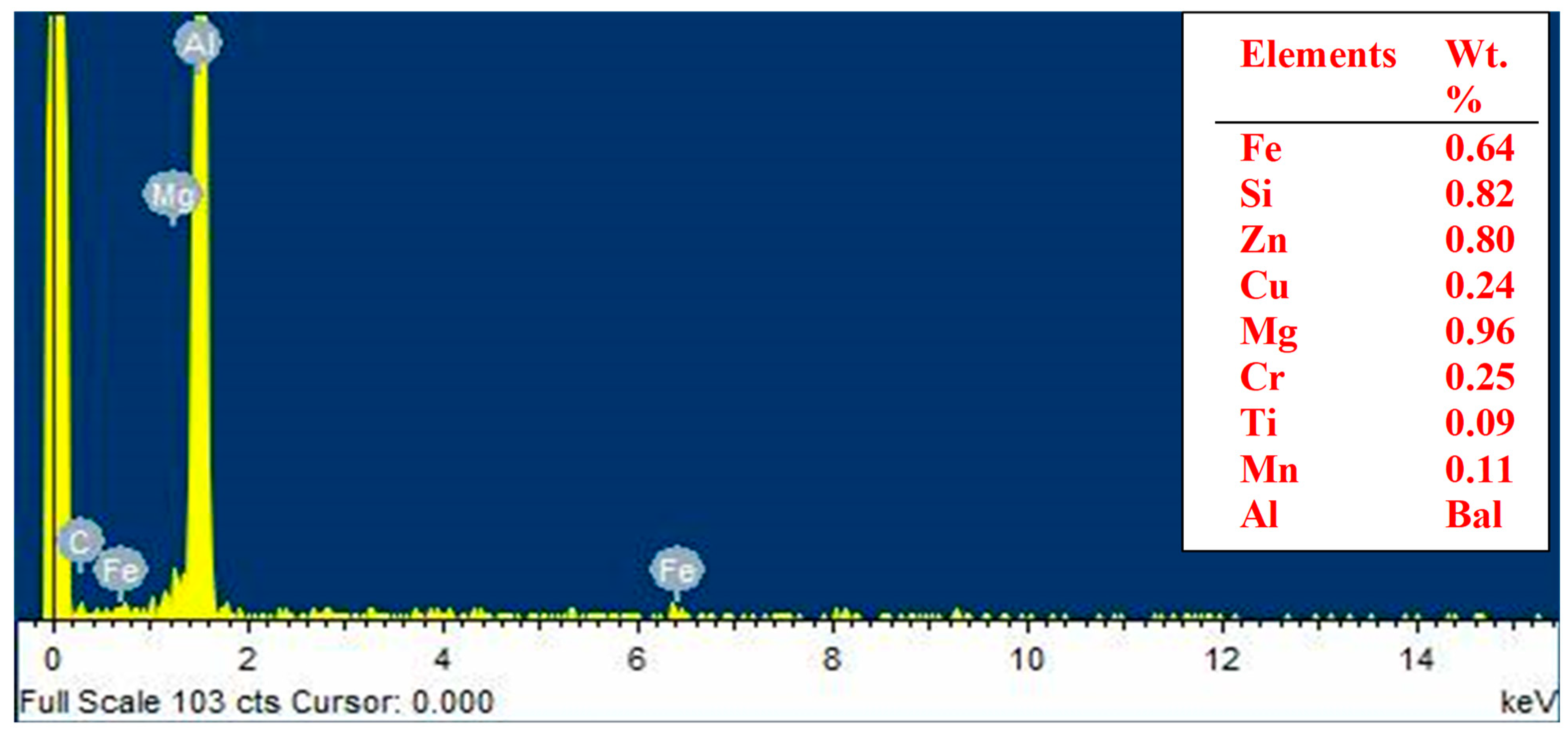
Figure 1, where Al, being the matrix, is the major element present. Other elements present in the matrix are Fe, Si, Zn, Cu, Mg, Cr, and Mn. The obtained elemental composition affirmed the elements present in Al6061 [
46].
Furthermore, the chemical and elemental compositions of palm kernel shell ash (PKA) and rice husk ash (RHA) are showcased in
Table 4. Going by the presented values, silica is the highest compound present in the two ashes, though its percentage in RHA is almost 90.6%, a value higher than that of PKA. MgO, Al
2O
3, Fe
2O
3, MnO
2, and CaO are other compounds present.
As indicated in
Table 5, iron remains the major element in the waste steel powder, indicating the particles are iron-based. Other minor elements are also indicated in the Table.
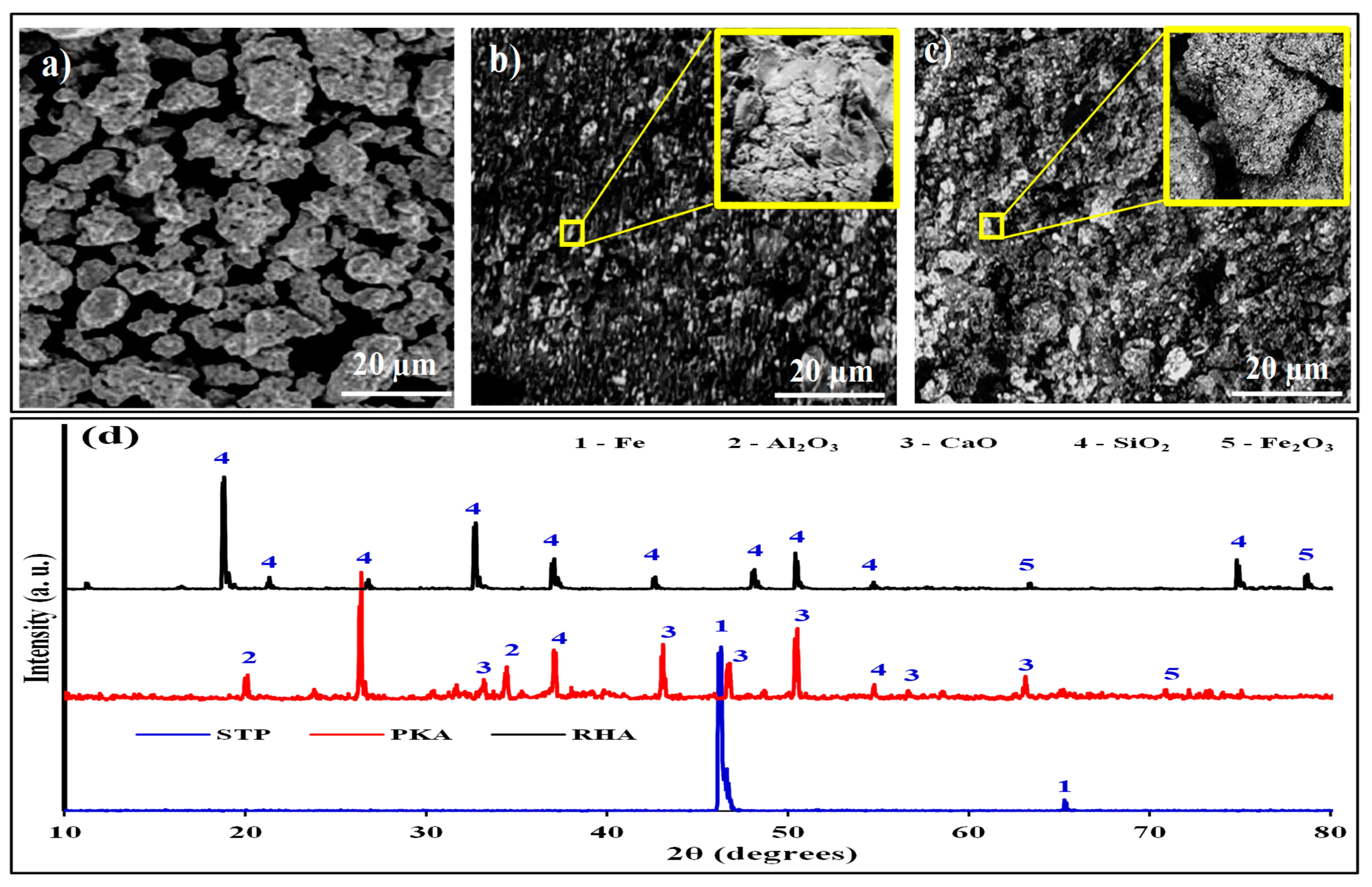
Figure 2 presents the morphological images of STP, PKA, and RHA (
Figure 2a–c) and the X-ray dispersive pattern (
Figure 2d).
Figure 2a presents the SEM image of the steel powder with irregular spherical-like shapes.
Figure 2a,b shows they have clustered particles with porous structures. Based on the energy dispersive result in
Figure 2d, the steel reflects Fe peaks depicting the powder as an alloy of iron (in blue line). Other phases were not identified because of their low contents. This is confirmed in
Table 5 as other elements are less than 0.5%.
Figure 2d depicts high peaks of silica observed at 26.2, 38.0, 46.3, and 54.8 degrees. CaO was revealed at positions of 33.2, 51.8, 56.2, and 63.8 degrees corroborating the results displayed in
Table 4 and also confirming the findings of Imoisili et al. [
47] and Imoisili et al. [
48]. The silica and CaO content observed in their findings for PKA is at almost the same positions noted in our findings.
As observed in
Figure 2c, SiO
2 is the most occurring phase, attaining positions of 19.5, 24.7, 32.8, 37.6, 42.5, 47.1, and 50.1 degrees, hence, agreeing with the observation of Farooque et al. [
49]. The results are consistent with
Table 4, which shows a SiO
2 proportion of 90.21% in RHA. Significantly, the strengthening of an aluminium base matrix with RHA has been on account of the rich silica content, which serves as a substitute for synthetic silica. By the chemical composition of variables, they are fit for the reinforcement of Al-6061.
The corresponding values for the evaluated properties for each experimental run are highlighted in
Table 6 with their respective designations. Experimental run 1 is designated 2-4-900 indicating a sample prepared with 2% PKA and 4% RHA at 900 °C. Other samples are also designated following the same pattern.
Table 6 presents the property value for specimens prepared at varying conditions according to the experimental runs in
Table 2.
3.2. Microstructural and Phase Characterization
The phases contained in the selected specimens are depicted in
Figure 3.
Figure 3a,b shows XRD results for specimens cast at 700 °C and prepared with 2% PKA, 4% RHA, and 4% PKA, 4% RHA in that order. SiO
2, Fe, and the Al phase were recognized in the specimens. The highest peak in aluminium is typical of aluminium-based alloys. The presence of SiO
2 is due to the reinforcement of PKA and RHA. Fe is also present owing to intrinsic Fe in Al6061 as well as assimilated steel particles included in the melt.
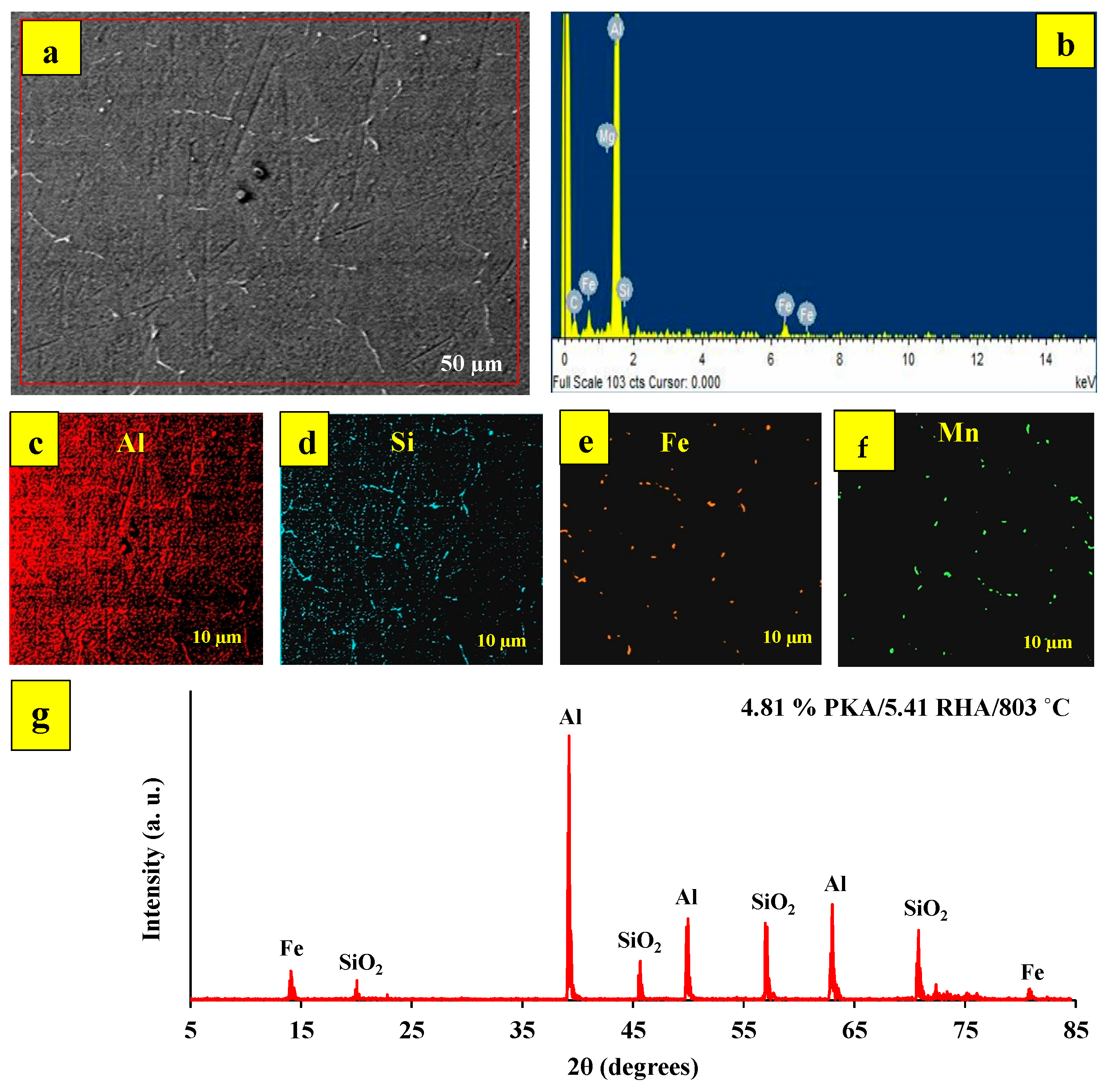
Figure 3c,d shows XRD data for specimens cast at 800 °C and prepared with 2% PKA/6% RHA and 6% PKA/2% RHA, respectively. From the XRD plot, there is a higher presence of silica, as depicted by the higher peak of the silica phase. The phases present in specimens prepared at 900 °C with 6% PKA/4% RHA and 4% PKA/6% RHA, respectively, are depicted in
Figure 3e,f. As featured in the results, intermetallic phases of Mg
2Si, Al
5FeSi, and Al
15Si
2(FeMn) are revealed. The implication of this is that 900 °C features the existence of intermetallic phases attributed to high-temperature reactions.
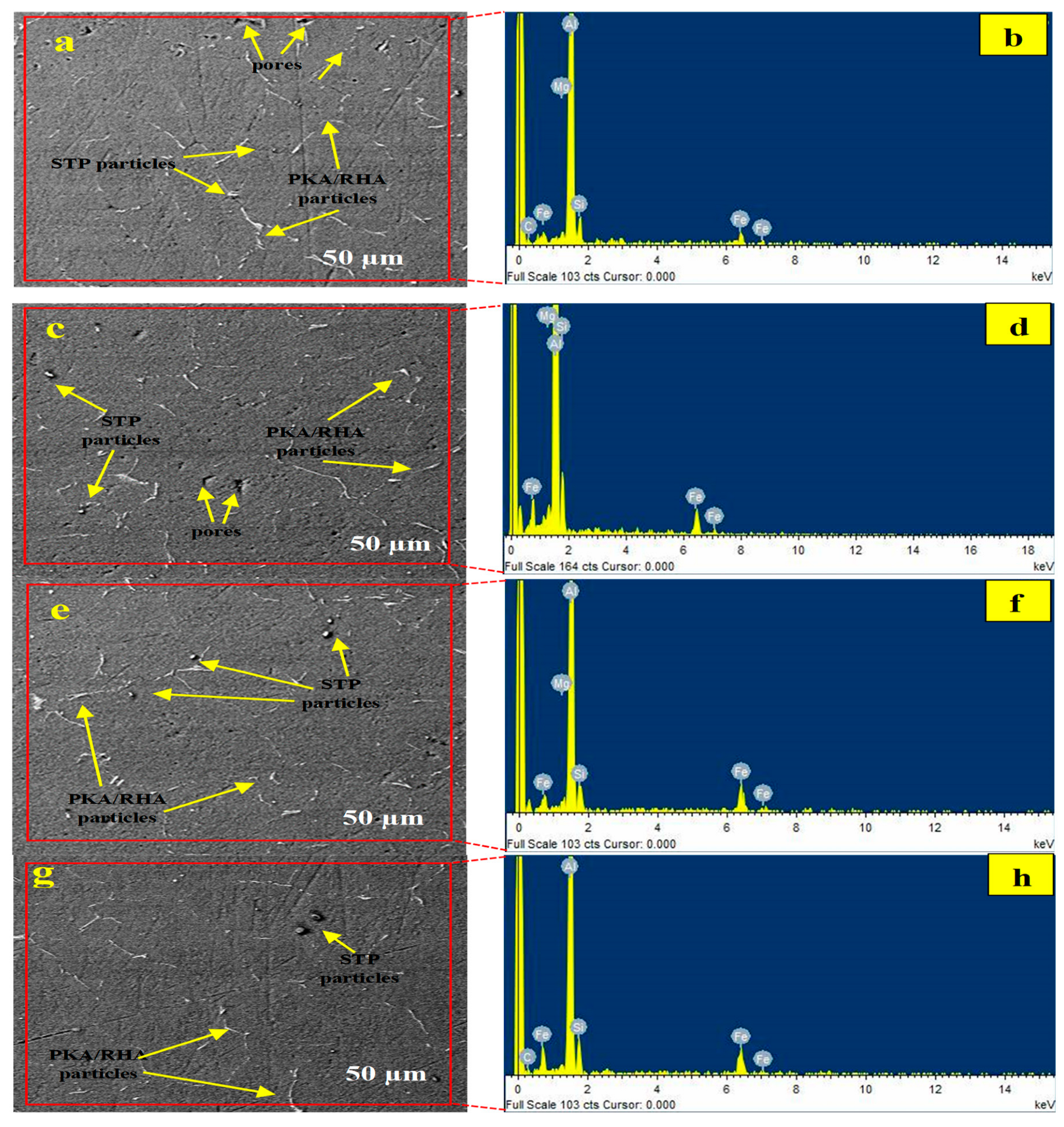
Figure 4a–h highlights the microstructural and elemental characteristics of specimens processed at 700 and 800 °C. While
Figure 4a,c,e,g contains the morphological images,
Figure 4b,d,f,h is the respective EDX result of the specimen.
Figure 4a,c shows the morphology of specimens processed at 700 °C with 2% PKA/4% RHA and 4% PKA/2% RHA. The reinforcing particles were revealed to be dispersed within the matrix. The relevance of the stir casting process in particle dispersion in aluminium melt has been demonstrated by authors [
50,
51,
52]. Microvoids have also been discovered in the microstructure represented by
Figure 4a,c. Infilling of intrinsic pores in matrixes is one of the effects of particle reinforcement of composites. The presence of pores in
Figure 4a,c may be as a result of lower viscosity in the melt, which did not permit even dispersion of the particles within the matrix. The lower viscosity of the melt is associated with a relatively low stirring temperature [
53,
54].
Figure 4b,d identifies the elements present in the phases as Al (matrix), Mg, Si, and Fe (as a result of steel particle addition). According to the XRD pattern (
Figure 3), there are no intermetallic phases identified in specimens (b) 2% PKA/4% RHA and (d) 4% PKA/2% RHA. The microstructure of the specimen also exhibits the absence of an intermetallic phase. The major defect, therefore, identified in the microstructure is the set of micropores.
The property values (
Table 6) of the specimens prepared at 700 °C represent the effect of the dispersion. In comparison to properties of pure aluminium 6061 (
Table 3), specimens fabricated with 2% PKA/4% RHA at 700 °C yielded 12.4, 11.1, 4.9, and 9.2% increases in tensile strength, tensile modulus, compressive strength, and flexural strength, respectively. Similarly, at the same 700 °C, specimens doped with 4% PKA/2.0% RHA showed a 3.7, 7.8, 1.0, and 6.20% increase in the respective properties over pure Al6061 values. This demonstrates that particle distribution played a significant influence in improving the properties of the developed composites as compared with the base material. Furthermore, a 2% PKA/4% RHA specimen displayed improved tensile strength, tensile modulus, compressive strength, and compressive modulus by 8.4, 3.1, 5.8, and 8.1% over a 4% PKA/2% RHA specimen in that order. Despite the two having the same particle count (6%), the specimen with the higher RHA content performed better. This is based on the higher silica content of RHA as compared with PKA (
Table 4).
Figure 4e,g demonstrates the morphology of specimens containing 2% PKA/6% RHA and 6% PKA/2% RHA, respectively, synthesized at an 800 °C stirring temperature. The presence of reinforcing agents PKA and RHA as well as steel particles can be featured in the microstructure. The particles are dispersed within the matrix, as observed in the figures. Micropores are significantly minimized when compared to specimens processed at 700 °C. This contributed significantly to the performance of the specimen at 800 °C.
The specimen generated with 2% PKA/6% RHA at 800 °C displayed a 24.2, 70.8, 39.2, and 26.0% boost in tensile strength, tensile modulus, compressive strength, and flexural strength when compared with the pure Al6061 used in the study. Specimens prepared with 6% PKA/2% RHA at 800 °C ensued 17.5, 50.9, 29.6, and 10% improvement over pure Al6061 as regards the same properties, respectively. The improvement is attributed to the dispersion observed within the microstructure. As a result of this even dispersion, there is even stress distribution within the matrix, thereby improving strength [
27,
55,
56]. The findings of this study as regards the improvement of properties on account of particle dispersion are exemplified in the works of authors Chaubey et al. [
57]. A similar investigation involving the use of palm kernel ash as reinforcing particles in Al6063 with improvement in the mechanical performance of the developed composite was earlier reported [
18]. The infilling of pores by the reinforcing palm kernel ash consequently improves densification and by extension the mechanical properties of the composite.
The even dispersion of the particles revealed in the microstructure at 800 °C (
Figure 4e,g) is based on the moderate viscosity of the melt, engendering even dispersion of the particles and strong interfacial adhesion with the matrix [
30,
55]. Further observation has shown that specimen 2% PKA/6% RHA performed better than specimen 6% PKA/2% RHA (despite the same particle count of 8%) by 5.6, 13.2, 8.6, and 14.6% as par tensile strength, tensile modulus, compressive strength, and flexural strength. The findings are associated with higher silica content in RHA as compared with PKA (
Table 4).
In
Figure 4e,g, the microstructure of specimens 6% PKA/2% RHA and 2% PKA/6% RHA revealed the presence of no cluster, dispersed particles, and reduced pores. This is responsible for superior performance noted at 800 °C than at 700 °C. The presence of elements Si, Fe, and Mg in the matrix’s EDX results confirmed the existence of the reinforcing phase (
Figure 4f,h). The major phases identified in the XRD for specimens 6% PKA/2% RHA and 2% PKA/6% RHA (
Figure 4c,d) were SiO
2 and Al, with no intermetallic phase present.
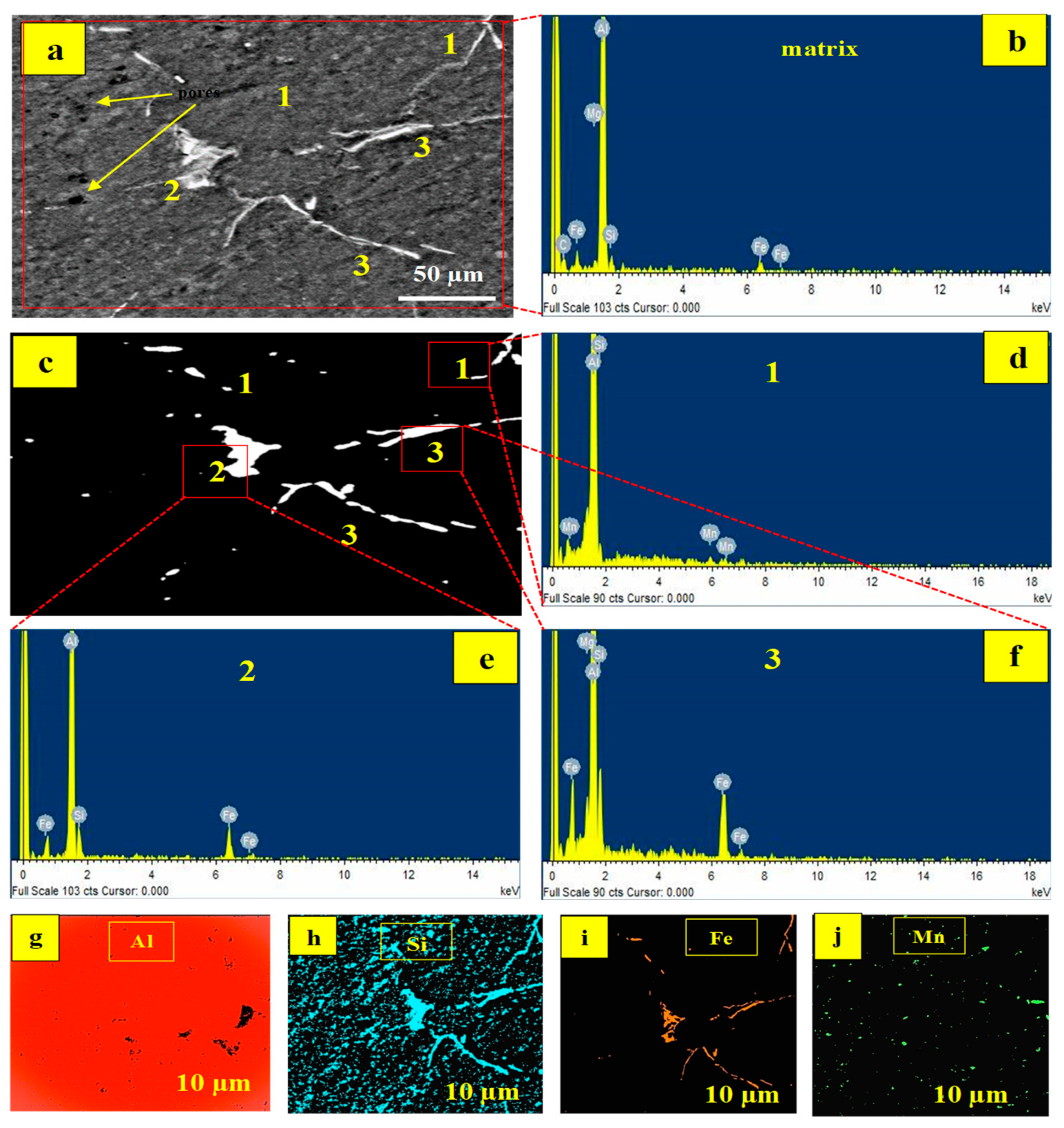
The microstructural characteristics and EDX results of specimens processed at 900 °C are demonstrated in
Figure 5. The appearance of micropores may be seen in the micrograph. Akinwande et al. [
29] and Adediran et al. [
55] also confirmed that microvoids and blow holes are common features at high-temperature casting. There is a tendency for greater air entrapment at such temperatures, and during solidification, the entrapped air is expelled, resulting in pores in the matrix [
58].
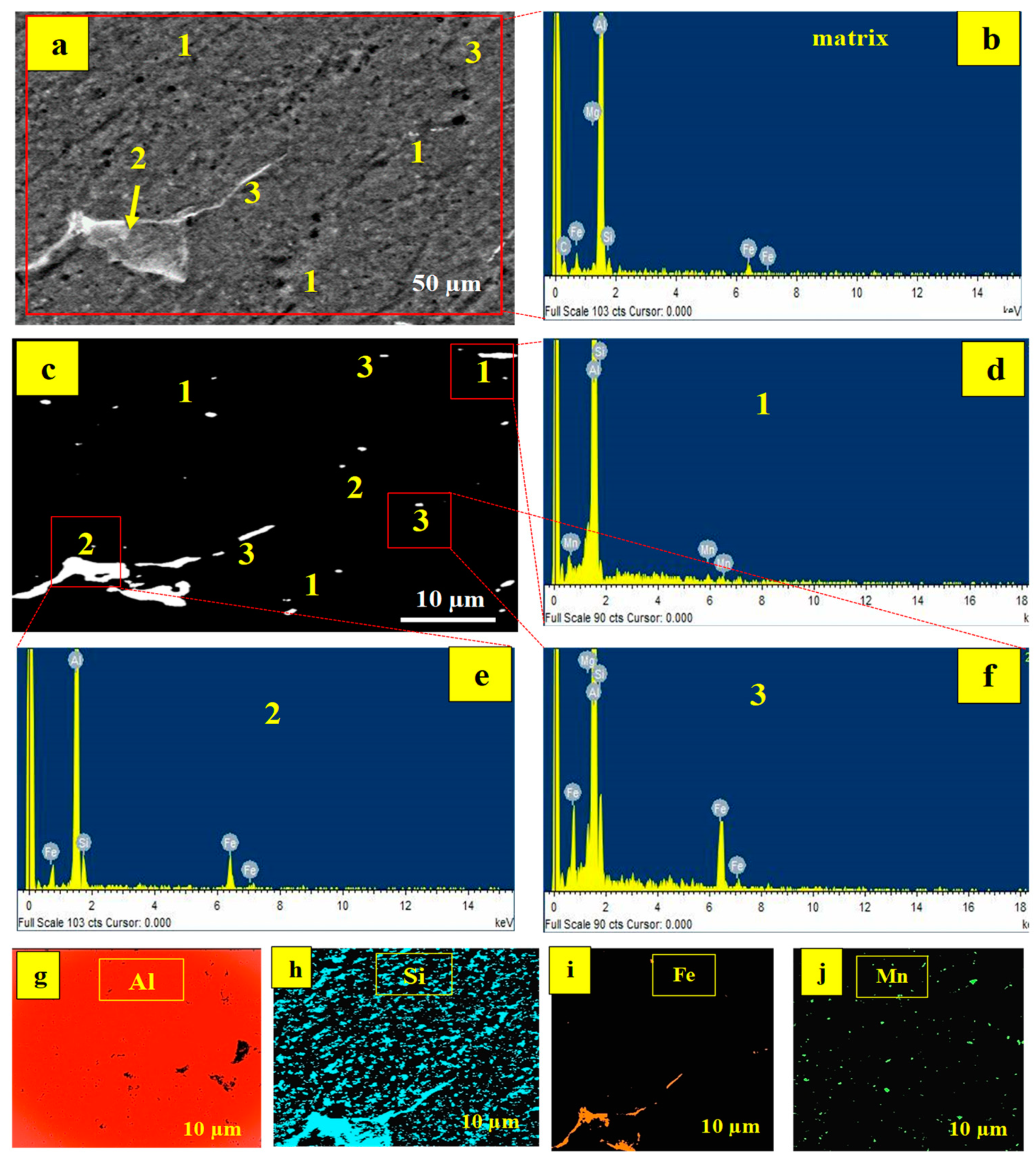
The EDS result of the matrix is illustrated in
Figure 5b, which detects the presence of elements such as Al, Si, Fe, Mn, and Mg, similar to the EDX result for Al6061 shown in
Figure 1. In
Figure 5a, different points are labelled spots 1, 2, and 3. Under polarized illumination,
Figure 5c highlights the detected spots even more clearly.
Figure 5d–f shows the spot EDS results for points 1, 2, and 3. For spot 1, the elements detected are Mg and Si, as indicated in
Figure 4d. While Al, Fe, Mn, and Si are found in spot 2 (
Figure 5e), spot 3 contains Al, Fe, Mn, and Si (
Figure 5f). The phases Mg
2Si, Al
5FeSi, and Al
15Si
2(FeMn) are the phases identified, which correlate to the literature references [
59,
60,
61]. The phases are also visible in the XRD (
Figure 3) for a specimen of formulation 4% PKA and 6% RHA cast at 900 °C.
The intermetallic phases are formed at a high temperature of 900 °C. Fe is present in spots 2 and 3, as evidenced by EDX and XRD results. Steel particles were added to increase the composite’s ductility. At higher temperatures, however, interactions between the active elements occur, resulting in the production of intermetallic phases. The elemental map of the specimen generated at 900 °C (4% PKA/6% RHA) is pictured in
Figure 5g–j. Aluminium’s map is featured in
Figure 5g. Based on it being the matrix, the aluminium element is established in practically every section of the matrix. The majority of the microstructure is noted to be dispersed with Si (
Figure 5h) on account of the inclusion of silica-based reinforcing particles. The silicon element is also found in the intermetallic phases, confirming the existence of silica in the phases displayed in
Figure 5d–f.
The distribution of Fe in the microstructure is shown in
Figure 5i. The majority of the Fe element is found in intermetallic phases, with minor amounts found elsewhere in the matrix. Its predominant presence in the intermetallic is due to the reaction that took place at that temperature (900 °C), resulting in the absorption of Fe into the phases. According to Lin et al. [
61] and according to the alloy phase diagram of Al-Fe, Fe has low solubility in the aluminium matrix, hence at high proportions, Fe tends to precipitate, forming Fe-rich intermetallic phases. The elemental distribution of manganese is depicted in
Figure 5j. It is sparsely dispersed within the matrix on account of its reduced amount in the mix.
The microstructural characteristics and EDS results of a specimen generated at 900 °C and containing 6% PKA/4% RHA are shown in
Figure 6. The presence of voids can be seen in
Figure 6a owing to the evolution of entrapped air and gases during solidification. This is a common occurrence in high-temperature casting [
29,
55].
Figure 6b depicts the elemental composition of the matrix, indicating the presence of Al, Si, Fe, Mn, and Mg; identical to the EDS result of the specimen, 6% PKA/4% RHA generated at the same temperature. Various spots numbered 1, 2, and 3 are also recognized and shown under polarized light (
Figure 6c).
Figure 6d–f highlight the EDS-determined composition of the spots. The elements discovered in spot 1 are Mg and Si, as in specimen 6% PKA and 4% RHA; spot 2 is Al, Fe, and Si; and spot 3 is Al, Fe, Mn, and Si. The spots are recognized as Mg
2Si, Al
5FeSi, and Al
5S
12(FeMn) intermetallic phases, respectively. The elemental maps in the specimen are featured in
Figure 6g–j. Due to the fact that it is the basic element, Al dominated the element distribution within the matrix (
Figure 6g). It is also among the intermetallic elements, according to the EDS elemental composition of the discovered spots.
Figure 6h depicts the Si distribution, while
Figure 6i and
Figure 7j depict the Fe and Mn elemental distributions, respectively.
In terms of property values, the specimen performed better than pure Al6061. Furthermore, for tensile strength, tensile modulus, compressive strength, and flexural strength, specimen 4% PKA/6% RHA outperformed its 6% PKA/6% RHA counterpart by 0.7, 5.6, 24.2, and 0.7%, respectively. The disparity is still associated with the difference in PKA and RHA content, as revealed in
Table 4. This is also linked to the microstructure of the specimen at 4% PKA/6% RHA having more area of coverage for intermetallic phases than its counterpart obtained with 6% PKA/4% RHA. The results for tensile strength and flexural strength are fairly close, with no appreciable differences. In comparison with specimens prepared at 800 °C with the ones at 900 °C, there is a clear disparity. In terms of tensile strength, tensile modulus, compressive strength, and flexural strength, specimens 2% PKA/6% RHA cast at 800 °C outperformed specimens 4% PKA/6% RHA cast at 900 °C by 3.09, 35.5, 2.3, and 11.4%, respectively. Similarly, and regarding the same set of responses, specimens of 6% PKA/2% RHA cast at 800 °C beat specimens of 6% PKA/4% RHA cast at 900 °C by 1.7, 26.4, 13.5, and 2.4% serially. This is a clear indication that the specimens cast at 800 °C performed better than the ones cast at 900 °C. The findings are linked to the moderate viscosity of the melt, which engendered even dispersion of the particles as noted in the microstructure. As a result of the dispersion, even the distribution of stress is kindled, thereby improving performance under loading. More so, the intermetallics present in the specimen cast at 900 °C are detrimental to the matrix, resulting in lower strength performance under loading owing to their brittle nature.
3.5. Process Mapping
As explained by Akinwande et al. [
62,
63] process maps for a given response are 2D views in which design points are connected by contour lines. The process of mapping the responses investigated in this study is presented in
Figure 14,
Figure 15,
Figure 16 and
Figure 17. The contour plots show different sections where different ranges of responses are obtained at different combinations of the independent variables. Important sections are the sections tagged “optimum region” where optimum response ranges are realizable at a diverse combination of the parameters.
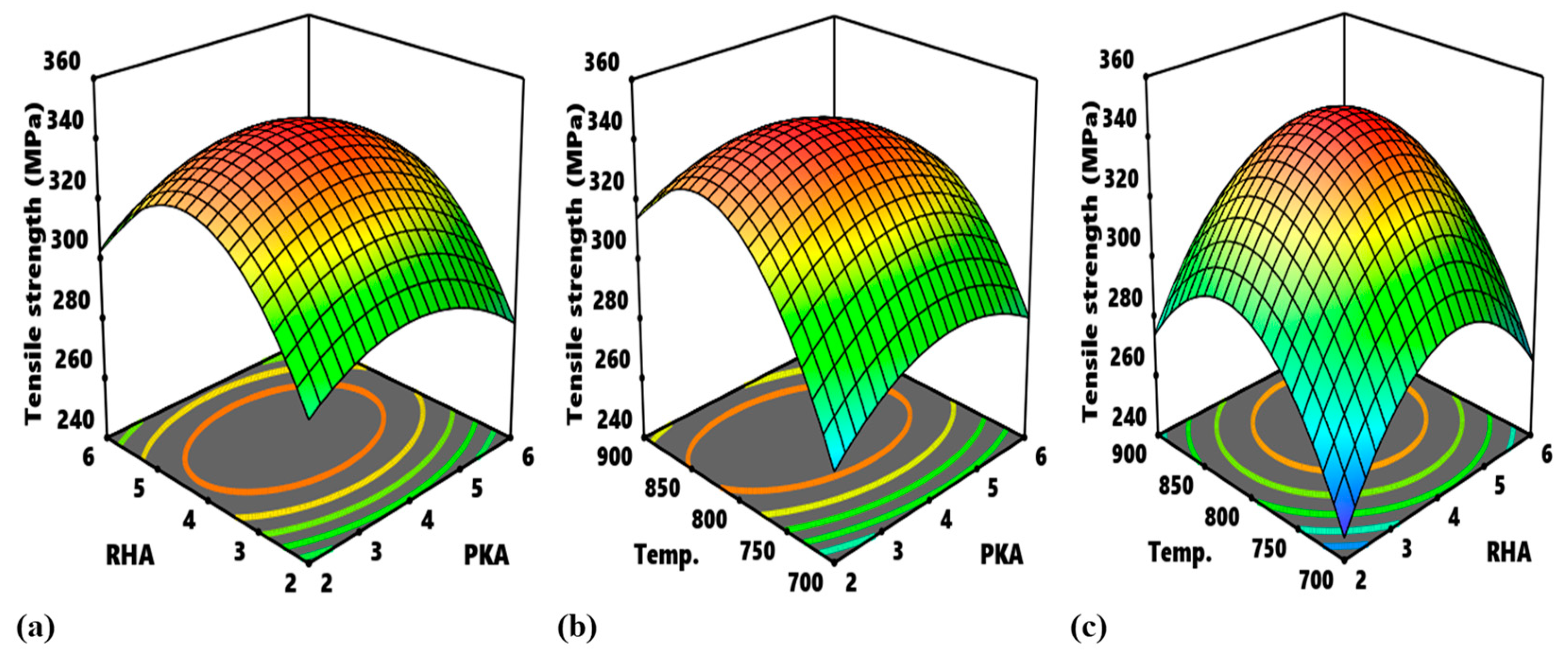
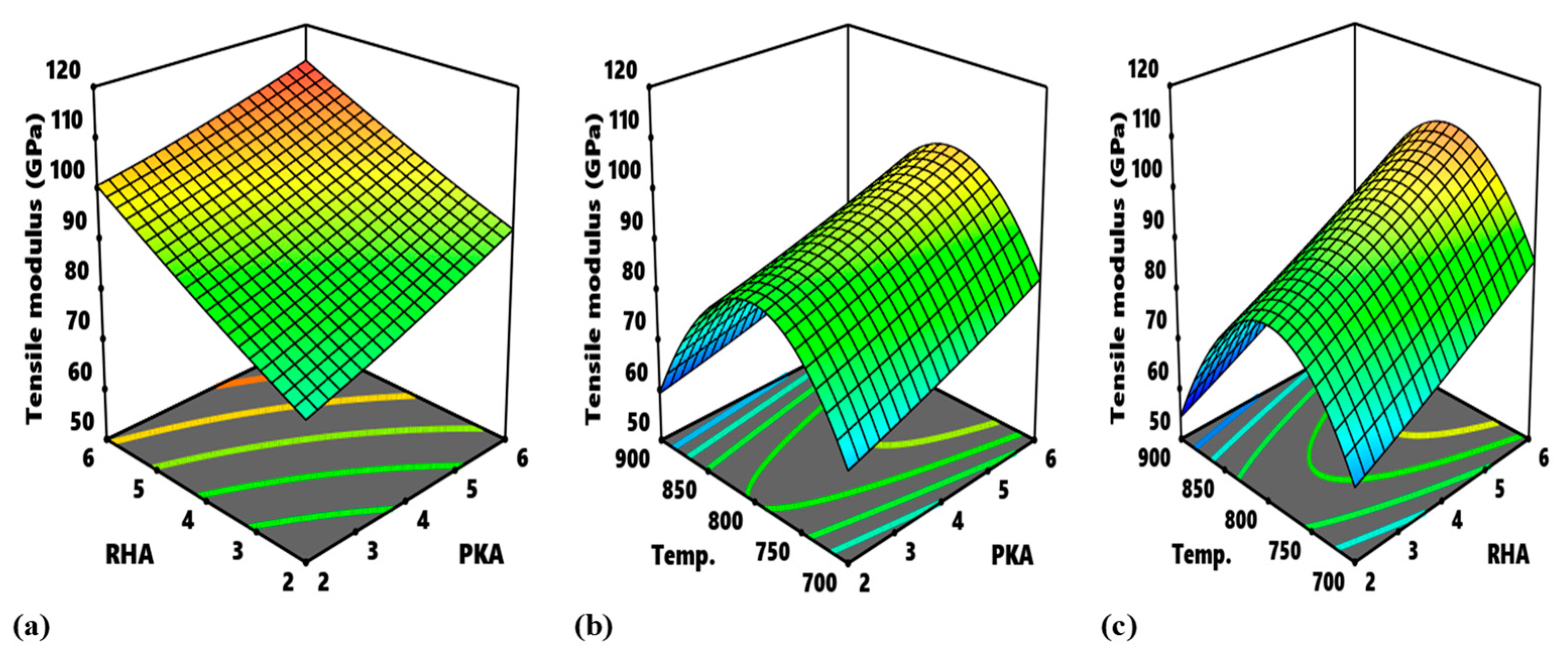
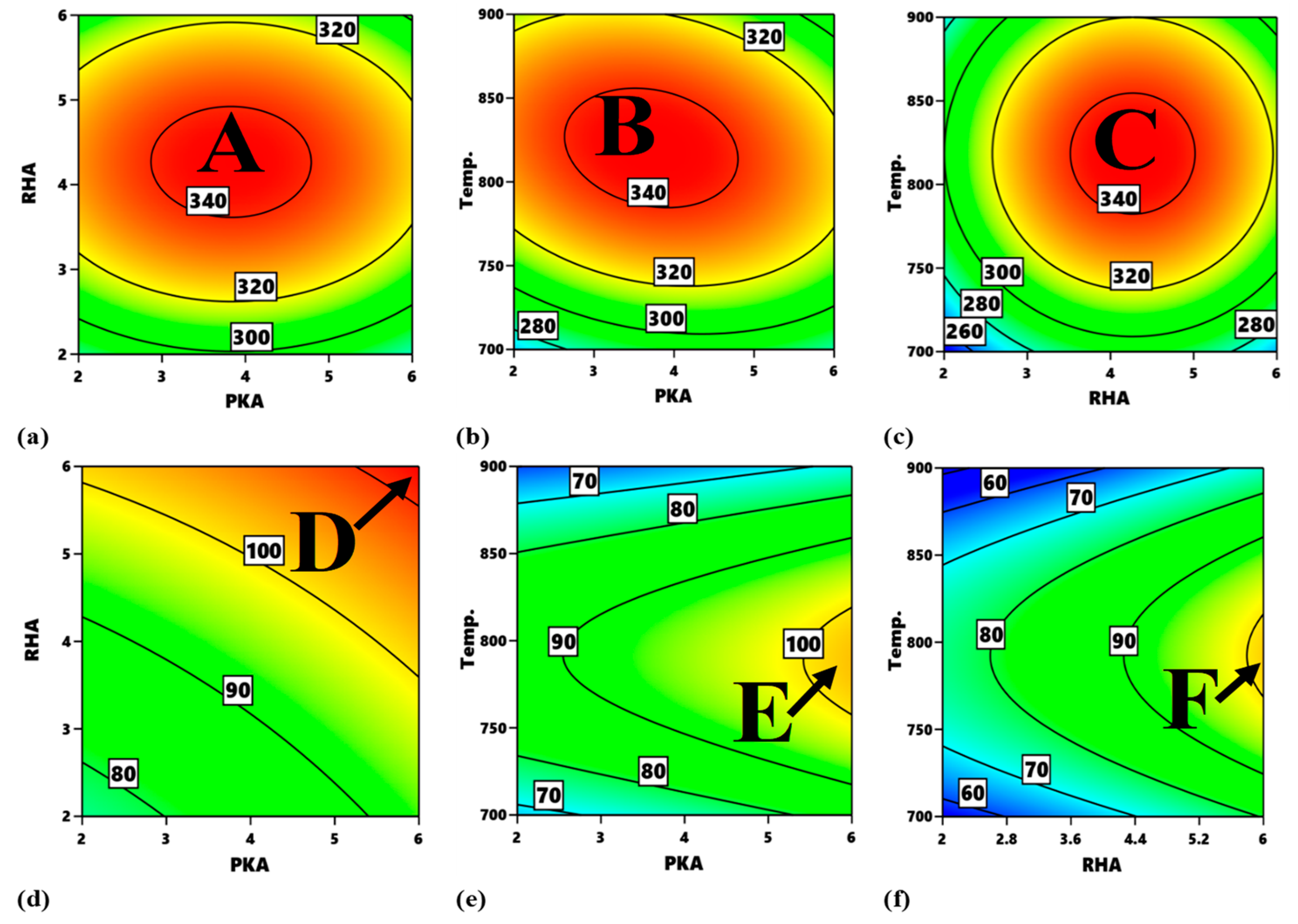
Figure 14 highlights the process map for the tensile strength and tensile modulus. In
Figure 14a, the portions, labelled A, are the optimum zones for the interaction of PKA and RHA when maintaining a stirring temperature of 800 °C. The contour intervals in ascending order are indicated as 280–300 MPa, 300–320 MPa, 320–340 MPa, and 340–360 MPa. The interval of 340–360 MPa is the optimum zone for the optimization of flexural strength at a constant 800 °C. The corresponding input variables for the optimization are 2.75–4.7% PKA and 3.6–4.8% RHA. In the same way, in
Figure 14b, as regards the interplay between PKA and temperature, the region labelled B is the optimum zone for the optimization of tensile strength when holding RHA constant at 4 wt.%. Hence, in optimizing tensile strength, the blend of 2.55–3.85 PKA with 4% (constant) RHA at the temperature of 780–865 °C is recommended. The contour plot for tensile strength at a constant value of 4 wt.% for the relationship “RHA vs. temperature” is identified in
Figure 14c. Point C is the portion of the attainment of optimum tensile strength in the range of values of 340–360 MPa. That means that, for the optimization of tensile strength for that interaction, 3.6–4.9% RHA combined with 4% PKA can be employed at a temperature range of 785–860 °C.
The contour plot for the interaction between PKA and RHA at an unchanging temperature of 800 °C with regard to tensile modulus is exhibited in
Figure 14d and outlines the regions in which varying response values can be obtained. The optimal domain is marked D, and it’s the region for optimization of tensile modulus in-range value of 110–120 GPa. The input variable for the region is 5.5–6.0% PKA and 5.6–6.0% RHA.
Figure 14e highlights the contour map for the interaction of PKA vs. temperature when sustaining RHA at 4%. The region marked E is the optimal area and values of 5.5–6.0% for PKA and 760–815 °C for temperature will suffice in the optimization of tensile modulus. In
Figure 14f, the region labelled “F” is regarded as the optimal zone for the interaction between RHA and temperature. In optimizing flexural modulus within the identified region, input variables are stated to be in the range of 5.65–6% for RHA and 770–820 °C for temperature.
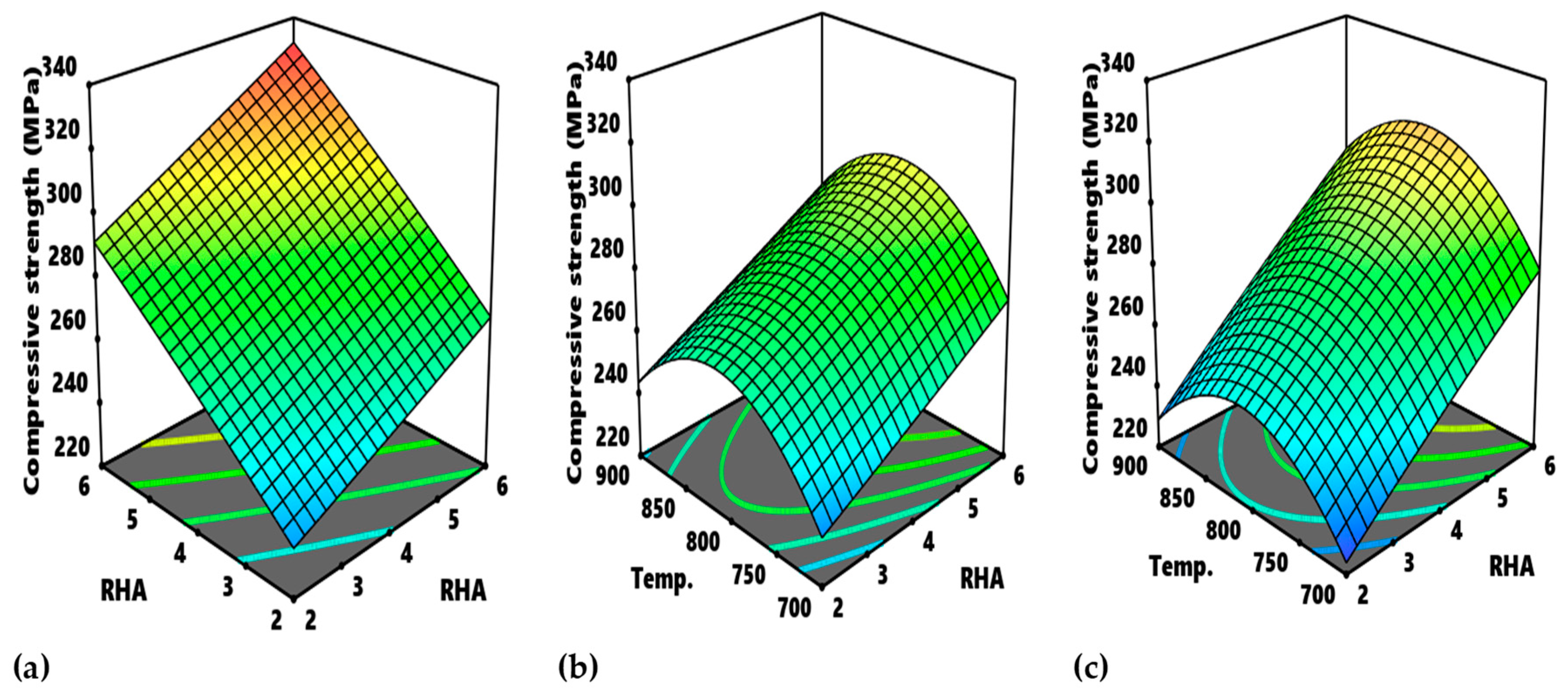
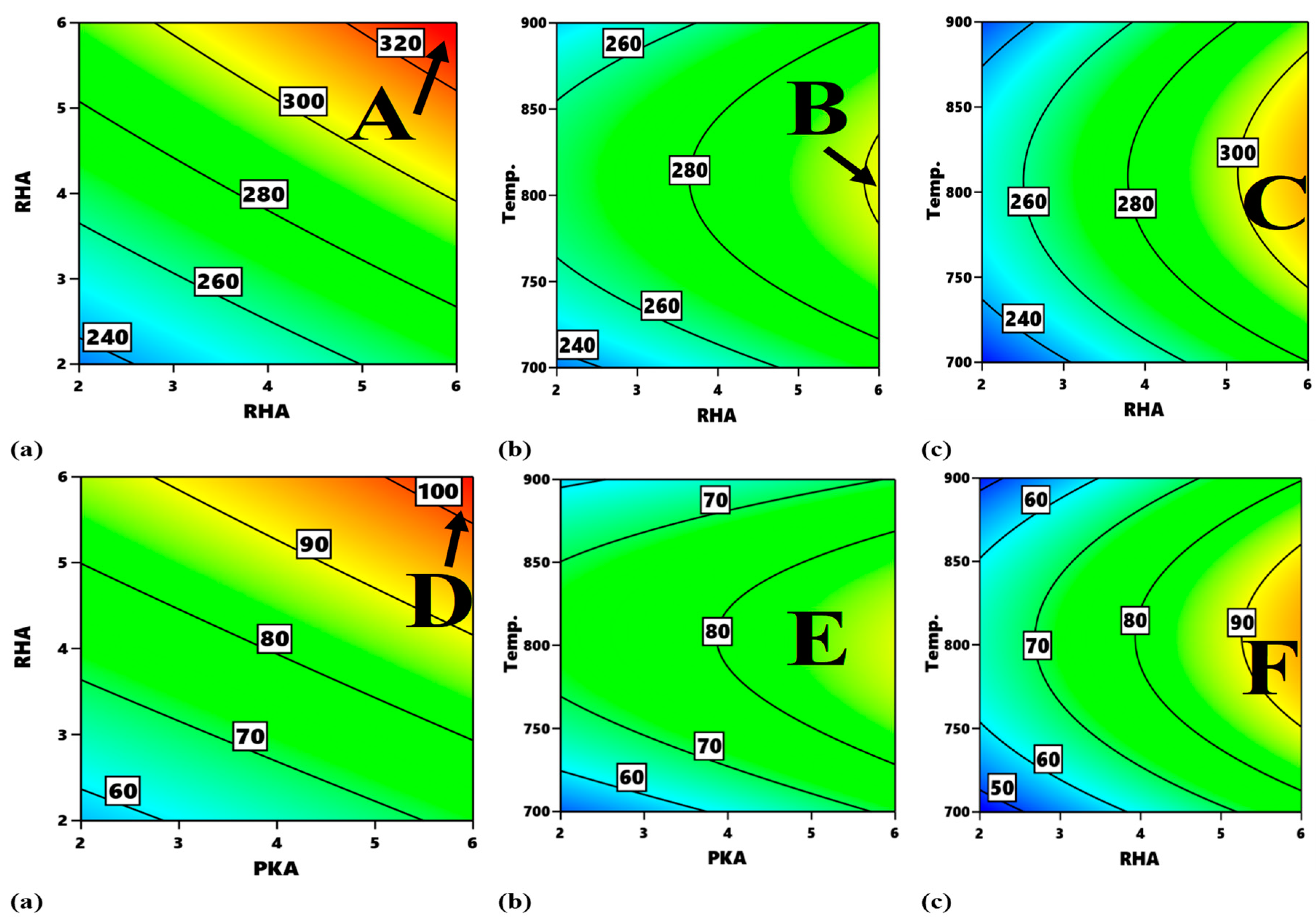
Figure 15 presents the contour plot for different interactions with respect to compressive strength and modulus.
Figure 15a refers to the contour plot of the interaction between PKA vs. RHA pertaining to compressive strength. The optimum zone for the optimization of compressive strength for such interaction is indicated as “A”, corresponding to 4.6–6.0% PKA and 5.3–6.0% RHA. It, therefore, means that optimal compressive strength between 320 and 340 MPa is visible within that range of values. Likewise, in
Figure 15b, as regards the interplay between PKA and temperature, the region labelled B is the optimum zone for the optimization of compressive strength when holding RHA constant at 4 wt.%. Therefore, in maximizing compressive strength response, the blend of 5.8–6.0% PKA with 4% (constant) RHA at a temperature of 725–825 °C is recommended. The contour plot for compressive strength at a constant value of 4 wt.% for the relationship “RHA vs. temperature” is showcased in
Figure 15c. Point C is the portion of the attainment of optimum compressive strength in the range of values of 300–320 MPa. That means that, for optimization of the strength response for that interaction, 5.4–6.4% RHA combined with 4% PKA can be employed at a temperature range of 740–870 °C.
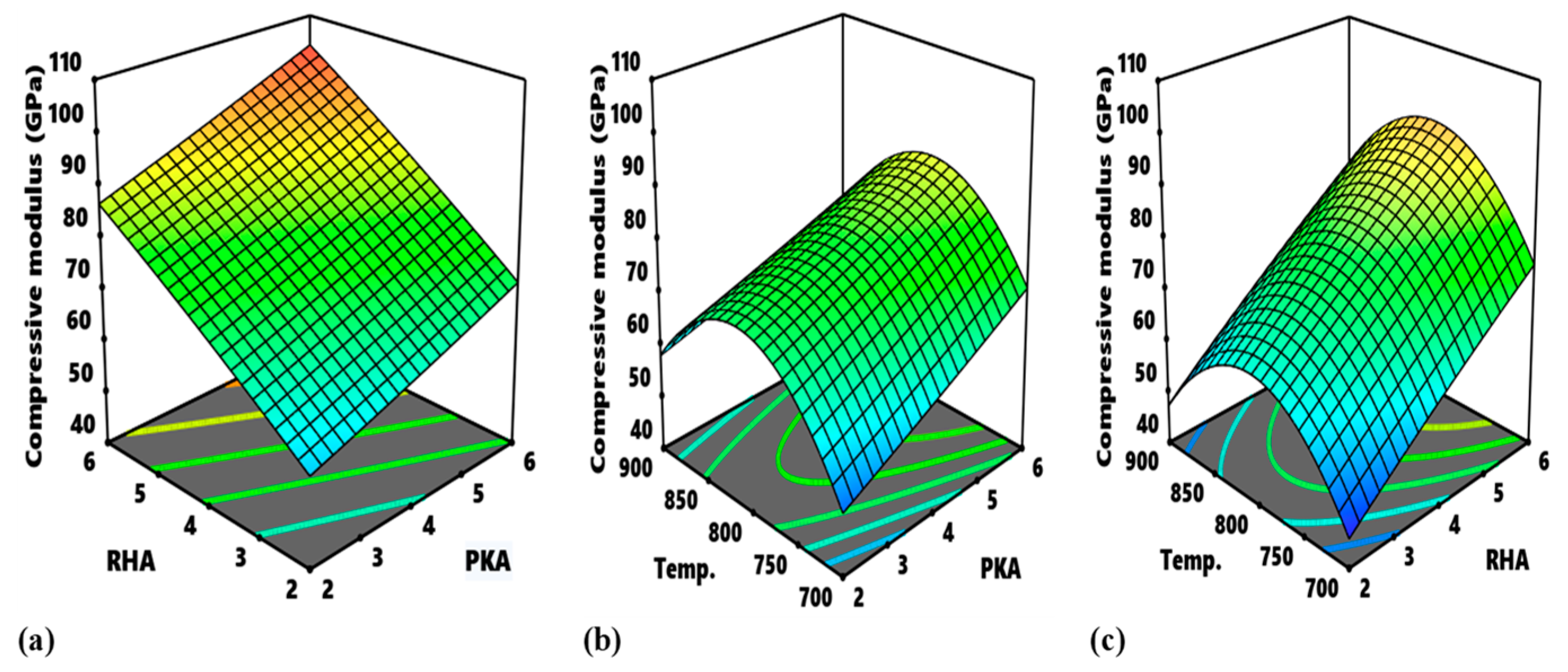
With respect to compressive modulus, the contour map for the relationship between PKA and RHA at an uninterrupted temperature of 800 °C is exhibited in
Figure 15d, outlining the regions in which varying response values can be obtained. The optimal domain is marked D, and it’s the region for optimization of the modulus in-range value of 100–110 GPa. The input variable for the region is 5.3–6.9% PKA and 5.3–6.9% RHA.
Figure 15e highlights the contour map for the interaction of PKA vs. temperature when sustaining RHA at 4%. The region marked E is the optimal area and values of 4.1–6.1% for PKA and 730–860 °C for temperature will suffice in the optimization of compressive modulus. In
Figure 15f, the region labelled “F” is regarded as the optimal zone for the interaction between RHA and temperature. In optimizing compressive modulus within the identified region, input variables are stated to be in the range of 5.55–6.0% for RHA and 775–855 °C for temperature.
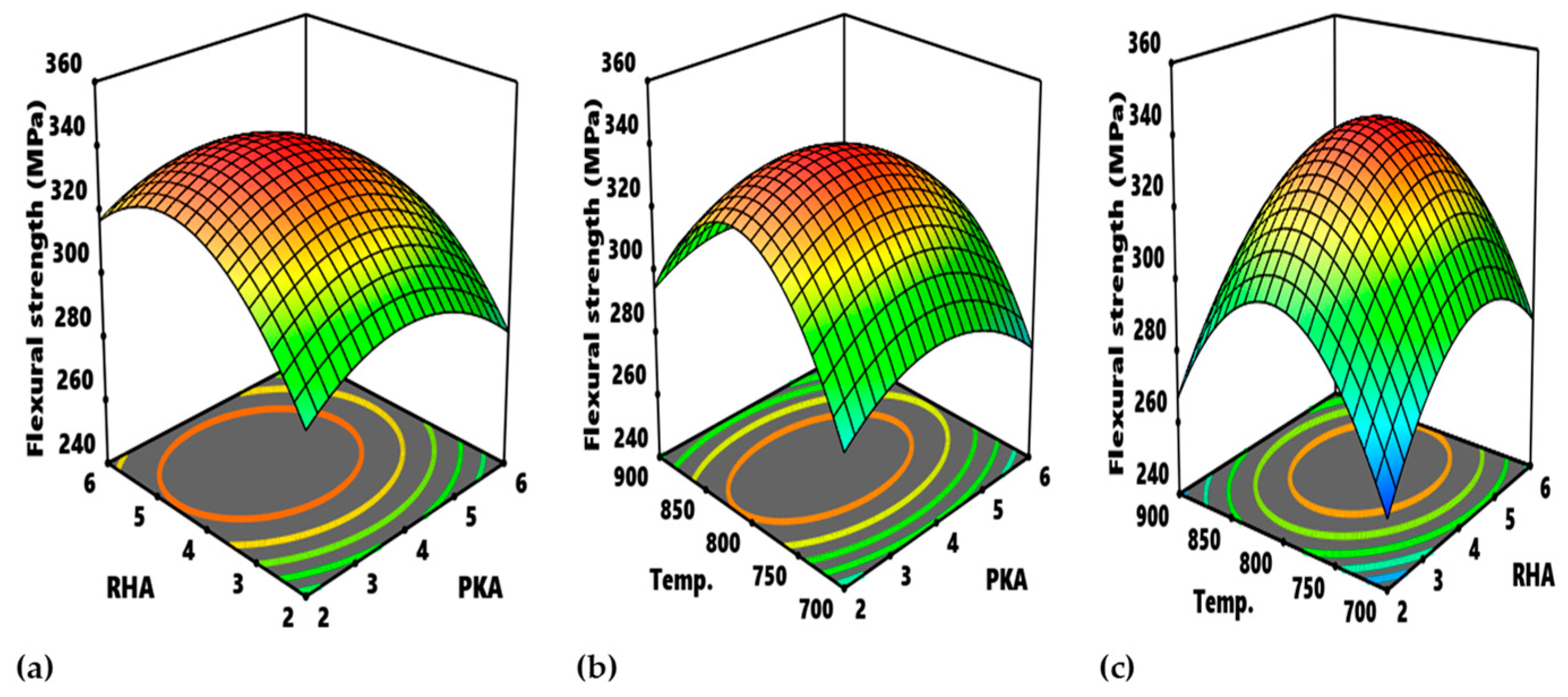
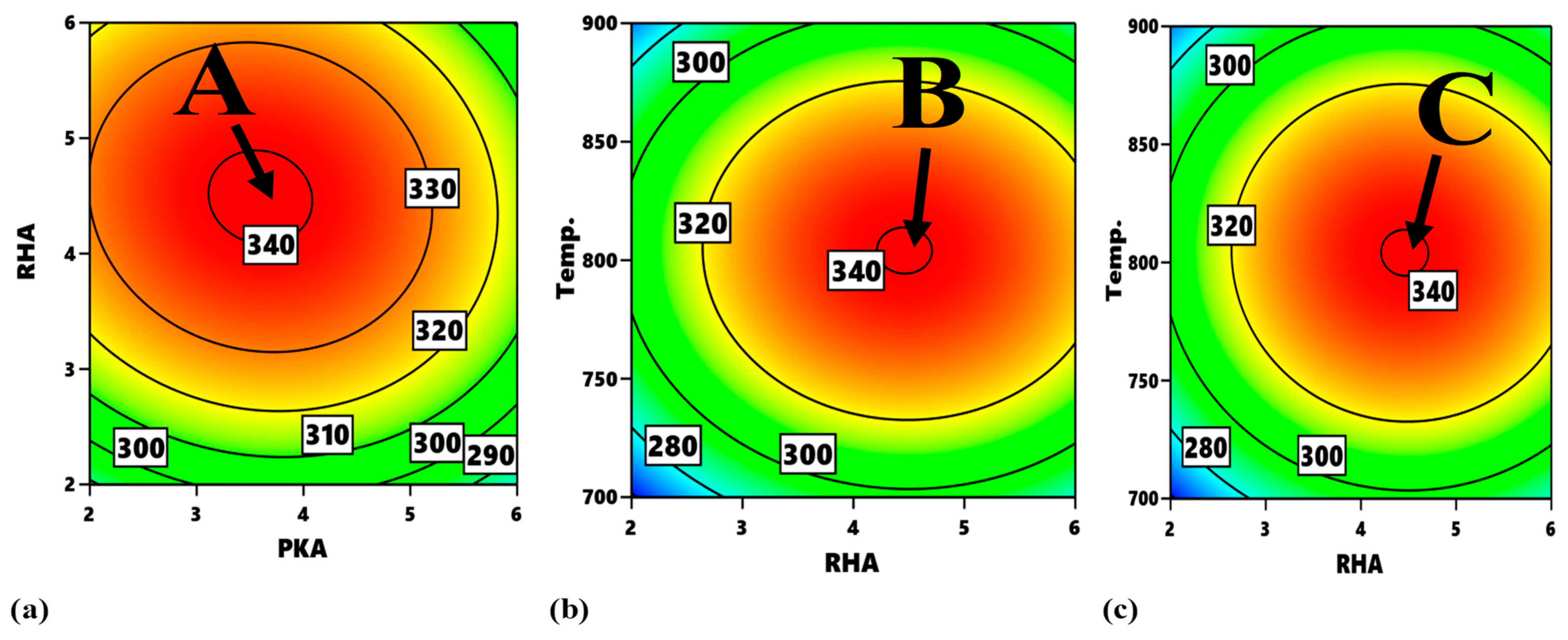
In
Figure 16, the contour plot of interactions between experimental variables with regard to flexural strength is identified. With reference to
Figure 16a, the portions, labelled A, are the optimum zones for the interaction of PKA and RHA when maintaining the temperature at 800 °C. The corresponding input variables for the optimization are 4.2–4.83% PKA and 3.4–4.3% RHA. Similarly,
Figure 16b displays the contour plot for the interplay between PKA and temperature. When RHA is held constant at 4 wt.%, the region labelled B is the optimum zone for optimizing flexural strength. When RHA is held constant at 4 wt.%, the region labelled B is the optimum zone for optimizing flexural strength. It is therefore estimated that the blend of 4.4–4.8 PKA with 4% (constant) RHA at a temperature of 792–817 °C will beget optimum flexural performance. The contour plot for the relationship “RHA vs. temperature” is identified in
Figure 16c (at a constant value of 4 wt.%). Point C is the division of the plot maximizing flexural strength response within the range of values of 340–360 MPa. That means that, for optimization of flexural strength as regards the interaction, 4.4–4.6% RHA combined with 4% PKA can be employed at a temperature range of 790–823 °C.