1. Introduction
Calcium phosphates are biocompatible, bioactive, and effective for bone tissue regeneration [
1,
2]. Low-temperature ceramics based on calcium phosphate cements were formed according to cement technology by the interaction of the initial components in an aqueous medium due to setting and hardening. The high specific surface area and increased resorption rate compared to matrices obtained by high-temperature treatment make low-temperature ceramics a promising material for bone tissue restoration [
3,
4,
5,
6].
Brushite cement is one of the main types of biocements for the production of low-temperature ceramics. Dicalcium phosphate dihydrate CaHPO
4·2H
2O (brushite) is usually obtained by the interaction of tricalcium phosphate (TCP) and monocalcium phosphate monohydrate (MCPM) with the formation of a metastable phase under physiological conditions [
7,
8,
9]. Due to its metastability, brushite-based ceramics have a high resorption rate and high potential as drug delivery systems (vancomycin, doxycycline, chlorhexidine, trolox), protein growth factors [
10,
11,
12], and as a material for bone replacement [
10,
13].
It is known that the presence of the bioactive Mg
2+ ion in the structure of calcium phosphates plays an essential role in the biological process and stimulates bone formation [
14,
15,
16,
17]. Magnesium is an obligate cofactor of alkaline phosphatase and many enzymatic reactions, which participates in the synthesis of bone collagen in cell proliferation and differentiation, cell–matrix interaction, and the normal functioning of organs [
18,
19,
20,
21].
One of the important features of the presence of the magnesium ion Mg
2+ in the composition of brushite when replacing the calcium ion Ca
2+ is the stabilization of the phase composition [
20,
21,
22,
23,
24]. Magnesium ions inhibit the formation of less soluble calcium phosphates (hydroxyapatite, tricalcium phosphate, octacalcium phosphate) in vivo in brushite materials [
24,
25,
26,
27,
28,
29,
30], since magnesium acts as a strong inhibitor of crystal growth in less soluble calcium phosphates. In this regard, magnesium calcium phosphate ceramics do not recrystallize over time, unlike brushite, during a long-term in vivo experiment. The high rate of resorption is maintained [
21,
31].
The formation of an additional phase of dimagnesium phosphate MgHPO
4·3H
2O trihydrate (newberyite) in addition to the main phase of brushite CaHPO
4·2H
2O can increase the rate of resorption. When high concentrations of Mg
2+ ions were introduced into the composition of brushite cement, newberyite was formed. Its solubility is at 25 °C-log(Ksp) is 5.51–5.82, whereas the solubility of brushite-log(Ksp) = 6.59 [
7,
16,
17,
23,
32,
33].
Volumetric resorption of newberyite and superficial resorption of brushite were observed by Klammert et al. using a model of subcutaneous implantation in rats [
17].
We assumed that the advantage of a two-phase product of a controlled composition may be a different rate of volumetric dissolution of the phases with the preservation of the geometric shape of the matrix, followed by its germination by newly formed bone tissue and gradual complete resorption of the matrix.
Brushite cement has a very short setting time, as is known. Despite the fact that rheological properties are not crucial for the production of ceramic matrices using cement technology, the setting time should be sufficient to form scaffolds of the required geometry. Additives of substances containing P
2O
74− pyrophosphate- and C
6H
5O
73− citrate-ions are the most common and effective regulators of the setting of brushite cements [
34,
35,
36,
37]. After adsorption, these ions form poorly soluble compounds on the particles of the initial components and reduce the electrostatic attraction. The reaction is slowed down. It is also known that when calcium ions are replaced with magnesium in TCP-based brushite cements, the setting time increases, and the mechanical properties of calcium phosphates improve [
33].
However, none of the abovementioned reports have focused on the study of the effect of Mg substitutions in TCP, leading to the production of ceramics based on brushite-newberyite cement containing set-retarding additives, on the properties of ceramics, including solubility and biocompatibility.
Therefore, the main purpose of this study was to investigate the effect of Mg substitutions in TCP in the production of magnesium calcium phosphate ceramics based on brushite-newberyite cement containing set-retarding additives.
2. Materials and Methods
Mg
xCa
(3−x)(PO
4)
2 was obtained during solid-phase synthesis of a homogeneous mixture of the precursors CaHPO
4, CaCO
3, Mg
2P
2O
7, and Mg(OH)
2 in accordance with
Table 1 at a temperature of 1100 °C for 6.5 h. The sintered material was crushed in a planetary ball mill for 20 min in an acetone medium with zirconium oxide balls with a diameter of 10 mm at a ratio of powder weight/ball weight = 1/3. The powder was dried at a temperature of 50 °C.
Magnesium-calcium phosphate ceramics were obtained during acid–base interaction by mixing synthesized MgxCa(3−x)(PO4)2 (x = 0–2.25—compositions Mg0, Mg0.75, Mg1.5, Mg2.25) and monocalcium phosphate monohydrate Ca(H2PO4)2·H2O with liquid content in a ratio of 55/45. The mixing liquid was distilled water or a solution of sodium pyrophosphate hexadydrate (SPP) salt or a solution of citric acid monohydrate (CA). The liquid/powder ratio was (L/P) 0.6 or 0.7.
The mechanical compressive strength of the matrices was determined on samples using cylinders with a diameter of 10 mm and a height of 10 mm by means of the universal testing machine LFV 10–50 T (Walter + Bai AG, Switzerland) at a loading speed of 8 mm/min on the 1st day after molding. Samples were stored in a 100% humidity box at 37 °C and dried. The results of the compressive strength test were expressed as the mean ± standard error of the mean of fivefold determinations.
The setting time of the cement pastes was determined using a small Vicat needle technique. Half a minute after placing the pastes into a metallic ring with a height of 9 mm, the indenter (136 gain mass, 1 mm diameter of the needle) was lowered vertically onto the surface of the cement. The indentation was repeated at intervals of 5 s. The time at needle penetrations of 1 mm was taken as the initial setting time. The time at needle penetrations of 9 mm was taken as the finishing time. The results of the determination of the setting time are the average value of the threefold definitions.
The pH of the medium in contact with the materials was determined using the laboratory pH meter Seven Compact S210 (Mettler-Toledo GmbH, Greifensee, Switzerland). The ratio of the weight of the sample granules with a diameter of 4 mm and a height of 2 mm to the mass of distilled water was 1:10. The pH of the contact medium was measured after 10 min while keeping samples in distilled water. The results of the pH determination were recorded as the mean value ± standard error of the mean value of the fivefold definitions.
The granulometric composition was studied using the particle size and shape analyzer CAMSIZER® X2 (MICROTRAC RETSCH GmbH, Haan, Germany). The results of the determination of the particle size and surface area were expressed as the average value ± standard error of the average value of threefold definitions.
The porosity was determined on samples using cylinders with a diameter of 10 mm and a height of 10 mm by hydrostatic weighing in an inert liquid (kerosene) on the 1st day after molding. Samples were stored in a 100% humidity box at 37 °C and dried. The results of the porosity determination were expressed as the average value ± standard error of the average value of the fivefold definitions.
X-ray powder diffraction (XRD) analysis used an ARL Equinox 1000 X-ray diffractometer (Thermo Fisher Scientific INEL SAS, Saint-Herblain, France). The survey was carried out in the reflection mode using CuKa radiation (angle interval 2Θ: from 5° to 45° or 68°, step 2Θ 0.03°). In the qualitative analysis of the resultant X-ray diffraction patterns, we used WinXPOW software and the ICDD PDF-2 database.
In the quantitative analysis, we used the reference intensity ratio method (with the I/Ic intensity ratio of the strongest lines of the substance and corundum (α-Al
2O
3) in a mixture containing 50 wt. % of both components). (Chung method) [
38]. The weight fraction was calculated using the relation
where
IiA is the measured intensity of the
ith reflection from phase A;
IreliA is the relative intensity of this reflection in the database;
I/
Ic(
A) is the corundum number for the phase
A being determined; and
IiK,
IreliK, and
I/
Ic(
K) are the corresponding quantities for all components of the mixture (including
A). The calculation was carried out on characteristic lines, on which the lines of other phases were not superimposed. XRD measurements for all the investigated compositions were repeated two or three times in two or three independent samples. The results of the XRD determination were expressed as the average value ± standard error of the average value of the threefold definitions in in vitro experiments and of the twofold definitions in in vivo experiments.
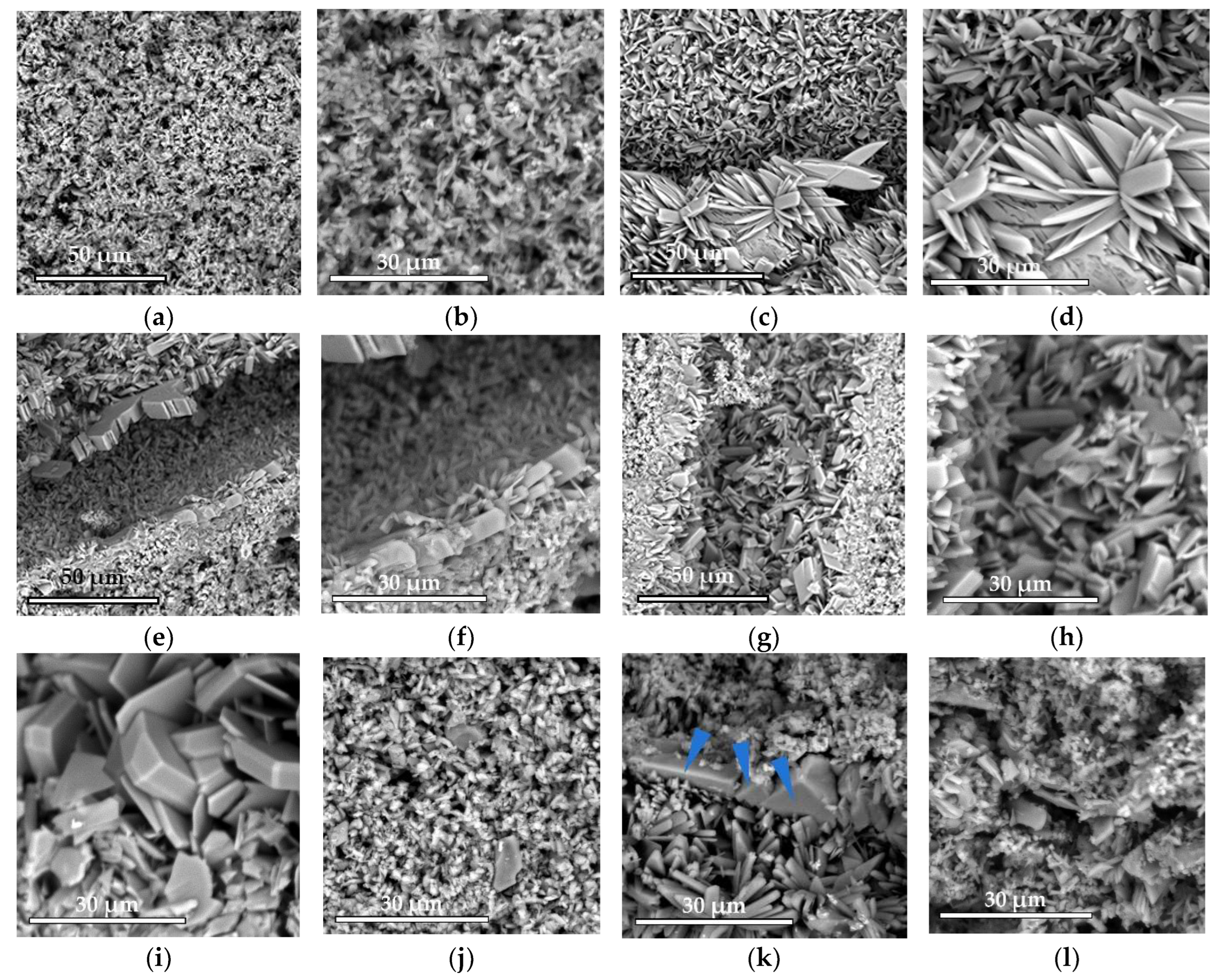
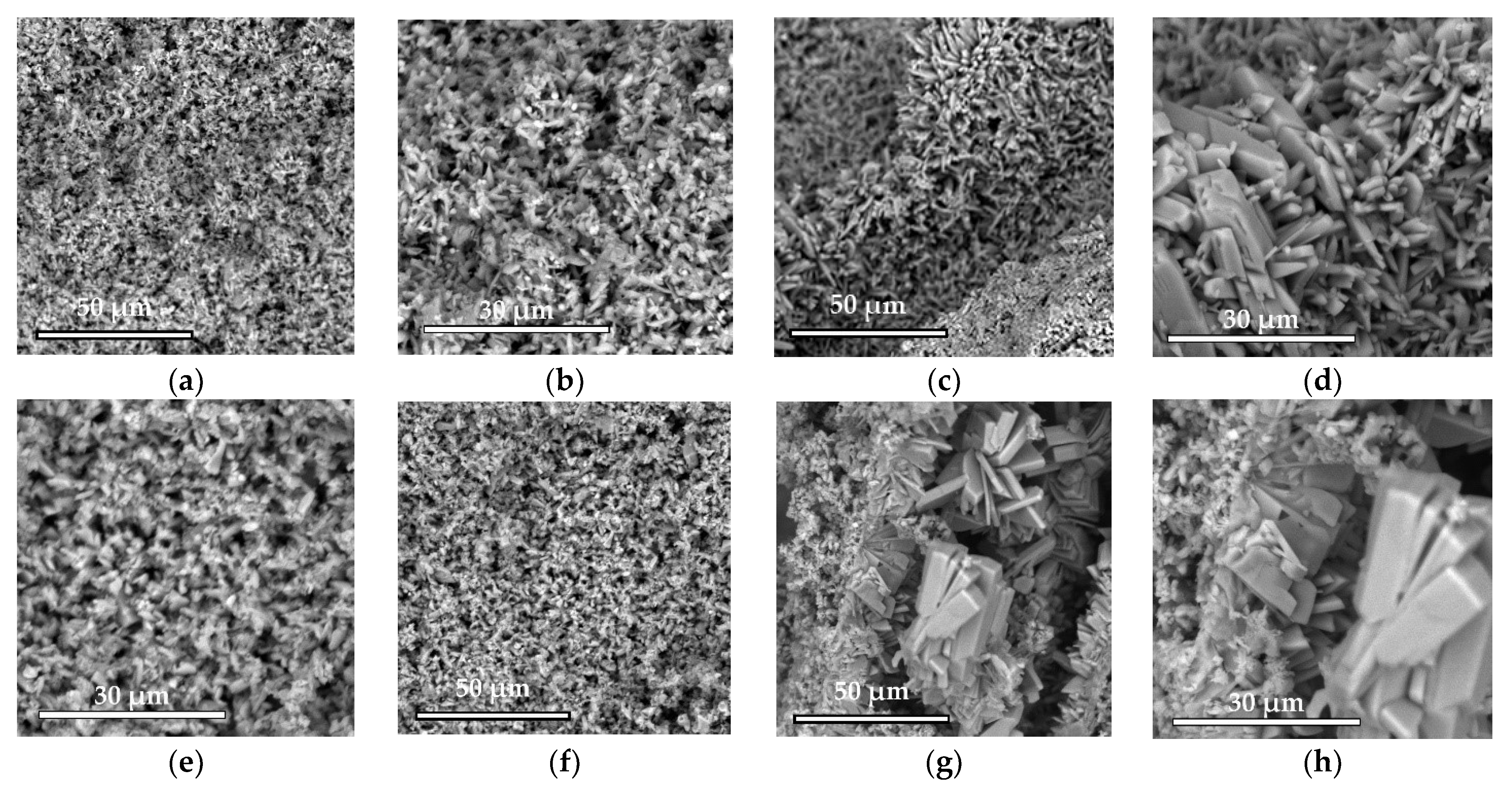
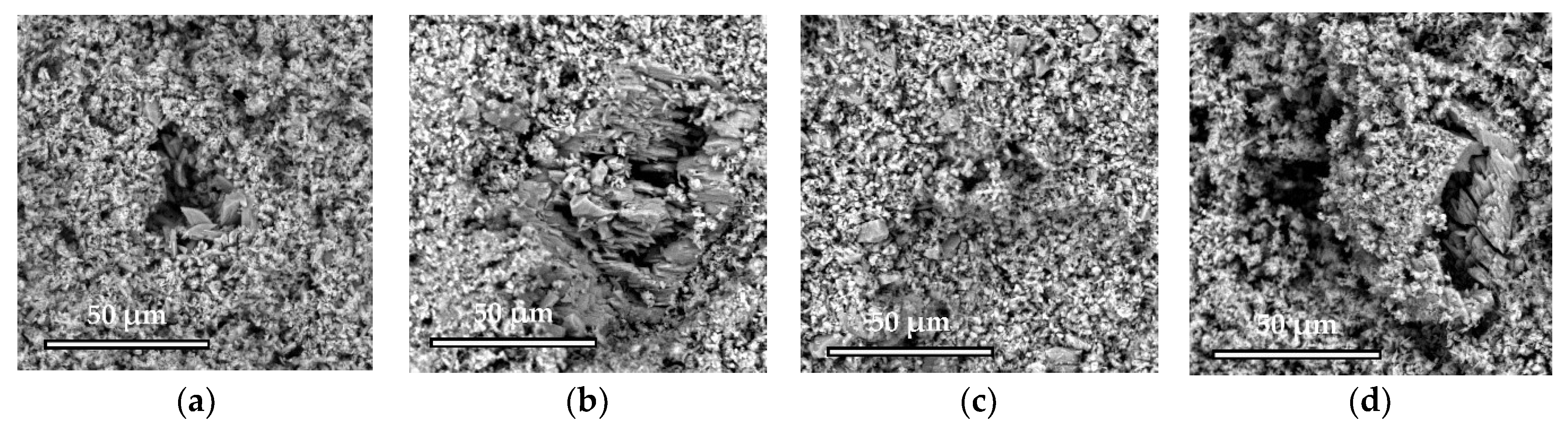
The microstructure was studied using a scanning electron microscope Phenom XL G2 (Thermo Fisher Scientific, Breda, Netherlands). The accelerating voltage was 15 kV. Silver was sprayed with the magnetron sprayer Cressington 108auto (Cressington Scientific Instruments Ltd., Watford, UK).
Elemental analysis was carried out by means of a special detector (energy dispersion spectrometer); data analysis was carried out in the INCA—Point & ID program. The results of EDS determination were expressed as the value of the onefold definition.
The solubility of ceramics samples with a diameter of 4 mm and a height of 2 mm was evaluated by measuring the concentration of calcium and magnesium ions in saline solution. The ratio of granules to liquid was 1/10 mL. Samples were kept in a shaker incubator IKA KS 3000 (IKA®-Werke GmbH & Co. KG, Staufen, Germany) at 37 °C for 5 days. When choosing an aliquot, it was replaced with a fresh solution. The concentration of calcium and magnesium ions in the solution was determined on a PE540054005400 HA spectrophotometer (Ekros-Analytics LLC, Saint Petersburg, Russia) at a wavelength of 570 nm and 540 nm, respectively, during the formation of colored complexes of calcium ions with o-cresolphthalein complexone and of magnesium ions with xylidine blue. The results of the determination of the concentrations of calcium and magnesium in the solution were expressed as the average value ± standard error of the average value of threefold definitions.
Statistical analysis. The data were recorded as average ± standard error and analyzed using the Origin Pro 2021 software. One-way ANOVA tests (p < 0.05) were used to determine the significance of the differences between the groups. Significant differences (p < 0.05) between different materials at one time point are labeled with *.
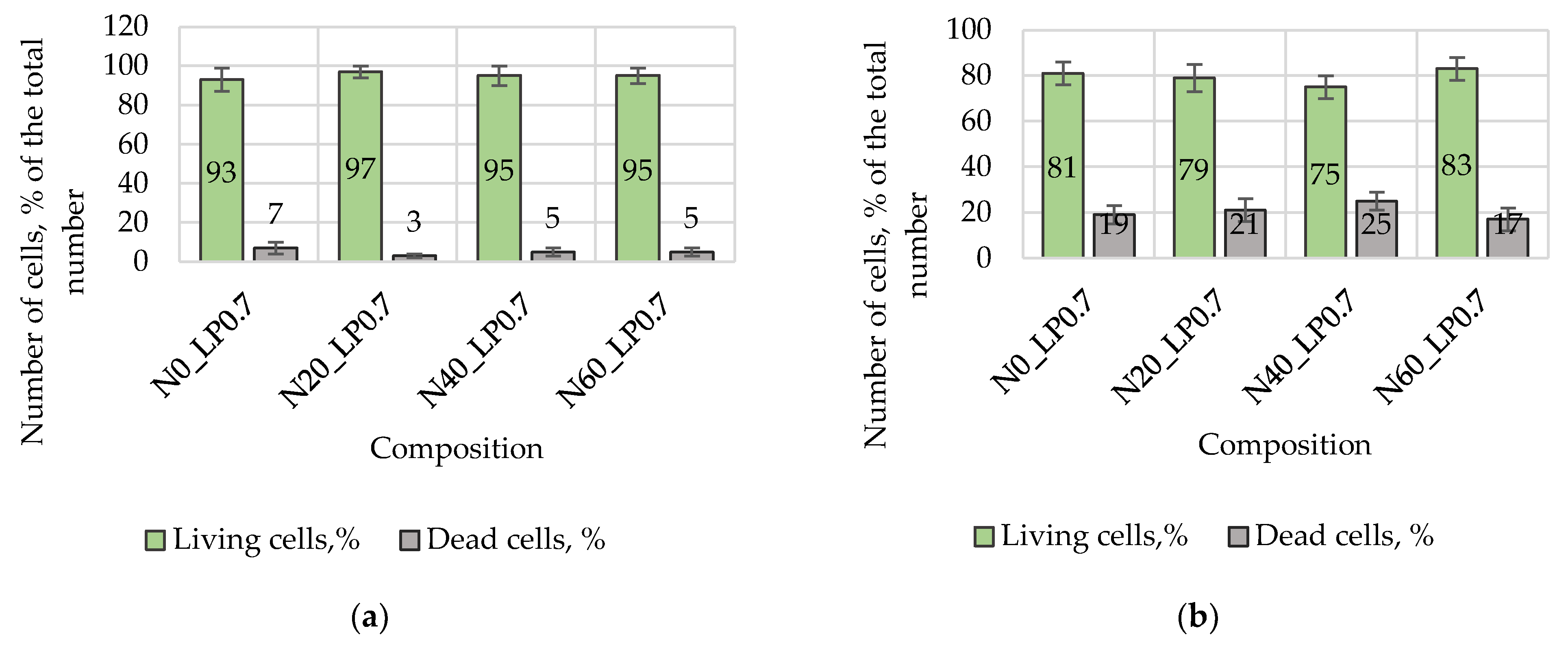
A cell viability test was carried out on samples of magnesium calcium phosphate matrixes with a diameter of 4 mm and a height of 2 mm. Mesenchymal stromal cells of human bone marrow were used. The cells were cultured in alfa MEM (Sigma-Aldrich, St. Louis, MO, USA) with the addition of 10% embryonic calf serum (Gibco, Carlsbad, CA, USA) and 40 mcg/mL gentamicin sulfate (Sigma-Aldrich, USA), at 37 °C, under conditions of 5% CO2 content in the air. Cultured cells of 1–4 passages were used in the experiments. Subcultivation of the cells was carried out in alfa MEM medium with 10% embryonic calf serum and 40 mcg/mL of gentamicin sulfate, at 37 °C, under conditions of 5% CO2 content in the air. The number of living and dead cells was determined by staining cells with fluorescent dyes calcein AM (Sigma-Aldrich, St. Louis, MO, USA) and propidium iodide (Sigma-Aldrich, St. Louis, MO, USA). The cells were detached from the surface of the samples using an Accutase cocktail. Cells were stained in L-15 medium with 1% embryonic calf serum containing 1 mcg/mL of calcein AM and 2 mcg/mL of propidium iodide for 25 min at 37 °C. The analysis of living and dead cells was carried out using an Accuri C6 flow cytometer (BD Bioscience, San Jose, CA, USA). The results of the experiments were presented as an average ± standard error.
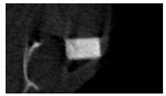
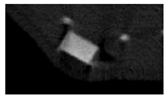
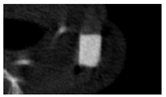
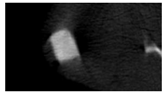
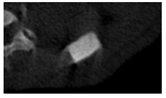
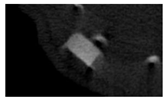
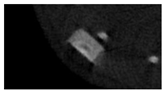
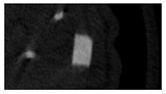
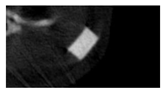
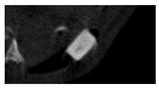
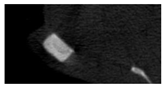
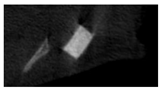
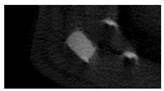
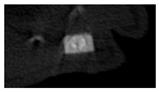
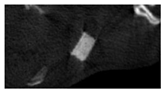
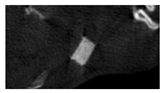
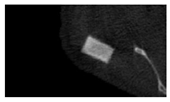
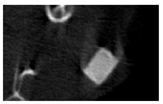
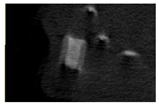
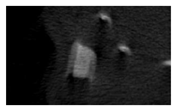
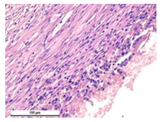
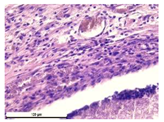
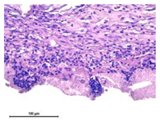
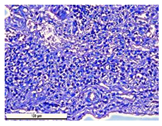
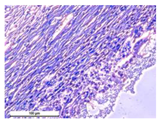
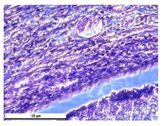
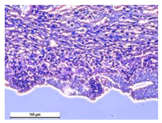
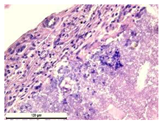
The study of the biological response of magnesium calcium phosphate matrixes was carried out with a Wistar rat model (a subcutaneous implantation into the hypodermis of male rats with a mass of ≈250–300 g). The total number of animals was 15. The animals were divided into three groups of 5. The animals in the first group were implanted with ceramic samples N0, N20, N40, and N60 containing PPS. Animals of the second group were implanted with ceramic samples N0, N20, N40, and N60 containing CA. Animals of the third group were implanted with ceramic samples N0, N20, N40, and N60 containing CA_PPS. The surgical inventions were fulfilled under intramuscular anesthesia with Zoletil (7 mg/kg) and Xylazine (13 mg/kg). The procedure was performed under aseptic conditions. Before the treatment, the surgical field was shaved and sanitized with an antiseptic. The animal was placed on its stomach. An incision was made along the spine. In the hypodermis, 4 caverns were formed by pushing the tissues apart. The samples were implanted in these caverns. The implantation period was 11 weeks. Allen surgical procedures and conditions of keeping animals complied with the ethical rules for experiments with animals, including the European Directive FELASA-2010. Tomographic examination was performed in vivo after surgery and then weekly after implantation.
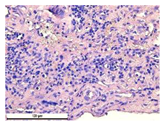
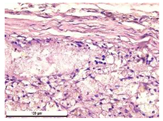
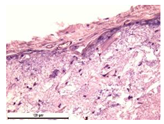
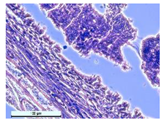
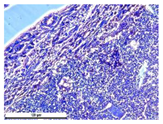
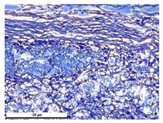
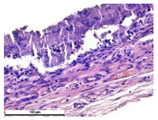
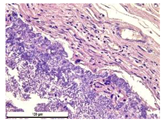
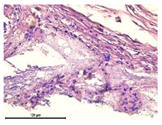
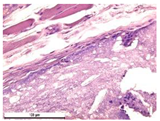
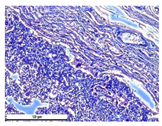
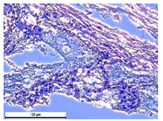
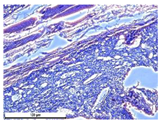
For morphological study, samples of connective tissue capsules were fixed in neutral buffered formalin (10%), dehydrated, and paraffin embedded. Sections (with a thickness of 4 μm) were prepared and stained with hematoxylin-eosin. They were studied with a Leica DM 4000 B LED (Leica Microsystems Wetzlar GmbH, Wetzlar, Germany) microscope with a Leica DFC 7000 T camera under standard light and phase-contrast visualization modes. A maturity of scar tissues around the samples was evaluated with its thickness and a relative area of blood vessels. The high maturity corresponds to a minimal inflammation in the low-thickness tissues with a small amount of the vessels. In the ImageJ program, the thickness of the capsules (at 50× magnification) and the relative area of the vessels (at 200× magnification) were measured from microphotographs of histological sections. Statistical analysis of the results was performed using an unpaired Student’s t-test and one-way ANOVA test followed by Tukey’s comparison test. The results were considered reliable at p ≤ 0.05. The procedure was carried out with GraphPad Prism 7.00. The results were presented in terms of the mean ± standard error.
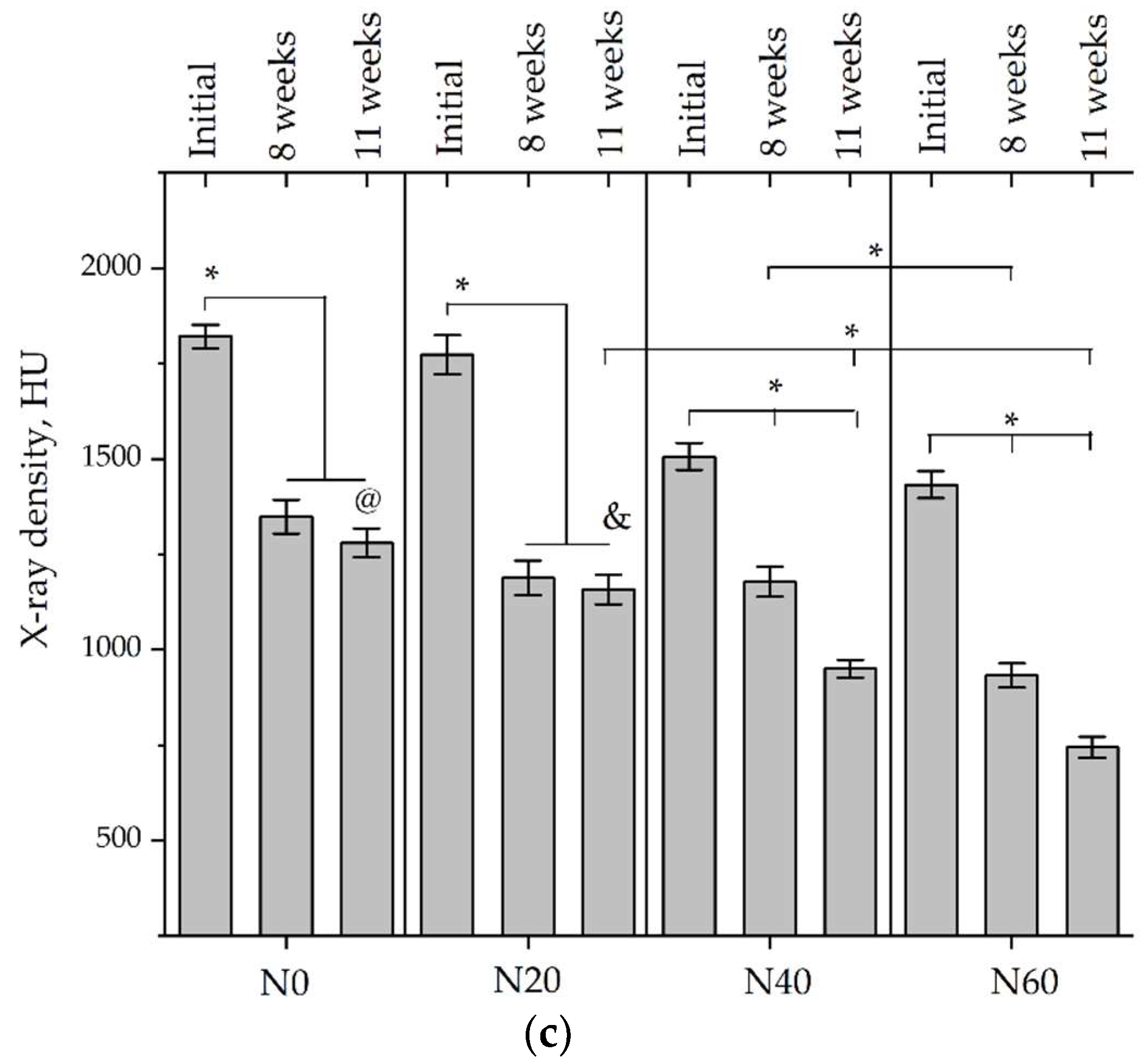
Micro-CT of the samples was performed on an X-ray microtomograph Bruker SkyScan 1178 (BRUKER BELGIUM, Kontich, Belgium) at a voltage of 65 Kv and a current of 615 Ma, with an Al 0.5 mm filter. The spatial resolution was 84 microns/pixel. The reconstruction of the slices was carried out with NRecon v.1.6.10.4 software, and densitometric measurements were made in the CTAn v.1.20.3.0 program. The data were recorded as the average ± standard error and analyzed using Origin Pro 2021 software. One-way ANOVA tests (p < 0.05) were used to determine the significance of the differences between the groups. Significant differences (p < 0.05) between different materials at one time point were labeled with *, and significant differences at a specific time point between different groups were labeled with #, @, and &.
4. Discussion
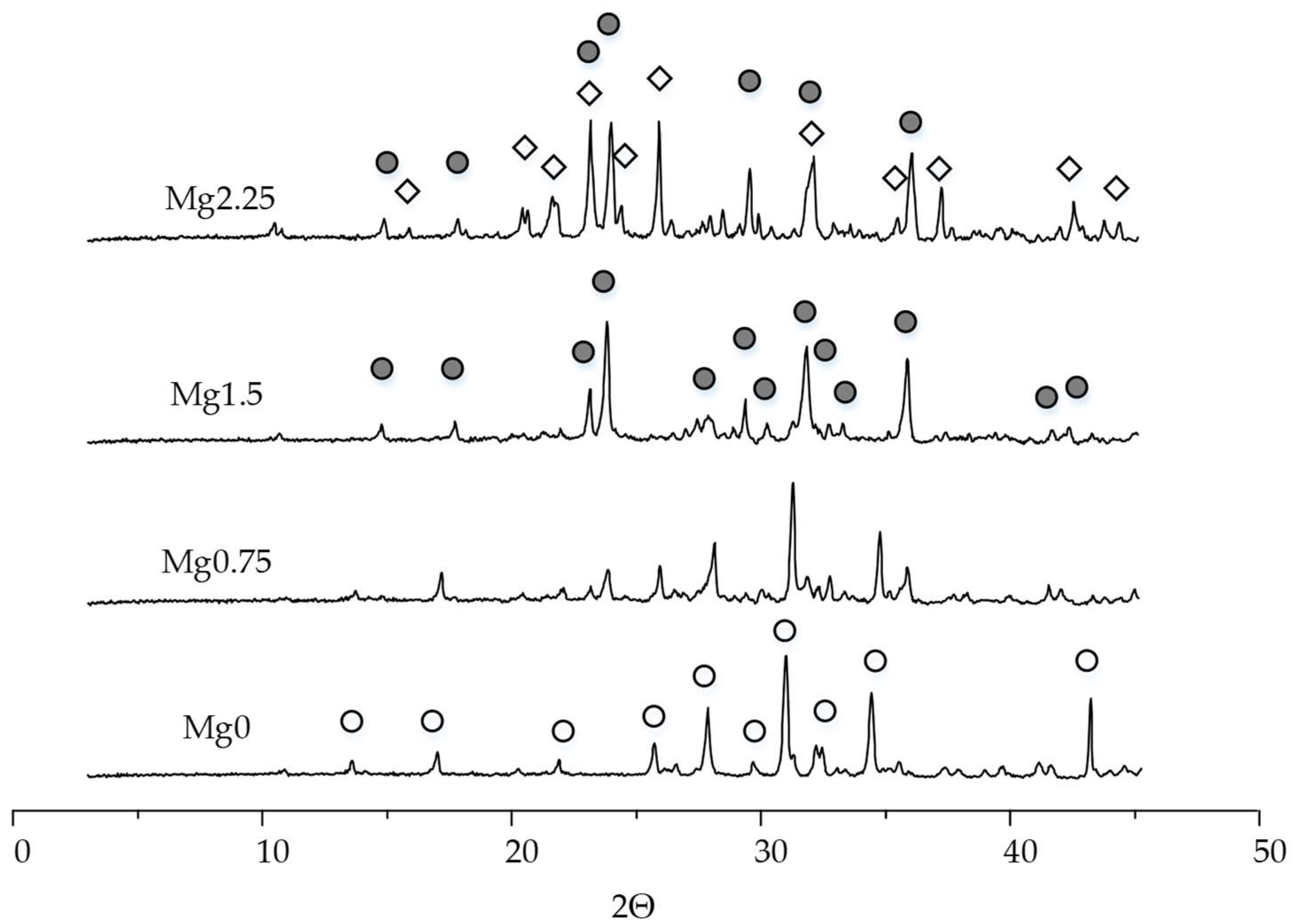
Magnesium was introduced into the initial component of TCP brushite cement until MgxCa(3−x)(PO4)2 was obtained at x = 0, 0.75, 1.5, 2.25 in order to obtain uniformly magnesium-doped brushite and newberyite.
The diffraction peaks of the Mg0.75 composition shift towards larger values of the angle 2Θ compared to Mg0, and new peaks of low intensity appear, related to Mg3Ca3(PO4)4.
Saleh et al. did not find phases other than β-TCP in the composition of Ca
(8.88−x)Mg
x(PO
4)
6 when x was less than 1 [
33]. However, the incorporation of Mg
2+ into the β-TCP lattice led to a shift in the diffraction peaks d = 2.61, 2.88, 3.21 Å to higher values of 2Θ [
41], as well as a decrease in the intensity of X-ray peaks, which was attributed to the substitution of Ca
2+ ions of larger size (1.00 Å) for Mg
2+ ions of smaller size (0.72 Å) [
42].
According to the literature data, the maximum amount of magnesium capable of replacing calcium in the positions of Ca(4) and Ca(5) in the TCP structure is 14–15 mol.% [
42,
43]. Exceeding 15.00 mol.% Mg
2+ leads to the formation of a magnesium-rich phase, such as stanfieldite [
42].
In our case, the concentration of doped magnesium was higher than 25 mol.% (Mg0.75). In this regard, magnesium ions were embedded in the TCP structure, and an additional phase of magnesium calcium phosphate Mg3Ca3(PO4)4 was formed, which became the only phase when the magnesium increased to 1.5. A further increase in magnesium leads to the formation of Mg3(PO4)2 trimagnesium phosphate.
Low-temperature magnesium calcium phosphate ceramics were obtained by cement technology through the acid–base interaction of MgxCa(3−x)(PO4)2 and monocalcium phosphate monohydrate Ca(H2PO4)2·H2O (MCPM).
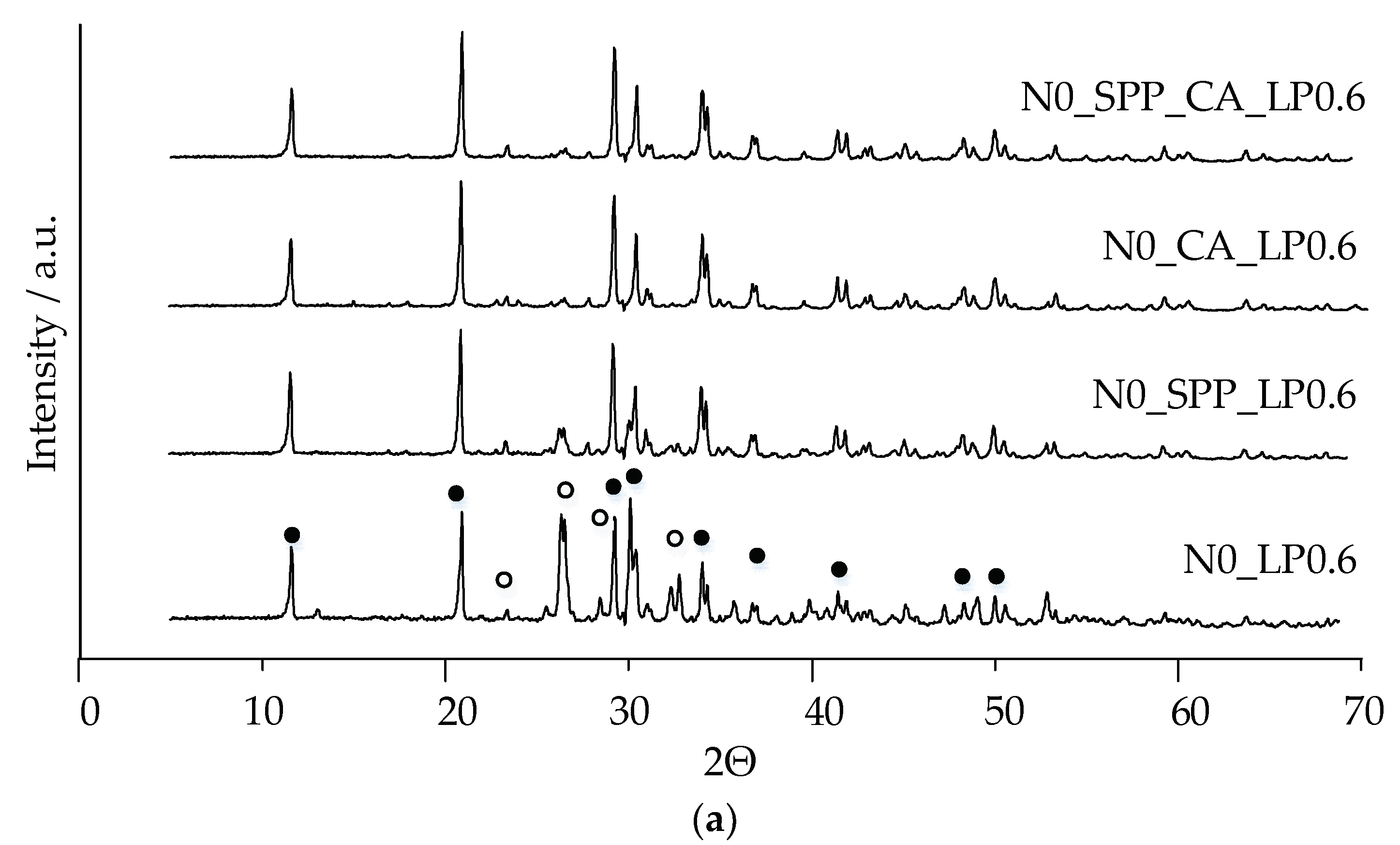
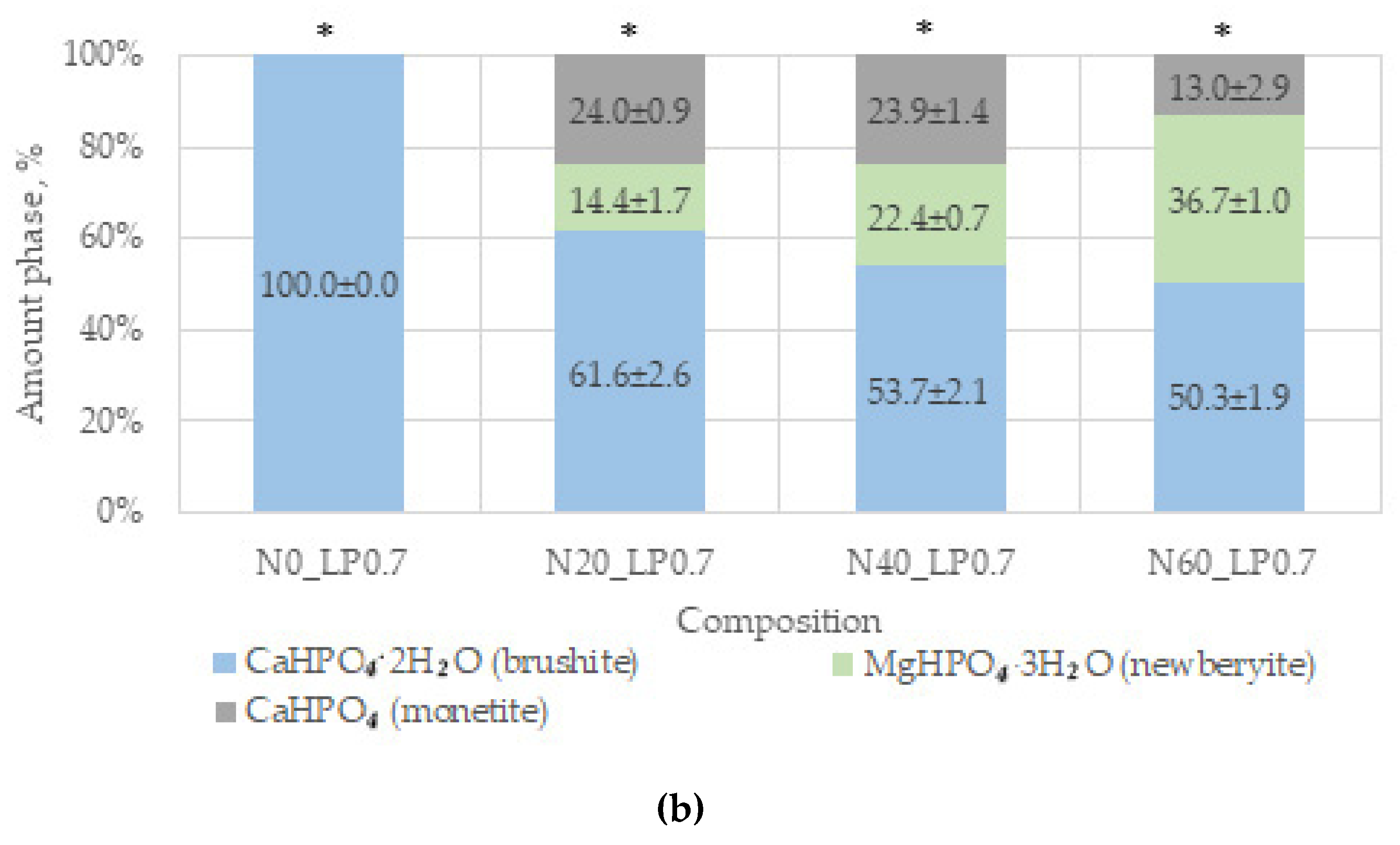
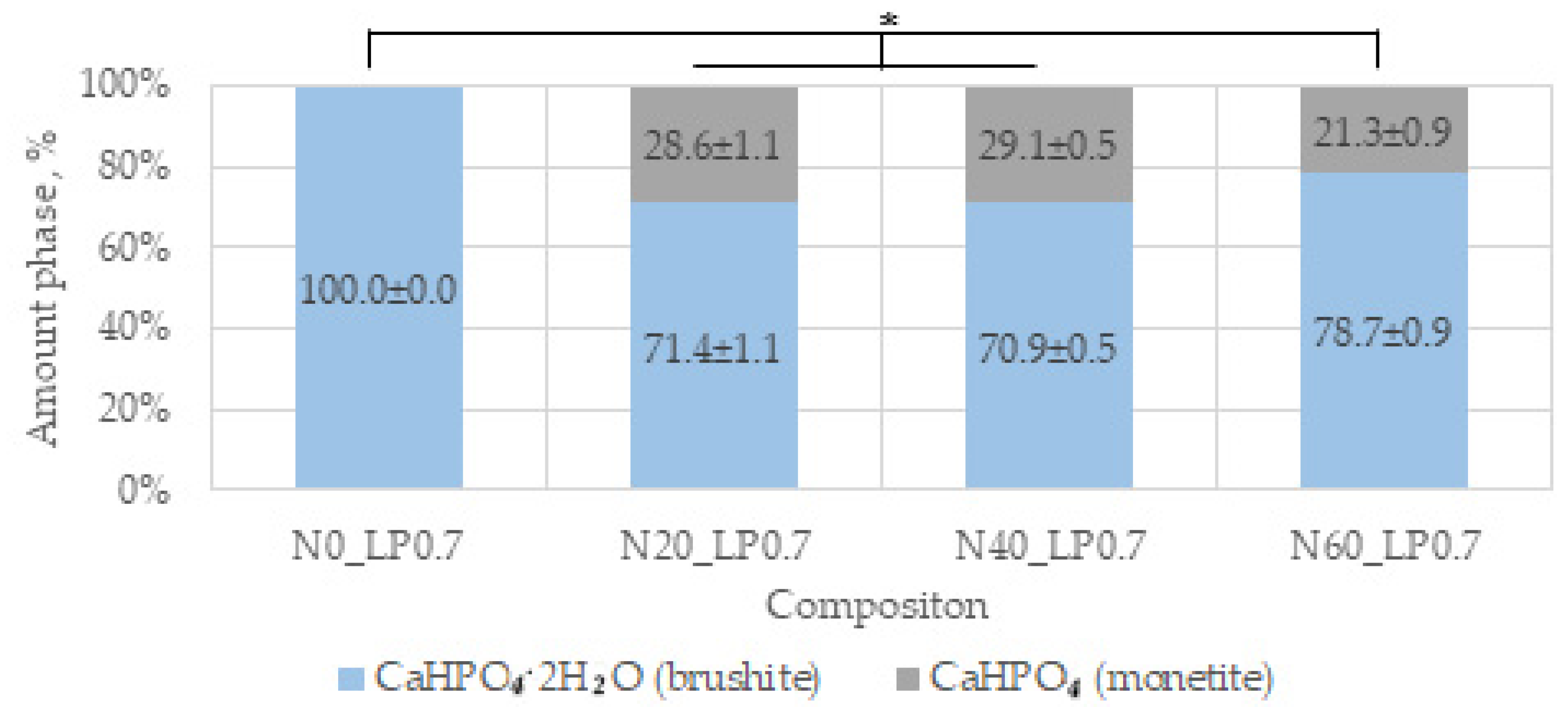
The main phases of the magnesium-free ceramics are brushite CaHPO
4·2H
2O and monetite CaHPO
4, in accordance with the equations:
Brushite is more soluble (−log(Ksp) = 6.59) than monetite (−log(Ksp) = 6.90), and it formed faster as a result of the reaction [
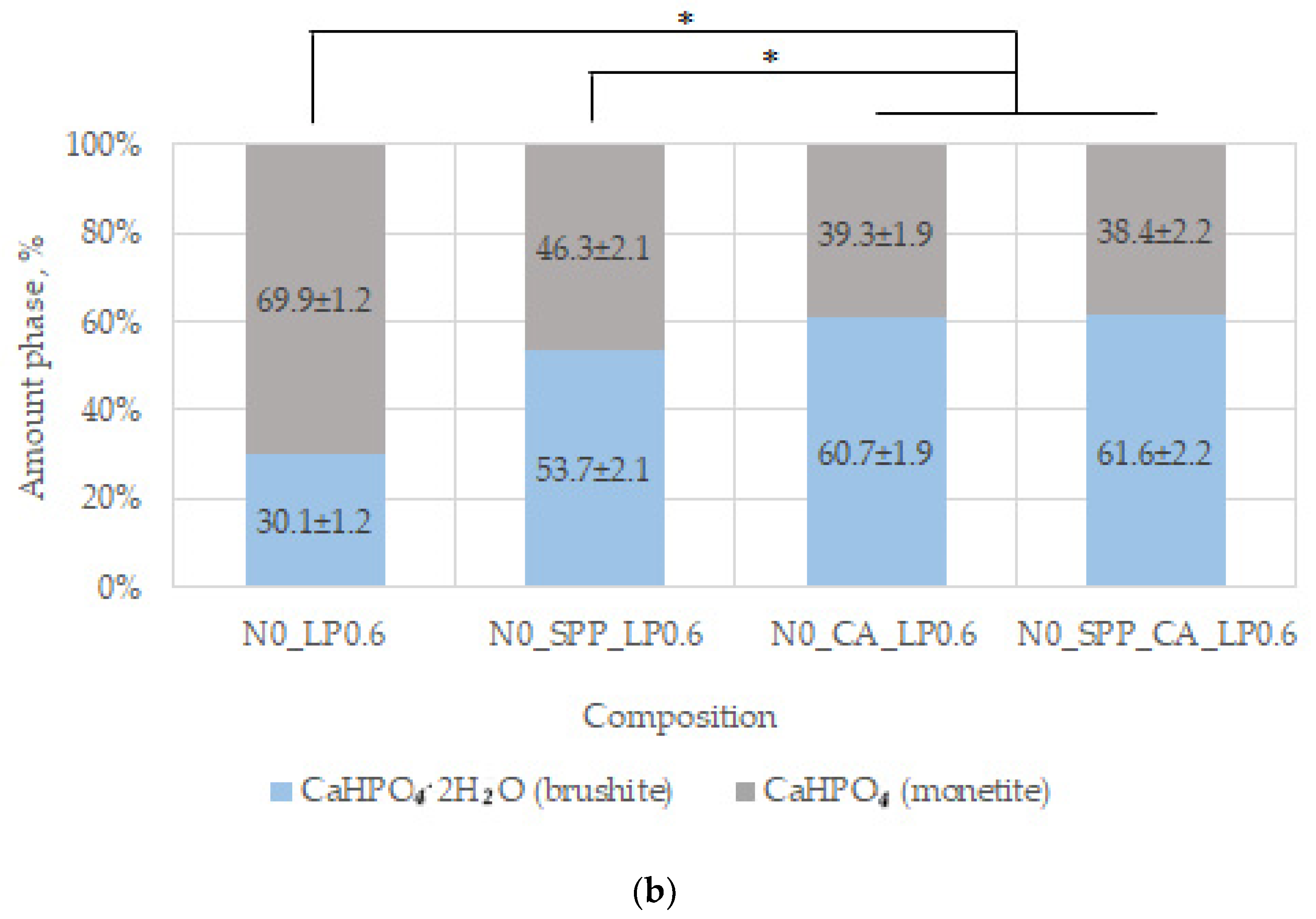
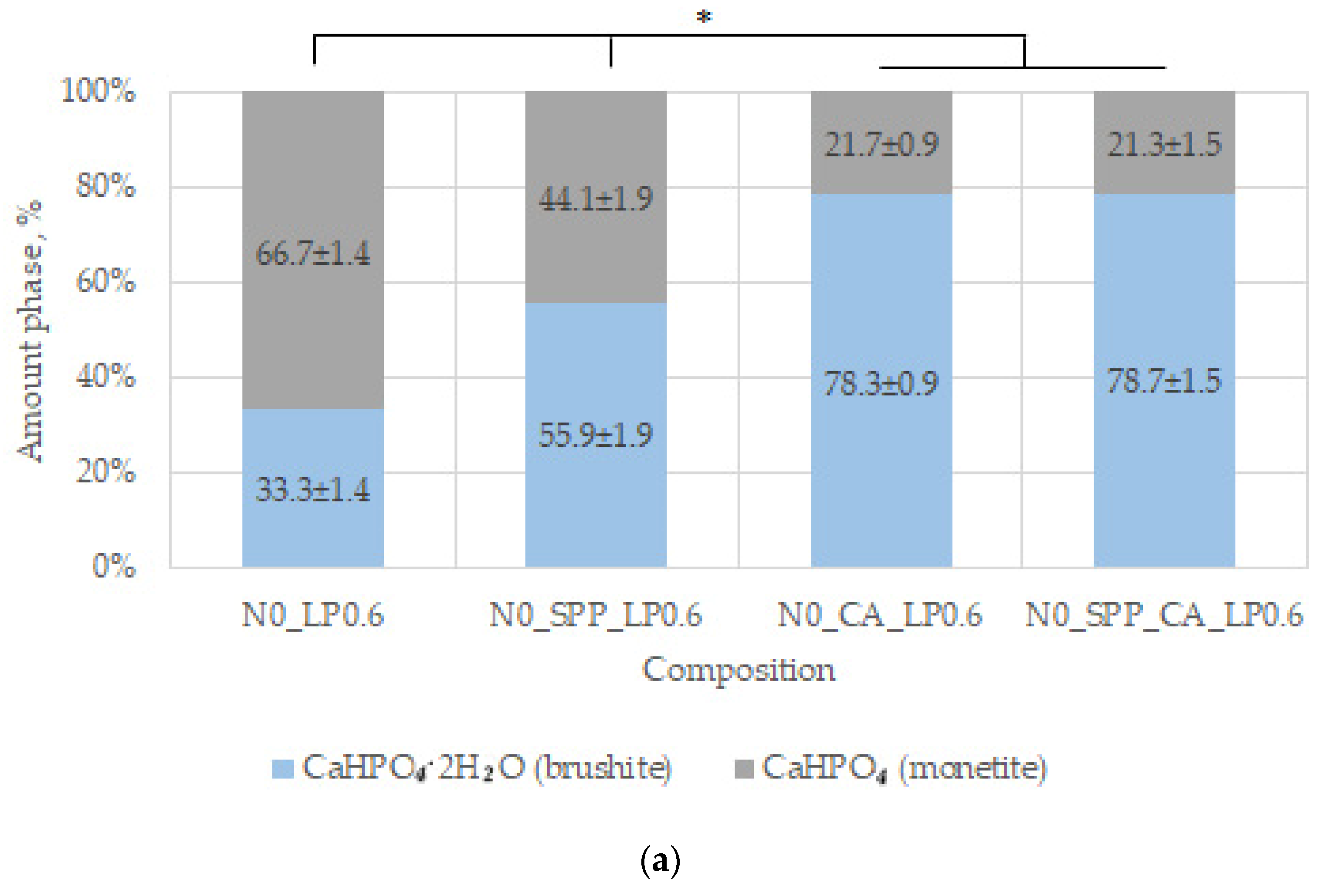
44]. In our work, additive-free ceramics contain about 30% brushite, which we attribute to a lack of cement-mixing fluid due to the small particle size of the initial component, which increases the water requirement.
With the introduction of set-retarding additives and increasing the water/solid ratio, the amount of the brushite phase increases.
Monetite, on the one hand, is an undesirable phase of ceramics due to a decrease in strength in its presence and a lower dissolution rate [
45]. On the other hand, when aging in an aqueous medium or subcutaneously, monetite, unlike brushite, does not transform into other poorly soluble phases [
27,
46], and therefore, it was characterized by a higher rate of resorption [
31,
44].
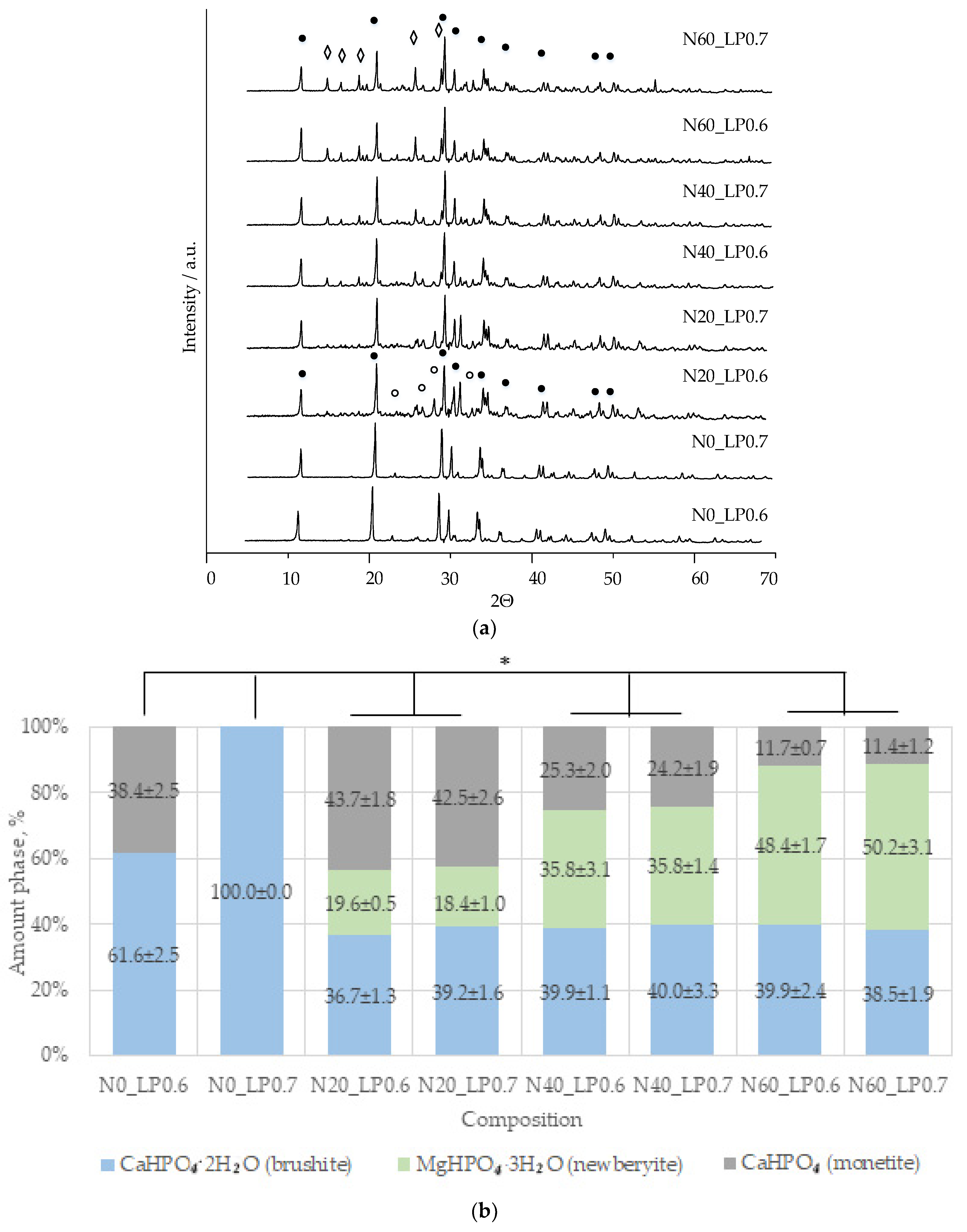
A slight difference in the amount of newberyite formed in the compositions of N20, N40, and N60 from the target values was associated, in our opinion, with the expenditure of magnesium on the doping of the brushite phase.
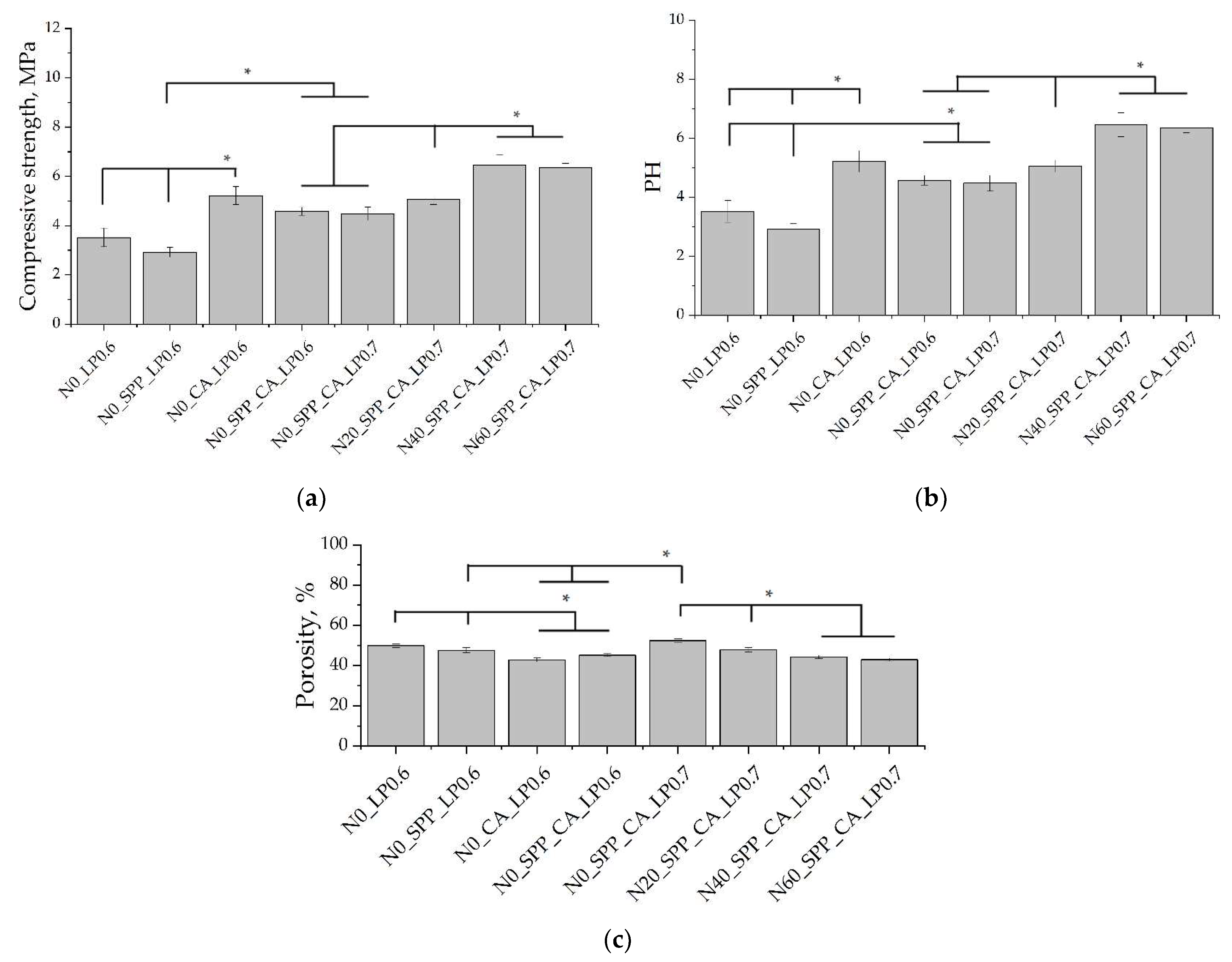
An increase in setting time and compressive strength was observed by Saleh et al. with an increase in the magnesium content in brushite cement. The initial setting time increased to 33 min with a magnesium content of up to 10% in the composition of TCP [
33]. In our work, the beginning of setting of the additive-free cement was less than 30 s for all Mg-substituted compounds. However, we found that the number of setting inhibitors was required less with an increase in the amount of magnesium in the cement composition. This may be due to the faster formation of poorly soluble Mg
2P
2O
7 on the surface of the new phases. An increase in the amount of magnesium in the composition of ceramics leads to a slight increase in strength and a decrease in porosity. This is consistent with the results of Alkhraisat et al., and it is connected with the formation of newberyite in the composition of ceramics, which compacts the matrix [
23].
In general, SPP has a negative effect on mechanical strength, although porosity was slightly reduced. SPP probably leads to the formation of calcium and magnesium pyrophosphates on the surface of the particles, partially preventing the formation of contacts of the mineral component of the consolidated matrix, which leads to a decrease in mechanical strength. CA seals the ceramic structure, reduces porosity, and significantly reduces pH. The combined use of additives reduces the negative effect when used separately and is preferable.
An increase in the liquid/solid ratio leads to an increase in porosity to values without additional ceramics. The phase composition of ceramics containing newberyite was not affected by an increase in L/P.
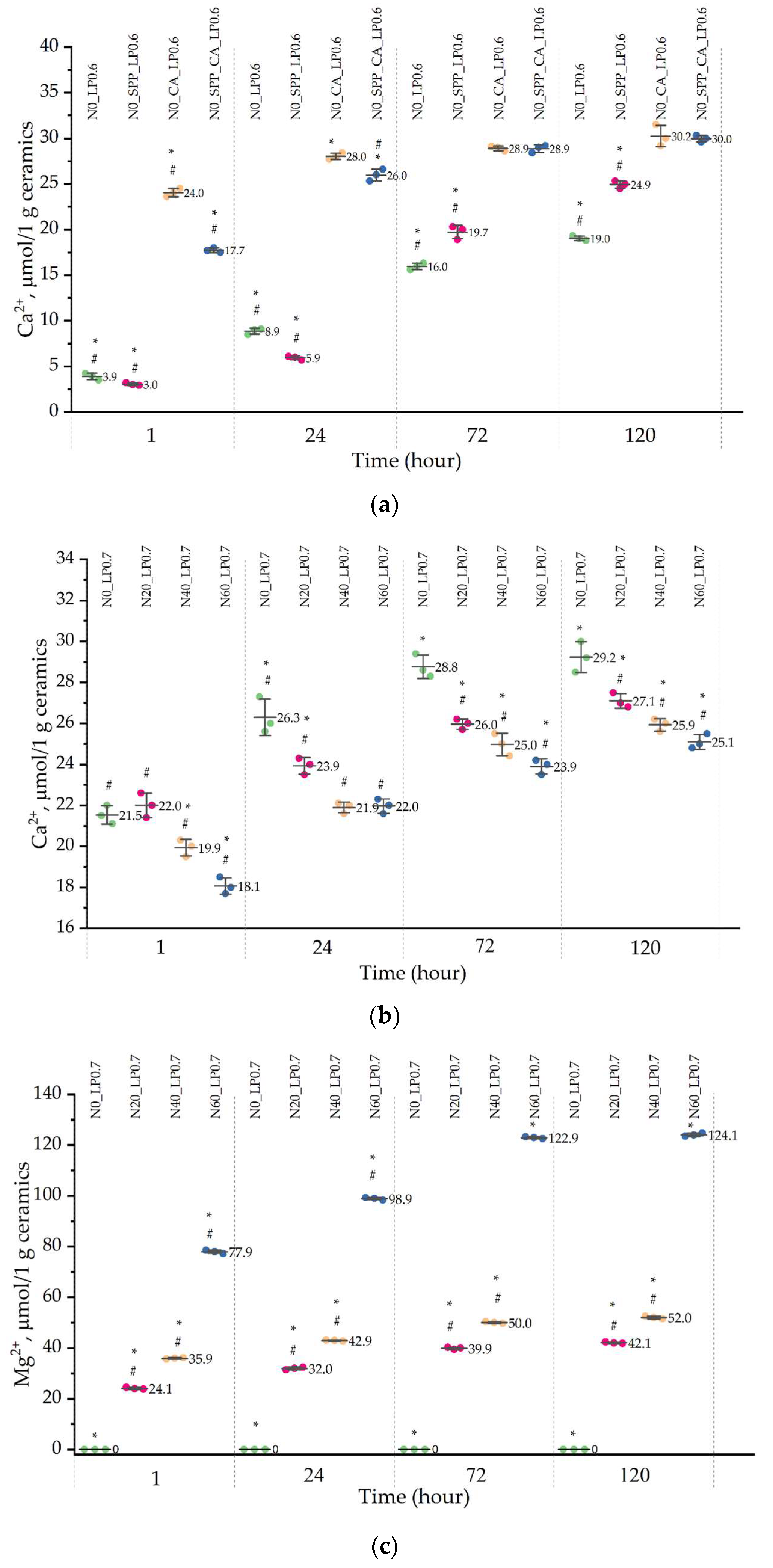
Set-retarding additives and magnesium doping have an effect on the solubility of ceramics. The amount of calcium ions in the contact medium was less than the amount of magnesium ions and almost did not differ for all compositions. CA increases the solubility of ceramics by six times in the initial period and by one and a half times by 5 days. Perhaps this is due to the formation of calcium citrate and a decrease in the size of ceramic particles. SPP reduces the level of calcium released into the solution in the first 24 h, after which the amount of calcium ions increases and reaches values greater than those for non-additive ceramics.
The release of Mg
2+ ions from ceramic samples N20, N40, and N60 for 5 days increased with an increase in the amount of magnesium in the precursor due to a lower degree of crystallinity of alloyed ceramic samples and the content of newberite, which has a greater chemical solubility (−log(Ksp) = 5.51–5.82) than brushite and monetite. After 7 days, the amount of newberite in the ceramic composition decreased significantly. The higher dissolution rate of magnesium-substituted brushite is consistent with the data of Alkhraisat et al. They observed an excess of Mg
2+ magnesium ions in SBF solution over Ca
2+ calcium ions and an increase in solubility in general with an increase in the magnesium content in the cement composition [
23]. A more intense dissolution of newberyite compared to brushite was observed by Klammert et al. during subcutaneous implantation in rats in in vivo experiments [
17].
In our study, a model of subcutaneous implantation in rats was also chosen. Since the implants were surrounded only by soft tissues, it was easy to carry out X-ray monitoring of the solubility of the implant without harming animals in long-term experiments. Changes in the phase composition can be investigated due to the absence of ingrown (and mineral-containing) hard tissues [
17].
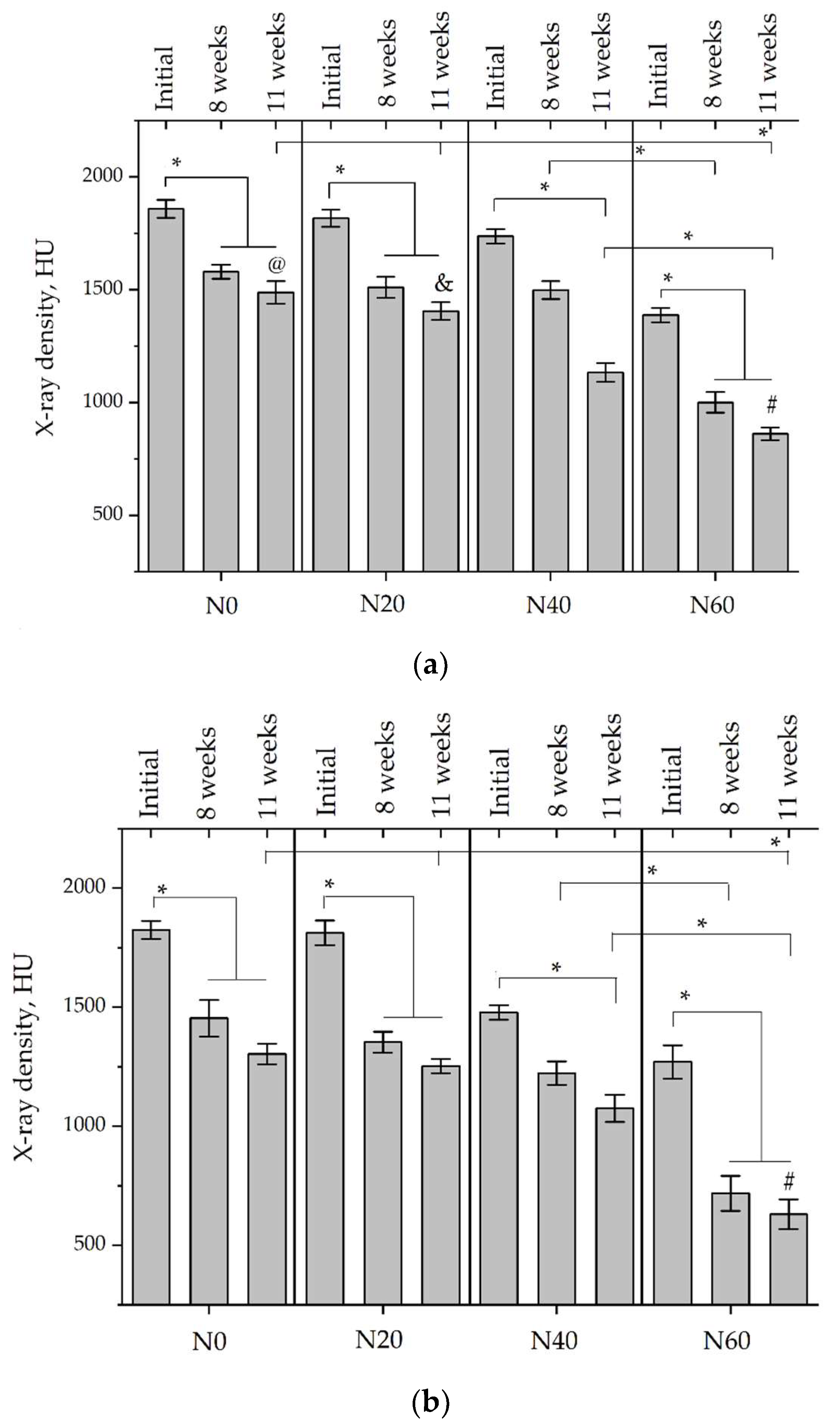
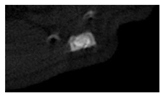
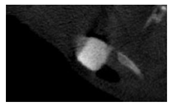
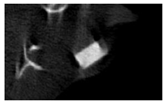
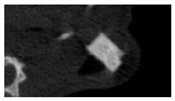
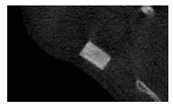
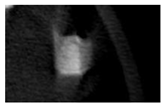
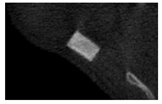
According to micro-CT, the surface resorption of ceramic granules was very insignificant, whereas dissolution was observed throughout the entire volume of ceramics, as can be judged by the X-ray density data. In our studies, the X-ray density of ceramics by the 8th and 11th days of implantation was less than the initial density. The mechanism of reducing the X-ray density of ceramics based on brushite and newberyite is associated with more intensive dissolution of the newberyite phase (Mg2+ and HPO42−) and less intensive dissolution of the brushite/monetite phase (Ca2+ and HPO42−) throughout the volume and leaching from the implant.
At week 8 and 11, there is some compaction inside the samples N60_CA and N60_SPP_CA. The formation of radiologically denser regions is probably associated with the re-deposition of brushite during ionic supersaturation into less soluble phases of calcium phosphate [
17]. However, no such compounds were found on radiographs. X-ray phase analysis of ceramic samples showed strong differences in the phase composition before and after implantation of ceramic granules. The absence of the newberyite phase in all modifications of ceramics confirms its higher dissolution rate.
Recrystallization in the center of the sample is most likely due to the highest saturation with Ca
2+ and HPO
42− ions due to the smallest boundary with the environment [
47]. Bonner et al. observed the formation of apatite at 6–8 weeks. The absence of magnesium in the composition of ceramics can contribute to recrystallization. It should be noted that the X-ray density of samples N60_CA and N60_SPP_CA was lower than N60_SPP. Ceramics containing SPP did not show areas with a higher X-ray density in the center of the ceramics. SPP forms magnesium and calcium pyrophosphate, which are less soluble than brushite, monetite, and newberyite [
47]. The literature data on the prevention of brushite recrystallization with the introduction of pyrophosphate are in contradiction to each other [
48,
49].
In addition to passive resorption due to chemical dissolution, active (cell-mediated) resorption due to the presence of macrophages was also observed in in vivo experiments [
45,
50,
51]. Further, the presence of macrophages indirectly confirms the absence of brushite recrystallization, in which active resorption occurs exclusively by osteoclasts [
31].
Animal studies using a subcutaneous implantation model have confirmed the assumption of volumetric dissolution of magnesium calcium phosphate ceramics and their biocompatibility. The rate of dissolution directly depends on the amount of magnesium in the composition and on the setting-inhibitor additive used. It was found that SPP reduces the rate of dissolution and biocompatibility, but not critically. The joint use of SPP and CA is optimal in terms of the physical and chemical properties of ceramics and biocompatibility. Optimal concentrations of SSP and CA additives when used together for compositions with different Mg2+ magnesium ion content in ceramics were found in our work. The resorption rate of ceramics based on brushite-newberyite cement can be controlled by the amount of Mg2+ ions. The main advantage of the resulting multiphase ceramics is volumetric resorption due to the different dissolution rates of the phases and the possibility of germination of the surrounding tissue into the internal space formed by dissolution. This was confirmed by the germination of fibroblasts during subcutaneous implantation.
An in vivo study of subcutaneous implantation confirms the biocompatibility and different rates of resorption of magnesium-calcium phosphate ceramics containing various setting inhibitors and determines the vector of further studies of magnesium-calcium phosphate ceramics, including in orthotopic implantation models.
 is magnesium calcium phosphate Mg3Ca3(PO4)4; and ◇ is trimagnesium phosphate Mg3(PO4)2.
is magnesium calcium phosphate Mg3Ca3(PO4)4; and ◇ is trimagnesium phosphate Mg3(PO4)2.
 is magnesium calcium phosphate Mg3Ca3(PO4)4; and ◇ is trimagnesium phosphate Mg3(PO4)2.
is magnesium calcium phosphate Mg3Ca3(PO4)4; and ◇ is trimagnesium phosphate Mg3(PO4)2.