Optimization of Plasma Electrolytic Oxidation Technological Parameters of Deformed Aluminum Alloy D16T in Flowing Electrolyte
Abstract
1. Introduction
- Develop a mathematical model of oxide coating forming and study the influence of the PEO technological parameters on the physical and mechanical properties of oxide coatings and the geometric dimensions of parts;
- Determine the optimal technological parameters to provide the maximum microhardness, minimal wear with low cone-likeness of the cylindrical surface of the coated part, as well as justify the placement of parts taking into account their shape in the electrochemical cell during PEO;
- Conduct microscopic studies of the oxide coating;
- Investigate the frictional heat resistance of the oxide coating.
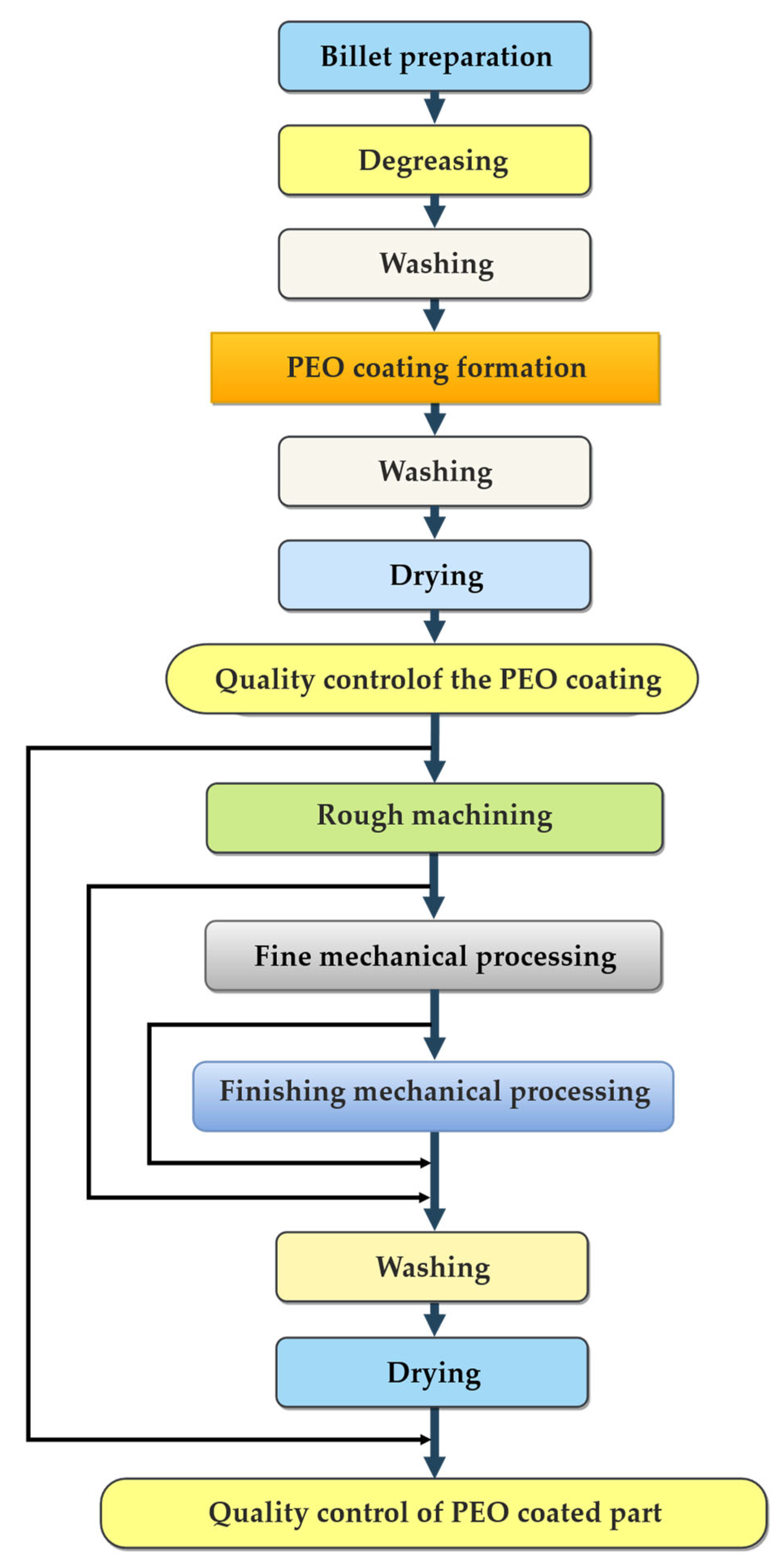
2. Materials and Methods
2.1. Research Materials and Equipment
2.2. Microstructure Observation and Mechanical Properties Measurement
2.3. Wear Test
2.4. Methods of Planning Experimental Research
3. Results and Discussion
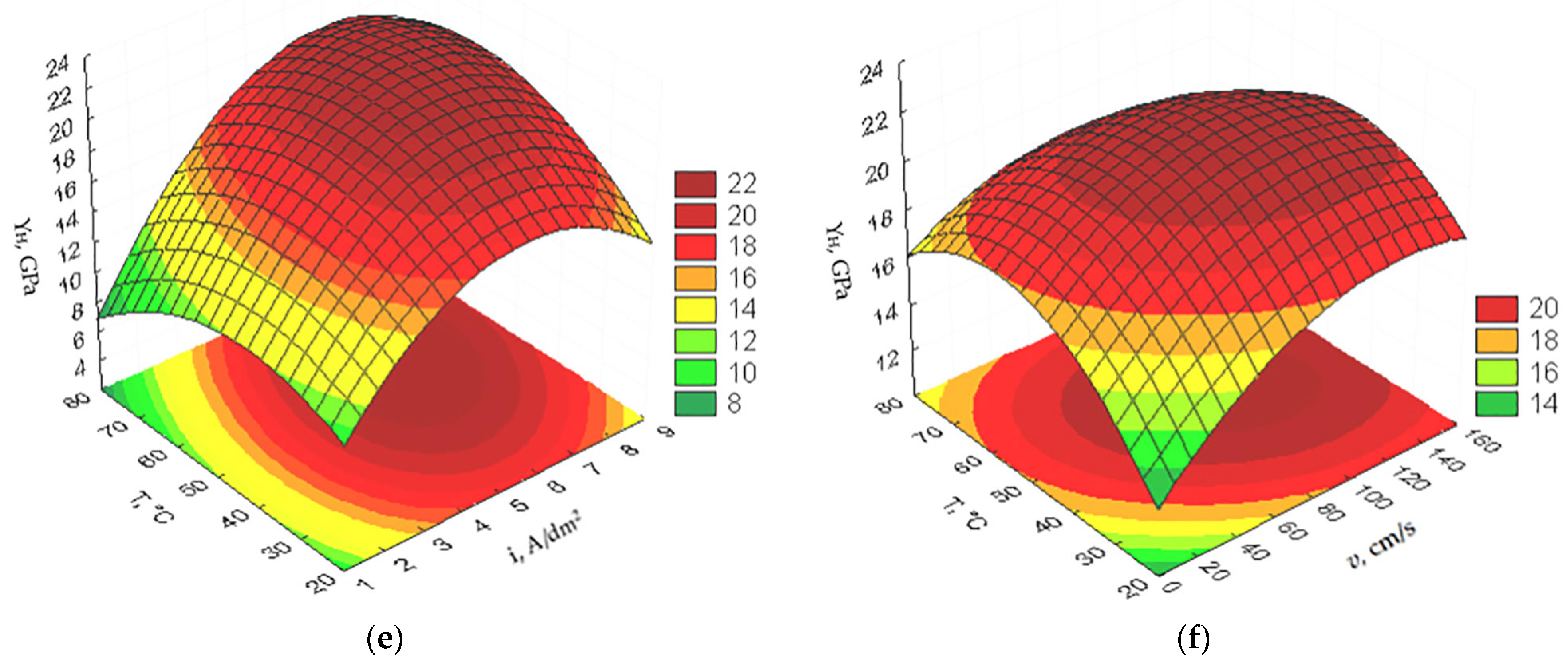
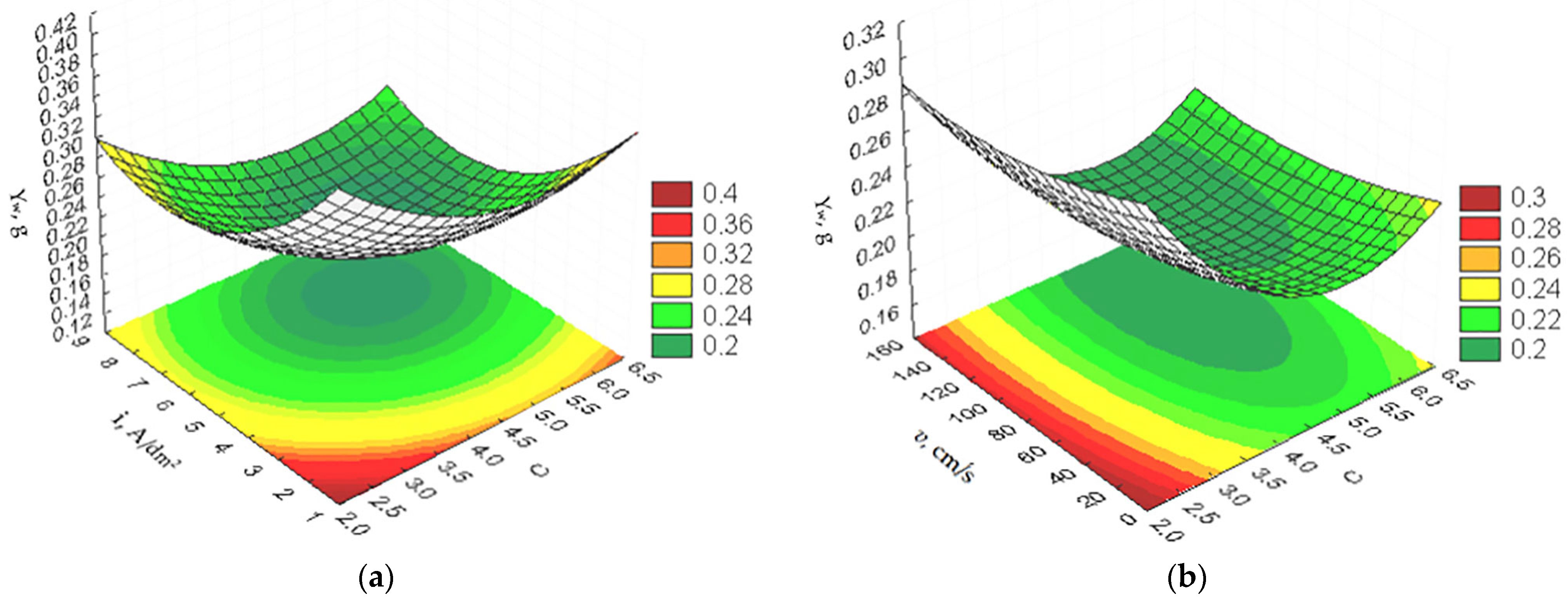
3.1. Optimization of the PEO Process Technological Parameters
- microhardness :
- wear :
- cone-likeness :
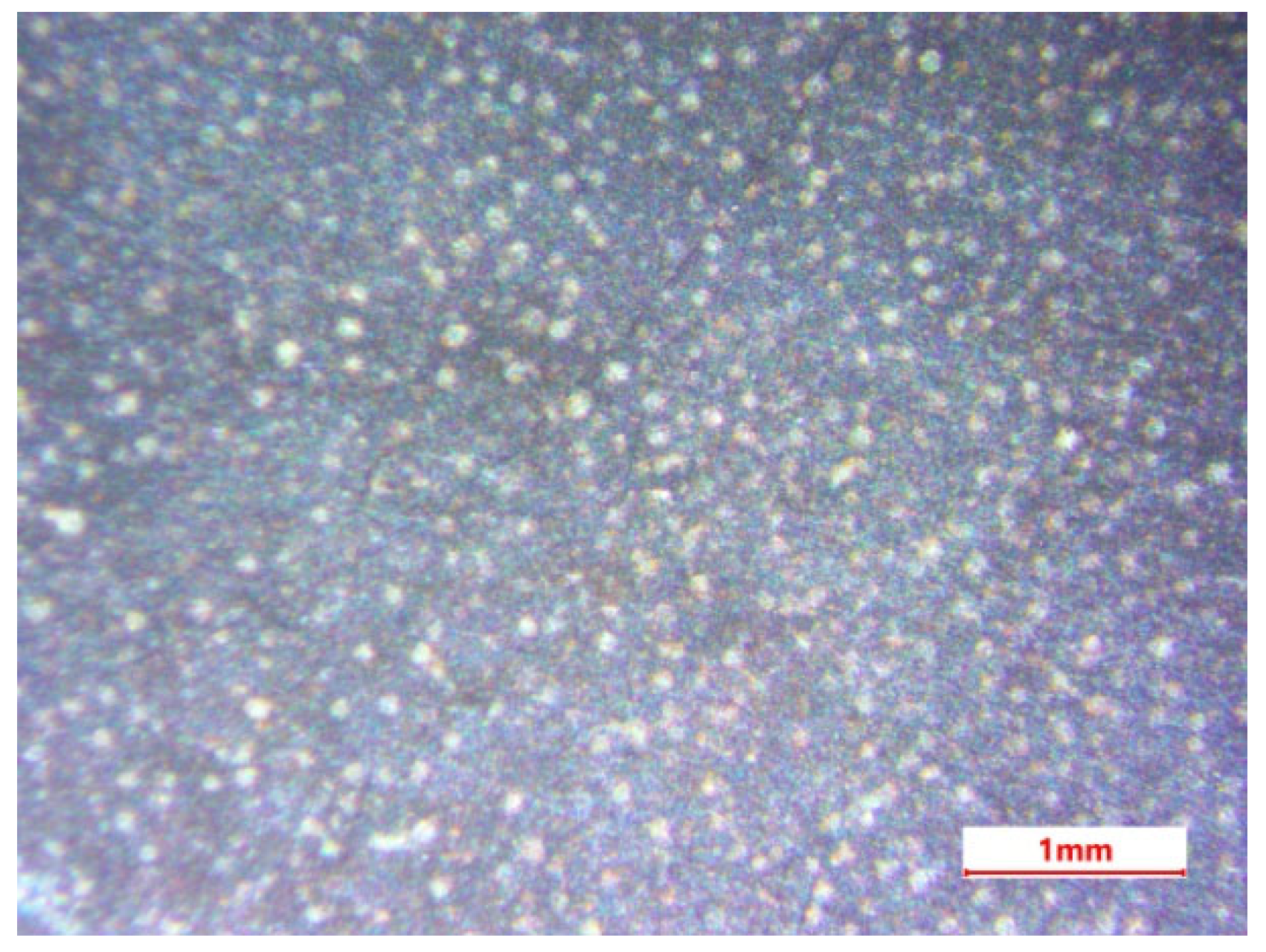
3.2. Microscopic Studies
3.3. Frictional Heat Resistance
4. Conclusions
- A mathematical model of the PEO process was built, based on which it was established that the optimal values of the technological parameters to ensure obtaining the maximum microhardness and minimal wear practically coincide, and, for cone-likeness, they do not coincide either with the optimal technological parameters for microhardness and wear or with each other;
- It was established that, in order to ensure high operational performance of parts with an oxide coating, optimization should be carried out according to minimum wear, which is achieved with the following values of the optimal technological parameters of the PEO process: mass ratio of concentrations of electrolyte components C = 4.98; current density i = 6.61 A/dm2; electrolyte flow rate in the electrochemical cell v = 103.96 cm/s; temperature of the electrolyte T = 53.59 °C; and necessary cone-likeness of the surface of the aluminum part should be obtained during further machining operations;
- Vertical mounting of cylindrical parts in the electrochemical cell is justified in order to eliminate the cone-likeness in the PEO process that arose during the previous operations of mechanical processing of the workpiece; for shafts, the minimum diameter of the surface on which the oxide coating is formed is from below, and, for bushings, respectively, from below, the maximum internal diameter to increase the accuracy of parts with oxide coatings since during formation of the oxide coating there is a maximum increase in diameter for external surfaces and a decrease for internal surfaces from bottom to top;
- The results of the SEM analysis of the microstructure of the oxide ceramic coating indicate the presence in the composition of oxides formed from the chemical elements of the alloy material and electrolyte components, the presence of pores, as well as the absence of through cracks;
- Research results showed high friction heat resistance of the oxide ceramic coating, which provides protection of aluminum alloy parts during dry friction, while the temperature stabilization time in the friction zone for a pair of samples with an oxide ceramic coating on the D16T alloy is twice as long as for a pair of samples with steel 40ChN.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooke, K.O. Introductory Chapter: Structural Aluminum Alloys and Composites. In Aluminium Alloys and Composites; Cooke, K.O., Ed.; IntechOpen: London, UK, 2020; pp. 1–14. [Google Scholar] [CrossRef]
- Bhatta, L.; Pesin, A.; Zhilyaev, A.P.; Tandon, P.; Kong, C.; Yu, H. Recent Development of Superplasticity in Aluminum Alloys: A Review. Metals 2020, 10, 77. [Google Scholar] [CrossRef]
- Georgantzia, E.; Gkantou, M.; Kamaris, G. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar] [CrossRef]
- Vlasiy, O.; Mazurenko, V.; Ropyak, L.; Rogal, O. Improving the aluminum drill pipes stability by optimizing the shape of protector thickening. East. Eur. J. Enterp. Technol. 2017, 1, 25–31. [Google Scholar] [CrossRef]
- Varshney, D.; Kumar, K. Application and use of different aluminium alloys with respect to workability, strength and welding parameter optimization. Ain Shams Eng. J. 2021, 12, 1143–1152. [Google Scholar] [CrossRef]
- Bazaluk, O.; Velychkovych, A.; Ropyak, L.; Pashechko, M.; Pryhorovska, T.; Lozynskyi, V. Influence of Heavy Weight Drill Pipe Material and Drill Bit Manufacturing Errors on Stress State of Steel Blades. Energies 2021, 14, 4198. [Google Scholar] [CrossRef]
- Czerwinski, F. Thermal Stability of Aluminum Alloys. Materials 2020, 13, 3441. [Google Scholar] [CrossRef]
- Timelli, G.; Fabrizi, A.; Vezzù, S.; De Mori, A. Design of Wear-Resistant Diecast AlSi9Cu3(Fe) Alloys for High-Temperature Components. Metals 2020, 10, 55. [Google Scholar] [CrossRef]
- Udoye, N.E.; Fayomi, O.S.I.; Inegbenebor, A.O. Assessment of Wear Resistance of Aluminium Alloy in Manufacturing Industry-A Review. Procedia Manuf. 2019, 35, 1383–1386. [Google Scholar] [CrossRef]
- Chokshi, A.H.; Meyers, M. The prospects for superplasticity at high strain rates: Preliminary considerations and an example. Scr. Metall. Mater. 1990, 24, 605–610. [Google Scholar] [CrossRef]
- Sergueeva, A.V.; Mara, N.A.; Mukherjee, A.K. Grain Size Distribution Effect on Mechanical Behavior of Nanocrystalline Materials. MRS Online Proc. Libr. 2004, 821, 336–342. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Ma, H.; Wang, H.; Hua, L.; Fu, S. Analysis of Hydrogen Embrittlement on Aluminum Alloys for Vehicle-Mounted Hydrogen Storage Tanks: A Review. Metals 2021, 11, 1303. [Google Scholar] [CrossRef]
- Shatskyi, I.; Vytvytskyi, I.; Senyushkovych, M.; Velychkovych, A. Modelling and improvement of the design of hinged centralizer for casing. IOP Conf. Ser. Mater. Sci. Eng. 2019, 564, 12073. [Google Scholar] [CrossRef]
- Barrak, F.N.; Li, S.; Muntane, A.M. Particle release from implantoplasty of dental implants and impact on cells. Int. J. Implant. Dent. 2020, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Bazaluk, O.; Chuzhak, A.; Sulyma, V.; Velychkovych, A.; Ropyak, L.; Vytvytskyi, V.; Mykhailiuk, V.; Lozynskyi, V. Determining the Tightrope Tightening Force for Effective Fixation of the Tibiofibular Syndesmosis during Osteomeatal Synthesis of Fibula Injuries. Appl. Sci. 2022, 12, 4903. [Google Scholar] [CrossRef]
- Pelekhan, B.; Dutkiewicz, M.; Shatskyi, I.; Velychkovych, A.; Rozhko, M.; Pelekhan, L. Analytical Modeling of the Interaction of a Four Implant-Supported Overdenture with Bone Tissue. Materials 2022, 15, 2398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, C.; Xu, X.; Liu, L. Surface quality and forming characteristics of thin-wall aluminium alloy parts manufactured by laser assisted MIG arc additive manufacturing. Int. J. Lightweight Mater. Manuf. 2018, 1, 89–95. [Google Scholar] [CrossRef]
- Zinchenko, A.; Baiul, K.; Krot, P.; Khudyakov, A.; Vashchenko, S.; Banasiewicz, A.; Wróblewski, A. Materials Selection and Design Options Analysis for a Centrifugal Fan Impeller in a Horizontal Conveyor Dryer. Materials 2021, 14, 6696. [Google Scholar] [CrossRef]
- Levchuk, K.G.; Moisyshyn, V.M.; Tsidylo, I.V. Influence of mechanical properties of a material on dynamics of the stuck drilling pipes. Metallofiz. Noveishie Tekhnologii 2016, 38, 1655–1668. [Google Scholar] [CrossRef]
- Dean, J.; Gu, T.; Clyne, T.W. Evaluation of residual stress levels in plasma electrolytic oxidation coatings using a curvature method. Surf. Coat. Technol. 2015, 269, 47–53. [Google Scholar] [CrossRef]
- Tatsiy, R.M.; Pazen, O.Y.; Vovk, S.Y.; Ropyak, L.Y.; Pryhorovska, T.O. Numerical study on heat transfer in multilayered structures of main geometric forms made of different materials. J. Serb. Soc. Comput. Mech. 2019, 13, 36–55. [Google Scholar] [CrossRef]
- Tatsiy, R.; Stasiuk, M.; Pazen, O.; Vovk, S. Modeling of Boundary-Value Problems of Heat Conduction for Multilayered Hollow Cylinder. In Proceedings of the 2018 International Scientific-Practical Conference on Problems of Infocommunications Science and Technology, PIC S and T 2018, Kharkiv, Ukraine, 9–12 October 2018; pp. 21–25. [Google Scholar]
- Shaloo, M.; Schnall, M.; Klein, T.; Huber, N.; Reitinger, B. A Review of Non-Destructive Testing (NDT) Techniques for Defect Detection: Application to Fusion Welding and Future Wire Arc Additive Manufacturing Processes. Materials 2022, 15, 3697. [Google Scholar] [CrossRef] [PubMed]
- Bembenek, M.; Mandziy, T.; Ivasenko, I.; Berehulyak, O.; Vorobel, R.; Slobodyan, Z.; Ropyak, L. Multiclass Level-Set Segmentation of Rust and Coating Damages in Images of Metal Structures. Sensors 2022, 22, 7600. [Google Scholar] [CrossRef] [PubMed]
- Student, M.M.; Ivasenko, I.B.; Posuvailo, V.M.; Veselivs’ka, H.H.; Pokhmurs’kyi, A.Y.; Sirak, Y.Y.; Yus’kiv, V.M. Influence of the Porosity of a Plasma-Electrolytic Coating on the Corrosion Resistance of D16 Alloy. Mater. Sci. 2019, 54, 899–906. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, K.; Lu, X.; Lam, Y.C. A review on the mechanical methods for evaluating coating adhesion. Acta Mech. 2014, 225, 431–452. [Google Scholar] [CrossRef]
- Abadias, G.; Chason, E.; Keckes, J.; Sebastiani, M.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.E.; Stoessel, C.H.; Martinu, L. Review Article: Stress in thin films and coatings: Current status, challenges, and prospects. J. Vac. Sci. Technol. A 2018, 36, 020801. [Google Scholar] [CrossRef]
- Ropyak, L.Y.; Velychkovych, A.S.; Vytvytskyi, V.S.; Shovkoplias, M.V. Analytical study of “crosshead-slide rail” wear effect on pump rod stress state. J. Phys. Conf. Ser. 2021, 1741, 12039. [Google Scholar] [CrossRef]
- Dubei, O.Y.; Tutko, T.F.; Ropyak, L.Y.; Shovkoplias, M.V. Development of Analytical Model of Threaded Connection of Tubular Parts of Chrome-Plated Metal Structures. Metallofiz. Noveishie Tekhnologii 2022, 44, 251–272. [Google Scholar] [CrossRef]
- Szczepankowski, A.; Przysowa, R.; Perczyński, J.; Kułaszka, A. Health and Durability of Protective and Thermal Barrier Coatings Monitored in Service by Visual Inspection. Coatings 2022, 12, 624. [Google Scholar] [CrossRef]
- Duriagina, Z.A.; Kulyk, V.V.; Filimonov, O.S.; Trostianchyn, A.M.; Sokulska, N.B. The role of stress–strain state of gas turbine engine metal parts in predicting their safe life. Prog. Phys. Met. 2021, 22, 643–677. [Google Scholar] [CrossRef]
- Dolgov, N.A. Analytical Methods to Determine the Stress State in the Substrate–Coating System Under Mechanical Loads. Strength Mater. 2016, 48, 658–667. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Curran, J.A.; Shrestha, S. Microstructure and multi-scale mechanical behavior of hard anodized and plasma electrolytic oxidation (PEO) coatings on aluminum alloy 5052. Surf. Coat. Technol. 2012, 207, 480–488. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Cheng, C. Properties and Structure of PEO Treated Aluminum Alloy. J. Wuhan Univ. Technol. Mat. Sci. Ed. 2021, 36, 424–432. [Google Scholar] [CrossRef]
- Shatskyi, I.P.; Makoviichuk, M.V.; Shcherbii, A.B. Equilibrium of cracked shell with flexible coating. In Shell Structures: Theory and Applications; CRC Press: Leiden, The Netherlands, 2018; Volume 4, pp. 165–168. [Google Scholar] [CrossRef]
- Shatskyi, I.P.; Makoviichuk, M.V.; Shcherbii, A.B. Influence of flexible coating on the limit equilibrium of a spherical shell with meridional crack. Mater. Sci. 2020, 55, 484–491. [Google Scholar] [CrossRef]
- Shats’kyi, I.P.; Makoviichuk, M.V. Contact interaction of the crack edges in the case of bending of a plate with elastic support. Mater. Sci. 2003, 39, 371–376. [Google Scholar] [CrossRef]
- Nobili, A.; Radi, E.; Lanzoni, L. A cracked infinite Kirchhoff plate supported by a two-parameter elastic foundation. J. Eur. Ceram. Soc. 2014, 34, 2737–2744. [Google Scholar] [CrossRef]
- Shatskyi, I.P.; Ropyak, L.Y.; Makoviichuk, M.V. Strength optimization of a two-layer coating for the particular local loading conditions. Strength Mater. 2016, 48, 726–730. [Google Scholar] [CrossRef]
- Ropyak, L.Y.; Makoviichuk, M.V.; Shatskyi, I.P.; Pritula, I.M.; Gryn, L.O.; Belyakovskyi, V.O. Stressed state of laminated interference-absorption filter under local loading. Funct. Mater. 2020, 27, 638–642. [Google Scholar] [CrossRef]
- Bembenek, M.; Makoviichuk, M.; Shatskyi, I.; Ropyak, L.; Pritula, I.; Gryn, L.; Belyakovskyi, V. Optical and Mechanical Properties of Layered Infrared Interference Filters. Sensors 2022, 22, 8105. [Google Scholar] [CrossRef]
- Shyrokov, V.V.; Maksymuk, O.V. Analytic Methods of Calculation of the Contact Interaction of Thin-Walled Structural Elements (Review). Mater. Sci. 2002, 38, 62–73. [Google Scholar] [CrossRef]
- Kul’chyts’kyi-Zhyhailo, R.; Bajkowski, A. Elastic Coating with Inhomogeneous Interlayer Under the Action of Normal and Tangential Forces. Mater. Sci. 2014, 49, 650–659. [Google Scholar] [CrossRef]
- Ropyak, L.Y.; Shatskyi, I.P.; Makoviichuk, M.V. Influence of the Oxide-Layer Thickness on the Ceramic–Aluminium Coating Resistance to Indentation. Metallofiz. Noveishie Tekhnol. 2017, 39, 517–524. [Google Scholar] [CrossRef]
- Kusyi, Y.M.; Kuk, A.M. Investigation of the technological damageability of castings at the stage of design and technological preparation of the machine Life Cycle. J. Phys. Conf. Ser. 2020, 1426, 12034. [Google Scholar] [CrossRef]
- Kusyi, Y.; Onysko, O.; Kuk, A.; Solohub, B.; Kopei, V. Development of the Technique for Designing Rational Routes of the Functional Surfaces Processing of Products. In New Technologies, Development and Application V; Lecture Notes in Networks and Systems; Springer: Berlin/Heidelberg, Germany, 2022; Volume 472, pp. 135–143. [Google Scholar] [CrossRef]
- Sathish, M.; Radhika, N.; Saleh, B. A critical review on functionally graded coatings: Methods, properties, and challenges. Compos. Part B Eng. 2021, 225, 109278. [Google Scholar] [CrossRef]
- Kusyj, J.; Kuk, A. Acentrifugal vibration strengthening method devised to improve technological reliability of machine parts. East. Eur. J. Enterp. Technol. 2015, 1, 41–51. [Google Scholar] [CrossRef]
- Mordyuk, B.N.; Silberschmidt, V.V.; Prokopenko, G.I.; Nesterenko, Y.V.; Iefimov, M.O. Ti particle-reinforced surface layers in Al: Effect of particle size on microstructure, hardness and wear. Mater. Charact. 2010, 61, 1126–1134. [Google Scholar] [CrossRef]
- Pokhmurs’ka, H.V.; Student, M.M.; Chervins’ka, N.R.; Smetana, K.R.; Wank, A.; Hoenig, T.; Podlesak, H. Structure and properties of aluminum alloys modified with silicon carbide by laser surface treatment. Mater. Sci. 2005, 41, 316–323. [Google Scholar] [CrossRef]
- Gaponova, O.; Kundera, C.; Kirik, G.; Tarelnyk, V.; Martsynkovskyy, V.; Konoplianchenko, I.; Dovzhyk, M.; Belous, A.; Vasilenko, O. Estimating qualitative parameters of aluminized coating obtained by electric spark alloying method. In Advances in Thin Films, Nanostructured Materials, and Coatings; Lecture Notes in Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2019; pp. 249–266. [Google Scholar] [CrossRef]
- Hutsaylyuk, V.; Student, M.; Zadorozhna, K.; Student, O.; Veselivska, H.; Gvosdetskii, V.; Maruschak, P.; Pokhmurska, H. Improvement of wear resistance of aluminum alloy by HVOF method. J. Mater. Res. Technol. 2020, 9, 16367–16377. [Google Scholar] [CrossRef]
- Peltier, F.; Thierry, D. Review of Cr-Free Coatings for the Corrosion Protection of Aluminum Aerospace Alloys. Coatings 2022, 12, 518. [Google Scholar] [CrossRef]
- Student, M.M.; Pohrelyuk, I.M.; Hvozdetskyi, V.M.; Veselivska, H.H.; Zadorozhna, K.R.; Mardarevych, R.S.; Dzioba, Y.V. Influence of the Composition of Electrolyte for Hard Anodizing of Aluminum on the Characteristics of Oxide Layer. Mater. Sci. 2021, 57, 240–247. [Google Scholar] [CrossRef]
- Mohedano, M.; Lu, X.; Matykina, E.; Blawert, C.; Arrabal, R.; Zheludkevich, M.L. Plasma electrolytic oxidation (PEO) of metals and alloys. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 423–438. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Qin, J.; Shi, X.; Li, H.; Zhao, R.; Li, G.; Zhang, S.; Ding, L.; Cui, X.; Zhao, Y.; Zhang, R. Performance and failure process of green recycling solutions for preparing high degradation resistance coating on biomedical magnesium alloys. Green Chem. 2022, 24, 8113–8130. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bobrova, L.S.; Baskevich, A.S.; Korniy, S.A.; Danilov, F.I. Electrodeposition of chromium coatings from a choline chloride based ionic liquid with the addition of water. J. Chem. Technol. Metall. 2018, 53, 906–915. [Google Scholar]
- Protsenko, V.S.; Bobrova, L.S.; Golubtsov, D.E.; Korniy, S.A.; Danilov, F.I. Electrolytic Deposition of Hard Chromium Coatings from Electrolyte Based on Deep Eutectic Solvent. Russ. J. Appl. Chem. 2018, 91, 1106–1111. [Google Scholar] [CrossRef]
- Bazaluk, O.; Dubei, O.; Ropyak, L.; Shovkoplias, M.; Pryhorovska, T.; Lozynskyi, V. Strategy of Compatible Use of Jet and Plunger Pump with Chrome Parts in Oil Well. Energies 2022, 15, 83. [Google Scholar] [CrossRef]
- Tang, Q.; Qiu, T.; Ni, P.; Zhai, D.; Shen, J. Soft Sparking Discharge Mechanism of Micro-Arc Oxidation Occurring on Titanium Alloys in Different Electrolytes. Coatings 2022, 12, 1191. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Dudek, Ł. Phosphate Porous Coatings Enriched with Selected Elements via PEO Treatment on Titanium and Its Alloys: A Review. Materials 2020, 13, 2468. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Wang, Y.L.; Jiang, Z.H.; Yao, Z.P. Microstructure, bonding strength and thermal shock resistance of ceramic coatings on steels prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2009, 256, 650–656. [Google Scholar] [CrossRef]
- Mahmoud, E.R.I.; Algahtani, A.; Tirth, V. Study on Microstructure Characterisation of Three Different Surface Coating Techniques on 6082-T6 Aluminum Alloy. Met. Mater. Int. 2021, 27, 4002–4013. [Google Scholar] [CrossRef]
- Berladir, K.; Hovorun, T.; Gusak, O.; Reshetniak, Y.; Khudaybergenov, D. Influence of Modifiers-Ligatures on the Properties of Cast Aluminum Alloy AK5M2 for the Automotive Industry. In Advances in Design, Simulation and Manufacturing III, DSMIE 2020; Ivanov, V., Trojanowska, J., Pavlenko, I., Zajac, J., Peraković, D., Eds.; Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2020; pp. 473–482. [Google Scholar] [CrossRef]
- Pokhmurskii, V.I.; Zin, I.M.; Vynar, V.A.; Khlopyk, O.P.; Bily, L.M. Corrosive wear of aluminium alloy in presence of phosphate. Corros. Eng. Sci. Technol. 2012, 47, 182–187. [Google Scholar] [CrossRef]
- Vynar, V.A.; Pokhmurs’kyi, V.I.; Zin’, I.M.; Vasyliv, K.B.; Khlopyk, O.P. Determination of the Mechanism of Tribocorrosion of D16T Alloy According to the Electrode Potential. Mater. Sci. 2018, 53, 717–723. [Google Scholar] [CrossRef]
- Wang, Z.J.; Nie, X.Y.; Hu, H.; Hussein, R.O. In situ fabrication of blue ceramic coatings on wrought Al alloy 2024 by plasma electrolytic oxidation. J. Vac. Sci. Technol. A 2012, 30, 021302. [Google Scholar] [CrossRef]
- Melnick, A.B.; Soolshenko, V.K.; Levchuk, K.H. Thermodynamic Prediction of Phase Composition of Transition Metals High-Entropy Alloys. Metallofiz. Noveishie Tekhnologii 2020, 42, 1387–1400. [Google Scholar] [CrossRef]
- Radchenko, T.M.; Tatarenko, V.A.; Zapolsky, H.; Blavette, D. Statistical-thermodynamic description of the order-disorder transformation of D0(19)-type phase in Ti-Al alloy. J. Alloy. Compd. 2008, 452, 122–126. [Google Scholar] [CrossRef]
- Shihab, T.; Prysyazhnyuk, P.; Semyanyk, I.; Anrusyshyn, R.; Ivanov, O.; Troshchuk, L. Thermodynamic Approach to the Development and Selection of Hardfacing Materials in Energy Industry. Manag. Syst. Prod. Eng. 2020, 28, 84–89. [Google Scholar] [CrossRef]
- Prysyazhnyuk, P.; Shlapak, L.; Semyanyk, I.; Kotsyubynsky, V.; Troshchuk, L.; Korniy, S.; Artym, V. Analysis of the effects of alloying with Si and Cr on the properties of manganese austenite based on AB INITIO modelling. East. Eur. J. Enterp. Technol. 2020, 6, 28–36. [Google Scholar] [CrossRef]
- Karnaukhov, I.M.; Levchuk, K.H. Electronic band structure of Dirac materials with Hubbard interaction. Metallofiz. Noveishie Tekhnologii 2022, 5, 565–585. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yin, X.; Du, N. Influence of electrolyte components on the microstructure and growth mechanism of plasma electrolytic oxidation coatings on 1060 aluminum alloy. Surf. Coat. Technol. 2020, 381, 125214. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, K.H.; Kim, S.J. Characterization of ceramic oxide coatings prepared by plasma electrolytic oxidation using pulsed direct current with different duty ratio and frequency. Appl. Surf. Sci. 2020, 516, 146049. [Google Scholar] [CrossRef]
- Li, X.-J.; Zhang, M.; Wen, S.; Mao, X.; Huo, W.-G.; Guo, Y.-Y.; Wang, Y.-X. Microstructure and wear resistance of micro-arc oxidation ceramic coatings prepared on 2A50 aluminum alloys. Surf. Coat. Technol. 2020, 394, 125853. [Google Scholar] [CrossRef]
- Rogov, A.B.; Huang, Y.; Shore, D.; Matthews, A.; Yerokhin, A. Toward rational design of ceramic coatings generated on valve metals by plasma electrolytic oxidation: The role of cathodic polarisation. Ceram. Int. 2021, 47, 34137–34158. [Google Scholar] [CrossRef]
- Mengesha, G.A.; Chu, J.P.; Lou, B.-S.; Lee, J.-W. Effects of Processing Parameters on the Corrosion Performance of Plasma Electrolytic Oxidation Grown Oxide on Commercially Pure Aluminum. Metals 2020, 10, 394. [Google Scholar] [CrossRef]
- Pokhmurs’kyi, V.I.; Dovhunyk, V.M.; Student, M.M.; Klapkiv, M.D.; Posuvailo, V.M.; Kytsya, A.R. Influence of silver nanoparticles added to lubricating oil on the tribological behavior of combined metal-oxide ceramic layers. Mater. Sci. 2013, 48, 636–641. [Google Scholar] [CrossRef]
- Student, M.M.; Dovhunyk, V.M.; Posuvailo, V.M.; Koval’chuk, I.V.; Hvozdets’kyi, V.M. Friction Behavior of Iron-Carbon Alloys in Couples with Plasma-Electrolytic Oxide-Ceramic Layers Synthesized on D16T Alloy. Mater. Sci. 2017, 53, 359–367. [Google Scholar] [CrossRef]
- Kotsyubynsky, V.; Shyyko, L.; Shihab, T.; Prysyazhnyuk, P.; Aulin, V.; Boichuk, V. Multilayered MoS2/C nanospheres as high performance additives to lubricating oils. Mater. Today Proc. 2019, 35, 538–541. [Google Scholar] [CrossRef]
- Hutsaylyuk, V.; Student, M.; Dovhunyk, V.; Posuvailo, V.; Student, O.; Maruschak, P.; Koval’chuck, I. Effect of Hydrogen on the Wear Resistance of Steels upon Contact with Plasma Electrolytic Oxidation Layers Synthesized on Aluminum Alloys. Metals 2019, 9, 280. [Google Scholar] [CrossRef]
- Shen, X.; Nie, X.; Hu, H.; Tjong, J. Effects of coating thickness on thermal conductivities of alumina coatings and alumina/aluminum hybrid materials prepared using plasma electrolytic oxidation. Surf. Coat. Technol. 2012, 207, 96–101. [Google Scholar] [CrossRef]
- Akatsu, T.; Kato, T.; Shinoda, Y.; Wakai, F. Thermal barrier coating made of porous zirconium oxide on a nickel-based single crystal superalloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2013, 223, 47–51. [Google Scholar] [CrossRef]
- Curran, J.A.; Kalkanci, H.; Magurova, Y.; Clyne, T.W. Mullite-rich plasma electrolytic oxide coatings for thermal barrier applications. Surf. Coat. Technol. 2006, 201, 8683–8687. [Google Scholar] [CrossRef]
- Shirani, A.; Joy, T.; Rogov, A.; Lin, M.; Yerokhin, A.; Mogonye, J.-E.; Korenyi-Both, A.; Aouadi, S.M.; Voevodin, A.A.; Berman, D. PEO-Chameleon as a potential protective coating on cast aluminum alloys for high-temperature applications. Surf. Coat. Technol. 2020, 397, 126016. [Google Scholar] [CrossRef]
- Shen, D.J.; Wang, Y.L.; Nash, P.; Xing, G.Z. Microstructure, temperature estimation and thermal shock resistance of PEO ceramic coatings on aluminum. J. Mater. Process. Technol. 2008, 205, 477–481. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, X.; Li, C.; Yao, G. Effect of thermal conductivity on micro-arc oxidation coatings. Surf. Eng. 2022, 38, 44–53. [Google Scholar] [CrossRef]
- Pakseresht, A.H.; Saremi, M.; Omidvar, H.; Alizadeh, M. Micro-structural study and wear resistance of thermal barrier coating reinforced by alumina whisker. Surf. Coat. Technol. 2019, 366, 338–348. [Google Scholar] [CrossRef]
- Kamalan Kirubaharan, A.M.; Kuppusami, P. Corrosion behavior of ceramic nanocomposite coatings at nanoscale. In Corrosion Protection at the Nanoscale; Micro and Nano Technologies; Rajendran, S., Nguyen, T.A., Kakooei, S., Yeganeh, M., Li, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 295–314. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Optimisation of the plasma electrolytic oxidation process efficiency on aluminium. Surf. Interface Anal. 2010, 42, 221–226. [Google Scholar] [CrossRef]
- Vakili-Azghandi, M.; Fattah-alhosseini, A.; Keshavarz, M.K. Optimizing the electrolyte chemistry parameters of PEO coating on 6061 Al alloy by corrosion rate measurement: Response surface methodology. Measurement 2018, 124, 252259. [Google Scholar] [CrossRef]
- Bembenek, M.; Popadyuk, O.; Shihab, T.; Ropyak, L.; Uhryński, A.; Vytvytskyi, V.; Bulbuk, O. Optimization of Technological Parameters of the Process of Forming Therapeutic Biopolymer Nanofilled Films. Nanomaterials 2022, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Tu, X.; Yu, J.; Zhang, Y.; Miao, C.; Xu, Y.; Fu, R.; Li, J. Optimization of AZ31B Magnesium Alloy Anodizing Process in NaOH-Na2SiO3-Na2B4O7 Environmental-Friendly Electrolyte. Coatings 2022, 12, 578. [Google Scholar] [CrossRef]
- An, L.; Ma, Y.; Yan, X.; Wang, S.; Wang, Z. Effects of electrical parameters and their interactions on plasma electrolytic oxidation coatings on aluminum substrates. Trans. Nonferrous Met. Soc. China 2020, 30, 883–895. [Google Scholar] [CrossRef]
- Kaseem, M.; Dikici, B. Optimization of Surface Properties of Plasma Electrolytic Oxidation Coating by Organic Additives: A Review. Coatings 2021, 11, 374. [Google Scholar] [CrossRef]
- Songur, F.; Arslan, E.; Dikici, B. Taguchi optimization of PEO process parameters for corrosion protection of AA7075 alloy. Surf. Coat. Technol. 2022, 434, 128202. [Google Scholar] [CrossRef]
- Hladkyi, S.I.; Palazhchenko, S.P. Development of equipment for the study of friction nodes operating in the mode of reciprocating motion. Sci. Bull. Ivano-Frankivsk Natl. Tech. Univ. Oil Gas 2015, 2, 89–100. [Google Scholar]
- Bandura, A.; Skaskiv, O. Analog of Hayman’s theorem and its application to some system of linear partial differential equations. J. Math. Phys. Anal. Geom. 2019, 15, 170–191. [Google Scholar] [CrossRef]
- Premchand, C.; Hariprasad, S.; Saikiran, A.; Lokeshkumar, E.; Manojkumar, P.; Ravisankar, B.; Venkataraman, B.; Rameshbabu, N. Assessment of Corrosion and Scratch Resistance of Plasma Electrolytic Oxidation and Hard Anodized Coatings Fabricated on AA7075-T6. Trans. Indian Inst. Met. 2021, 74, 1991–2002. [Google Scholar] [CrossRef]





| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Ti+Zr | Others Elements | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.50 | 0.50 | 3.8–4.9 | 0.3–0.9 | 1.2–1.8 | 0.10 | 0.25 | 0.15 | 0.20 | 0.15 | The rest |
| σUTS, MPa | σY, MPa | δ5 | E, GPa | HB | α, degree–1 | λ, W/(m·degree) | ρ, kg/m3 | C, J/(kg·degree) |
|---|---|---|---|---|---|---|---|---|
| 390–420 | 255–275 | 10–12 | 72 | 105 | 0.0000229 | 130 | 2770 | 0.922 |
| Levels of Factors | Coded Values | Natural Values | ||||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | Concentration Ratio (Na2SiO3/KOH), C | Current Density, i, A/dm2 | Flow Rate, v, cm/s | Electrolyte Temperature, T, °C | |
| The main level | 0 | 0 | 0 | 0 | 4.4 | 5 | 75 | 50 |
| Interval of variation | 1 | 1 | 1 | 1 | 1.3 | 2 | 40 | 15 |
| The upper level | +1 | +1 | +1 | +1 | 5.7 | 7 | 115 | 65 |
| The lower level | −1 | −1 | −1 | −1 | 3.1 | 3 | 45 | 35 |
| Star points (+) | +1.4826 | +1.4826 | +1.4826 | +1.4826 | 6.3 | 8 | 141.7 | 72.2 |
| Star points (−) | −1.4826 | −1.4826 | −1.4826 | −1.4826 | 2.6 | 2 | 8.3 | 27.8 |
| Experiment Number | Levels of Factors | The Average Value of the Optimization Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| x0 | x1 | x2 | x3 | x4 | Microhardness , GPa | Wear , g | Cone-likeness , μm | |
| 1 | +1 | −1 | −1 | −1 | −1 | 13.93 | 0.277 | 15.8 |
| 2 | +1 | −1 | −1 | −1 | +1 | 14.84 | 0.287 | 15.3 |
| 3 | +1 | −1 | −1 | +1 | −1 | 15.11 | 0.260 | 10.4 |
| 4 | +1 | −1 | −1 | +1 | +1 | 14.99 | 0.272 | 10.9 |
| 5 | +1 | −1 | +1 | −1 | −1 | 13.89 | 0.238 | 13.1 |
| 6 | +1 | −1 | +1 | −1 | +1 | 17.73 | 0.215 | 11.4 |
| 7 | +1 | −1 | +1 | +1 | −1 | 16.32 | 0.234 | 10.4 |
| 8 | +1 | −1 | +1 | +1 | +1 | 19.00 | 0.207 | 8.2 |
| 9 | +1 | +1 | −1 | −1 | −1 | 15.37 | 0.241 | 13.1 |
| 10 | +1 | +1 | −1 | −1 | +1 | 13.53 | 0.258 | 15.3 |
| 11 | +1 | +1 | −1 | +1 | −1 | 17.44 | 0.230 | 9.3 |
| 12 | +1 | +1 | −1 | +1 | +1 | 15.47 | 0.245 | 8.7 |
| 13 | +1 | +1 | +1 | −1 | −1 | 19.40 | 0.201 | 10.4 |
| 14 | +1 | +1 | +1 | −1 | +1 | 19.86 | 0.198 | 11.4 |
| 15 | +1 | +1 | +1 | +1 | −1 | 21.95 | 0.182 | 7.1 |
| 16 | +1 | +1 | +1 | +1 | +1 | 20.29 | 0.196 | 6.5 |
| 17 | +1 | −1.4826 | 0 | 0 | 0 | 13.91 | 0.268 | 12.1 |
| 18 | +1 | +1.4826 | 0 | 0 | 0 | 19.65 | 0.207 | 7.4 |
| 19 | +1 | 0 | −1.4826 | 0 | 0 | 14.08 | 0.287 | 11.1 |
| 20 | +1 | 0 | +1.4826 | 0 | 0 | 21.51 | 0.192 | 8.8 |
| 21 | +1 | 0 | 0 | −1.4826 | 0 | 18.91 | 0.213 | 14.2 |
| 22 | +1 | 0 | 0 | +1.4826 | 0 | 19.86 | 0.205 | 7.6 |
| 23 | +1 | 0 | 0 | 0 | −1.4826 | 18.68 | 0.215 | 7.7 |
| 24 | +1 | 0 | 0 | 0 | +1.4826 | 19.63 | 0.207 | 8.8 |
| 25 | +1 | 0 | 0 | 0 | 0 | 21.51 | 0.192 | 8.3 |
| 26 | +1 | 0 | 0 | 0 | 0 | 21.98 | 0.188 | 8.4 |
| Optimization Parameters | Technological Parameters | ||||
| Name | Optimal Value | Concentration Ratio (Na2SiO3/KOH), C | Current Density, i, A/dm2 | Flow Rate, v, cm/s | Electrolyte Temperature,T, °C |
| Minimum Values | |||||
| 3.1 | 3 | 45 | 35 | ||
| Optimal Values | |||||
| , GPa | 18.56 | 5.04 | 6.63 | 106.77 | 49.92 |
| , g | 0.185 | 4.98 | 6.61 | 103.96 | 53.59 |
| , μm | 10.6 | 5.07 | 5.82 | 119.52 | 55.16 |
| − | Maximum Values | ||||
| 5.7 | 7 | 115 | 65 | ||
| Optimization Parameters | Technological Parameters | Optimization Parameters Deviation, % | ||||
| Name | Optimal Value | Concentration Ratio(Na2SiO3/KOH), C | Current Density, i, A/dm2 | Flow Rate, v, cm/s | Electrolyte Temperature, T, °C | |
| Optimal Values | ||||||
| 4.98 | 6.61 | 103.96 | 53.59 | |||
| The Values of the Optimization Parameters are Calculated | ||||||
| , GPa | 18.56 | 17.96 | 3.35 | |||
| , g | 0.185 | 0.185 | 0 | |||
| , μm | 10.6 | 10.9 | 2.91 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ropyak, L.; Shihab, T.; Velychkovych, A.; Bilinskyi, V.; Malinin, V.; Romaniv, M. Optimization of Plasma Electrolytic Oxidation Technological Parameters of Deformed Aluminum Alloy D16T in Flowing Electrolyte. Ceramics 2023, 6, 146-167. https://doi.org/10.3390/ceramics6010010
Ropyak L, Shihab T, Velychkovych A, Bilinskyi V, Malinin V, Romaniv M. Optimization of Plasma Electrolytic Oxidation Technological Parameters of Deformed Aluminum Alloy D16T in Flowing Electrolyte. Ceramics. 2023; 6(1):146-167. https://doi.org/10.3390/ceramics6010010
Chicago/Turabian StyleRopyak, Liubomyr, Thaer Shihab, Andrii Velychkovych, Vitalii Bilinskyi, Volodymyr Malinin, and Mykola Romaniv. 2023. "Optimization of Plasma Electrolytic Oxidation Technological Parameters of Deformed Aluminum Alloy D16T in Flowing Electrolyte" Ceramics 6, no. 1: 146-167. https://doi.org/10.3390/ceramics6010010
APA StyleRopyak, L., Shihab, T., Velychkovych, A., Bilinskyi, V., Malinin, V., & Romaniv, M. (2023). Optimization of Plasma Electrolytic Oxidation Technological Parameters of Deformed Aluminum Alloy D16T in Flowing Electrolyte. Ceramics, 6(1), 146-167. https://doi.org/10.3390/ceramics6010010









