Abstract
Microporous ceramic material, based on β-tricalcium phosphate β-Ca3(PO4)2 with grain size 2–5 μm, pore size smaller than 10 mm, and density 1.22 g/cm3 corresponding to ~40% of the theoretical density (3.07 g/cm3) of β-Ca3(PO4)2, was obtained from a powder mixture with a given molar ratio Ca/P = 1.5 after firing at 1100 °C. A homogenized powder mixture of synthetic dicalcium hydrogen phosphates with the molar ratio Ca/P = 1 and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O with the molar ratio Ca/P = ∞ was used for microporous ceramic preparation. The phase composition of calcium phosphate powder, synthesized from an aqueous solution of phosphoric acid H3PO4 and calcium carbonate CaCO3 powder, included brushite CaHPO4·2H2O as the predominant phase. Formation of β-tricalcium phosphate β-Ca3(PO4)2 during firing occurred due to the heterophase interaction of the products of thermal decomposition of the components of the starting powder mixture, namely, calcium pyrophosphate Ca2P2O7 and calcium oxide CaO. The formation of arch-like structures from β-tricalcium phosphate β-Ca3(PO4)2 grains, which were tightly sintered together, hindered the shrinkage of ceramics. The microporous ceramics obtained, based on β-tricalcium phosphate β-Ca3(PO4)2, can be recommended as a biocompatible and biodegradable material for treatment of bone defects and as a substrate for bone-cell cultivation.
1. Introduction
β-tricalcium phosphate can be treated as one of the leading inorganic materials in bone-tissue engineering [1]. β-tricalcium phosphate in the form of micro- and macroporous ceramic matrices, granules, or as filler for composites with polymer or hydrogel matrices, is widely used as a biocompatible and bioresorbable material in regenerative methods of bone-defect treatment [2,3].
The synthesis of β-tricalcium phosphate in the form of a powder or ceramic can be performed using two different methods. One of them uses thermal transformation of amorphous calcium phosphate, Ca-deficient hydroxyapatite, or carbonate-substituted hydroxyapatite [4,5,6,7,8,9]. The other method uses a heterophase reaction between the components of the powder mixture [8,10,11,12,13,14,15,16]. The use of the powders of amorphous calcium phosphate, Ca-deficient hydroxyapatite, carbonate-substituted hydroxyapatite, or powder mixtures of various components with the molar ratio Ca/P = 1.5 and heat treatment in the interval from 650–750 °C [2,4,8] to 1125 °C (temperature of β−α phase transformation [17]) provide a successful synthesis of β-tricalcium phosphate. There have been attempts to synthesize fine powders of tricalcium phosphate using synthesis in non-aqueous media. Since these attempts cannot be considered successful [18] or scaled for the production of large quantities of powder [19], thermal conversion of synthetic powder precursors and heterophase synthesis of tricalcium phosphate remain in demand.
Taking into account the CaO-P2O5, CaO-P2O5-H2O, and CaO-P2O5-(NH4)2O phase diagrams, it is possible to admit that it is convenient for synthesis of tricalcium phosphate to use powder mixtures containing two components—one with the molar ratio Ca/P = 1 and the other with a molar ratio Ca/P >1.5 [10,20]. There are several calcium phosphate salts with the molar ratio Ca/P = 1. These are calcium pyrophosphate Ca2P2O7, monetite CaHPO4 [21,22,23], brushite CaHPO4·2H2O [24], and hydrated calcium pyrophosphate Ca2P2O7·xH2O [25]. Calcium pyrophosphate Ca2P2O7 forms during heating from hydrated calcium pyrophosphate Ca2P2O7·xH2O, brushite CaHPO4·2H2O, and monetite CaHPO4. So, all of these calcium phosphates can be treated as precursors of calcium pyrophosphate Ca2P2O7. When planning the synthesis of calcium phosphates using aqueous solutions, it is necessary to take into account the possible influence of reaction synthesis byproducts adsorbed at the surface of the powder particles on the formation of phase composition of the ceramics [26]. It is possible to find CO2 and H2O as reaction synthesis byproducts by using an aqueous solution of phosphoric acid H3PO4 and CaCO3, CaO, or Ca(OH)2 as starting reagents. Byproducts such as CO2 and H2O can hardly affect the phase composition of calcium phosphate ceramics. In this work, this served as the basis for using the reaction between the aqueous solution of phosphoric acid and CaCO3 for the synthesis of brushite as a precursor of calcium pyrophosphate.
Powder mixtures with a given molar ratio Ca/P = 1.5 intended for the synthesis of tricalcium phosphate Ca3(PO4)2, in addition to the calcium phosphates with Ca/P = 1 (i.e., calcium pyrophosphate Ca2P2O7, hydrated calcium pyrophosphate Ca2P2O7·xH2O, brushite CaHPO4·2H2O, or monetite CaHPO4) may contain tetracalcium phosphate Ca4(PO4)2O, hydroxyapatite Ca10(PO4)6(OH)2 [27], calcium oxide CaO, or precursors of calcium oxide CaO, such as calcium carbonate CaCO3 [28,29] or calcium salts of carboxylic acids [30,31,32]. Calcium carbonate CaCO3 or calcium salts of carboxylic acids can be treated as reagents convenient for synthesis due to the stability of their chemical composition in storage.
Ceramics based on β-tricalcium phosphate can be prepared pre-synthesized via high-temperature solid-state reaction powder of β-tricalcium phosphate as a direct phase precursor [33,34]. It is well known that powders prepared using a high-temperature solid-state reaction have lower sintering activity than powders synthesized via condensation methods for creation of dispersed systems, sometimes referred as bottom-up methods [35]. Powders of Ca-deficient hydroxyapatite with a molar ratio Ca/P = 1.5, synthesized via the wet precipitate method, have higher sintering activity in the preparation of tricalcium phosphate ceramics. The wide application of this method of powder synthesis for the production of ceramics is limited by the complexity of controlling the molar ratio of Ca/P in synthesized powders. This limitation can be overcome, however, by combining the synthesis of the target phase and the sintering of ceramics at the firing stage during heating of the pre-ceramic powder compact prepared from the powder mixture of the components. To provide sintering activity, at least one of them should be synthesized using one of the bottom-up methods, for example, precipitation from a solution. To enhance the homogeneity of the powder mixture for ceramic production, the second component should have solubility, at least to a small extent, to provide an opportunity to rearrange on the surface of particles of the synthesized powder.
All of the combinations of calcium phosphates with the molar ratio Ca/P = 1 and other components with a molar ratio of Ca/P higher than 1.5 assume the necessity of initial powder mixture homogenization and the heterophase reaction of components for the tricalcium phosphate synthesis, which can take place during heating. It should be noted that creating the possibility for heterophase reaction on the surface of ceramic grains can be treated as one of the ways for inhibiting grain growth [36].
Microporosity of ceramic materials based on calcium phosphates intended for use as bone implants is required to create better conditions for bone-cell adhesivity and osteogenesis [37,38]. Various methods can be used to achieve microporosity of ceramic materials. Among them are incomplete sintering [39], addition of small particles of sacrificial organic or inorganic components to the starting powder mixture [40,41], addition of components having the ability to release of sufficiently large volumes of gases when heated [42], using the sol-gel method of precursor preparation [43], or using a powder mixture containing columnar-like [44] or plate-like [45] particles restraining densification. Synthesized powders of dicalcium phosphate dihydrate CaHPO4·2H2O and/or dicalcium phosphate anhydrate CaHPO4 (i.e., brushite and/or monetite) usually consist of plate-like or petal-like particles [46,47,48,49]. So, the use of synthetic brushite CaHPO4·2H2O or monetite CaHPO4 with special particle morphology as components of a powder mixture for tricalcium phosphate ceramics production can contribute to microporosity creation. Calcium salts of carboxylic acids as precursors of calcium carbonate and calcium oxide, present in the starting powder mixture for the preparation of tricalcium phosphate, can release a considerable volume of gaseous products during heating. Thermal treatment of calcium salts of carboxylic acids gives an opportunity to prepare calcium carbonate with a smaller particle size [32], which can help to reach higher homogeneity of component distribution in the powder mixture.
It should be noted that the development of ceramics based on β-tricalcium phosphate β-Ca3(PO4)2 has a very long history. Most articles during the recent decade with titles and keywords including “microporous calcium phosphate” are devoted to surface modification, creation of composites with polymer matrices, investigation of biocompatibility, or methods of implantation [50,51]. It appears that the topic devoted to the synthesis of β-tricalcium phosphate β-Ca3(PO4)2 and sintering of β-tricalcium phosphate β-Ca3(PO4)2 is closed. Nevertheless, combining the synthesis of target phase composition and sintering with restricted densification at the firing stage in the production of ceramics based on β-tricalcium phosphate β-Ca3(PO4)2 can be treated as a new, non-trivial approach.
The purpose of this study was to create microporous ceramics based on β-tricalcium phosphate β-Ca3(PO4)2 by using a powder mixture with a given molar ratio Ca/P = 1.5, including synthetic dicalcium hydrogen phosphates powder with the molar ratio Ca/P = 1 and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O powder with the molar ratio Ca/P = ∞.
2. Materials and Methods
The scheme for preparing microporous ceramic samples is presented in Figure 1. The sequence of main steps included dicalcium hydrogen phosphates powder synthesis, homogenization of the powder mixture, pressing and firing of samples.
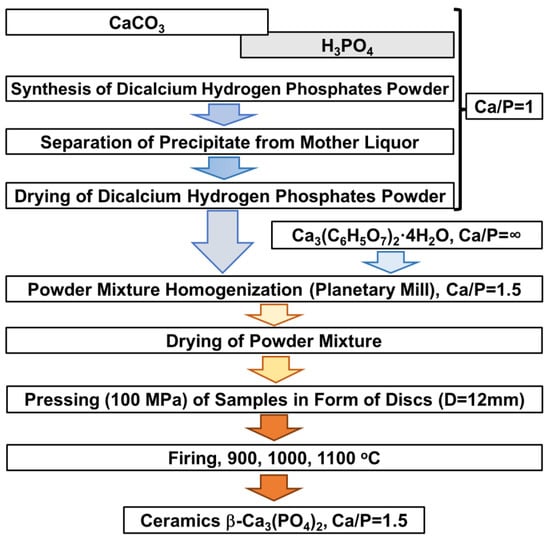
Figure 1.
Scheme of microporous ceramic sample preparation.
Powder of dicalcium hydrogen phosphates with the molar ratio Ca/P = 1 was synthesized by adding 1 M (100 g) of calcium carbonate CaCO3 powder (GOST 4530-76, “Labteh”, Moscow, Russia) to 1 L of 1 M aqueous solution of phosphoric acid H3PO4 prepared from phosphoric acid with density 1.71 g/cm3 (GOST 6552-80, “Chimmed”, Moscow, Russia). Quantities of calcium carbonate CaCO3 and phosphoric acid H3PO4 were calculated according to the reaction of brushite CaHPO4·2H2O synthesis (1):
CaCO3 + H3PO4 + H2O = CaHPO4·2H2O + CO2
The suspension of precipitate prepared according Reaction (1) was kept in the mother liquor under stirring for 1 week. Then, the precipitate was separated from the mother liquor and dried for 1 week at room temperature.
Reaction (2) was used to calculate quantities of initial components, i.e., powder of dicalcium hydrogen phosphate dihydrate (brushite CaHPO4·2H2O), and powder of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O for preparation of powder mixture with the molar ratio Ca/P = 1.5:
6CaHPO4·2H2O + Ca3(C6H5O7)2·4H2O + 9O2 = 3Ca3(PO4)2 + 24H2O + 12CO2
Dicalcium hydrogen phosphate (brushite CaHPO4·2H2O) powder (11.09 g), calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O powder (6.13 g) (CAS 5785-44-4, Shandong Zhishang New Materials Co., Ltd., Jinan, Shandong, China), 86.10 g of grinding media (zirconia balls with diameter ~8 mm), and 40 mL of acetone were placed in container made from agate and homogenized under mechanical activation conditions in a planetary mill (Fritch Pulverisette, Idar-Oberstein, Germany) at a rotation speed of 500 rpm for 15 min. Then, the resulting suspension after homogenization was placed in a porcelain cup and dried in air until the acetone was completely evaporated.
The powder mixture based on the synthesized dicalcium hydrogen phosphates powder and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O after homogenization in a planetary mill was passed through a sieve with a mesh size of 200 µm. Pre-ceramic powder compacts in the form of discs with a diameter of 12 mm and a height of 2–3 mm were made from the prepared powder mixture using a steel mold and a manual press (Carver Laboratory Press model C, Fred S. Carver, Inc., Wabash, IN, USA) at 100 MPa. Then, the samples were fired in air in a furnace at 900, 1000, and 1100 °C with exposure at the specified temperatures for 2 h (the heating rate of the furnace was 5 °C/min). The mass and the linear dimensions of the samples were measured with accuracy ± 0.001 g and ± 0.01 mm, respectively, before and after firing.
Then, the relative diameter after firing and the density of the samples before and after firing were calculated using Equations (3) and (4), respectively.
where:
Drel = Dfiring/Dpress × 100, %,
Drel—relative diameter of the sample after firing, %;
Dfiring—diameter of the sample after firing, cm;
Dpress—diameter of the sample after pressing, cm.
where:
ρ = m/(h × πD2/4), g/cm3,
ρ—density of the sample, g/cm3;
m—weight of the sample, g;
h—thickness of the sample, cm
D—diameter of the sample, cm.
The phase composition of the synthesized powder, powder of calcium citrate tetrahydrate, homogenized powder mixture, and ceramics samples were studied by X-ray diffraction analysis (XRD) using DRON-4M and DRON-3M diffractometers (Joint Stock Company «Bourevestnik», Saint Petersburg, Russia), CuKα, λ = 1.5406, with SiO2 cuvette. Phase analysis was performed at a 2Θ angle range of 5–70° with a step of 0.1° using the ICDD PDF2 database [52].
Thermal analysis was performed using an STA 409 PC Luxx thermal analyzer (NETZSCH, Selb, Germany) during heating in air (10 °C/min, 40–1000 °C), the specimen mass being at least 10 mg. The gas-phase composition was monitored by a Netzsch QMS 403C Aëolos quadrupole mass spectrometer (NETZSCH, Selb, Germany) coupled with a Netzsch STA 409 PC Luxx thermal analyzer. The mass spectra were registered for the following m/Z values: 18 (H2O); 44 (CO2).
Scanning electron microscopy (SEM) images of synthesized powder and powder mixture were characterized by SEM on an NVision 40 microscope (Carl Zeiss, Jena, Germany), and SEM images of ceramic samples were taken with Tescan Vega II (Inca, Oxford Instruments, Abingdon, UK) at accelerating voltages from 1 to 20 kV in secondary electron imaging mode (SE2 detector). A gold layer (≤10 nm in thickness) on the surface of the synthesized powder and powder mixture (synthesized powder + calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O) and chromium layer (≤10 nm in thickness) on the surface of ceramic sample were grown by sputter deposition.
3. Results and Discussion
XRD data for the starting powders and powder mixture with the molar ratio Ca/P = 1.5, prepared from synthesized powder, and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O are shown in Figure 2.
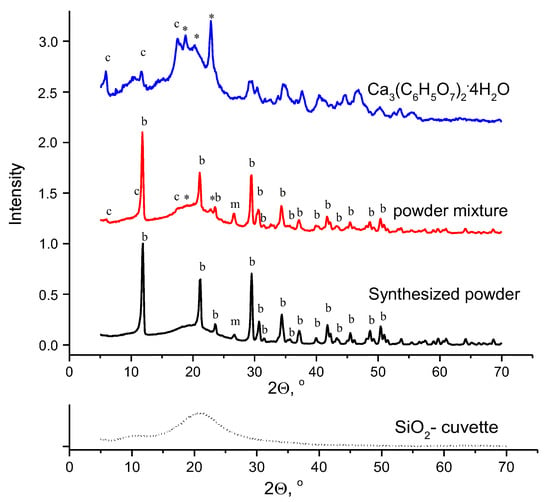
Figure 2.
XRD data for synthesized powder (in black), calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O (in blue), and their powder mixture (in red); (c)—calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O (PDF card 25-1568), *—reflexes belonged to the XRD data for the calcium citrate tetrahydrate reagent, (b)—brushite CaHPO4·2H2O (PDF card 72-713), m—monetite CaHPO4 (PDF card 71-1759).
According to XRD data (Figure 2), the phase composition of synthesized powder was presented by brushite CaHPO4·2H2O (PDF card 72-713). A single reflex (2Θ = 26.6°) matching PDF card 71-1759 corresponding to monetite CaHPO4 was detected on the XRD graph for synthesized powder. High acidity of the aqueous solution of phosphoric acid at the beginning of the synthesis made it possible to form monetite CaHPO4 [53]. Several reflexes matching those of PDF card 25-1568 and several unidentified reflexes (marked *) were found in the XRD graph of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O. The XRD graph of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O used in this work is very close to the XRD graph of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O described previously [31,54]. According to XRD data, the powder mixture after homogenization in acetone media using the planetary mill consisted of synthesized powder of brushite CaHPO4·2H2O as a predominated phase, monetite CaHPO4 as a minor phase, and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O. It should be noted that the reflex (2Θ = 26.6°) of monetite in the XRD graph of the powder mixture after homogenization of the powder mixture in acetone became more apparent than its reflex in the XRD graph of the synthesized powder. Probably, the amplification of the reflex occurred because of the action of acetone as a hygroscopic liquid during homogenization in the planetary mill. As was shown earlier, the powder of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O retained its phase composition after grinding in the acetone medium [55].
SEM images of the synthesized powder with phase composition presented by brushite CaHPO4·2H2O as a predominant phase and the powder mixture of synthesized powder and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O are shown in Figure 3.
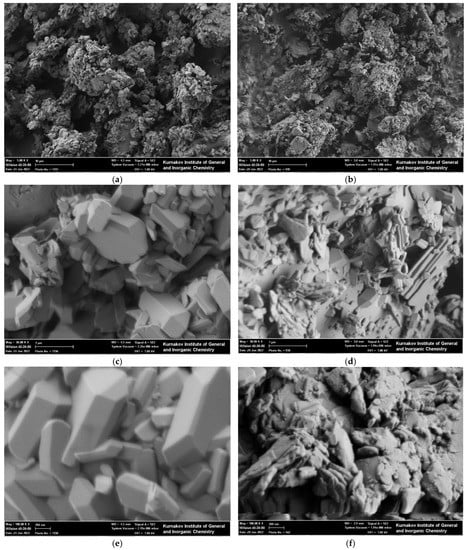
Figure 3.
SEM images of the synthesized powder presented by brushite CaHPO4·2H2O as a predominant phase (a,c,e) and the powder mixture of synthesized powder and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O after homogenization in the planetary mill (b,d,f).
Both the synthesized powder (Figure 3a) and powder mixture (Figure 3b) after homogenization consisted of aggregates of particles with dimensions not exceeding 10 μm. The size of the particles of synthesized powder, consisting mainly of brushite CaHPO4·2H2O (Figure 2), according to SEM images (Figure 3a,c,e), was about 0.5–1.0 µm. The morphology of brushite particles synthesized in this work differs from the plate-like morphology of brushite presented in most articles devoted to brushite synthesis [56,57]. The near-prismatic habit of the brushite crystals can be formed due to the high concentration of aqueous solution of phosphoric acid and saturated water solution of calcium carbonate in the reaction zone [58]. Moreover, the presence of carbonate ions in the reaction zone can also be the reason for the formation of prismatic habit of the brushite crystals [59]. The change in the size and morphology of the particles was observed after homogenization of the powder mixture (Figure 3b,d,f). After homogenization, at least on the surface of the brushite particles, one can observe smaller particles with a shape close to lamellar. The larger dimension of these lamellar particles can be estimated as 0.5–1.0 µm and the smaller dimension can be estimated as 50–100 nm. Particles with an isometric shape and a dimension of about 100 nm can also be observed in the SEM image of the powder mixture after homogenization (Figure 3f). SEM images of the powder mixture after homogenization suggest a conclusion about possible redistribution of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O on the surface of the brushite CaHPO4·2H2O particles. All initial components of the powder mixture are hydrates. With the rather strong mechanical impact of the grinding media, more soluble substance can dissolve into local microdrops of water originating from their own hydrated water or from the water present in the commercially available acetone that was used. This possibility of dissolution can facilitate the rearrangement of more soluble substance on the surface of particles of less soluble substances. More water-soluble calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O (solubility in water—0.95 g/100 mL) could find their place on the surface of less soluble brushite CaHPO4·2H2O particles (solubility in water—0.02 g/100 mL). Bulk densities of synthetic powder, consisting mainly of brushite CaHPO4·2H2O and a powder mixture after homogenization in conditions of mechanical activation, were about 0.56 and 0.33 g/cm3, respectively.
Thermal analysis data for initial components and the powder mixture are shown in Figure 4. According to the XRD data, the expected mass loss of synthesized powder containing brushite CaHPO4·2H2O as the predominant phase should be about 26.2% according to Reaction (5).
2CaHPO4·2H2O = Ca2P2O7 + 5H2O
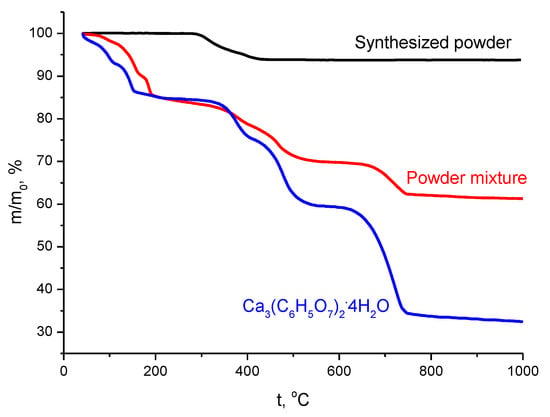
Figure 4.
Thermal analysis data for the synthesized powder (in black), calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O (in blue), and their powder mixture (in red).
The mass loss of synthesized powder according to thermal analysis data was determined as 6.3%, which is close to the mass loss of monetite CaHPO4, 6.6%, as calculated according to Reaction (6).
2CaHPO4 = Ca2P2O7 + H2O
This contradiction between mass loss expected according to XRD analysis data (Figure 2) and a certain mass loss can be explained if we assume that the synthesized dicalcium hydrogen phosphates powder consisted mainly of metastable brushite CaHPO4·2H2O prepared in this work, which was transformed into monetite CaHPO4 (Reaction 7) before recording started. This transformation could take place during the mandatory thermostating stage of thermal analysis, when the sample was maintained at 40 °C for 30 min.
CaHPO4·2H2O = CaHPO4 + 2H2O
It was shown that thermal conversion of brushite CaHPO4·2H2O to monetite CaHPO4 might begin at this temperature [60]. This temperature was marked as a factor that makes formation of monetite CaHPO4 more preferable than the formation of brushite CaHPO4·2H2O during solution synthesis [23,58]. Probably, the presence of some quantity of monetite crystals as the nucleus for a more stable phase could accelerate the transformation. Thermal transformation of dicalcium hydrogen phosphates powder prepared in this work according to Reaction (6) occurred in the 214 °C interval, from 276 to 490 °C, with two maxima at 327 and 403 °C, according to mass spectra data (Figure 5, Table 1).
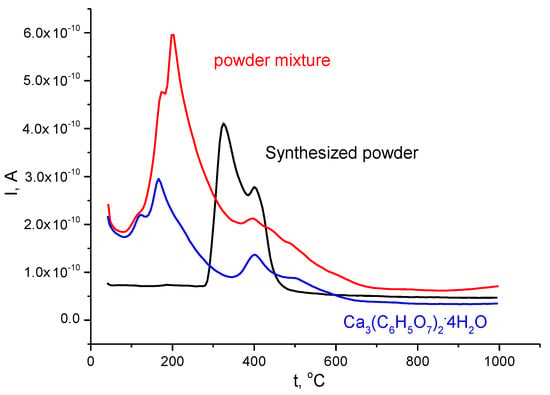
Figure 5.
Mass spectra for evolving gases with m/Z = 18 for synthesized powder (in black), calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O (in blue), and their powder mixture (in red).

Table 1.
Comparison of mass spectra data for synthesized powder, calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O, and their powder mixture.
Monetite CaHPO4, synthesized from the same pair of precursors at 80 °C during 24 h and dried at 60 °C, transformed to the calcium pyrophosphate Ca2P2O7 at the same heating rate in a narrower 70 °C interval, from 420 to 490 °C [61]. Monetite CaHPO4, synthesized in conditions of mechanical activation from hydroxyapatite Ca10(PO4)6(OH)2 and monocalcium phosphate monohydrate Ca(H2PO4)2·H2O converted to calcium pyrophosphate Ca2P2O7 in a 174 °C interval, from 300 to 475 °C [62]. Probably, the morphology, size of particles, and chemical prehistory were the reasons for the slight difference in thermal characteristics of powders of synthesized dicalcium hydrogen phosphates. A slightly noticeable maximum at 547 °C is visible at m/Z = 44 on the mass spectra graph of synthesized powder (Figure 6, Table 1). This maximum can hardly be attributed to the decomposition of calcium carbonate; no mass loss was detected at temperatures higher than 490 °C. Probably, the release of CO2 from carbonate ions adsorbed in minor quantity during synthesis on the particle surfaces of the dicalcium hydrogen phosphates was captured.
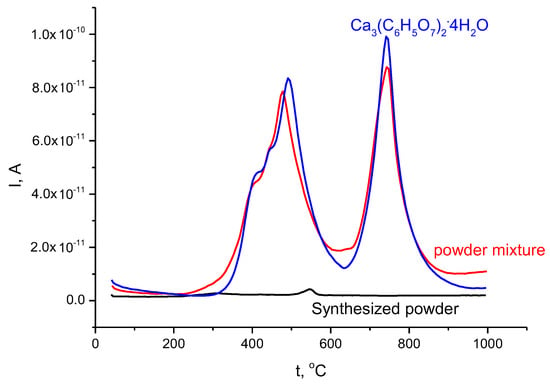
Figure 6.
Mass spectra for evolving gases with m/Z = 44 for synthesized powder (in black), calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O (in blue), and their powder mixture (in red).
The curve for the temperature dependence of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O mass (Figure 4) correlates well with thermal analysis data published before [31,54].
The total mass loss of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O according to thermal analysis data was determined as 67.6%. This mass loss is close to that calculated (70.5%) for the transformation of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O into calcium oxide CaO, according to Reaction (8).
Ca3(C6H5O7)2·4H2O + 9O2 = 3CaO + 12CO2 + 9H2O
The process of thermal decomposition of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O can be described by following Reactions (9)–(12).
Ca3(C6H5O7)2·4H2O = Ca3(C6H5O7)2·2H2O + 2H2O
Ca3(C6H5O7)2·2H2O = Ca3(C6H5O7)2 + 2H2O
Ca3(C6H5O7)2 + 9O2 = 3CaCO3 + 9CO2 + 5H2O
CaCO3 = 3CaO + CO2
According to the m/Z = 18 mass spectrum graph of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O, evolution of H2O occurred in the interval 80–650 °C with maxima at 120, 167, and 403 °C (Figure 5). According to the m/Z = 44 mass spectrum graph of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O, evolution of CO2 occurred in the interval 310–940 °C with maxima at 491 and 742 °C (Figure 6). Decomposition of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O according to Reaction (10) is a rather complicated process, since two additional steps can be found in the m/Z = 44 mass spectrum graph before the first maximum (491 °C)—at 410 and 445 °C.
Total mass loss of the powder mixture according thermal analysis data was determined as 38.7% (Figure 4). This mass loss is between mass loss calculated for the powder mixture prepared using monetite (33%) and a powder mixture prepared using brushite (42%). It is possible to assume that brushite present in the powder mixture may partially lose water in the same way as synthesized dicalcium hydrogen phosphates powder itself during the thermostating stage, as described above. The mass loss of the powder mixture due to evolving H2O (m/Z = 18) occurred in the interval 80–705 °C with maxima at (120—slightly visible), 175, 200, and 401 oC (Figure 5, Table 1). The mass loss due to evolving CO2 (m/Z = 44) occurred in the interval 230–900 °C with maxima at 475 and 742 °C, and two steps before the first maximum at 403 and 435 °C, according to mass spectra data (Figure 6, Table 1). The character of the m/Z = 44 mass spectrum curve for the powder mixture is very close to that of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O. It can be noted that the interval of CO2 evolving from the powder mixture has a slight shift toward lower temperatures (Figure 6, Table 1).
The evolution of H2O (m/Z = 18) from the powder mixture during heating occurred in a broader interval (Figure 5, Table 1), and had all maxima attributable to calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O, and two maxima, which can be explained by the decomposition of brushite (200 °C, Reaction (7)) and monetite (~400 °C, Reaction (6)). All chemical reactions listed above, both for synthesized dicalcium hydrogen phosphates powder and for powder of calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O, occurred in the powder mixture of those components. Moreover, the reaction between products of thermal decomposition of synthesized dicalcium hydrogen phosphates powder and calcium citrate tetrahydrate was expected. However, signs of this reaction cannot be found at the curve of mass loss.
XRD data for the ceramic samples after firing at 900, 1000, and 1100 °C (Figure 7) showed no significant differences in the peaks of the diffraction patterns.
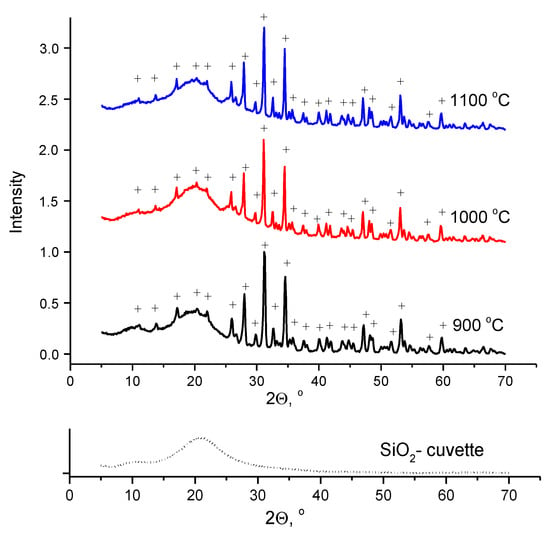
Figure 7.
XRD data for ceramic samples fired at different temperatures with 2 h holding time; (+)—β-tricalcium phosphate (PDF card 9-169).
According to XRD data (Figure 7), the phase composition of ceramic samples after firing at 900, 1000, and 1100 °C was represented by β-tricalcium phosphate. This means that Reaction (13) was completed before 900 °C during firing at these temperatures.
Ca2P2O7 + CaO = Ca3(PO4)2
The formation of the phase composition of ceramic samples, represented by β-Ca3(PO4)2, occurred during the heterogeneous interaction of the products of thermal decomposition of the components of the powder mixtures, namely, calcium pyrophosphate Ca2P2O7 and calcium oxide CaO. Reaction (13) may reflect the chemical processes of formation of β-tricalcium phosphate β-Ca3(PO4)2 during firing of the ceramic samples. Mass loss of the ceramic samples after firing at 900, 1000, 1100 °C was about 40%. Additionally, this mass loss of the samples, determined by weighting before and after firing, is close to the mass loss of the powder mixture determined by means of thermal analysis (38.7%, Figure 4).
SEM images of ceramic samples prepared from the powder mixture of dicalcium hydrogen phosphates (mainly brushite CaHPO4·2H2O) and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O after firing at 900 °C (a), 1000 °C (b), 1100 °C (c) are shown in Figure 8.
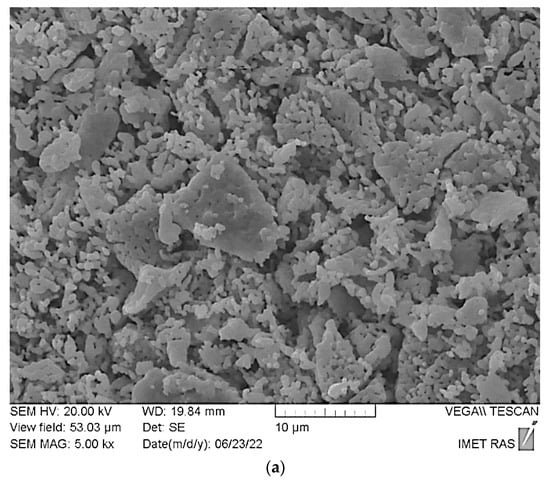
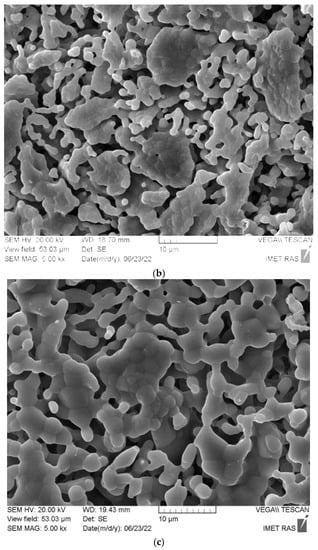
Figure 8.
SEM images of ceramic samples prepared from the powder mixture of synthesized dicalcium hydrogen phosphates (mainly brushite CaHPO4·2H2O) and calcium citrate tetrahydrate (Ca3(C6H5O7)2·4H2O) after firing at 900 °C (a), 1000 °C (b), 1100 °C (c).
Microstructure of the ceramic sample after firing at 900 °C (Figure 8a) appears undersintered. Grain dimensions are less than 1 μm. One can see plate-like sintered fragments of irregular form with the dimensions 3–10 μm. After firing at 1000 °C (Figure 8b), grain dimensions are in the interval 1–2 μm. Plate-like fragments are connected with each other with arc–grain segments. Ceramics have permeable porosity. After firing at 1100 °C (Figure 8c), the ceramics remain porous with dimensions of the pores at less than 10 μm. Grain dimensions are in the interval 2–5 μm. The formation of arch-like structures from densely sintered β-tricalcium phosphate grains hindered the shrinkage of the ceramics.
Density and relative diameter of the ceramic samples prepared from the powder mixture of synthesized dicalcium hydrogen phosphates (mainly brushite CaHPO4·2H2O) and calcium citrate tetrahydrate (Ca3(C6H5O7)2·4H2O) before (1.20–1.25 g/cm3) and after firing at 900, 1000, 1100 °C are shown in Figure 9. Linear shrinkage of ceramic samples increased from ~1% after firing at 900 °C up to 13% after firing at 1100 °C. Owing to mass loss of 40%, the density of the ceramic samples after firing at 900 °C was ~0.83 g/cm3. The density of the ceramic samples increased with increasing firing temperature, and after firing at 1100 °C, the density was 1.22 g/cm3 (~40%—in comparison to the theoretical density of β-Ca3(PO4)2 = 3.07 (PDF card 9-169, [63,64]).
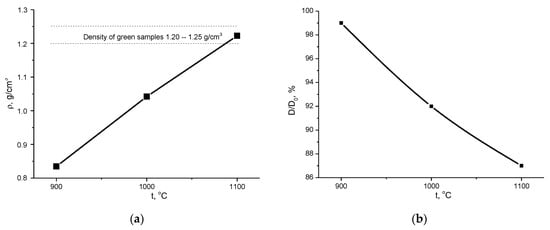
Figure 9.
Density ρ, g/cm3 (a), and relative diameter D/D0, % (b), of ceramic samples prepared from the powder mixture of dicalcium hydrogen phosphates powder (mainly brushite) and calcium citrate tetrahydrate after firing at 900, 1000, 1100 °C.
The powder mixture including dicalcium hydrogen phosphates and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O can potentially be used for the preparation of not only monophase powder and ceramics with a preset molar ratio Ca/P = 1.5, but also for preparation of monophase hydroxyapatite Ca10(PO4)6(OH)2 powder and ceramics with a preset molar ratio Ca/P = 1.67, and for ceramic composite materials containing both β-tricalcium phosphate β-Ca3(PO4)2 and hydroxyapatite Ca10(PO4)6(OH)2. According to the CaO-P2O5 and CaO-P2O5-H2O phase diagrams, the β-tricalcium phosphate β-Ca3(PO4)2 phase can be combined with β-calcium pyrophosphate β-Ca2P2O7 and/or hydroxyapatite Ca10(PO4)6(OH)2. The possibility for creating microporosity in ceramic materials based on a powder mixture including dicalcium hydrogen phosphates and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O with a preset molar ratio in interval 1 < Ca/P ≤ 1.67 requires additional research. Moreover, the creation of ceramic materials with bimodal character of porosity based on the powder mixture considered in this present article can be a task for future investigation. The powder mixture considered in this present article can be used for the preparation of macroporous ceramics via paste extrusion 3D printing or via use of sacrificed additives.
4. Conclusions
The unique starting powder mixture with a composition including dicalcium hydrogen phosphates (mainly brushite CaHPO4·2H2O) powder and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O powder was used for preparing microporous ceramics with a density of 1.22 g/cm3 (~40%—in comparison with theoretical density of β-Ca3(PO4)2, = 3.07 g/cm3) with a phase composition presented by β-tricalcium phosphate β-Ca3(PO4)2 via firing at 1100 °C.
The formation of the phase composition of ceramic samples, represented by β-Ca3(PO4)2, occurred during firing at 900–1100 °C due to the heterophase interaction of the products of thermal decomposition of the components of the starting powder mixture, namely, calcium pyrophosphate Ca2P2O7 and calcium oxide CaO. The powder mixture consisting of dicalcium hydrogen phosphates powder with the molar ratio Ca/P = 1 and calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O with the molar ratio Ca/P = ∞ was, for the first time, used for the preparation of β-tricalcium phosphate β-Ca3(PO4)2 ceramics.
According to our knowledge, the possibility to use calcium citrate tetrahydrate Ca3(C6H5O7)2·4H2O as the precursor of calcium oxide CaO in the heterophase reaction of β-tricalcium phosphate β-Ca3(PO4)2 synthesis was investigated for the first time. A rather uniform distribution of calcium citrate tetrahydrate (precursor of CaO) on the surface of dicalcium hydrogen phosphate particles (mainly brushite CaHPO4·2H2O), due to homogenization under conditions of mechanical activation, provided tight sintering of the grains in the arc-like structures in the microstructure of the ceramics based on β-tricalcium phosphate β-Ca3(PO4)2.
It was first demonstrated that there was inheritance in the ceramic microstructure of a multi-level rigid structure of prismatic particle aggregates of dicalcium hydrogen phosphates powder with phase composition presented mainly by brushite CaHPO4·2H2O. This multi-level rigid structure of brushite CaHPO4·2H2O aggregates was the reason for formation of arch-like constructions of tightly sintered grains of β-tricalcium phosphate β-Ca3(PO4)2, and one of the factors restraining densification. Gas evolution during heating due to thermal decomposition of the powder mixture components could also be an additional factor that restrained densification of ceramics and caused microporosity formation.
The investigation presented was mainly devoted to the creation of the target phase composition (β-tricalcium phosphate β-Ca3(PO4)2, molar ratio Ca/P = 1.5) and microporous structure of ceramics. The development of this study will consist of an investigation of properties that are important for the implementation of this material in the clinical practice of regenerative medicine.
Author Contributions
Conceptualization, T.S. (Tatiana Safronova); methodology, T.S. (Tatiana Safronova); investigation, G.G., T.S. (Tatiana Safronova), T.S. (Tatiana Shatalova), I.R., V.P. and D.K.; writing—original draft preparation, T.S. (Tatiana Safronova) and G.G; writing—review and editing, T.S. (Tatiana Safronova); visualization, G.G., T.S. (Tatiana Safronova), I.R., V.P. and D.K.; supervision, T.S. (Tatiana Safronova); project administration, T.S. (Tatiana Safronova); funding acquisition, T.S. (Tatiana Safronova). All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with financial support from the Russian Foundation for Basic Research (RFBR) (grant no. 20-03-00550).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was carried out using the equipment of the MSU Shared Research Equipment Center “Technologies for obtaining new nanostructured materials and their complex study”, and purchased by MSU in the frame of the Equipment Renovation Program (National Project “Science”), and in the frame of the MSU Program of Development. Some of the SEM images were recorded using scientific equipment at the Joint Research Center for Physical Methods of Research, located in the Kurnakov Institute of General and Inorganic Chemistry RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Stahl, A.; Yang, Y.P. Regenerative approaches for the treatment of large bone defects. Tissue Eng. Part B Rev. 2021, 27, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Eanes, E.D. Thermochemical studies on amorphous calcium phosphate. Calcif. Tissue Res. 1970, 5, 133–145. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Avramenko, O.A.; Shekhirev, M.A.; Veresov, A.G. Ca-deficient hydroxyapatite powder for producing tricalcium phosphate based ceramics. Glass Ceram. 2011, 68, 28–32. [Google Scholar] [CrossRef]
- Gibson, I.R.; Rehman, I.; Best, S.M.; Bonfield, W. Characterization of the transformation from calcium-deficient apatite to β-tricalcium phosphate. J. Mater. Sci. Mater. Med. 2000, 11, 533–539. [Google Scholar] [CrossRef]
- Villet-Regi, M.; Rodriguez, L.M.; Salinas, A.J. Synthesis and characterization of calcium deficient apatite. Solid State Ion. 1997, 101–103, 1279–1285. [Google Scholar] [CrossRef]
- Barralet, J.; Knowles, J.C.; Best, S.; Bonfield, W. Thermal decomposition of synthesised carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 2002, 13, 529–533. [Google Scholar] [CrossRef]
- Safronova, T.; Putlayev, V.; Filippov, Y.; Shatalova, T.; Karpushkin, E.; Larionov, D.; Kazakova, G.; Shakhtarin, Y. Calcium phosphate powder synthesized from calcium acetate and ammonium hydrophosphate for bioceramics application. Ceramics 2018, 1, 375–392. [Google Scholar] [CrossRef]
- Safronova, T.V. Inorganic materials for regenerative medicine. Inorg. Mater. 2021, 57, 443–474. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, P.V.; Putlyaev, V.I.; Ivanov, V.K.; Garshev, A.V.; Shatalova, T.B.; Orlov, N.K.; Safronova, T.V. Phase equilibria in the tricalcium phosphate-mixed calcium sodium (potassium) phosphate systems. Russ. J. Inorg. Chem. 2014, 59, 1219–1227. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. Bioactive ceramics: Physical chemistry. In Comprehensive Biomaterials; Ducheyne, P., Healy, K., Hutmacher, D., Grainger, D.E., Kirkpatrick, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 187–221. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, J.-L.; Shao, C.Y. Preparation of β-TCP with high thermal stability by solid reaction route. J. Mater. Sci. 2003, 38, 1049–1056. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Ivanov, V.K.; Knot’ko, A.V.; Shatalova, T.B. Powders mixtures based on ammonium hydrophosphate and calcium carbonate for preparation of biocompatible porous ceramic in the CaO–P2O5 system. Refract. Ind. Ceram. 2016, 56, 502–509. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Besprozvannykh, V.K.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Smirnova, M.P.; Levin, I.S.; Ivchenko, P.V. Simple, efficient and reliable method for the preparation of β-tricalcium phosphate. Mendeleev Commun. 2021, 31, 379–381. [Google Scholar] [CrossRef]
- Hill, W.L.; Faust, G.T.; Reynolds, D.S. The binary system P2O5; 2CaO.P2O5. Am. J. Sci. 1944, 242, 457–477. [Google Scholar] [CrossRef]
- Larionov, D.S.; Kuzina, M.A.; Evdokimov, P.V.; Garshev, A.V.; Orlov, N.K.; Putlyaev, V.I. Synthesis of Calcium Phosphate Powders in Nonaqueous Media for Stereolithography 3D Printing. Russ. J. Inorg. Chem. 2020, 65, 312–322. [Google Scholar] [CrossRef]
- Galea, L.; Bohner, M.; Thuering, J.; Doebelin, N.; Aneziris, C.G.; Graule, T. Control of the size, shape and composition of highly uniform, non-agglomerated, sub-micrometer β-tricalcium phosphate and dicalcium phosphate platelets. Biomaterials 2013, 34, 6388–6401. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I. Powder systems for calcium phosphate ceramics. Inorg. Mater. 2017, 53, 17–26. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Imaniyyah, A.G.; Herda, E. Monetite as a potential ideal bone substitute: A short review on fabrication and properties. Mater. Today Proc. 2022, 66, 2762–2766. [Google Scholar] [CrossRef]
- Boanini, E.; Pagani, S.; Tschon, M.; Rubini, K.; Fini, M.; Bigi, A. Monetite vs. Brushite: Different Influences on Bone Cell Response Modulated by Strontium Functionalization. J. Funct. Biomater. 2022, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Safronova, T.V.; Shatalova, T.B.; Tikhonova, S.A.; Filippov, Y.Y.; Krut’ko, V.K.; Musskaya, O.N.; Kononenko, N.E. Synthesis of Calcium Pyrophosphate Powders from Phosphoric Acid and Calcium Carbonate. Inorg. Mater. Appl. Res. 2021, 12, 986–992. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Kurbatova, S.A.; Shatalova, T.B.; Larionov, D.S.; Kozlov, D.A.; Evdokimov, P.V. Properties of amorphous calcium pyrophosphate powder synthesized via ion exchange for the preparation of bioceramics. Inorg. Mater. 2015, 51, 1177–1184. [Google Scholar] [CrossRef]
- Safronova, T.V. Phase composition of ceramic based on calcium hydroxyapatite powders containing byproducts of the synthesis reaction. Glass Ceram. 2009, 66, 136–139. [Google Scholar] [CrossRef]
- Safronova, T.; Putlayev, V.; Shekhirev, M. Resorbable Calcium Phosphates Based Ceramics. Powder. Metall. Met. Ceram. 2013, 52, 357–363. [Google Scholar] [CrossRef]
- Jinlong, N.; Zhenxi, Z.; Dazong, J. Investigation of phase evolution during the thermochemical synthesis of tricalcium phosphate. J. Mater. Synth. Process. 2001, 9, 235–240. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Filippov, Y.; Kazakova, G.; Shatalova, T.; Rau, J.V. Powders Based on Ca2P2O7-CaCO3-H2O System as Model Objects for the Development of Bioceramics. Ceramics 2022, 5, 423–434. [Google Scholar] [CrossRef]
- Polat, S. Thermal degradation of calcium lactate pentahydrate using TGA/FTIR/MS: Thermal kinetic and thermodynamics studies. Indian Chem. Eng. 2021, 64, 1–14. [Google Scholar] [CrossRef]
- Mansour, S.A. Thermal decomposition of calcium citrate tetrahydrate. Thermochim. Acta 1994, 233, 243–256. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shatalova, T.B.; Boytsova, O.V.; Knotko, A.V.; Toshev, O.U.; Hotamov, S.M.; Odinaeva, A.T.; Azizian-Kalandaragh, Y. Chemical Transformations as a Tool for Controlling the Properties of Calcium Carbonate Powder. Glass Ceram. 2020, 77, 145–148. [Google Scholar] [CrossRef]
- Safronova, T.V.; Selezneva, I.I.; Tikhonova, S.A.; Kiselev, A.S.; Davydova, G.A.; Shatalova, T.B.; Larionov, D.S.; Rau, J.V. Biocompatibility of biphasic α, β-tricalcium phosphate ceramics in vitro. Bioact. Mater. 2020, 5, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Youn, H.J.; Hong, K.S.; Chang, B.S.; Lee, C.K.; Chung, S.S. An improvement in sintering property of β-tricalcium phosphate by addition of calcium pyrophosphate. Biomaterials 2002, 23, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2021, 200, 102597. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Z.; Wang, H. Densification and grain growth during sintering of nanosized particles. Int. Mater. Rev. 2008, 53, 326–352. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Shekhirev, M.A.; Kuznetsov, A.V. Disperse systems in calcium hydroxyapatite ceramics technology. Glass Ceram. 2007, 64, 22–26. [Google Scholar] [CrossRef]
- Biggemann, J.; Köllner, D.; Simon, S.; Heik, P.; Hoffmann, P.; Fey, T. Porous Functional Graded Bioceramics with Integrated Interface Textures. Ceramics 2021, 4, 681–695. [Google Scholar] [CrossRef]
- Hoelzle, D.J.; Svientek, S.R.; Alleyne, A.G.; Wagoner Johnson, A.J. Design and manufacture of combinatorial calcium phosphate bone scaffolds. J. Biomech. Eng. 2011, 133, 101001. [Google Scholar] [CrossRef]
- Beletskii, B.I.; Shumskii, V.I.; Nikitin, A.A.; Vlasova, E.B. Biocomposite calcium-phosphate materials used in osteoplastic surgery. Glas Ceram. 2000, 57, 322–325. [Google Scholar] [CrossRef]
- Layrolle, P.; Ito, A.; Tateishi, T. Sol-gel synthesis of amorphous calcium phosphate and sintering into microporous hydroxyapatite bioceramics. J. Am. Ceram. Soc. 1998, 81, 1421–1428. [Google Scholar] [CrossRef]
- Safronova, T.V.; Belokozenko, M.A.; Yahyoev, S.O.; Shatalova, T.B.; Kazakova, G.K.; Peranidze, K.K.; Toshev, O.U.; Khasanova, S.S. Ceramics based on CaSO4⋅2H2O powder synthesized from Ca(NO3)2 and (NH4)2SO4. Inorg. Mater. 2021, 57, 867–873. [Google Scholar] [CrossRef]
- Safronova, T.V.; Chichulin, S.N.; Shatalova, T.B.; Filippov, Y.Y. Powder mixture for the production of microporous ceramics based on hydroxyapatite. Ceramics 2022, 5, 108–119. [Google Scholar] [CrossRef]
- Arifuzzaman, S.M.; Rohani, S. Experimental study of brushite precipitation. J. Cryst. Growth 2004, 267, 624–634. [Google Scholar] [CrossRef]
- Hamai, R.; Toshima, T.; Tafu, M.; Masutani, T.; Chohji, T. Effect of anions on morphology control of brushite particles. Key Eng. Mater. 2013, 529, 55–60. [Google Scholar] [CrossRef]
- Toshima, T.; Hamai, R.; Tafu, M.; Takemura, Y.; Fujita, S.; Chohji, T.; Tanda, S.; Li, S.; Qin, G.W. Morphology control of brushite prepared by aqueous solution synthesis. J. Asian Ceram. Soc. 2014, 2, 52–56. [Google Scholar] [CrossRef]
- Issa, K.; Alanazi, A.; Aldhafeeri, K.A.; Alamer, O.; Alshaaer, M. Brushite: Synthesis, Properties, and Biomedical Applications. In Crystallization and Applications, 2nd ed.; Smida, Y., Marzouki, R., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Ressler, A.; Antunović, M.; Teruel-Biosca, L.; Ferrer, G.G.; Babić, S.; Urlić, I.; Ivanković, M.; Ivanković, H. Osteogenic differentiation of human mesenchymal stem cells on substituted calcium phosphate/chitosan composite scaffold. Carbohydr. Polym. 2022, 277, 118883. [Google Scholar] [CrossRef]
- Qi, C.; Musetti, S.; Fu, L.H.; Zhu, Y.J.; Huang, L. Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications. Chem. Soc. Rev. 2019, 48, 2698–2737. [Google Scholar] [CrossRef]
- CDD. PDF-4+ 2010 (Database); Kabekkodu, S., Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA, 2010; Available online: https://www.icdd.com/pdf-2/ (accessed on 18 July 2022).
- Minh, D.P.; Lyczko, N.; Sebei, H.; Nzihou, A.; Sharrock, P. Synthesis of calcium hydroxyapatite from calcium carbonate and different orthophosphate sources: A comparative study. Mater. Sci. Eng. B 2012, 177, 1080–1089. [Google Scholar] [CrossRef]
- Herdtweck, E.; Kornprobst, T.; Sieber, R.; Straver, L.; Plank, J. Crystal Structure, Synthesis, and Properties of tri-Calcium di-Citrate tetra-Hydrate [Ca3(C6H5O7)2(H2O)2]·2H2O. Z. Anorg. Allg. Chem. 2011, 637, 655–659. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shatalova, T.B.; Filippov, Y.Y.; Krut’ko, V.K.; Musskaya, O.N.; Safronov, A.S.; Toshev, O.U. Ceramics in the Ca2P2O7–Ca(PO3)2 System Obtained by Annealing of the Samples Made from Hardening Mixtures Based on Calcium Citrate Tetrahydrate and Monocalcium Phosphate Monohydrate. Inorg. Mater. Appl. Res. 2020, 11, 777–786. [Google Scholar] [CrossRef]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and hydrolysis of brushite (DCPD): The role of ionic substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Ucar, S.; Bjørnøy, S.H.; Bassett, D.C.; Strand, B.L.; Sikorski, P.; Andreassen, J.P. Nucleation and Growth of Brushite in the Presence of Alginate. Cryst. Growth Des. 2015, 15, 5397–5405. [Google Scholar] [CrossRef]
- Abbona, F.; Christensson, F.; Angela, M.F.; Madsen, H.L. Crystal habit and growth conditions of brushite, CaHPO4⋅ 2H2O. J. Cryst Growth 1993, 131, 331–346. [Google Scholar] [CrossRef]
- Kuz’mina, M.A.; Zhuravlev, S.V.; Frank-Kamenetskaya, O.V. The effect of medium chemistry on the solubility and morphology of brushite crystals. Geol. Ore Deposits. 2013, 55, 692–697. [Google Scholar] [CrossRef]
- Dosen, A.; Giese, R.F. Thermal decomposition of brushite, CaHPO4· 2H2O to monetite CaHPO4 and the formation of an amorphous phase. Am. Mineral. 2011, 96, 368–373. [Google Scholar] [CrossRef]
- Mulongo-Masamba, R.; El Kassri, T.; Khachani, M.; Arsalane, S.; Halim, M.; El Hamidi, A. Synthesis and thermal dehydroxylation kinetic of anhydrous calcium phosphate monetite CaHPO4. J. Therm. Anal. Calorim. 2016, 124, 171–180. [Google Scholar] [CrossRef]
- Safronova, T.V.; Sadilov, I.S.; Chaikun, K.V.; Shatalova, T.B.; Filippov, Y.Y. Synthesis of Monetite from Calcium Hydroxyapatite and Monocalcium Phosphate Monohydrate under Mechanical Activation Conditions. Russ. J. Inorg. Chem. 2019, 64, 1088–1094. [Google Scholar] [CrossRef]
- Dickens, B.; Schroeder, L.W.; Brown, W.E. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. The crystal structure of pure β-Ca3(PO4)2. J. Solid State Chem. 1974, 10, 232–248. [Google Scholar] [CrossRef]
- Calvo, C.; Gopal, R. The crystal structure of whitlockite from the Palermo Quarry. Am. Mineral. 1975, 60, 120–133. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).