Abstract
This paper is the continuation of our previous paper. In this work, we optimized the synthesis of aluminum borides by the SHS method. The purpose of the research was to develop the foundations of waste-free technology. The initial components were powders of boric anhydride (B2O3), aluminum (Al), the oxide-heating additive (KNO3), various fluxing additives, including mixed ones. The optimal ratios of the initial components for increasing the yield of aluminum boride with a high boron content and obtaining slag suitable for the production of high-alumina clinkers were determined. Studies have shown that the development of a waste-free technology for producing aluminum borides by the method of self-propagating high-temperature synthesis (SHS) is possible and yields target (alloy) and by-product (slag) products that meet the requirements for chemical and phase composition.
1. Introduction
The currently widespread methods for producing aluminum borides, such as the aluminothermal furnace [1,2,3,4], direct synthesis from pure elements [5,6,7], electrolysis of molten media [8], deposition from gas phases, electrochemical in halogen oxide melts, and reduction of boron oxide and potassium tetrafluoroborate in cryolite melts [9,10], are all energy-intensive and need long-term, complex hardware design. In addition, the waste generated during the implementation of these technologies requires disposal, which increases the costs.
In the present study, the synthesis of aluminum borides in the mode of self-propagating high-temperature synthesis (SHS) [11] was based on the recipe criteria for obtaining slag with certain physical and chemical properties—fluidity, low viscosity and density—which contribute to good phase separation and the formation of a solid aluminum boride alloy ingot. The proposed method is characterized by a short duration and environmental friendliness.
In addition to the target product, aluminum borides, the resulting by-product, aluminothermic slag, exhibits commercial properties that make it possible to use it as a raw material for the production of high-alumina clinkers. High-alumina clinkers are used in the production of cement used for the refractory concrete lining of furnaces of metallurgical, chemical, and machine-building enterprises and in the production of building materials.
Waste-free technology implies the most rational use of natural resources and energy, ensuring environmental protection through the use of waste in a parallel (adjacent) production. The attempt to develop a waste-free technology for the production of aluminum borides showed that the SHS method used makes it possible to obtain the target and by-products that meet industrial requirements for raw materials for the production of high-alumina clinkers concerning chemical and phase composition.
2. Materials and Methods
The initial components were powders of boric anhydride, B2O3, special purity grade 12-3 (Russian standard TU 6-09-3558-78); aluminum, brand PA-2, PA-3 (Russian standard GOST 6058-73); potassium nitrate, KNO3, grade pure for analysis (Russian standard GOST (TU) 4217-77); fluorspar, CaF2 (Russian standard GOST 7167-77); lime, CaO (Russian standard GOST 9179-2018); potassium tetraborofluorate, KBF4 (Russian standard GOST 9532-75).
X-ray diffraction analysis (XRD) of the synthesis products was carried out by using an automated diffractometer with CuKα-radiation (Bourevestnik, model DRON-3). X-ray phase analysis on a semi-quantitative basis was performed using the method of equal weights and artificial mixtures. Quantitative ratios of crystalline phases were determined. The interpretation of the diffraction patterns was carried out using the International Centre for Diffraction Data (ICDD) file repository (PDF 2 2022 database) and HighScorePlus XRD analysis software. The content was calculated for the main phases. The identification of possible impurities, which could be ambiguous due to their low contents, were not indicated.
Compositional analysis of the synthesis products was carried using the EXAFS (extended X-ray absorption fine structure) method, which yields information about the local environment of atoms, both in solids and individual molecules.
3. Results and Discussion
The synthesis of aluminum boride was carried out by the aluminothermic method in the SHS mode [2,12,13].
When obtaining aluminum borides from boric anhydride in this way, the formation of two types of aluminum boride is possible, as described by the reaction equations:
13Al + 6B2O3 → AlB12 + 6Al2O3
3Al + B2O3 → AlB2 + Al2O3
A feature of the aluminothermic process is a large slag ratio. The ingot of the alloy is formed as a result of the deposition of particles of the reduced metal through the thickness of the molten slag.
Therefore, the viscosity of the slag melt affects the rate of the metal ingot formation process and largely determines the technical and economic indicators of the process. The viscosity of the main component of aluminothermic slags, aluminum oxide, is relatively low near the melting point and amounts to 0.06 Pa∙s, and at 2373 K it is 0.05 Pa∙s [14]. Table 1 shows the compositions of boron-containing slags formed during aluminothermic processes for obtaining boron alloys and their viscosity at different temperatures.

Table 1.
Chemical composition and viscosity of boron-containing slags. Reprinted/adapted with permission from Ref. [14]. 1960, Kozakevitch, P.
At temperatures of 2023–2073 K, the slag viscosity does not exceed 0.15 Pa∙s. As the temperature of the melted slag decreases, its crystallization begins, and the formed droplets of the alloy “get stuck” in the slag.
The process of ingot formation and the completeness of extraction of the alloy are also affected by the density of the melts. The densities of some aluminothermic alloys and ligatures with elements such as calcium, barium, boron, titanium, silicon are as follows (g/cm3): Ba, 3.57; Ca, 1.44; Ti, 4.11; B, 2.3 [15]; Al2O3, 3.0 [16]; aluminum borides, 2.8–3, which is close to the density of the resulting slag 19 [17], which inhibits phase separation. A decrease in the density of alumina slag is possible with an increase in the temperature of the entire melt. Hence, an increase of 200 K reduces the density of the slag by 0.05–0.07 g/cm3 [18,19]. At the temperature of the boric anhydride reduction process, the slag density is 2.6–2.7 g/cm3 [20].
In the present study, a series of experiments was carried out to determine the effect of both the ratio of the heating agent (KNO3) and boric anhydride (B2O3) and the amount of reducing agent on the formation of the ingot in the yield of the aluminum boride alloy, the completeness of separation of the metal and slag phases, and the recovery of the reduced metal. One of the important components of metallothermic mixtures, in which hard-to-reduce oxides act as oxidizing agents, is heated additives that increase the energy content of the mixture and improve phase separation.
Potassium nitrate was chosen as a heating additive on the basis of a high thermal effect of the reaction with aluminum:
the absence of harmful substances in the products, the possibility of using the formed slags as a commercial product. Results are summarized in Table 2.
6KNO3 + 10Al = 5Al2O3 + 3K2O + 3N2; (Q = 4308.9 kJ/moll)

Table 2.
Compositions and results of experiments on the effect of the ratio of the heating additive and boric anhydride, the amount of reducing agent on the yield of aluminum boride alloy.
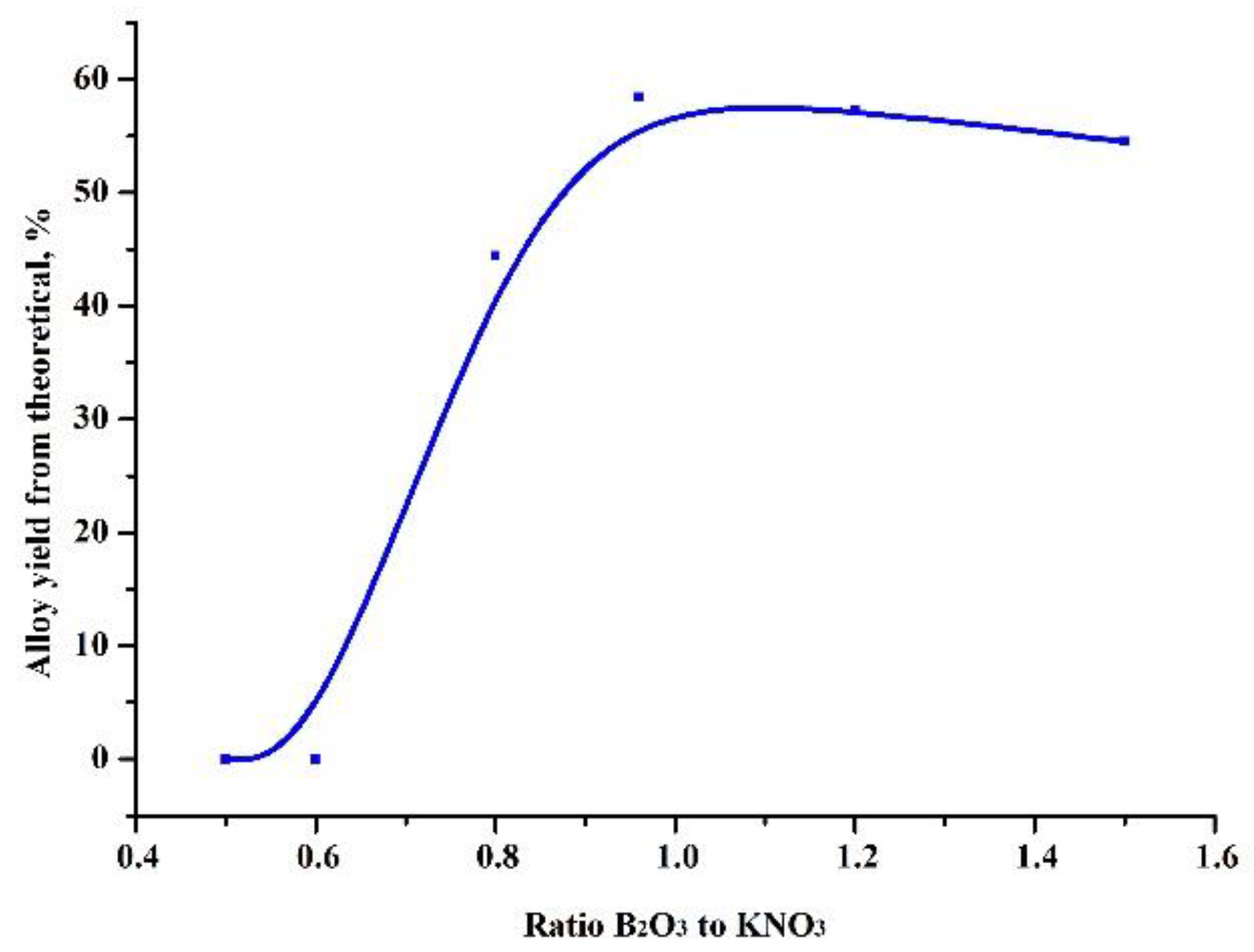
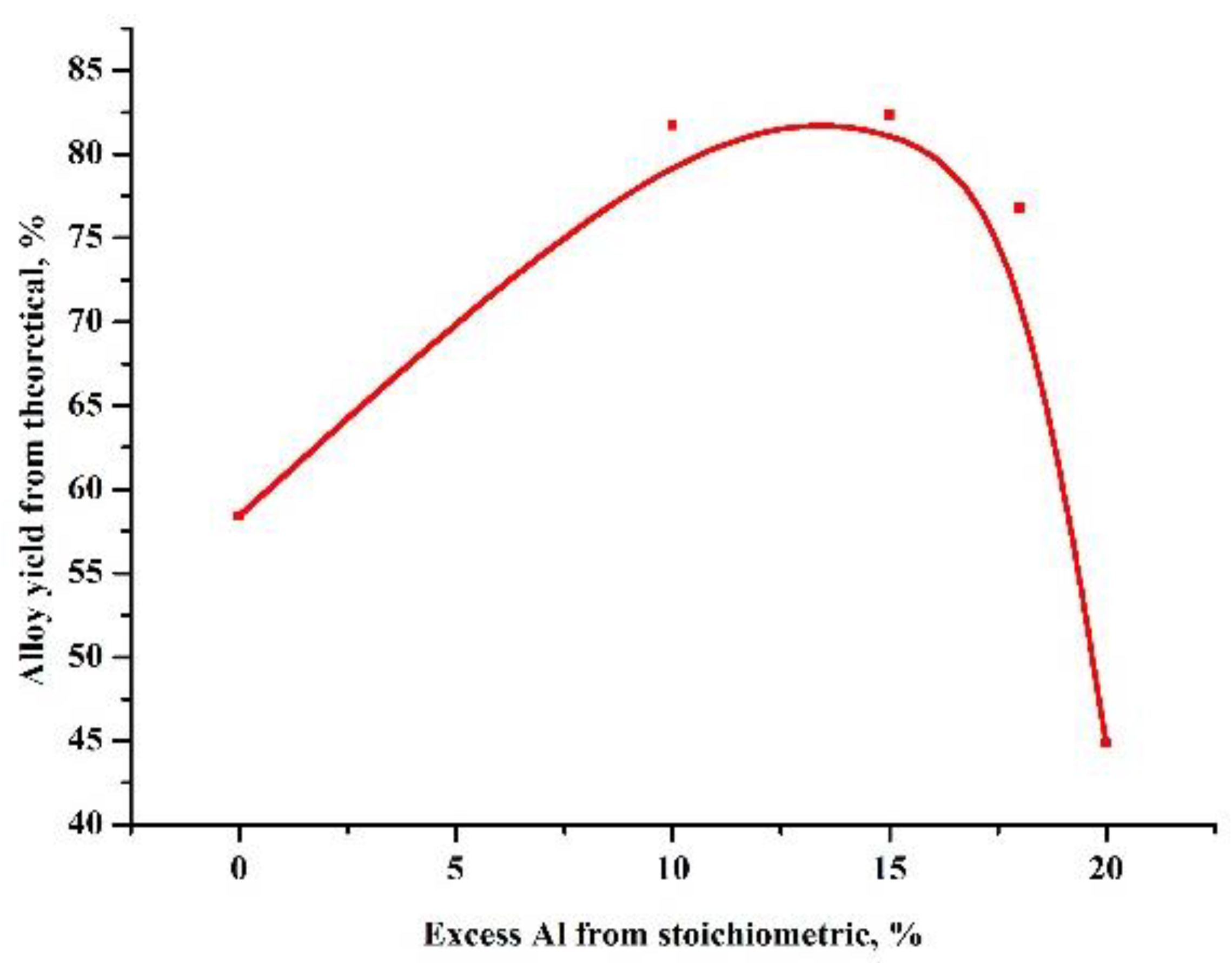
At the first stage, experiments were carried out to select the ratio of the amount of the heating additive and the oxide being reduced, in such a way that the highest yield of the alloy was obtained (Figure 1). Based on these results, further experiments were carried out to test the influence of the amount of the reducing agent (Figure 2).
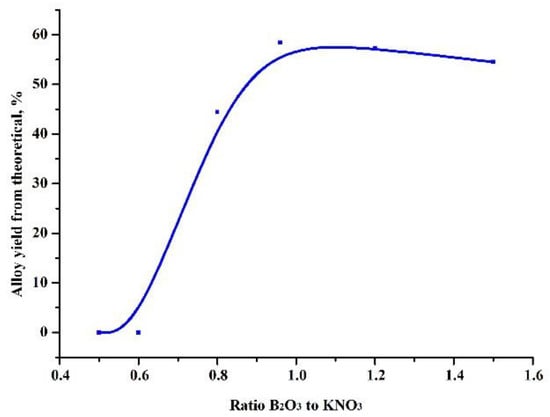
Figure 1.
Dependence of the yield of the alloy on the ratio of boric anhydride and preheating additive in the charge.
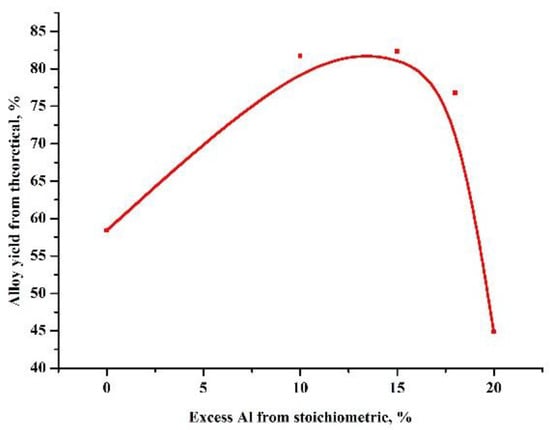
Figure 2.
Dependence of the yield of the alloy on the excess of the oxidizer.
As can be seen in Table 2, the maximum yield of the target product, an aluminum boride alloy, was obtained at an oxide-heating additive ratio of 0.96 and an excess of reducing agent of 15%. In this case, difficult phase separation was observed and the alloy formed into pellets without the coalescence of a single ingot.
Fluxing additives were introduced into the aluminothermic charge to obtain a melt with physicochemical properties that provide optimal density and viscosity of the slag and the surface tension of the metal component. The use of fluxes makes it possible to optimize the slag regime in the out-of-furnace aluminothermic process in the direction of improving phase separation and increasing process performance. Fluorides and chlorides of metals and some metal oxides are used as fluxes in metallothermic processes. Table 3 shows flux additives and their melting points.

Table 3.
Melting points of flux additives. Reprinted/adapted with permission from Ref [21]. 1964, Nikolsky, B.P.; Rabinovich, V.A.
Aluminum fluoride and cryolite additions influence the combustion parameters of aluminothermic charges and melt composition most effectively. Other additives are arranged in terms of effectiveness in the following sequence: chlorides and fluorides of alkaline earth metals, chlorides, and iodides and fluorides of alkali metals [22]. To intensify the processes of phase separation and increase the yield of the alloy in the production of aluminum alloys, mixed fluxes of salts of halides and alkaline earth metals are used. Salt compositions such as NaCl-KCl, NaF-AlF3, and KCl-MgCl2 are often used [23]. Based on them, it is possible to obtain compositions with controlled density and melting point.
In practice, common fluxing additives in metallothermy are calcium oxide and fluorspar. The use of calcium oxide as a flux does not lead to a sharp intensification of the initial stages of the process, which is obvious due to its melting temperature, which exceeds the temperature of most of the ongoing reactions. Calcium oxide passes into the melt, dissolving in the liquid high-alumina slag. The melting of lime during the process due to the heat of the exothermic reactions reduces the temperature of the melt, and hence the rate of penetration of the charge. This improves the conditions for the formation of an ingot but worsens the conditions for the occurrence of reduction reactions and reduces the yield of the alloy [22].
Halide salts (fluorides and chlorides) have a different effect on the performance of the aluminothermic process and differ from the role of calcium and magnesium oxides. Additives of fluorspar (calcium fluoride) to reduced oxides accelerate the migration of the reducing agent at a temperature of 1373–1573 K and improve the wettability of aluminum oxide, which accelerates the process of the low-temperature reduction of metals. This circumstance also leads to a decrease in the ignition temperature of the charge. The intensification of many metallothermic processes with the addition of chlorides and fluorides is evidenced [24] by the data given in Table 4.

Table 4.
The effect of salt additives on the ignition temperature of aluminothermic compositions.
The role of fluoride and chloride salts as activators of aluminothermal reduction is that they are capable of dissolving alumina with a decrease in the liquidus temperature of the melt compared to that of pure aluminum oxide. They act as surfactants that reduce the surface tension of slag melts and are able to penetrate into oxide films, leading to an increase in wettability. As a result, the temperature of the beginning of the interaction of liquid melts decreases and the diffusion of reagents to the boundary of their separation is facilitated, which, apparently, explains the increase in both the rate of charge penetration and the yield of the alloy [24,25].
Obtaining aluminum borides is a rather complex technological process, since boric anhydride is one of the hard-to-reduce oxides, and the resulting aluminum oxide has a high melting point. The duration of methods based on the furnace aluminothermal reduction of boric anhydride is 1.5 days [26]. Even when the charge is heated to 1773 K, a slag-like mass is formed, from which the extraction of aluminum borides is a laborious process. The use of the SHS technology and the selection of appropriate fluxing components makes it possible to optimize the process of obtaining borides with a high degree of extraction of the target component and commercial slag for further use.
To optimize the process of obtaining boron–aluminum alloys, mixtures of halides of alkali and alkaline earth metals can be used. Such mixtures are convenient, since they can be used to obtain alloys with a controlled density and melting temperature in the SHS mode by reducing the surface tension and improving the wettability of the melt.
The properties of mixed fluxes largely depend on the properties of the original salts. Of particular interest in recent years is the method of obtaining aluminum borides in molten fluoride fluxes [27]. To select the flux and determine its optimal amount, which provides the necessary properties of the slag melt, experiments were carried out using: calcium oxide, fluorspar, sodium and potassium chlorides, and potassium tetrafluoroborate in various combinations. In all experiments, the fineness and ratio of the main components of the charge, namely, B2O3, Al, KNO3, remained unchanged, and only the amount and composition of the fluxing additives was varied. Table 5 shows the composition of the charge and the results of these studies. The average values of the yield of the alloy compared to the theoretical one are given for three experiments. The process was carried out in cast iron crucibles. SHS was initiated from above using a thermite mixture.

Table 5.
Summary of experimental data for choosing the optimal composition of the fluxing additive and its effect on the yield of the alloy.
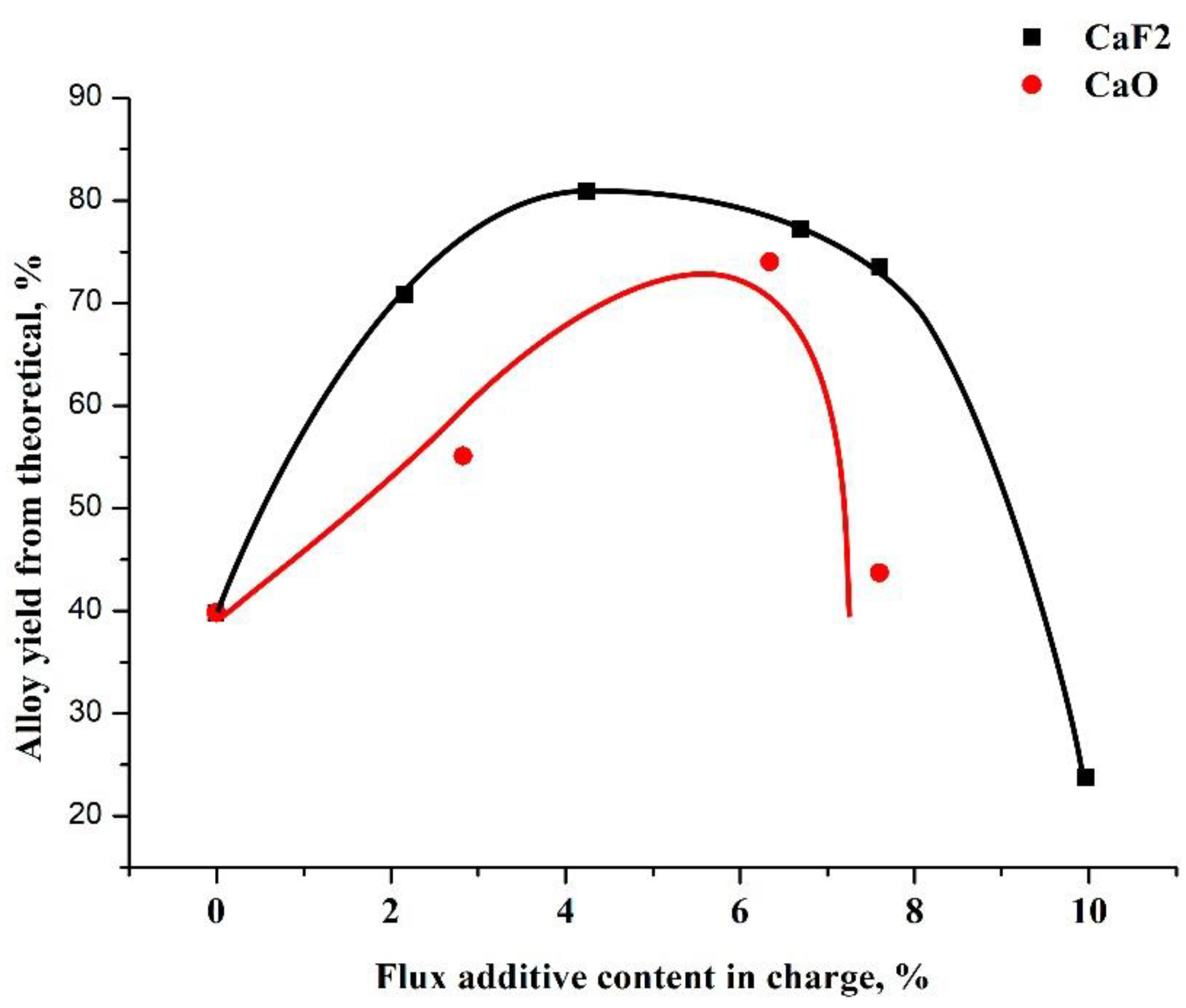
The use of calcium oxide as a flux for the Al-B system does not contribute to the phase separation of the reaction products or the formation of a single alloy ingot. During the melting due to the heat of the exothermic reactions of the process, calcium oxide reduces the overall temperature of the melt, which worsens the rheological properties of the slag. The introduction of fluorspar, to certain limits, into the charge as a flux improves the phase separation and the formation of an alloy ingot, as illustrated in Figure 3.
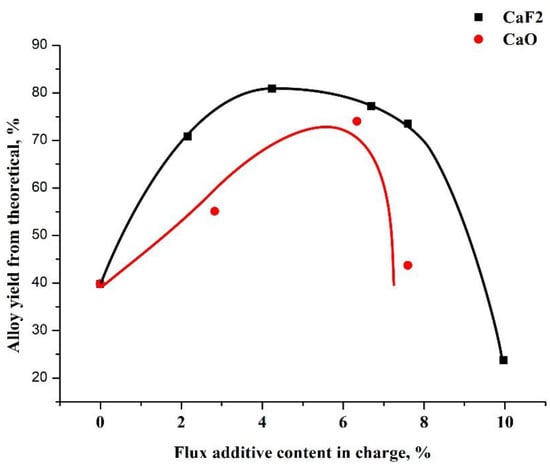
Figure 3.
Plots of the influence of the amount of flux on the yield of the alloy.
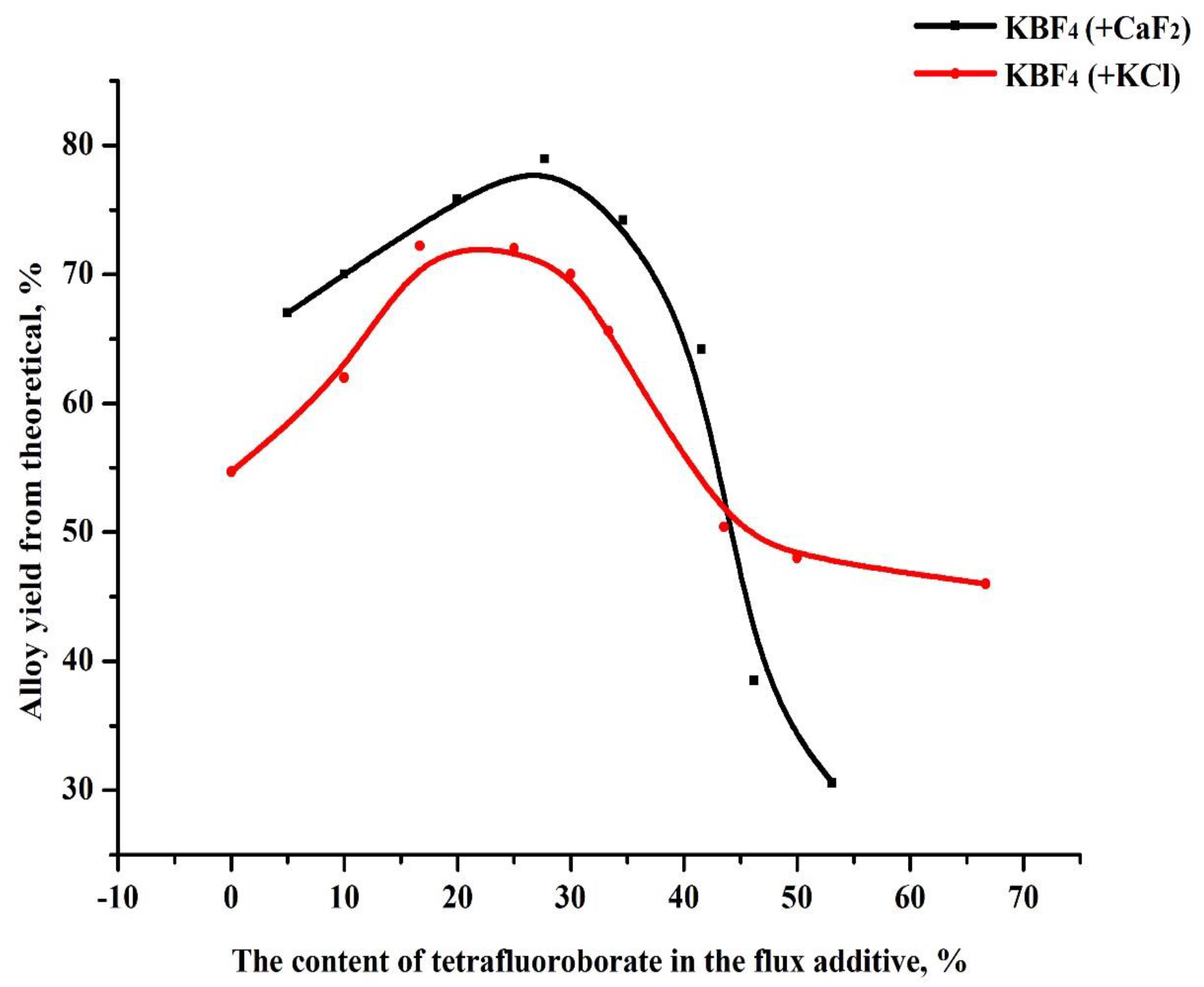
In order to increase the yield of the alloy and increase its boron content, the possibility of using potassium tetrafluoroborate mixed with fluorspar and potassium chloride as a fluxing additive was studied. The effect of chloride and fluoride salts of alkali metals as fluxes is shown in Figure 4. As observed in the figure, the addition of chloride salts, up to a certain limit, led to a decrease in surface tension in the melts, a decrease in the temperature at the beginning of the process, and an improvement in phase separation. However, at the same time, the use of chlorides is not economically profitable or environmentally safe.
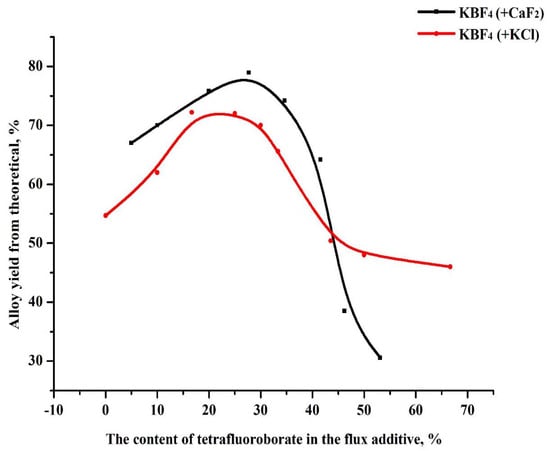
Figure 4.
Dependence of the output of the boride alloy on the use of mixed fluxing additives from chloride and fluoride salts.
Thus, the best effect was obtained when using a mixture of fluorspar and potassium tetrafluoroborate. The use of KBF4 makes it possible to obtain aluminum boride with a higher boron content in the alloy due to the production of an additional amount of boron by reacting KBF4 with aluminum at the temperature of the SHS process. The process of the interaction of KBF4 with liquid aluminum is described in [18] by the following reactions [5]:
KBF4 + Al → KF + AlF3 + B
2KBF4 + 3Al → AlB2 + 2KAlF4
The reaction products KF and AlF3 in Equation (4) form potassium cryolite KAlF4. During the reduction of KBF4 with aluminum according to the reactions of Equations (4) and (5), and amorphous boron can remain in the potassium cryolite melt. It was found in [10,28] that the addition of KBF4 increases the liquidus temperature of the melt, but despite this, the solubility of Al2O3 in these melts in the presence of KBF4 increases significantly (by more than two times).
We assume that this can be explained by the fact that AlF3 formed from alkaline and alkaline earth fluorides, which have the highest surface-active properties and relieve surface tension in the melt, which should ensure the complete separation of the slag and alloy.
Figure 5 shows a sample of an aluminum boride alloy obtained in the SHS mode. Alloy samples were analyzed for elemental composition by X-ray spectral analysis.

Figure 5.
Aluminum boride alloy.
Table 6 shows the elemental compositions of alloys using CaF2 and its mixture with KBF4 as a flux. The mean values of the spectra taken at four points of the alloy are presented.

Table 6.
Elemental composition of the alloy with various flux additives.
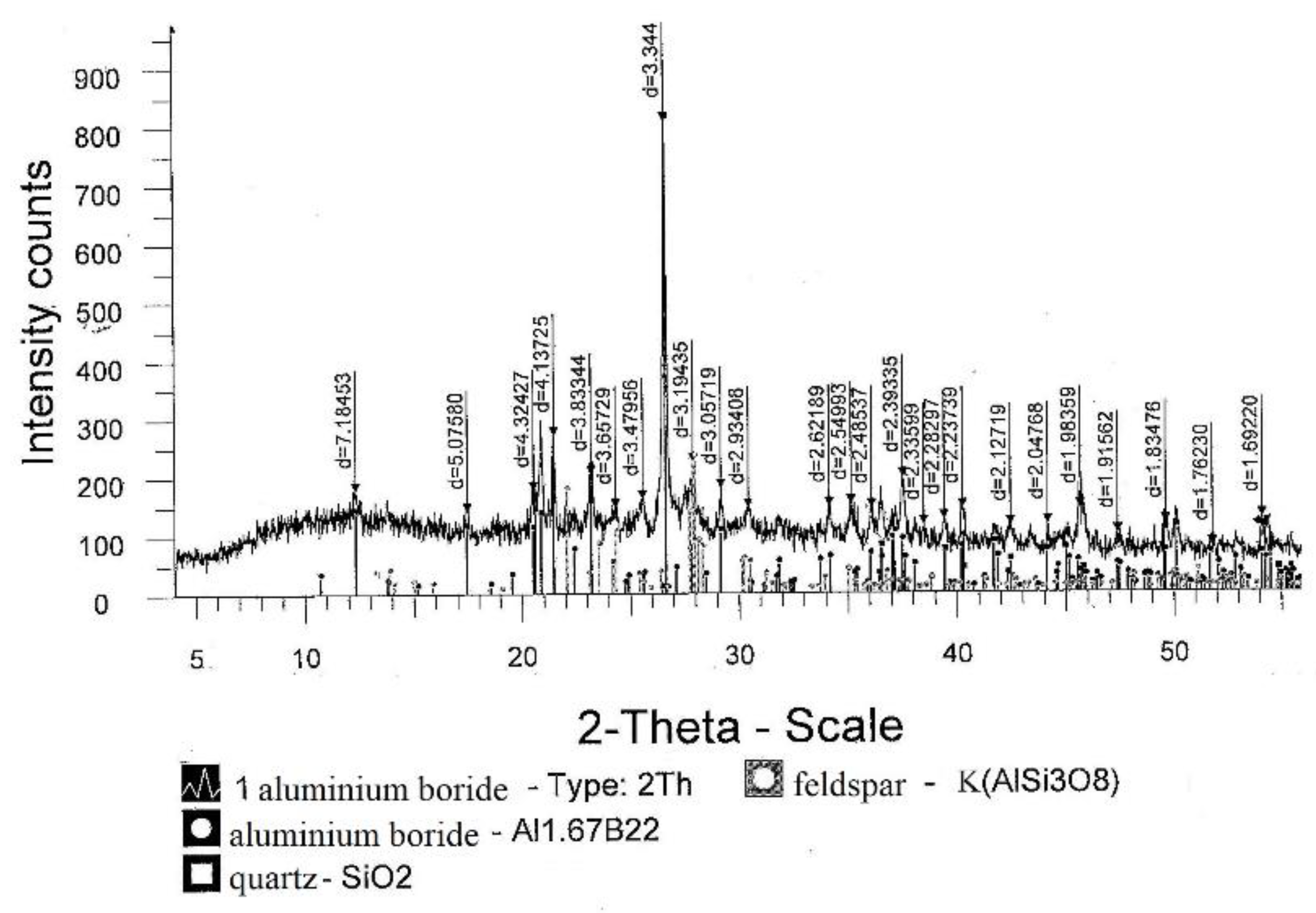
The results of the analyses showed that the use of the mixture makes it possible to increase the extraction of boron into the alloy. The maximum 100% recovery of boron from 50.0 g B2O3 should be 15.52 g boron. According to the analysis, ingots weighing 31.31–36.10 g contained 12.85–13.80 g of boron; therefore, the extraction of boron was 80.0–89.0%. According to XRD data of Figure 6, the main phase was aluminum boride at 92.5%, as reported in Table 7.
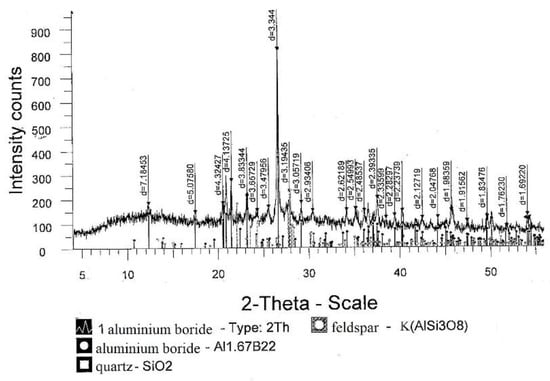
Figure 6.
Diffractogram of an aluminum boride alloy sample.

Table 7.
Results of semi-quantitative X-ray phase analysis of the boride alloy.
The analysis showed that the slag obtained as a result of the aluminothermic process for obtaining borides in the SHS mode met the requirements for raw materials for the production of high-alumina clinkers, namely, the content of aluminum oxides should be at least 60%. The objective of the study was the wastelessness of the synthesis method used, which consisted of the use of not only aluminum boride, but also aluminothermic slags in the production of by-products, for example, clinkers. The resulting slag samples were also analyzed for phase composition, and they are summarized in Table 8.

Table 8.
Results of semi-quantitative X-ray phase analysis of slag.
The disposal of slags from metallurgical production is an obligatory element of waste-free technology. Firstly, slag dumps have a negative impact on the air and water basins; they are harmful and environmentally unacceptable. Secondly, the utilization of slags can be an economically profitable production if they are used as raw materials for the cement and construction industries.
In the production of high-alumina clinkers, the content of impurities in them in the form of oxides of silicon, iron, alkali metals, chromium and magnesium is limited according to GOST TU 14-11-347-2000 standard (see Table 9).

Table 9.
Chemical composition of alumina slags for the production of clinkers according to GOST TU 14-11-347-2000 standard.
High-alumina clinkers are used in the production of cement used for the refractory concrete lining of furnaces of metallurgical, chemical, machine-building enterprises and in the production of building materials.
A content of iron oxides in the clinker is not desirable, since their presence reduces the fire resistance of the cement and, consequently, the technical properties of the heat-resistant concrete obtained from it. The iron content should not exceed 1–2%.
A titanium content of the order of 1–10% reduces the fire resistance of cement from 2043 to 1943 K, and the amount of chromium oxide in the same range, from 2043 to 1703 K. The presence of silicon oxide in cement leads to stress in concrete and the formation of the mineral gelenite, 2CaO∙Al2O3∙SiO2, which worsens the surface properties of concrete.
The formation of magnesium spinel MgO∙Al2O3 with a magnesium oxide content of more than 2% increases the fire resistance of cement to 2408 K, but at the same time negatively affects the activity of the cement, causing an uneven volume change during concrete hardening.
Alkali metal oxides at a content of more than 1% leads to variability in the setting time of cements and the formation of efflorescence on the surface of mortars and concretes [29]. Table 10 shows the composition of high-alumina clinkers according to TU 14-11-138-86 standard.

Table 10.
High-alumina clinkers of aluminothermic production according to TU 14-11-138-86 standard.
There are two ways to produce clinkers [30]. Namely:
- -
- Melting of the reaction mass, in which the components of the charge are mixed in a given proportion and subjected to melting at a temperature of 2073–2273 K in an oxidizing or reducing environment. After cooling, they are subjected to crushing and grinding.
- -
- Sintering of the charge in rotary or shaft furnaces, in which the materials preliminarily constituting the charge are jointly crushed and thoroughly homogenized, then fired at a temperature of 1473–1523 K. After cooling they are crushed into a fine powder.
In the process of heating the raw mixture at 723–1273 K, excess moisture and crystallization water are removed. At 1073–1173 K, CaCO3 decomposes and CaO and Al2O3 begin to interact with the formation of single-calcium aluminate according to the reaction equations:
CaCO3 → CaO + CO2,
CaO + Al2O3 → CaO∙Al2O3,
At 1000–1100 °C formed CaO∙2Al2O3:CaO + 2Al2O3 → CaO∙2Al2O3,
Above 1200 °C—5CaO∙3Al2O3 and 3CaO∙Al2O3:5CaO + 3Al2O3 → 5CaO∙3Al2O3,
3CaO + Al2O3 → 3CaO∙Al2O3
Roasting is the main operation in the production of clinkers. Under normal conditions, the components of the raw mixture—limestone, lime, aluminum oxide and other minerals—are inert. When heated, they become active and begin to mutually exhibit reactivity. The interaction reactions of calcium oxide with aluminum, iron, and silicon oxides present in the feedstock are exothermic [30]. As a result of firing in the exothermic zone, CaO compounds with aluminum are formed according to Equations (6)–(10), as well as other compounds such as 2CaO·SiO2 and 4CaO·Al2O3·Fe2O3:
2CaO + SiO2 → 2CaO∙SiO2,
4CaO + Fe2O3 + Al2O3 → 4CaO∙Al2O3∙Fe2O3.
The microstructure of a melted clinker is determined by the cooling mode. With slow cooling, the crystals grow under favorable conditions and reach large sizes. During rapid cooling, the clinker contains a significant amount of the glassy phase that did not have time to crystallize [31].
After cooling, the clinker is crushed. If there are metallic inclusions of iron and ferrosilicon in the clinker, then magnetic separation is carried out to remove them.
The charge is calculated based on the chemical composition of the final product and raw materials: as a rule, 75% slag and 25% lime [31]. Table 11 shows the mineralogical composition of the source materials according to XRD data.

Table 11.
Composition of starting materials.
The calculated mineralogical composition per 1 kg of charge, consisting of 75% slag and 25% lime, containing 5% impurities, includes: Al2O3, 615.02 g; MgO, 5.73 g; Fe2O3, 4.08 g; SiO2, 42.57 g; CaO, 244.04 g; K2O, 34.53 g; Na2O, 3.03 g; impurities, 50.00 g.
The slag obtained from the smelting was crushed, having previously selected the remaining metal pellets from it. Further, the mixture of purified slag and lime was subjected to joint grinding, combining the technological stages of grinding and mixing.
A ball mill was used for grinding. The size of the raw materials was 5.0–15.0 mm, and the finished product was 0.074–0.40 mm. Next, the ground and additionally mixed mixture was placed in a sintering oven at a temperature of 1423–1523 K for 1.0–1.5 h to cool down slowly. After cooling, the clinker was crushed in a ball mill.
The resulting clinker sample was analyzed for phase composition and the results are reported in Table 12.

Table 12.
Results of semi-quantitative analysis of clinker.
The percentage composition of clinker in terms of oxides was determined to be: Al2O3, 60.44%; CaO, 22.57%; Fe2O3, 1.40%; MgO, 1.56%; K2O+Na2O, 0.62%; B2O3, 0.085%; TiO2, 0.11%; SiO2, 0.62%; gaseous phase CO2, 12.60%. The content of limited impurities of titanium oxides, alkali metals and boron does not exceed the limits that have a negative impact on the properties of the resulting cements [29].
4. Conclusions
Aluminum boride alloy was successfully produced by the low-waste SHS method. It was found that the maximum yield of the aluminum boride alloy was obtained at a ratio of oxide–heating additive equal to 0.96 and an excess of reducing agent of 15%. The use of chloride salts as a component of a mixed flux up to 15–18% led to a decrease in surface tension in melts, a decrease in the temperature of the beginning of the process, and an improvement in phase separation. The use of a mixed flux of fluorspar and potassium tetrafluoroborate made it possible to obtain aluminum boride with a higher boron content in the alloy by obtaining an additional amount of boron during the interaction of KBF4 with aluminum at the temperature of the SHS process.
According to XRD data, the main phase of the resulting alloy was aluminum boride (92.5%).
The slag obtained as a result of the aluminothermic process for obtaining borides in the SHS mode met the requirements for raw materials for the production of high-alumina clinkers, namely, the content of aluminum oxides must be at least 60%. This composition corresponds to the brand of high-alumina clinker KVC-60. The attempt to develop technology for the production of aluminum borides showed that the SHS method used makes it possible to obtain the target and by-products that meet the requirements for chemical and phase composition.
5. Patents
Aknazarov S.K., Bayrakova O.S., Seisenova A.B., Golovchenko N.Y., Ponomareva E.A. Method for the production of aluminium diboride. Kazakh Patent No. 5774, 2021.
Bayrakova, O.S.; Ponomareva, E.A.; Aknazarov, S.H.; Mutushev, A.Zh.; Golovchenko, O.Y.; Allan, I.K. Method for the preparation of aluminum boride. Kazakh patent No. 7075, 2022.
Author Contributions
Conceptualization, S.K.A. and O.S.B.; methodology, O.Y.G. and J.M.G.-L.; software, N.Y.G.; validation, S.K.A., O.S.B. and A.Z.M.; formal analysis, A.Z.M.; investigation, O.S.B., A.Z.M. and E.A.P.; resources, S.K.A.; data curation, O.S.B. and A.Z.M.; writing—original draft preparation, A.Z.M.; writing—review and editing, J.M.G.-L. and A.Z.M.; visualization, O.S.B., E.A.P. and N.Y.G.; supervision, S.K.A.; project administration, S.K.A.; funding acquisition, S.K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan [Grant number: AP08857190 (2020–2022)].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Samsonov, G.V.; Serebryakova, T.I.; Neronov, V.A. Borides; Atomizdat: Moscow, Russia, 1975; 376p. [Google Scholar]
- Aknazarov, S.K.; Mutushev, A.Z.; Gonzalez-Leal, J.M.; Bairakova, O.S.; Golovchenko, O.Y.; Golovchenko, N.Y.; Ponomareva, E.A. Kinetics of the Synthesis of Aluminum Boride by the Self-Propagating High-Temperature Synthesis Method. Ceramics 2022, 5, 435–446. [Google Scholar] [CrossRef]
- Serebryakova, T.I.; Neronov, V.A.; Peshev, P.D. High Temperature Borides; Metallurgy: Moscow, Russia, 1991; 368p. [Google Scholar]
- Podergin, V.A. Metal Thermal Systems; Metallurgy: Moscow, Russia, 1972; 189p. [Google Scholar]
- Kislyi, P.S.; Neronov, V.A.; Prikhina, T.A.; Bevzo, Y.B. Aluminium Borides; Naukova Dumka: Kyiv, Ukraine, 1990; p. 192. [Google Scholar]
- Kayikci, R.; Kurtulus, O.; Gurbuz, H. The formation and growth behavior of aluminum for bide crystals in an Al-B alloy. Solid State Phenom. 2009, 144, 140–144. [Google Scholar] [CrossRef]
- Edward, J.F. The Preparation of Aluminium Diboride, AlB2. J. Am. Chem. Soc. 1956, 78, 5977–5978. [Google Scholar]
- Galievsky, G.V.; Neronov, V.A.; Rudneva, V.V. Plasma-chemical synthesis of boride powders, their properties and applications. Vestn. Gorn. Metall. Sektsii Ross. Akad. Yestestvennykh Nauk. Otd. Metall. 1997, 55–67. [Google Scholar]
- Abramov, A.A.; Shpakov, V.I.; Kryukovskii, V.A.A. Improving the technology of production of ligatures aluminum—Boron in electrolysis. Izv. Univ. Non Ferr. Metall. 1979, 22–24. [Google Scholar]
- Kataev, A.A.; Tkacheva, O.Y.; Molchanov, N.G.; Zaikina, Y.P. Obtaining Al-B ligature by aluminothermic reduction of KBF4 and B2O3 in molten salt fluxes. Izv. Vuzov Non Ferr. Metall. 2019, 9, 20–29. [Google Scholar]
- Levashov, A.E.; Rogachev, A.S.; Yukhvid, V.I.; Borovinskaya, I.P. Physico-Chemical and Technological Foundations of Self-Propagating High-Temperature Synthesis; BINOM: Moscow, Russia, 1989; 176p. [Google Scholar]
- Aknazarov, S.K.; Bayrakova, O.S.; Seisenova, A.B.; Golovchenko, N.Y.; Ponomareva, E.A. Method for the Production of Aluminium Diboride. Kazakh Patent No. 5774, 15 June 2021. [Google Scholar]
- Aknazarov, S.K.; Seisenova, A.B.; Golovchenko, O.Y.; Golovchenko, N.Y.; Gonzalez-Leal, J.M. Determination of Thermodynamic Characteristics of Phase-stabilized Ammonium Nitrate-Based High-energy Solid Combustible Materials. Combust. Sci. Technol. 2022, 4, 768–784. [Google Scholar] [CrossRef]
- Kozakevitch, P. Modelling viscosity of alumina-containing silicate melts. Rev. Metall. 1960, 57, 149–160. [Google Scholar] [CrossRef]
- Eliot, D.; Glaser, M.; Ramakrishna, V. Thermochemistry of Steelmaking Processes. Handbook; Metallurgy: Moscow, Russia, 1969; p. 252. [Google Scholar]
- Ravdel, A.A.; Ponomareva, A.M. Short Reference Book of Physical and Chemical Values, 10th ed.; Ivan Fedorov: Saint Petersburg, Russia, 1998; p. 232. [Google Scholar]
- Efimov, A.L.; Efimov, A.I.; Belorukova, L.P.; Vasilkova, I.V.; Chechev, V.P. Properties of Inorganic Compounds. Reference Book; Chemistry: Leningrad, Russia, 1983; p. 94. [Google Scholar]
- Levin, E.S.; Ayushina, G.D.; Shchipacheva, L.V.; Geld, P.V. Density and Surface Energy of Liquid Alloys of Aluminium-Chromium, Aluminium-Iron and Germanium-Iron Systems; Naukova Dumka: Kyiv, Ukraine, 1983; pp. 191–201. [Google Scholar]
- Popel, S.I. Surface Phenomena in Melts; Metallurgiya: Moscow, Russia, 1994; p. 440. [Google Scholar]
- Alchagirov, B.B.; Alchagirov, B.B.; Karamurzov, B.S.; Taova, T.M.; Khokonov, K.B. Density and Surface Properties of Liquid Alkali and Fusible Metals and Alloys; KBGU: Nalchik, Russia, 2011; p. 214. [Google Scholar]
- Nikolsky, B.P.; Rabinovich, V.A. The Basic Properties of Inorganic and Organic Compounds Chemistry. In Handbook of Chemist; Chemist: Moscow-Leningrad, Russia, 1964; Volume 2, pp. 28–117. [Google Scholar]
- Lyakishev, N.P.; Pliner, Y.L.; Ignatenko, G.F.; Lappo, S.I. Aluminothermy; Metallurgy: Moscow, Russia, 1978; p. 424. [Google Scholar]
- Napalkov, V.I.; Mahov, S.V. Alloying and Modification of Aluminum and Magnesium; MISIS: Moscow, Russia, 2002; p. 376. [Google Scholar]
- Dubrovin, A.S.; Kuznetsov, V.A.; Ezikov, V.I. Effect of salt additives on the rate of aluminothermic processes. Izv. Akad. Nauk SSSR Metally 1968, 5, 79–88. [Google Scholar]
- Podergin, V.A. Metallothermic Systems; Metallurgy: Moscow, Russia, 1992; p. 271. [Google Scholar]
- Lutz, A.R. Self-Propagating High-Temperature Synthesis of Modifying Ligatures and Composite Alloys in Aluminum Melt with Flux Impurities. Ph.D. Dissertation, Samara State Technical University, Samara, Russia, 2006. [Google Scholar]
- Kataev, L.A.; Tkachev, O.Y.; Zaikov, Y.P. Metallothermal production of Al-B alloys using fluoride fluxes. In Proceedings of the IV International Scientific and Technical Conference, Ekaterinburg, Russia, 4–5 October 2018. [Google Scholar]
- Bayrakova, O.S.; Ponomareva, E.A.; Aknazarov, S.H.; Mutushev, A.Z.; Golovchenko, O.Y.; Allan, I.K. Method for the Preparation of Aluminum Boride. Kazakh Patent No. 7075, 6 May 2022. [Google Scholar]
- Kuznetsova, T.V.; Talaber, J. Aluminous Cement; Stroyizdat: Moscow, Russia, 1988; p. 268. [Google Scholar]
- Rytvin, V.M.; Perepelitsin, V.A.; Abyzov, V.A. Practice of Processing and Application of Ferroalloy Aluminothermic Slags. Refract. Tech. Ceram. 2013, 10, 38–43. [Google Scholar]
- Sulimenko, L.M.; Akimova, T.N.; Makaeva, A.A. Production Technology of Mineral Binders: Textbook; Orenburg State University: Orenburg, Russia, 2016; p. 156. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).