Deterioration of Mortar Bars Using Binary and Ternary Mixtures Immersed in Sodium Sulfate Solutions
Abstract
1. Introduction
2. Research Significance
3. Materials and Experimental Procedures
3.1. Materials
3.2. Methods and Testing Procedures
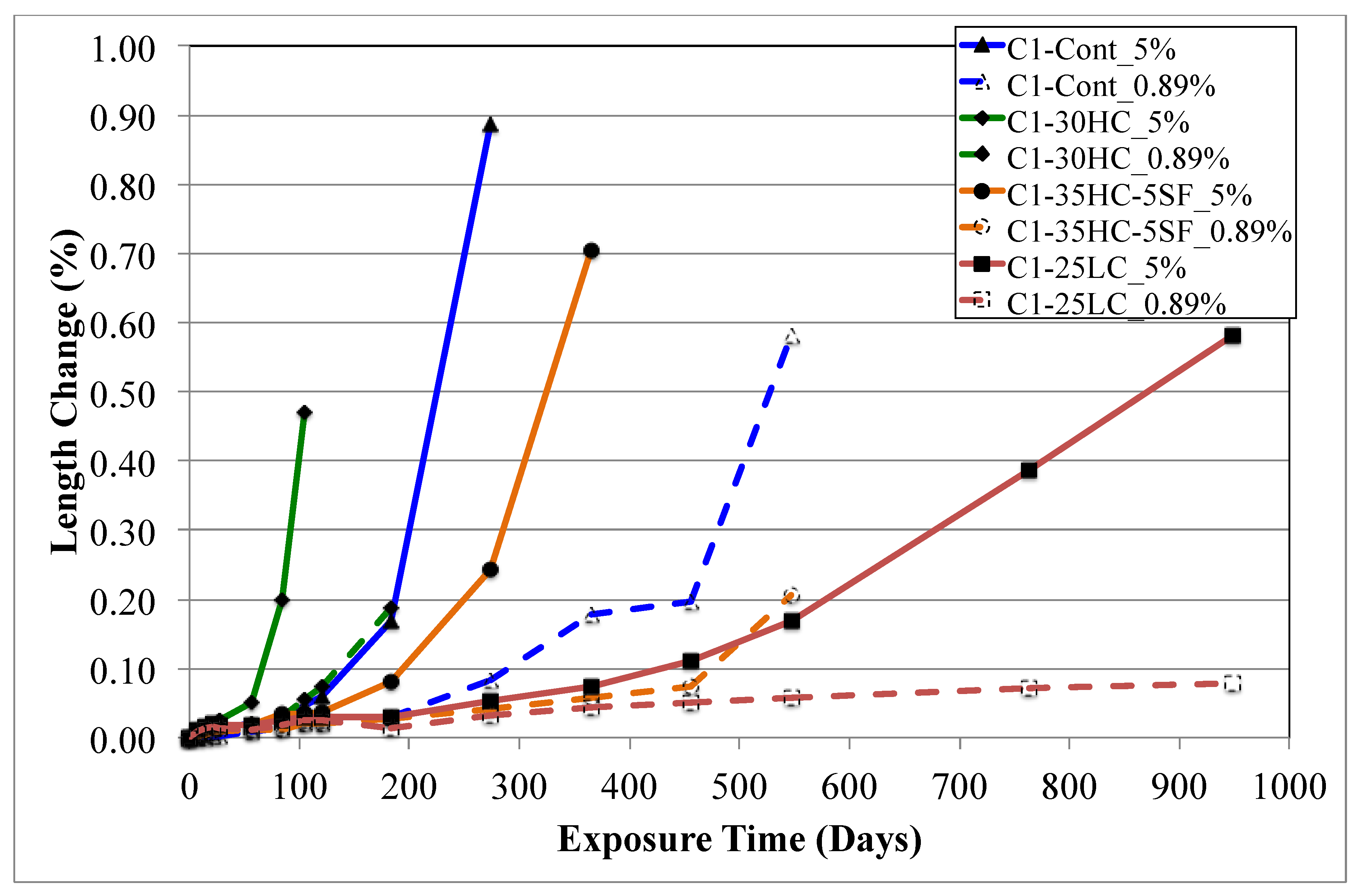
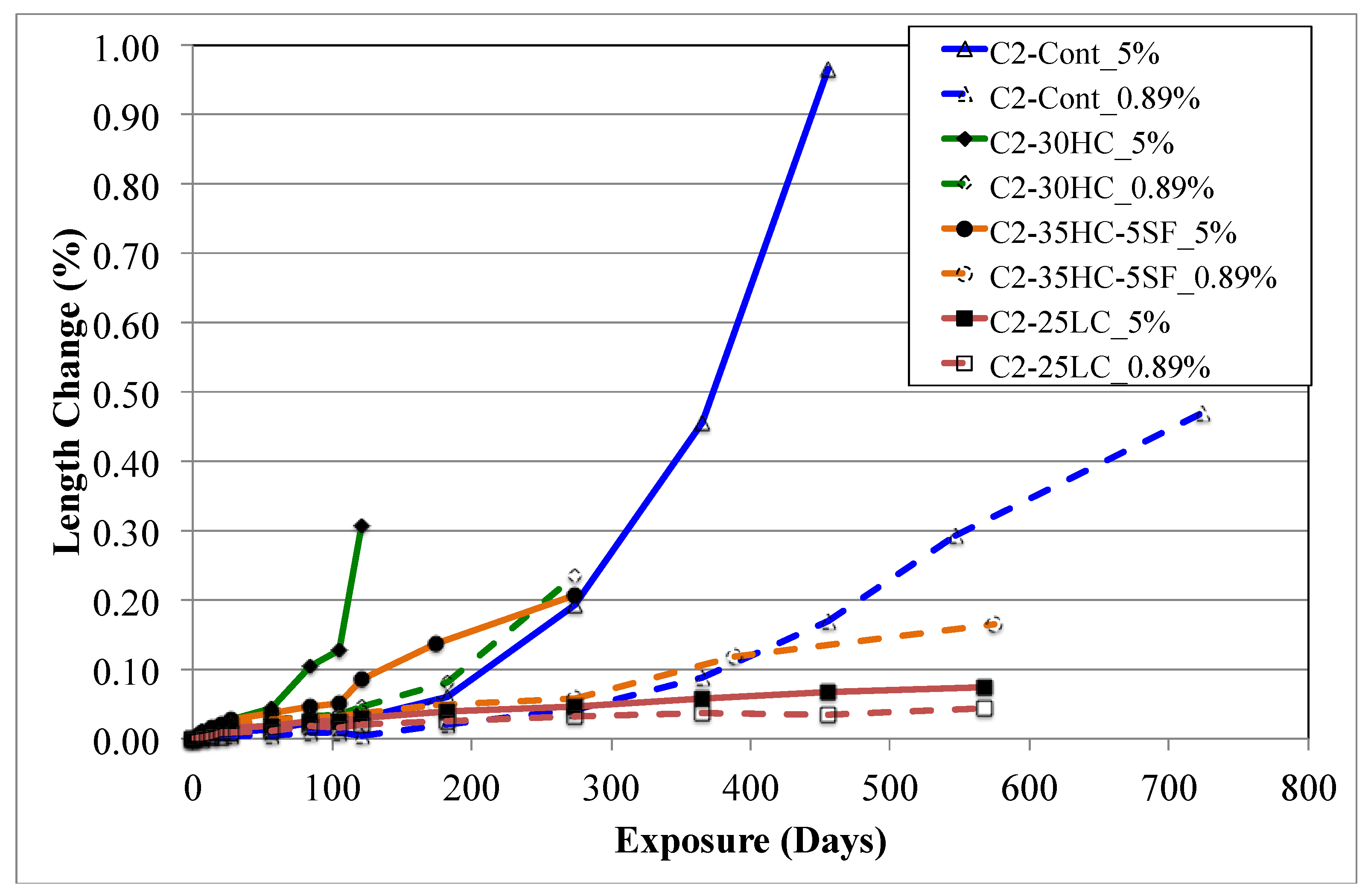
Length Changes
3.3. Scanning Electron Microscopy
3.4. X-ray Diffraction
4. Experimental Results and Discussion
4.1. Expansion and Visual Appearance
4.1.1. C1 Mixtures
- The mortar bars submerged in the 5% sodium sulfate solution clearly illustrates the aggressiveness from the concentrated sulfate solution showing severe damage and deterioration in the mortar bars;
- The mortar bars exposed to 0.89% sodium sulfate solution exhibited similar expansion values at later times; however, the noted deterioration is significantly less than those in 5% solution.
4.1.2. C2 Mixtures
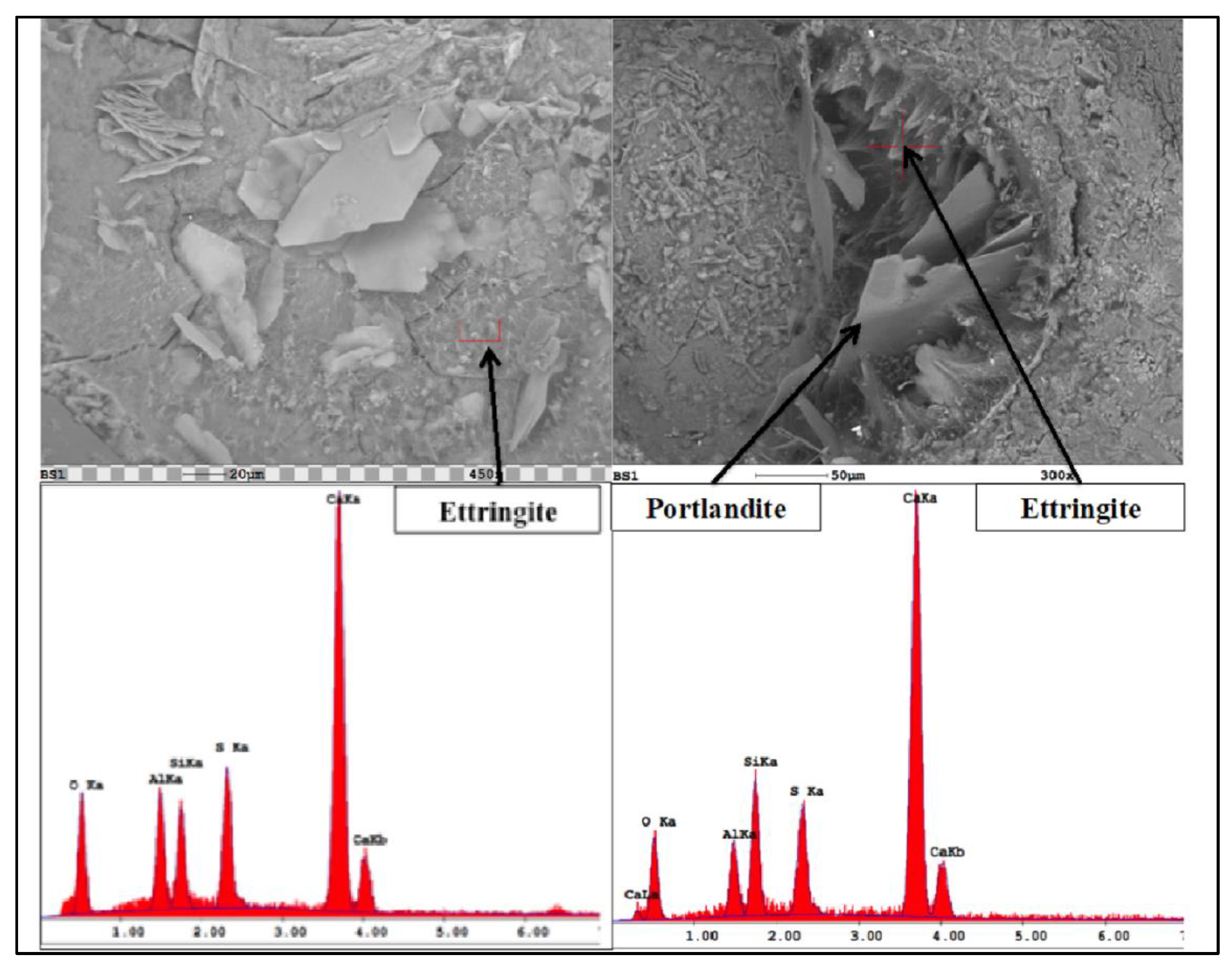
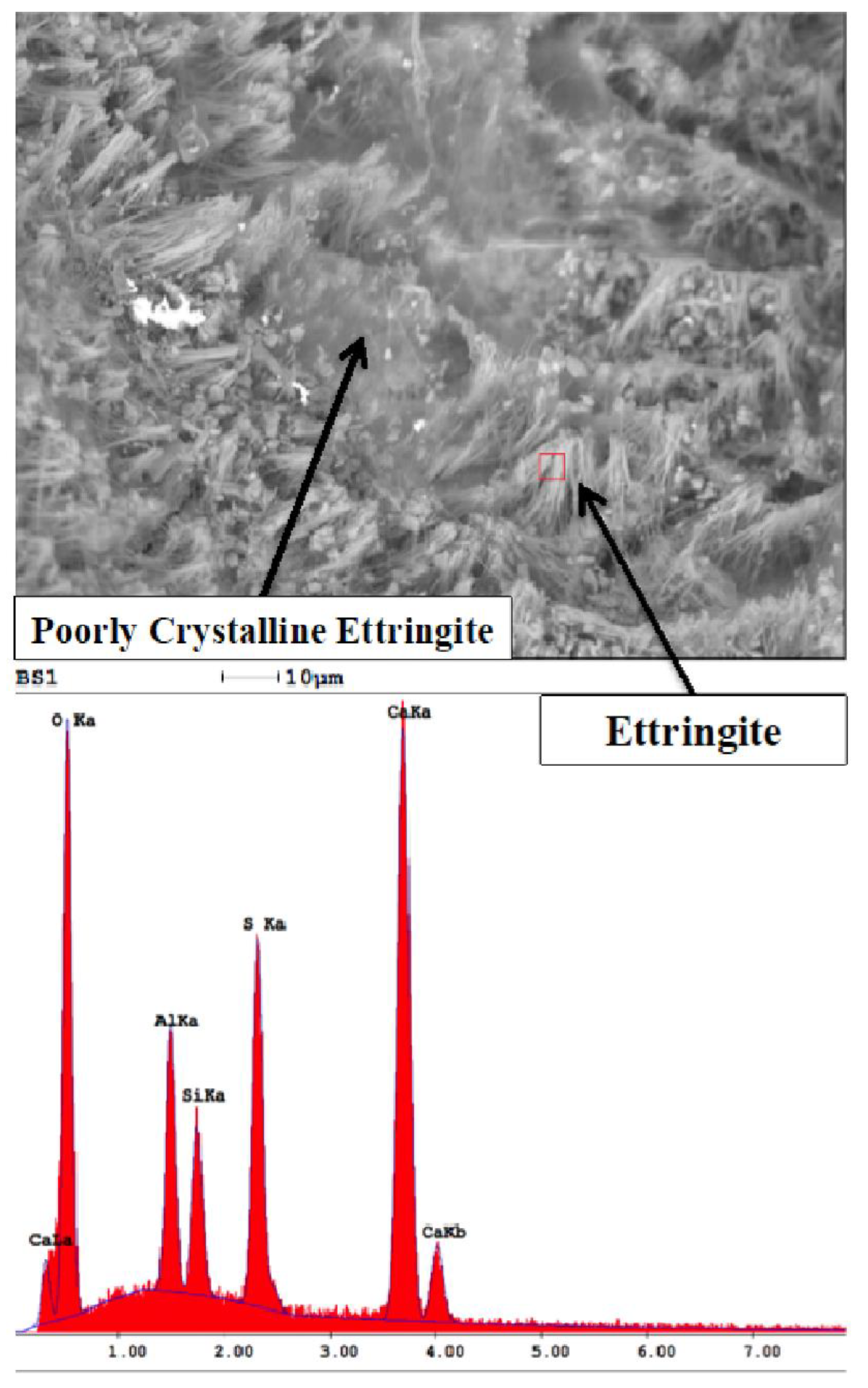
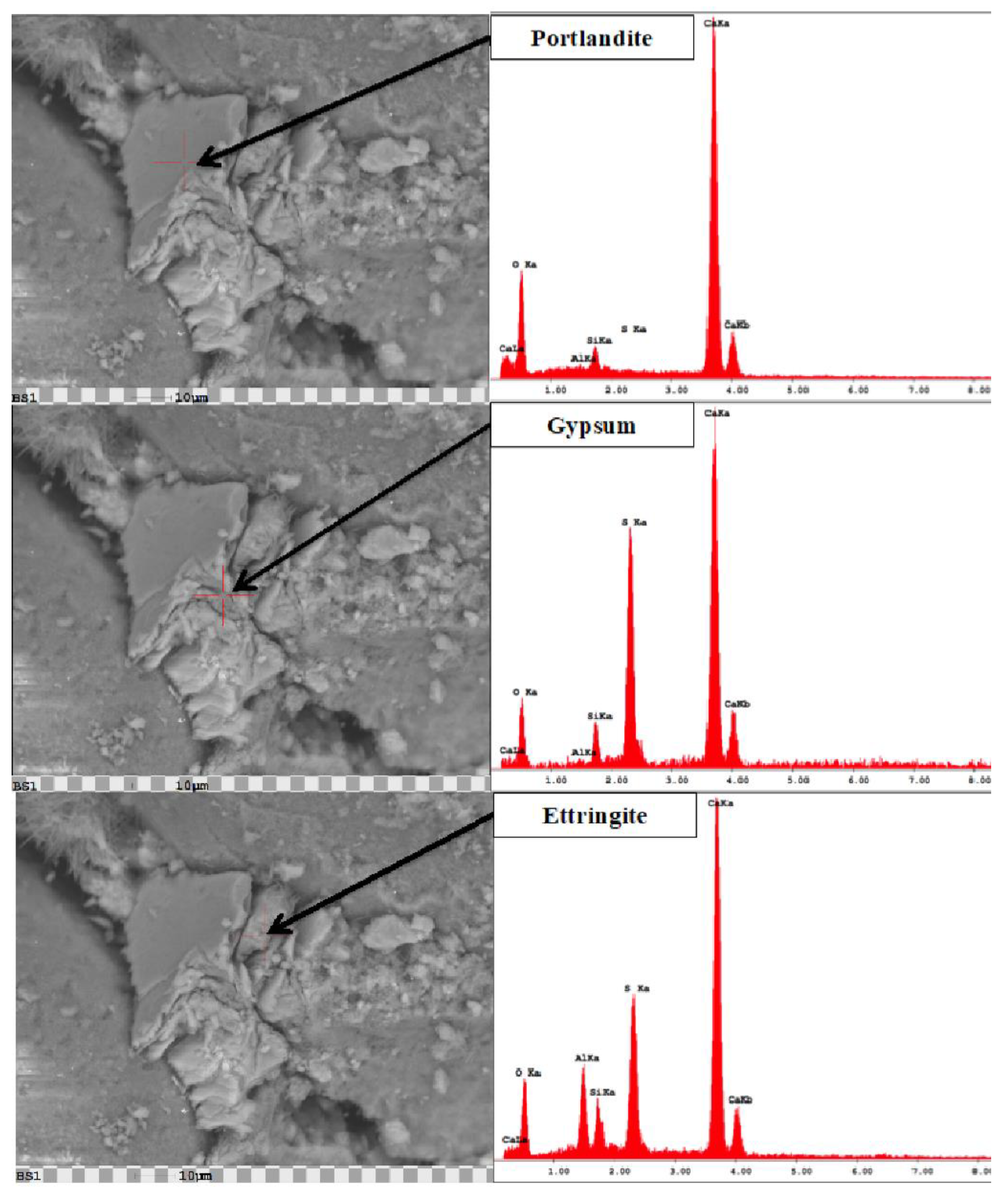
4.2. Microstructural Changes
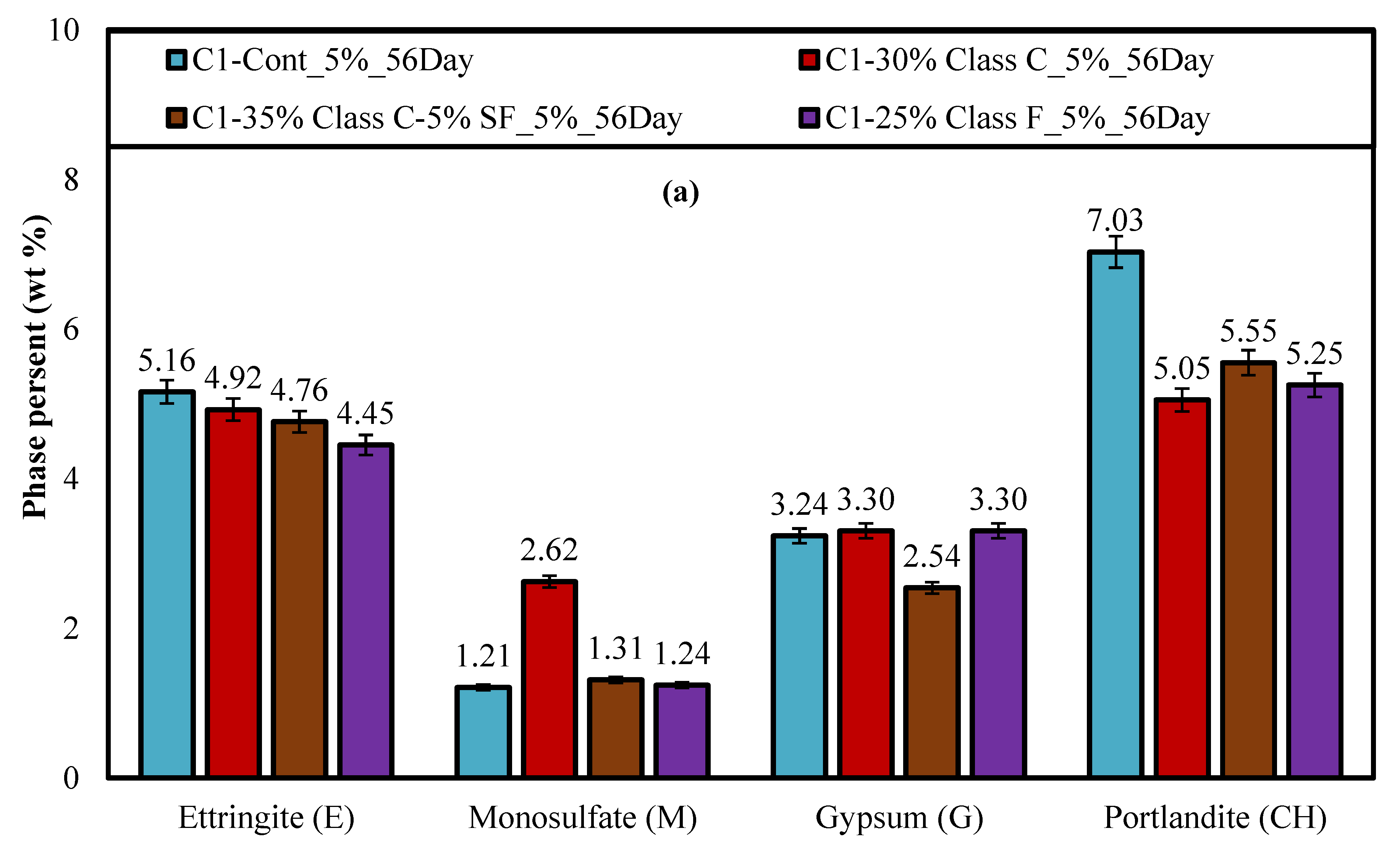
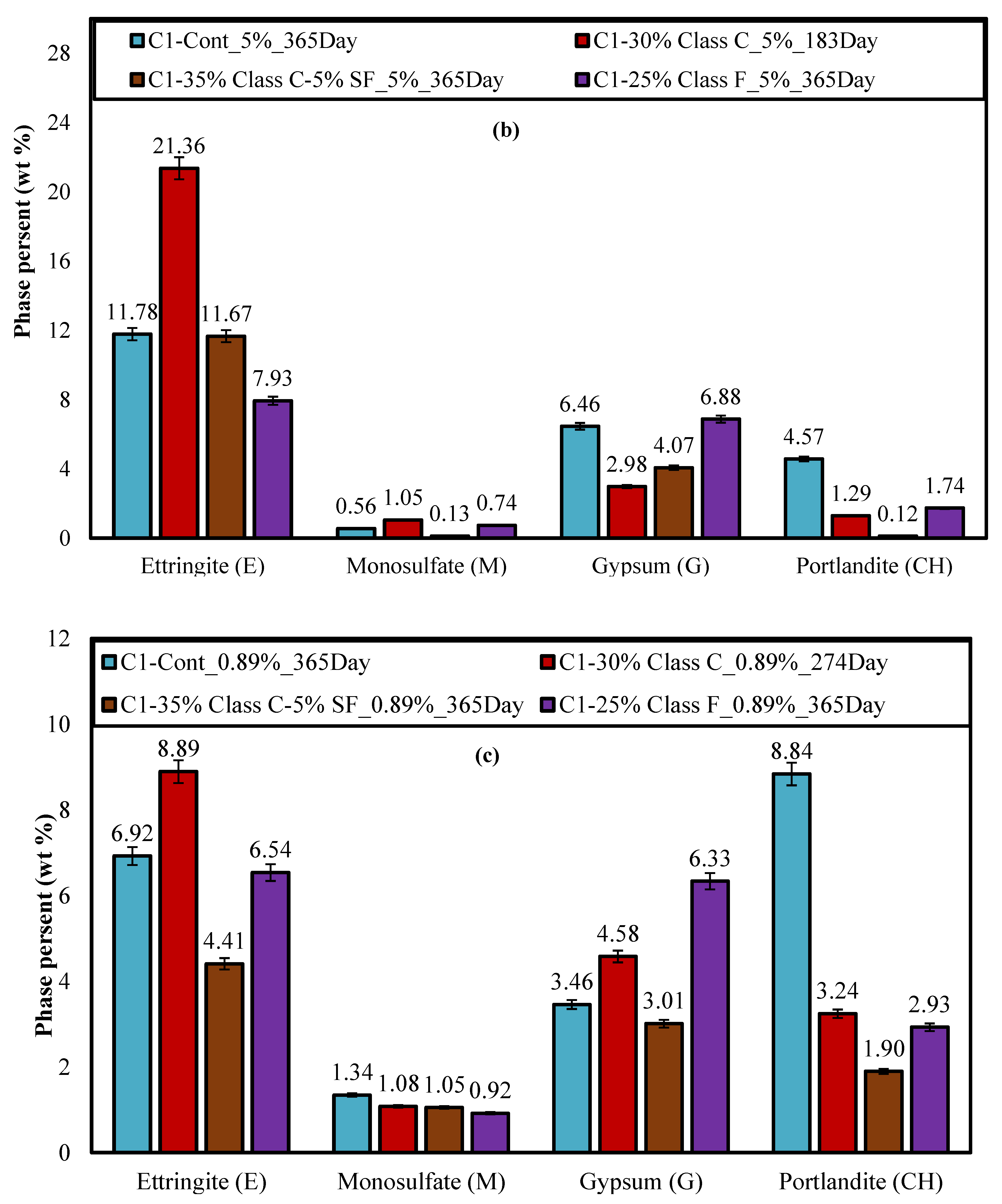
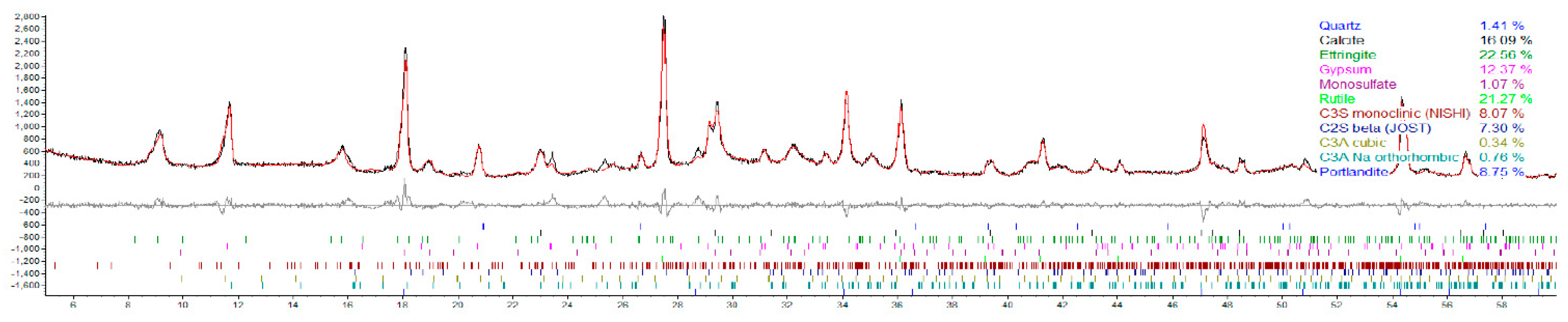
4.3. X-ray Diffraction (XRD)
- Ettringite and gypsum phases were also present in mortars immersed in the lower sulfate concentration after one year of exposure; however, ettringite was found in much smaller quantities. At 0.89% sodium sulfate, the control mixture (C1) observed over 70% less ettringite whereas, the binary mixture (C1-30HC) observed over a 140% less ettringite. It is interesting to note the similar ettringite quantities present in the ternary (C1-35HC-5SF) and LC binary (C1-25LC) mixture immersed in the 0.89% sodium sulfate solution. Both mixtures also observed very similar expansion values after one-year exposure. The formation of ettringite does not necessarily result in significant expansion or damage, depending on where and under what conditions it forms [36].
5. Conclusions and Future Study Recommendation
- Exposure to sodium sulfate shows that the test specimens are damaged primarily by the formation and ongoing crystal growth pressure of ettringite independent of sodium sulfate concentration. Microcracking was observed in the test samples, which led to an enhanced ingress of sulfate ions and consequently, accelerated the disintegration; however, the rate of attack is proportional to the concentration and supersaturation of the pores with sulfate ions.
- Gypsum was observed in very few instances when evaluated using the SEM independent of the solution concentration; however, traces of gypsum formation were evident in the XRD patterns and in relative amounts based on the Rietveld analysis of the diffraction patterns indicating that some deterioration some may have been attributed from gypsum formation.
- Significant differences in the mode of failure were evident between the two concentrations investigated. Larger cracks and in some cases warping and/or complete loss of cohesion were seen in samples placed in 5% sodium sulfate, especially high-calcium binary mixtures. Smaller cracks were observed in samples placed in 0.89% sodium sulfate with most deterioration only occurring at the ends and corners of the samples.
- SEM imaging revealed ettringite deposits were found within the paste matrix in both sodium sulfate concentrations; however, the higher concentration appeared more distinct and distributed throughout the matrix, while the amount and arrangement were discontinuous and significantly less dense in the lower concentration revealing little to no microcracking within the bulk paste matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nodehi, M. Epoxy, polyester and vinyl ester based polymer concrete: A review. Innov. Infrastruct. Solut. 2022, 7, 64. [Google Scholar] [CrossRef]
- Nodehi, M.; Aguayo, F.; Nodehi, S.E.; Gholampour, A.; Ozbakkaloglu, T.; Gencel, O. Durability properties of 3D printed concrete (3DPC). Autom. Constr. 2022, 142, 104479. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Messad, S.; Carcassès, M.; Linger, L.; Boutillon, L. Performance approach using accelerated test method for external sulfate attack. In Proceedings of the 3rd International fib Congress and Exhibition, Incorporating the PCI Annual Convention and Bridge Conference: Think Globally, Build Locally, Proceedings, Washington, DC, USA, 29 May–2 June 2010. [Google Scholar]
- ASTM C1012/C1012M-15; Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Dhole, R. Sulfate Resistance of High Calcium Fly Ash. Ph.D. Thesis, University of New Brunswick, Fredericton, NB, Canada, 2007. [Google Scholar] [CrossRef]
- Drimalas, T. Laboratory and Field Evaluations of External Sulfate Attack. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2007. [Google Scholar]
- Bonen, D.; Cohen, M.D. Magnesium sulfate attack on portland cement paste-I. Microstructural analysis. Cem. Concr. Res. 1992, 22, 169–180. [Google Scholar] [CrossRef]
- Marchand, J.; Odler, I.; Skalny, J.P. Sulfate Attack on Concrete; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Does gypsum formation during sulfate attack on concrete lead to expansion? Cem. Concr. Res. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Wright, L.; Khatib, J.M. Sustainability of Desulphurised (FGD) Waste in Construction, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Leemann, A.; Loser, R. Analysis of concrete in a vertical ventilation shaft exposed to sulfate-containing groundwater for 45years. Cem. Concr. Compos. 2011, 33, 74–83. [Google Scholar] [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and microanalytical studies of sulfate attack III. Sulfate-resisting portland cement: Reactions with sodium and magnesium sulfate solutions. Cem. Concr. Res. 1995, 25, 1581–1590. [Google Scholar] [CrossRef]
- Kunther, W.; Lothenbach, B.; Scrivener, K.L. Deterioration of mortar bars immersed in magnesium containing sulfate solutions. Mater. Struct. 2013, 46, 2003–2011. [Google Scholar] [CrossRef]
- Kurtoğlu, A.E.; Radhwan, A.; Omar, A.; Anil, N.; Eren, G.M.; Ghassan, H.; Abdulkadir, C. Mechanical and durability properties of fly ash and slag based geopolymer concrete. Adv. Concr. Constr. 2018, 6, 345–362. [Google Scholar] [CrossRef]
- Dehwah, H.A.F. Effect of sulfate concentration and associated cation type on concrete deterioration and morphological changes in cement hydrates. Constr. Build. Mater. 2007, 21, 29–39. [Google Scholar] [CrossRef]
- ACI Committee 201. 201.2R-08: Guide to Durable Concrete; ACI: Farmington Hills, MI, USA, 2008. [Google Scholar]
- ASTM C150/C150M-19a; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM C778; Standard Specification for Standard Colors for Polymer-Coated Chain Link Fence. ASTM: West Conshohocken, PA, USA, 2015.
- Humboldt Inc. Ottawa Test Sand for Cube Molds. Available online: https://www.humboldtmfg.com/ottawa-test-sand.html (accessed on 11 November 2022).
- Dhole, R.; Thomas, M.D.A.; Folliard, K.J.; Drimalas, T. Sulfate resistance of mortar mixtures of high-calcium fly ashes and other pozzolans. ACI Mater. J. 2011, 108, 645–654. [Google Scholar] [CrossRef]
- Dhole, R.; Thomas, M.D.A.; Folliard, K.J.; Drimalas, T. Characterization of fly ashes for sulfate resistance. ACI Mater. J. 2013, 110, 159–168. [Google Scholar] [CrossRef]
- Panesar, D.K. Supplementary cementing materials. In Developments in the Formulation and Reinforcement of Concrete; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–85. [Google Scholar] [CrossRef]
- A. C109/109M-16a; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-In. or Cube Specimens); Annual Book of ASTM Standards. ASTM: West Conshohocken, PA, USA, 2016. [CrossRef]
- C490/C490M; Standard Practice for Use of Apparatus for the Determination of Length Change of Hardened Cement Paste, Mortar, and Concrete, C 490; Annual Book ofASTM Standards. American Society for Testing and Materials: West Conshohocken, PA, USA, 2007. [CrossRef]
- Reynolds, R.C. Principles of Powder Diffraction. In Modern Powder Diffraction; De Gruyter: Berlin, Germany, 1989; pp. 1–18. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Shehata, M.H.; Shashiprakash, S.G.; Hopkins, D.S.; Cail, K. Use of ternary cementitious systems containing silica fume and fly ash in concrete. Cem. Concr. Res. 1999, 29, 1207–1214. [Google Scholar] [CrossRef]
- Tikalsky, P.J.; Carrasquillo, R.L. Fly Ash Evaluation and Selection for Use in Sulfate-Resistant Concrete. ACI Mater. J. 1993, 90, 545–551. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Vo, D.-H.; Hwang, C.-L. Engineering and durability properties of eco-friendly mortar using cement-free SRF binder. Constr. Build. Mater. 2018, 160, 145–155. [Google Scholar] [CrossRef]
- Sulfate Attack on Concrete: Research Needs. ACI Mater. J. 1991, 88, 62–69. [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and microanalytical studies of sulfate attack. I. Ordinary portland cement paste. Cem. Concr. Res. 1992, 22, 1027–1038. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Modeling the effects of solution temperature and concentration during sulfate attack on cement mortars. Cem. Concr. Res. 2002, 32, 585–592. [Google Scholar] [CrossRef]
- Basista, M.; Weglewski, W. Chemically Assisted Damage of Concrete: A Model of Expansion under External Sulfate Attack. Int. J. Damage Mech. 2009, 18, 155–175. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Pimraksa, K.; Chindaprasirt, P. Sulfoaluminate Cement-Based Concrete; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Biczok, I. Concrete Corrosion and Concrete Protection, 1st ed.; Chemical Publishing Co., Inc.: Gloucester, MA, USA, 2011; Volume 2. [Google Scholar]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]




| Cement Type | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Type I (C1) | 20.36 | 5.43 | 2.50 | 63.12 | 1.35 | 0.09 | 1.03 | 3.23 | 2.60 |
| Type I/II (C2) | 20.38 | 4.90 | 3.55 | 63.62 | 1.14 | 0.11 | 0.67 | 2.86 | 2.20 |
| Supplementary Cementitious Materials | |||||||||
| Class C Fly Ash (HC) | 30.76 | 17.75 | 5.98 | 28.98 | 6.55 | 2.15 | 0.3 | 3.64 | - |
| Class F Fly Ash (LC) | 48.48 | 25.01 | 3.56 | 15.92 | 2.5 | 0.3 | 0.71 | 0.72 | - |
| Silica Fume (SF) | 93.17 | - | 2.1 | 0.8 | 0.3 | - | - | 0.2 | - |
| Cements | C3S | C2S | C3A | C4AF |
|---|---|---|---|---|
| Type I | 62.12 | 11.52 | 10.16 | 7.61 |
| Type I/II | 66.06 | 8.60 | 6.98 | 10.80 |
| Mixture | W/CM | C1 (%) | C2 (%) | HC (%) | LC (%) | SF (%) |
|---|---|---|---|---|---|---|
| C1-Cont | 0.485 | 100 | - | - | - | - |
| C1-30HC | 0.485 | 70 | - | 30 | - | - |
| C1-35HC-5SF | 0.485 | 60 | - | 35 | - | 5 |
| C1-25LC | 0.485 | 75 | - | - | 25 | - |
| C2-Cont | 0.485 | - | 100 | - | - | - |
| C2-30HC | 0.485 | - | 70 | 30 | - | - |
| C2-35HC-5SF | 0.485 | - | 60 | 35 | - | 5 |
| C2-25LC | 0.485 | - | 75 | - | 25 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguayo, F.; Nodehi, M. Deterioration of Mortar Bars Using Binary and Ternary Mixtures Immersed in Sodium Sulfate Solutions. Ceramics 2022, 5, 991-1008. https://doi.org/10.3390/ceramics5040071
Aguayo F, Nodehi M. Deterioration of Mortar Bars Using Binary and Ternary Mixtures Immersed in Sodium Sulfate Solutions. Ceramics. 2022; 5(4):991-1008. https://doi.org/10.3390/ceramics5040071
Chicago/Turabian StyleAguayo, Federico, and Mehrab Nodehi. 2022. "Deterioration of Mortar Bars Using Binary and Ternary Mixtures Immersed in Sodium Sulfate Solutions" Ceramics 5, no. 4: 991-1008. https://doi.org/10.3390/ceramics5040071
APA StyleAguayo, F., & Nodehi, M. (2022). Deterioration of Mortar Bars Using Binary and Ternary Mixtures Immersed in Sodium Sulfate Solutions. Ceramics, 5(4), 991-1008. https://doi.org/10.3390/ceramics5040071










