Modelling the Mechanical Properties of Hydroxyapatite Scaffolds Produced by Digital Light Processing-Based Vat Photopolymerization †
Abstract
:1. Introduction
2. Materials and Methods
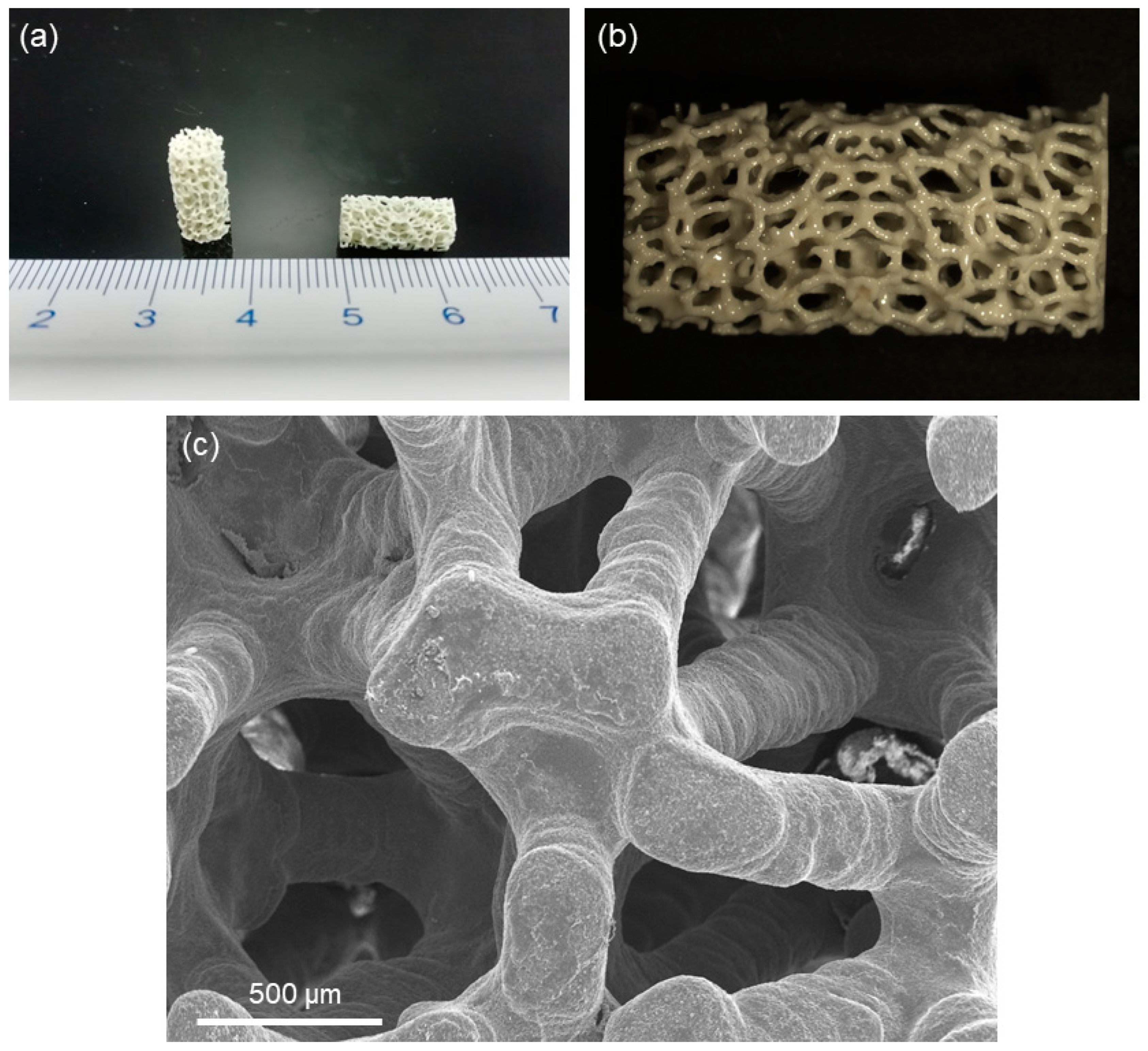
2.1. Fabrication and Characterization of Hydroxyapatite Scaffolds
2.2. Mechanical Modelling
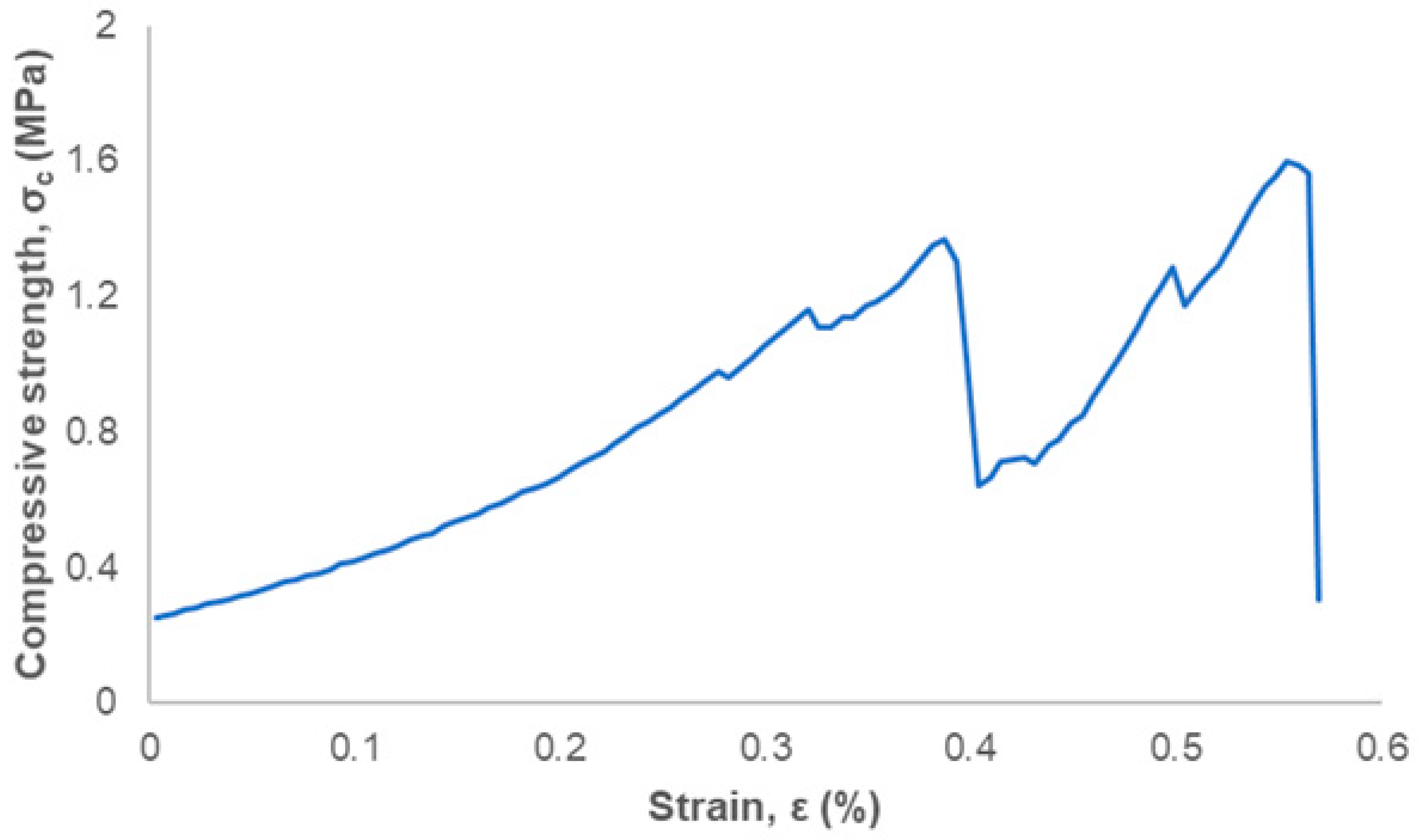
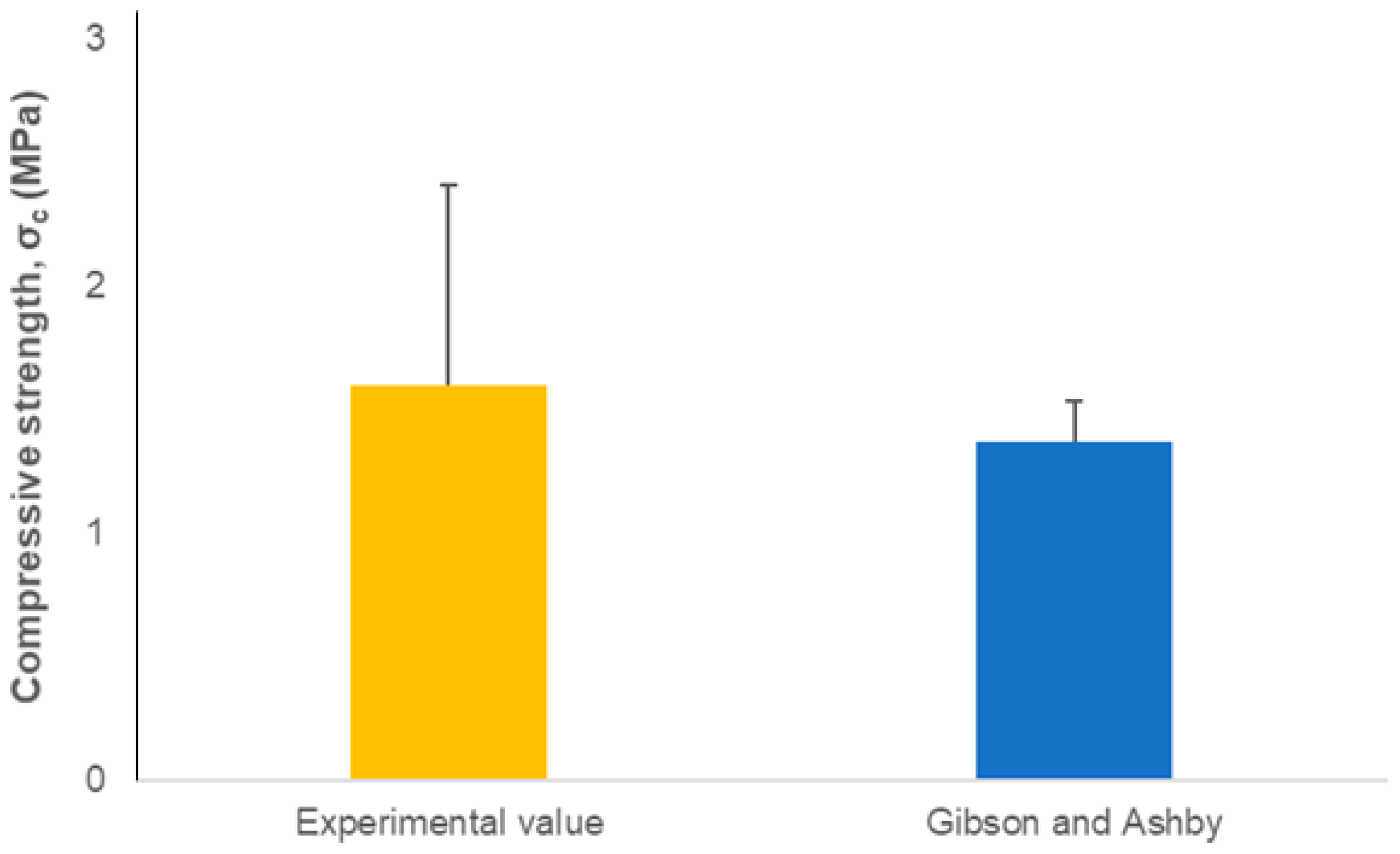
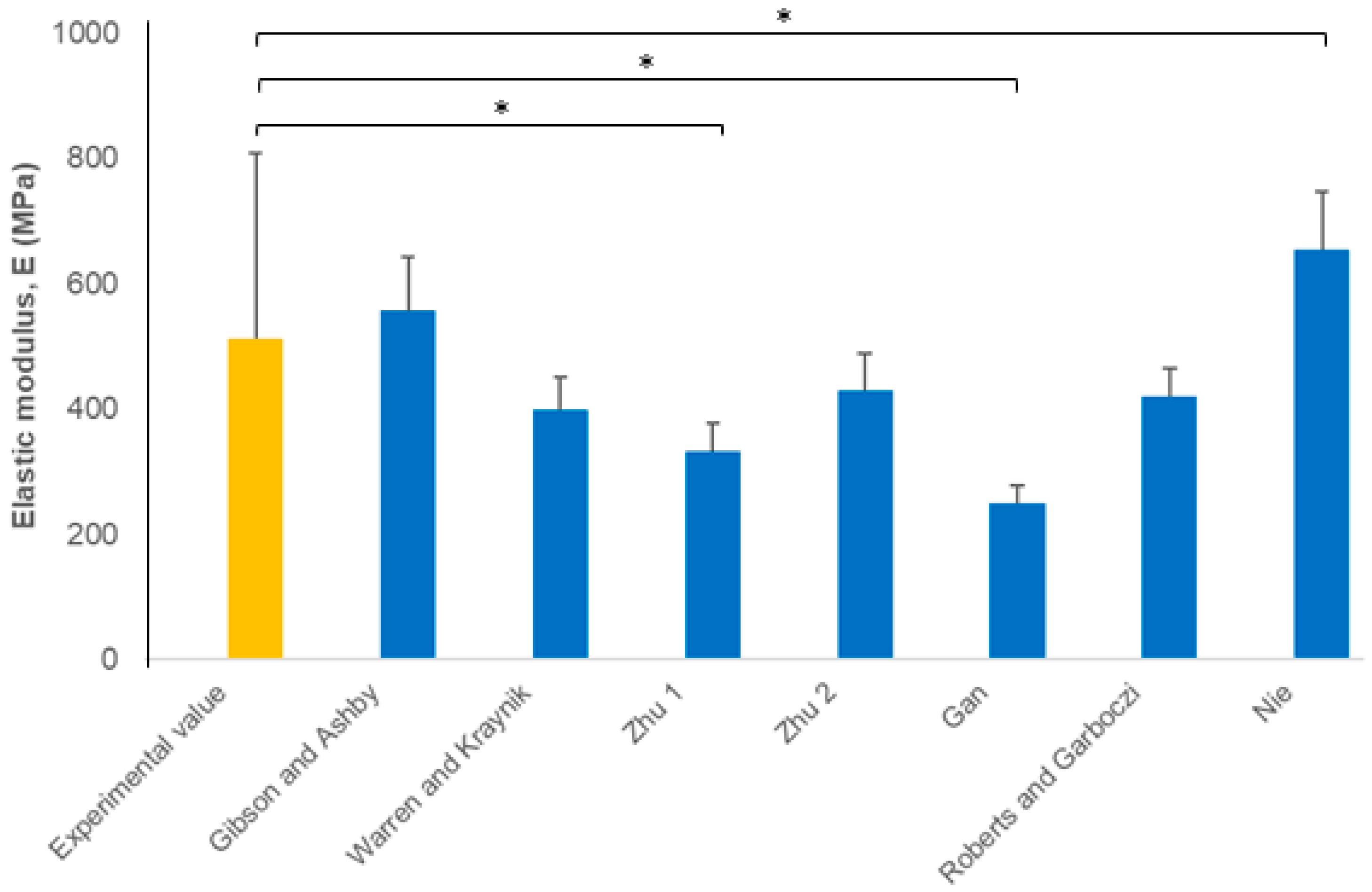
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hulsart Billström, G.; Blom, A.W.; Larsson, S.; Beswick, A.D. Application of scaffolds for bone regeneration strategies: Current trends and future directions. Injury 2013, 44, S28–S33. [Google Scholar] [CrossRef]
- Boskey, A.L. Mineralization of bones and teeth. Elements 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Gomes, D.S.; da Cunha Santos, A.M.; de Araújo Neves, G.; Menezes, R.R. A brief review on hydroxyapatite production and use in biomedicine. Ceramica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: A review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Potestio, I. Lithoz: How lithography-based ceramic AM is expanding the opportunities for technical ceramics. Powder Inject. Mould. 2019, 13, 2–5. [Google Scholar]
- Feng, C.; Zang, K.; He, R.; Ding, G.; Xia, M. Additive manufacturing of hydroxyapatite bioceramic scaffolds: Dispersion, digital light processing, sintering, mechanical properties, and biocompatibility. J. Adv. Ceram. 2020, 9, 360–373. [Google Scholar] [CrossRef]
- Fiume, E.; Ciavattini, S.; Verné, E.; Baino, F. Foam replica method in the manufacturing of bioactive glass scaffolds: Out-of-date technology or still underexploited potential? Materials 2021, 14, 2795. [Google Scholar] [CrossRef]
- Baino, F.; Magnaterra, G.; Fiume, E.; Schiavi, A.; Tofan, L.P.; Schwentenwein, M.; Verné, E. Digital light processing stereolithography of hydroxyapatite scaffolds with bone-like architecture, permeability, and mechanical properties. J. Am. Ceram. Soc. 2022, 105, 1648–1657. [Google Scholar] [CrossRef]
- Hing, K.A. Bioceramic bone graft substitutes: Influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar] [CrossRef]
- Karageorgiu, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Bhattacharya, I.; Guerrero, J.; Özcan, M.; Weber, F.E. 3D-Printed HA-Based Scaffolds for Bone Regeneration: Microporosity, Osteoconduction and Osteoclastic Resorption. Materials 2022, 15, 1433. [Google Scholar] [CrossRef] [PubMed]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, F. The mechanics of three-dimensional cellular materials. Proc. R. Soc. Lond. A 1982, 382, 43–59. [Google Scholar]
- Warren, W.E.; Kraynik, A.M. The linear elastic properties of open-cell foams. J. Appl. Mech. 1988, 55, 341–346. [Google Scholar] [CrossRef]
- Zhu, H.X.; Knott, J.F.; Mills, N.J. Analysis of the elastic properties of open-cell foams with tetrakaidecahedral cells. Mech. Phys. Solids 1997, 45, 319–343. [Google Scholar] [CrossRef]
- Kraynik, A.M.; Warren, W.E. The elastic behavior of low-density cellular plastics. In Low Density Cellular Plastics; Hilvard, N.C., Cunningham, A., Eds.; Chapman and Hall: London, UK, 1994; pp. 187–225. [Google Scholar]
- Gan, Y.X.; Chen, C.; Shen, Y.P. Three-dimensional modeling of the mechanical property of linearly elastic open cell foams. Int. J. Solids Struct. 2005, 42, 6628–6642. [Google Scholar] [CrossRef]
- Roberts, A.P.; Garboczi, E.J. Computation of the linear elastic properties of random porous materials with a wide variety of microstructure. Proc. R. Soc. Lond. A 2002, 458, 1033–1054. [Google Scholar] [CrossRef]
- Nie, Z.; Lin, Y.; Ton, Q. Computational modeling of the elastic property of three-dimensional open cell foams. Arch. Metall. Mater. 2018, 63, 1153–1165. [Google Scholar]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, A.; Fiume, E.; Orlygsson, G.; Schwentenwein, M.; Verné, E.; Baino, F. High-reliability data processing and calculation of microstructural parameters in hydroxyapatite scaffolds produced by vat photopolymerization. J. Eur. Ceram. Soc. 2022, 42, 6206–6212. [Google Scholar] [CrossRef]
- Gibson, L.J. Modelling the mechanical behavior of cellular materials. Mater. Sci. Eng. A 1989, 110, 1–36. [Google Scholar] [CrossRef]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Hydroxyapatite porous scaffold engineered with biological polymer hybrid coating for antibiotic Vancomycin release. J. Mater. Sci. Mater. Med. 2005, 16, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E. Elastic mechanical properties of 45S5-based bioactive glass-ceramic scaffolds. Materials 2019, 12, 3244. [Google Scholar] [CrossRef]
- Keller, T.S. Predicting the compressive mechanical behavior of bone. J. Biomech. 1994, 27, 1159–1168. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F.; Day, D.E. Mechanical and in vitro performance of 13–93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008, 4, 1854–1864. [Google Scholar] [CrossRef]
- Fritsch, A.; Dormieux, L.; Hellmich, C.; Sanahuja, J. Mechanical behavior of hydroxyapatite biomaterials: An experimentally validated micromechanical model for elasticity and strength. J. Biomed. Mater. Res. A 2009, 88, 149–161. [Google Scholar] [CrossRef]
- Tagliabue, S.; Rossi, E.; Baino, F.; Vitale-Brovarone, C.; Gastaldi, D.; Vena, P. Micro-CT based finite element models for elastic properties of glass-ceramic scaffolds. J. Mech. Behav. Biomed. Mater. 2017, 65, 248–255. [Google Scholar] [CrossRef]
- Farina, E.; Gastaldi, D.; Baino, F.; Verné, E.; Massera, J.; Orlygsson, G.; Vena, P. Micro computed tomography based finite element models for elastic and strength properties of 3D printed glass scaffolds. Acta Mech. Sin. 2021, 37, 292–306. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baino, F.; Schwentenwein, M.; Verné, E. Modelling the Mechanical Properties of Hydroxyapatite Scaffolds Produced by Digital Light Processing-Based Vat Photopolymerization. Ceramics 2022, 5, 593-600. https://doi.org/10.3390/ceramics5030044
Baino F, Schwentenwein M, Verné E. Modelling the Mechanical Properties of Hydroxyapatite Scaffolds Produced by Digital Light Processing-Based Vat Photopolymerization. Ceramics. 2022; 5(3):593-600. https://doi.org/10.3390/ceramics5030044
Chicago/Turabian StyleBaino, Francesco, Martin Schwentenwein, and Enrica Verné. 2022. "Modelling the Mechanical Properties of Hydroxyapatite Scaffolds Produced by Digital Light Processing-Based Vat Photopolymerization" Ceramics 5, no. 3: 593-600. https://doi.org/10.3390/ceramics5030044
APA StyleBaino, F., Schwentenwein, M., & Verné, E. (2022). Modelling the Mechanical Properties of Hydroxyapatite Scaffolds Produced by Digital Light Processing-Based Vat Photopolymerization. Ceramics, 5(3), 593-600. https://doi.org/10.3390/ceramics5030044








