Foam-Replicated Diopside/Fluorapatite/Wollastonite-Based Glass–Ceramic Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Glass
2.3. Fabrication of Scaffolds
2.4. Characterizations
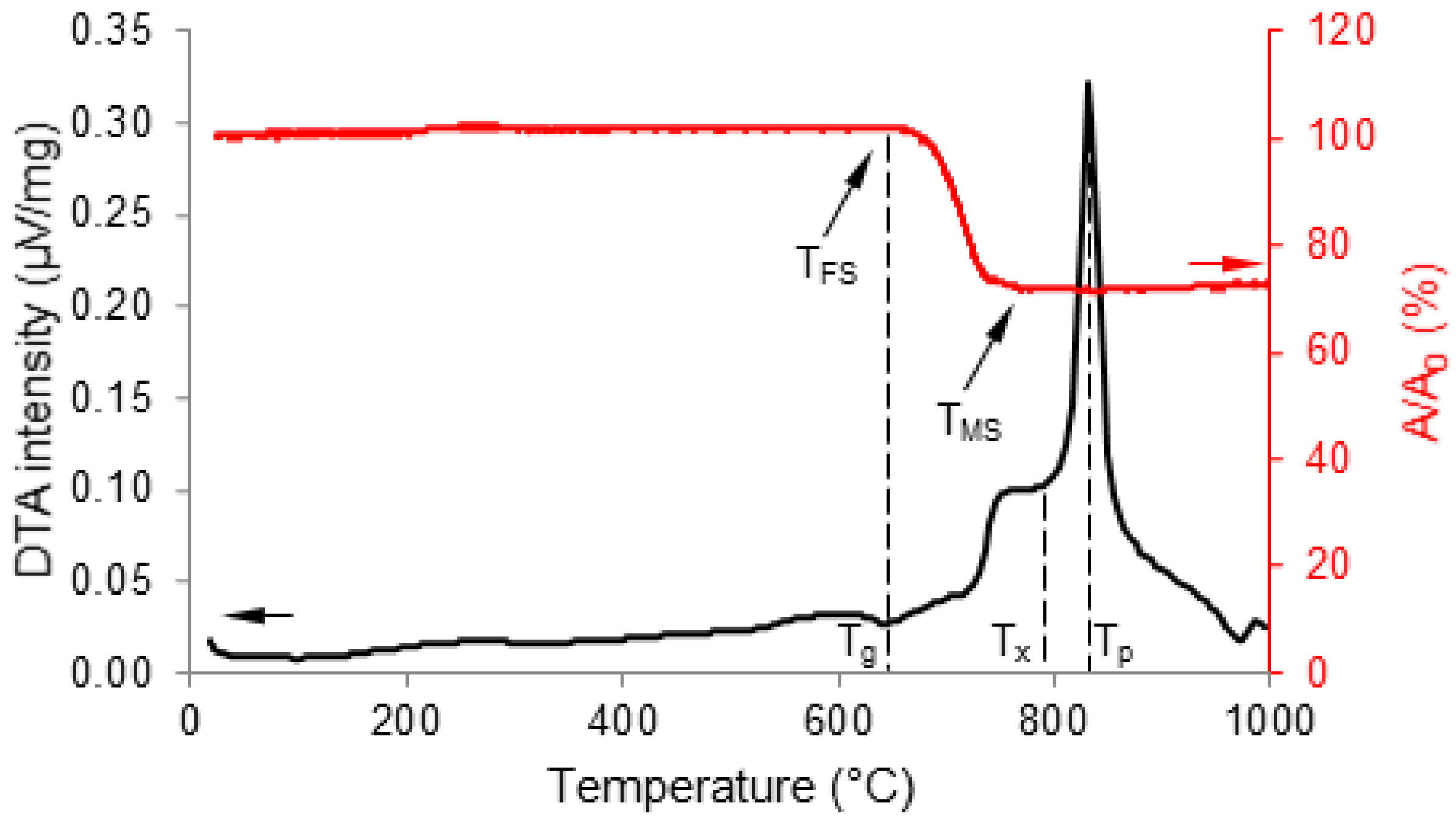
2.4.1. Thermal Analyses
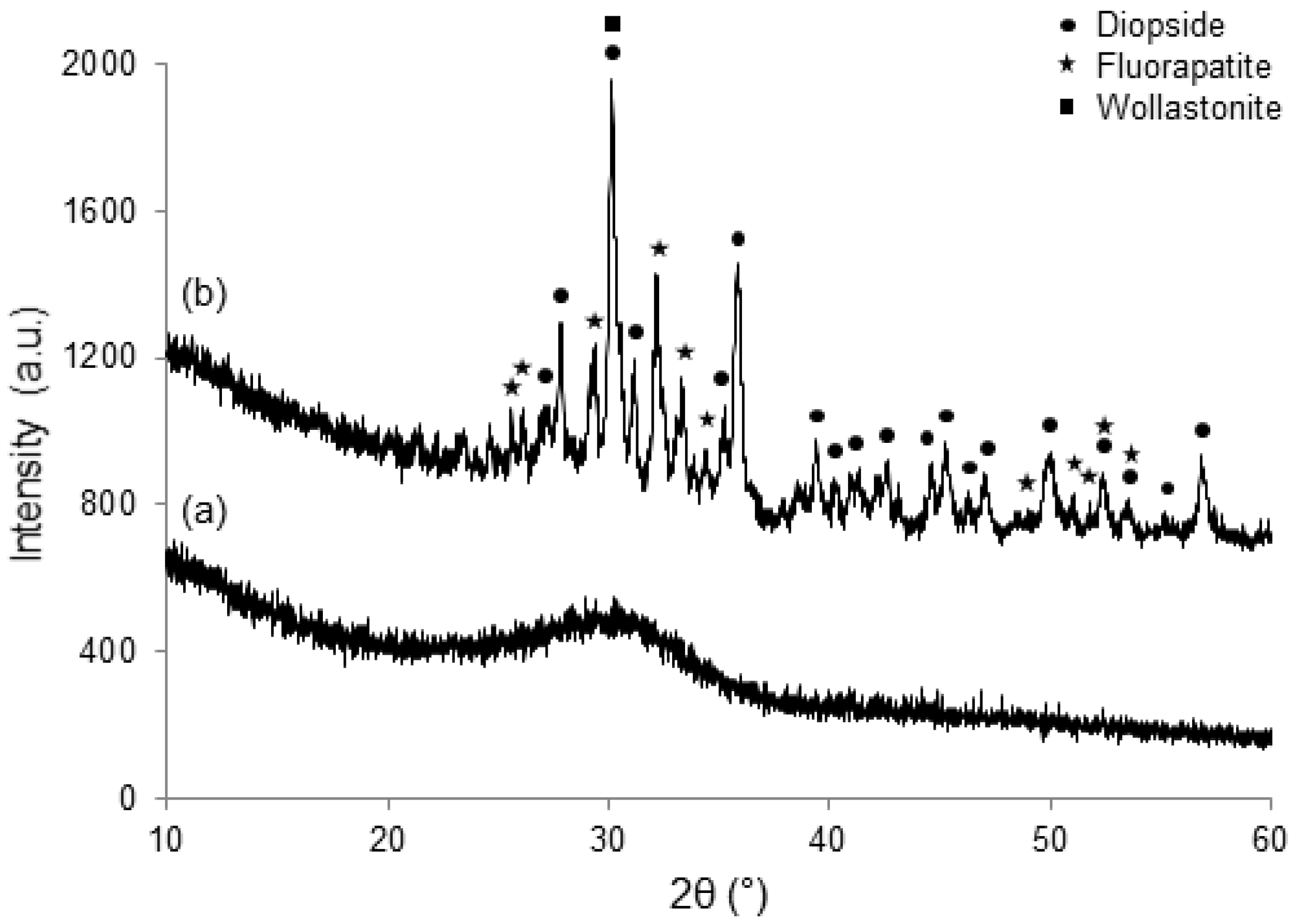
2.4.2. Crystallization
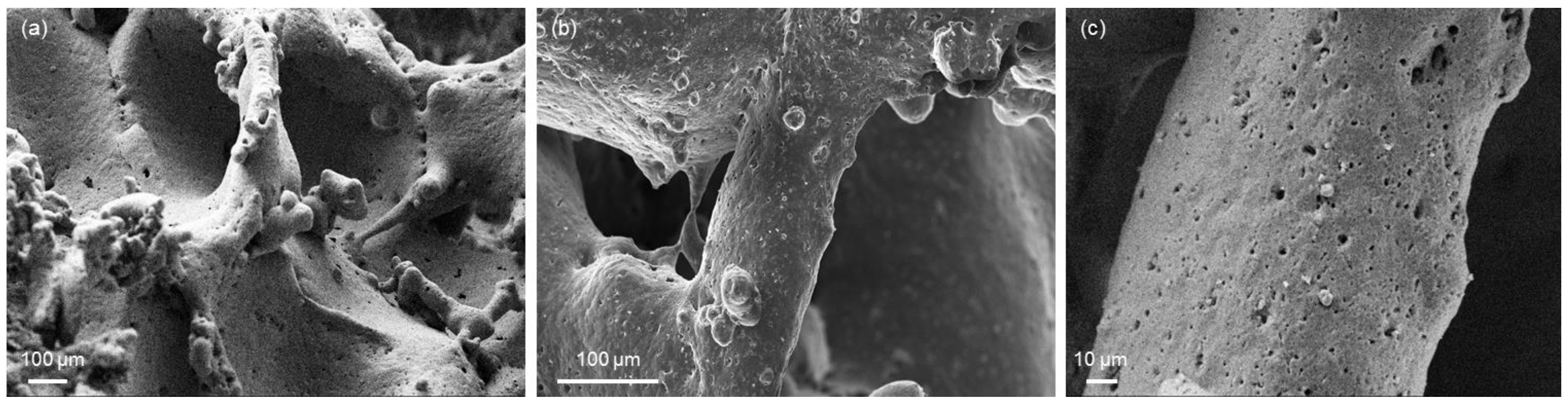
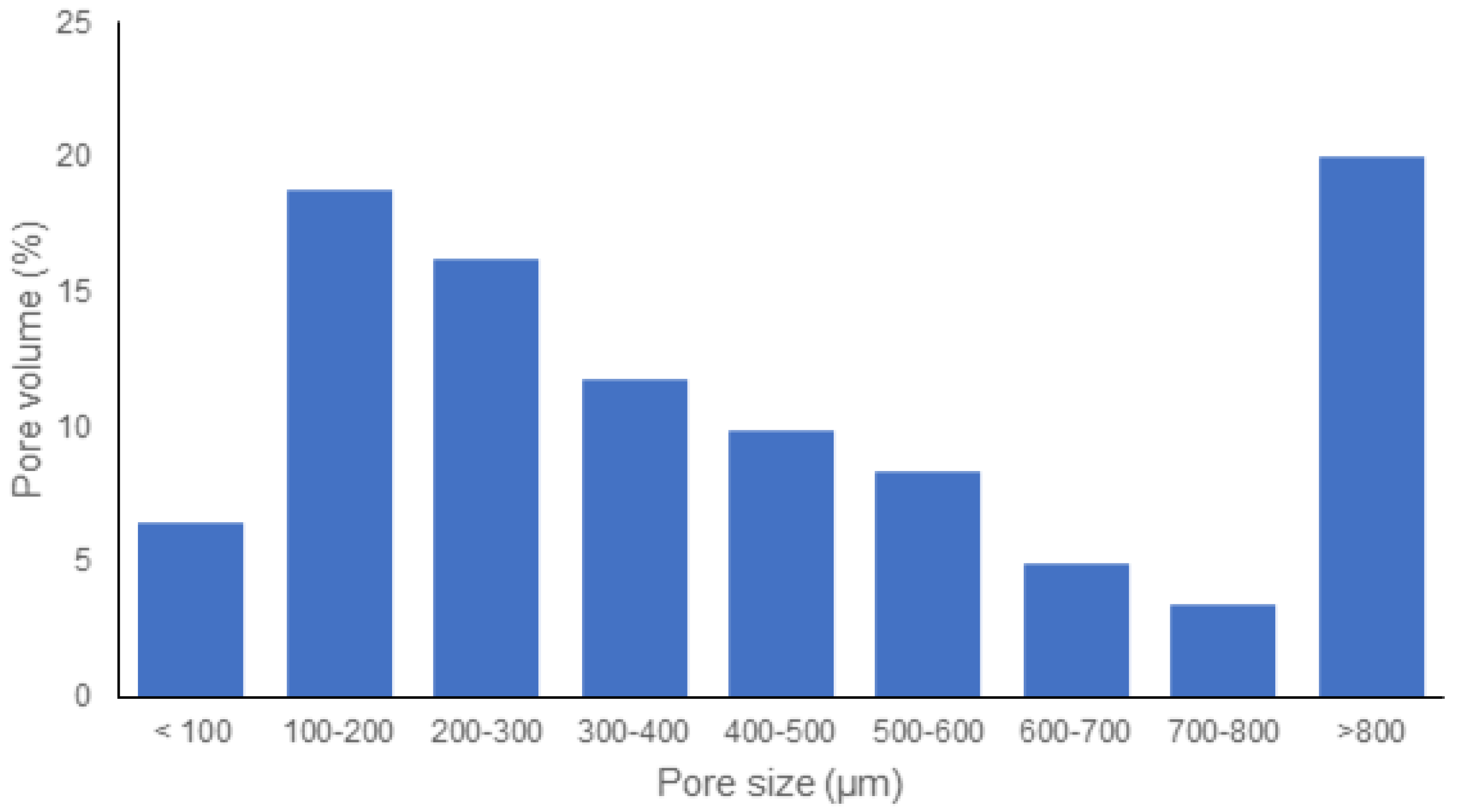
2.4.3. Morphology
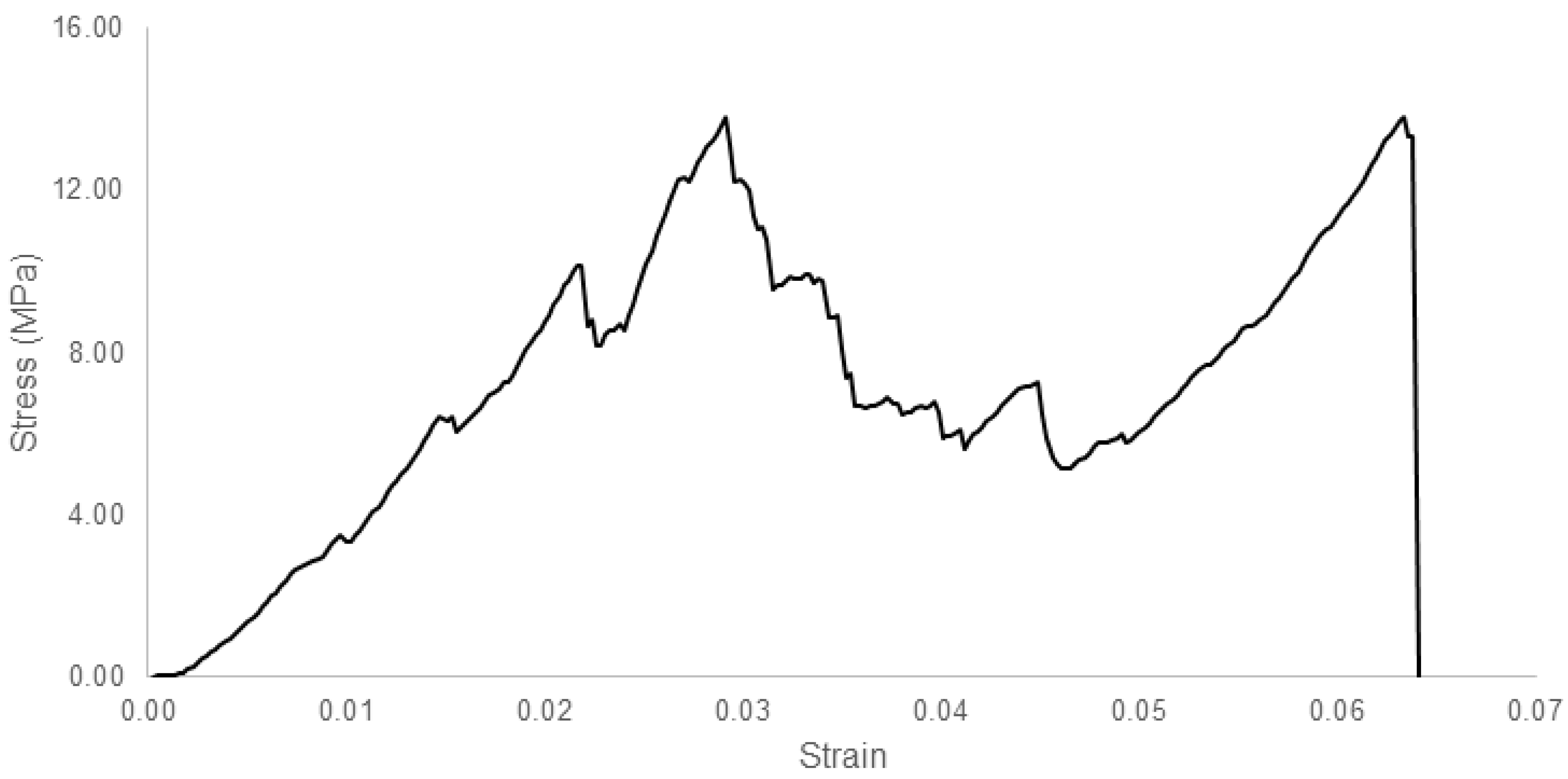
2.4.4. Mechanical Properties
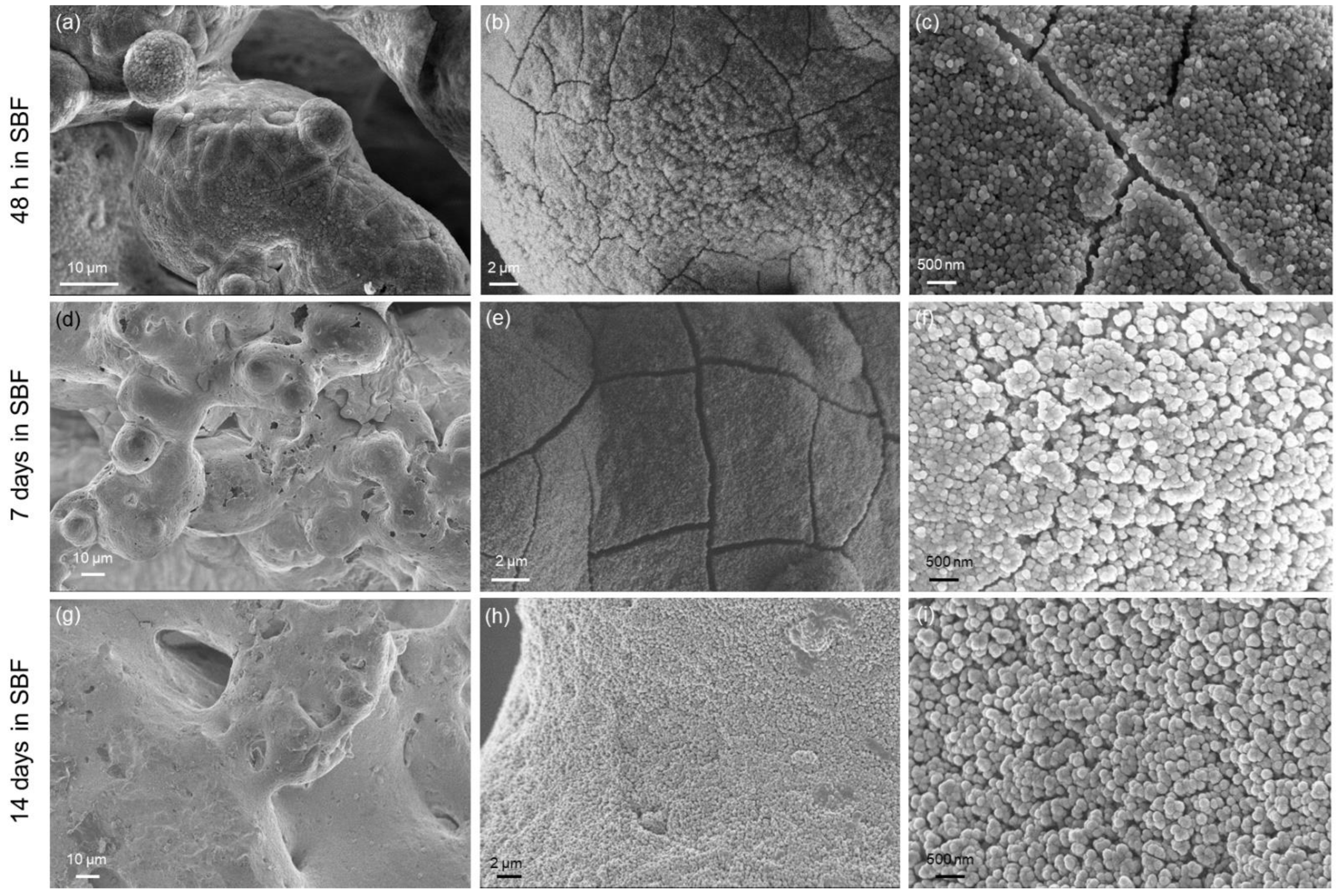
2.4.5. In Vitro Bioactivity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baino, F.; Ferraris, M. Learning from Nature: Using bioinspired approaches and natural materials to make porous bioceramics. Int. J. Appl. Ceram. Technol. 2017, 14, 507–520. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics—From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Boccardi, E.; Philippart, A.; Juhasz-Bortuzzo, J.A.; Novajra, G.; Vitale-Brovarone, C.; Boccaccini, A.R. Characterisation of Bioglass based foams developed via replication of natural marine sponges. Adv. Appl. Ceram. 2015, 114 (Suppl. 1), S56–S62. [Google Scholar] [CrossRef] [Green Version]
- Falvo D’Urso Labate, G.; Catapano, G.; Vitale-Brovarone, C.; Baino, F. Quantifying the micro-architectural similarity of bioceramic scaffolds to bone. Ceram. Int. 2017, 43, 9443–9450. [Google Scholar] [CrossRef]
- Baino, F.; Ferraris, M.; Bretcanu, O.; Verné, E.; Vitale-Brovarone, C. Optimization of composition, structure and mechanical strength of bioactive 3-D glass-ceramic scaffolds for bone substitution. J. Biomater. Appl. 2013, 27, 872–890. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass derived glass ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Chen, Q.; Mohn, D.; Stark, W.J. Optimization of Bioglass® scaffold fabrication process. J. Am. Ceram. Soc. 2011, 94, 4184–4190. [Google Scholar] [CrossRef]
- Studart, A.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Jones, J.; Hench, L.L. Regeneration of trabecular bone using porous ceramics. Curr. Opin. Solid State Mater. Sci. 2003, 7, 301–307. [Google Scholar] [CrossRef]
- Fukasawa, T.; Deng, Z.Y.; Ando, M.; Ohji, T.; Goto, Y. Pore structure of porous ceramics synthesized from water-based slurry by freeze-dry process. J. Mater. Sci. 2001, 36, 2523–2527. [Google Scholar] [CrossRef]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Barberi, J.; Baino, F.; Fiume, E.; Orlygsson, G.; Nommeots-Nomm, A.; Massera, J.; Verné, E. Robocasting of SiO2-based bioactive glass scaffolds with porosity gradient for bone regeneration and potential load-bearing applications. Materials 2019, 12, 2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Reyes, E.A.; Leon-Patino, C.A.; Jacinto-Diaz, B.; Lefebvre, L.P. Structural characterization and mechanical evaluation of bioactive glass 45S5 foams obtained by a powder technology approach. J. Am. Ceram. Soc. 2012, 95, 3776–3780. [Google Scholar] [CrossRef]
- Aguilar-Reyes, E.A.; Leon-Patino, C.A.; Jacinto-Diaz, B.; Lefebvre, L.P. Synthesis of 45S5 Bioglass® foams by a powder metallurgy approach. Mater. Sci. Technol. 2010, 17, 70–77. [Google Scholar]
- Schwartzwalder, K.; Somers, A.V. Method of Making Porous Ceramic Articles. U.S. Patent 3,090,094, 21 May 1963. [Google Scholar]
- Park, Y.S.; Kim, K.N.; Kim, K.M.; Choi, S.H.; Kim, C.K.; Legeros, R.Z.; Lee, Y.K. Feasibility of three-dimensional macroporous scaffold using calcium phosphate glass and polyurethane sponge. J. Mater. Sci. 2006, 41, 4357–4364. [Google Scholar] [CrossRef]
- Fiume, E.; Ciavattini, S.; Verné, E.; Baino, F. Foam replica method in the manufacturing of bioactiveglassscaffolds: Out-of-date technology or still underexploited potential? Materials 2021, 14, 2795. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Separovic, F.; Van Deventer, J.S.J. Effect of alkali cations on aluminum incorporation in geopolymeric gels. Ind. Eng. Chem. Res. 2005, 44, 832839. [Google Scholar] [CrossRef]
- Schmitz, S.I.; Widholz, B.; Essers, C.; Becker, M.; Tulyaganov, D.U.; Moghaddama, A.; Gonzalo de Juan, I.; Westhauser, F. Superior biocompatibility and comparable osteoinductive properties: Sodium-reduced fluoride-containing bioactive glass belonging to the CaO–MgO–SiO2 system as a promising alternative to 45S5 bioactive glass. Bioactive Mater. 2020, 5, 55–65. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Makhkamov, M.E.; Urazbaev, A.; Goel, A.; Ferreira, J.M.F. Synthesis, processing and characterization of a bioactive glass composition for bone regeneration. Ceram. Int. 2013, 39, 2519–2526. [Google Scholar] [CrossRef]
- Dimitriadis, K. Development of Glass-Ceramics for Dental Applications. Ph.D. Thesis, University of Ioannina, Ioannina, Greece, 2020. [Google Scholar]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Sintering behavior of a six-oxide silicate bioactive glass for scaffold manufacturing. Appl. Sci. 2020, 10, 8279. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansal, I.; Tulyaganov, D.U.; Goel, A.; Pascual, M.J.; Ferreira, J.M.F. Structural analysis and thermal behaviour of diopside-fluorapatite–wollastonite-based glasses and glass-ceramics. Acta Biomater. 2010, 6, 4380–4388. [Google Scholar] [CrossRef] [PubMed]
- Tulyaganov, D.U.; Agathopoulos, S.; Valerio, P.; Balamurugan, A.; Saranti, A.; Karakassides, M.A.; Ferreira, J.M.F. Synthesis, bioactivity and preliminary biocompatibility studies of glasses in the system CaO–MgO–SiO2–Na2O–P2O5–CaF2. J. Mater. Sci. Mater. Med. 2011, 22, 217–227. [Google Scholar] [CrossRef]
- Boskey, A.L. Mineralization of bones and teeth. Elements 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Kokubo, T.; Ito, S.; Sakka, S.; Yamamuro, T. Formation of a highstrength bioactive glass-ceramic in the system MgO-CaO-SiO2-P2O5. J. Mater. Sci. 1986, 21, 536–540. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Degradation, bioactivity, and cytocompatibility of diopside, akermanite, and bredigite ceramics. J. Biomed. Mater. Res. B 2007, 83, 153–160. [Google Scholar] [CrossRef]
- Ressler, S.A.; Zuzic, A.; Ivanisevic, I.; Kamboj, N.; Ivankovic, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and fluorapatite in conservative dentistry and oral implantology—A review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar Venkatraman, S.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. A 2020, 108, 1546–1562. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, N.; Ressler, A.; Hussainov, I. Bioactive ceramic scaffolds for bone tissue engineering by powder bed selective laser processing: A review. Materials 2021, 14, 5338. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lin, K.; Wang, Z.; Chang, J.; Wang, L.; Lu, J.; Ning, C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Hildebrand, T.; Laib, A.; Muller, R.; Dequeker, J.; Ruegsegger, P. Direct three-dimensional morphometric analysis of human cancellous bone: Microstructural data from spine, femur, iliac crest, and calcaneus. J. Bone Miner. Res. 1999, 14, 1167–1174. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.; Oh, S.; Yi, W.; Heo, M.; Lee, S.; Choi, S.; Huh, K. The three-dimensional microstructure of trabecular bone: Analysis of site-specific variation in the human jaw bone. Imaging Sci. Dent. 2013, 43, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing methods for making porous bioactive glass-based scaffolds—A state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Rice, R. Mechanical properties. In Cellular Ceramics: Structure, Manufacturing, Properties and Applications; Schaufer, M., Colombo, P., Eds.; Wiley: New York, NY, USA, 2005; pp. 291–312. [Google Scholar]
- Thompson, I.D.; Hench, L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 127–136. [Google Scholar] [CrossRef]
- Keaveny, T.M. Mechanistic approaches to analysis of trabecular bone. Forma 1997, 12, 267–275. [Google Scholar]
- Gibson, L.J.; Ashby, F. The mechanics of three-dimensional cellular materials. Proc. R. Soc. Lond. A 1982, 382, 43–59. [Google Scholar]
- Dimitriadis, K.; Moschovas, D.; Tulyaganov, D.U.; Agathopoulos, S. Development of novel bioactive glass-ceramics in the Na2O/K2O-CaO-MgOSiO2-P2O5-CaF2 system. J. Non-Cryst. Solids 2020, 533, 119936. [Google Scholar] [CrossRef]
- Clupper, D.C.; Hench, L.L. Crystallization kinetics of tape cast bioactive glass 45S5. J. Non-Cryst. Solids 2003, 318, 43–48. [Google Scholar] [CrossRef]
- Pantano, C.G.; Clark, A.E.; Hench, L.L. Multilayer corrosion films on Bioglass surfaces. J. Am. Ceram. Soc. 1974, 57, 412–413. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regi, M. Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Verné, E. Fe-doped bioactive glass-derived scaffolds produced by sol-gel foaming. Mater. Lett. 2019, 235, 207–211. [Google Scholar] [CrossRef]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Bread-derived bioactive porous scaffolds: An innovative and sustainable approach to bone tissue engineering. Molecules 2019, 24, 2954. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, M.; Banijamali, S.; Baino, F.; Kargozar, S.; Hill, R.G. Calcium carbonate: Adored and ignored in bioactivity assessment. Acta Biomater. 2019, 91, 35–47. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baino, F.; Tulyaganov, D.U.; Kahharov, Z.; Rahdar, A.; Verné, E. Foam-Replicated Diopside/Fluorapatite/Wollastonite-Based Glass–Ceramic Scaffolds. Ceramics 2022, 5, 120-130. https://doi.org/10.3390/ceramics5010011
Baino F, Tulyaganov DU, Kahharov Z, Rahdar A, Verné E. Foam-Replicated Diopside/Fluorapatite/Wollastonite-Based Glass–Ceramic Scaffolds. Ceramics. 2022; 5(1):120-130. https://doi.org/10.3390/ceramics5010011
Chicago/Turabian StyleBaino, Francesco, Dilshat U. Tulyaganov, Ziyodilla Kahharov, Abbas Rahdar, and Enrica Verné. 2022. "Foam-Replicated Diopside/Fluorapatite/Wollastonite-Based Glass–Ceramic Scaffolds" Ceramics 5, no. 1: 120-130. https://doi.org/10.3390/ceramics5010011
APA StyleBaino, F., Tulyaganov, D. U., Kahharov, Z., Rahdar, A., & Verné, E. (2022). Foam-Replicated Diopside/Fluorapatite/Wollastonite-Based Glass–Ceramic Scaffolds. Ceramics, 5(1), 120-130. https://doi.org/10.3390/ceramics5010011







