Structure and Properties of Piezoelectric Strontium Fresnoite Glass-Ceramics Belonging to the Sr–Ti–Si–Al–K–O System
Abstract
:1. Introduction
2. Materials and Methods
- 300 °C/h from RT to Tc
- Dwell time of tc hours at Tc
- Natural cooling in switched off furnace
3. Results
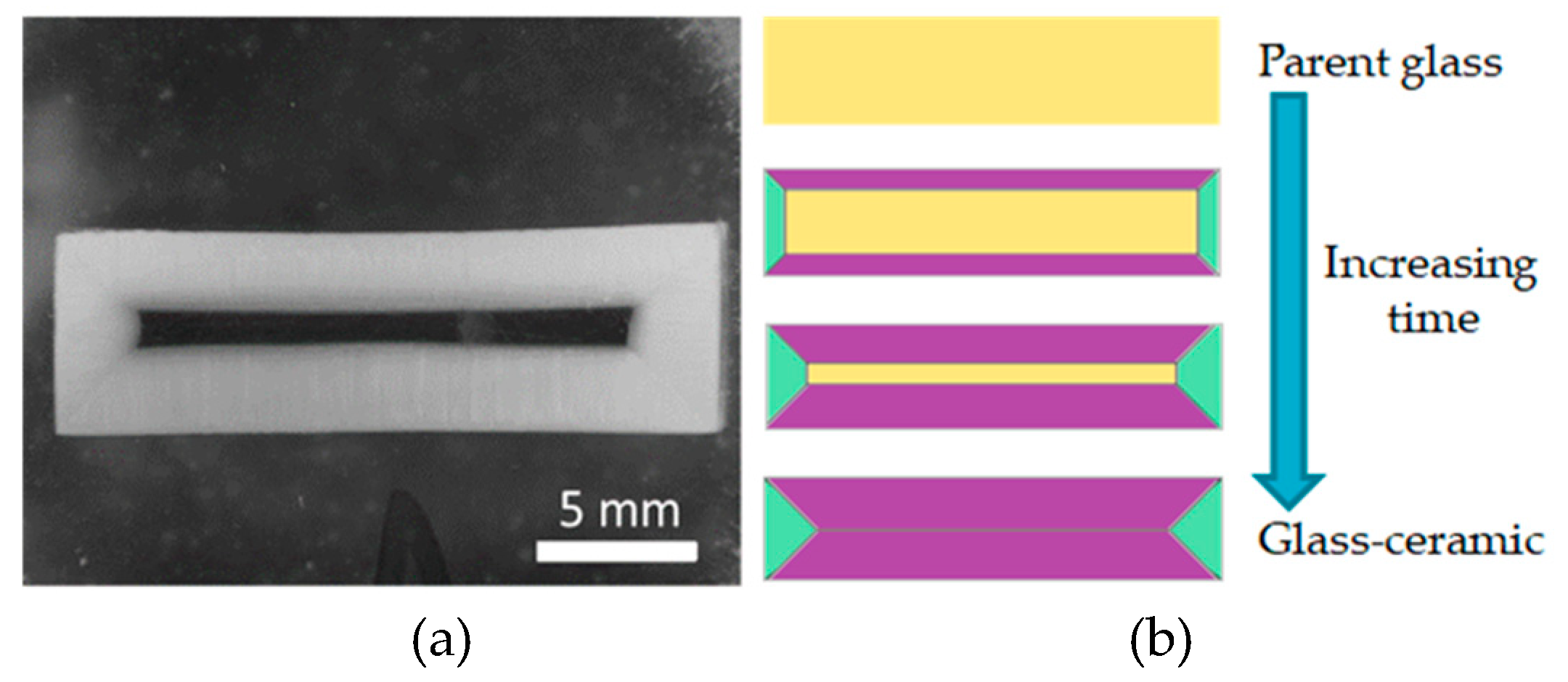
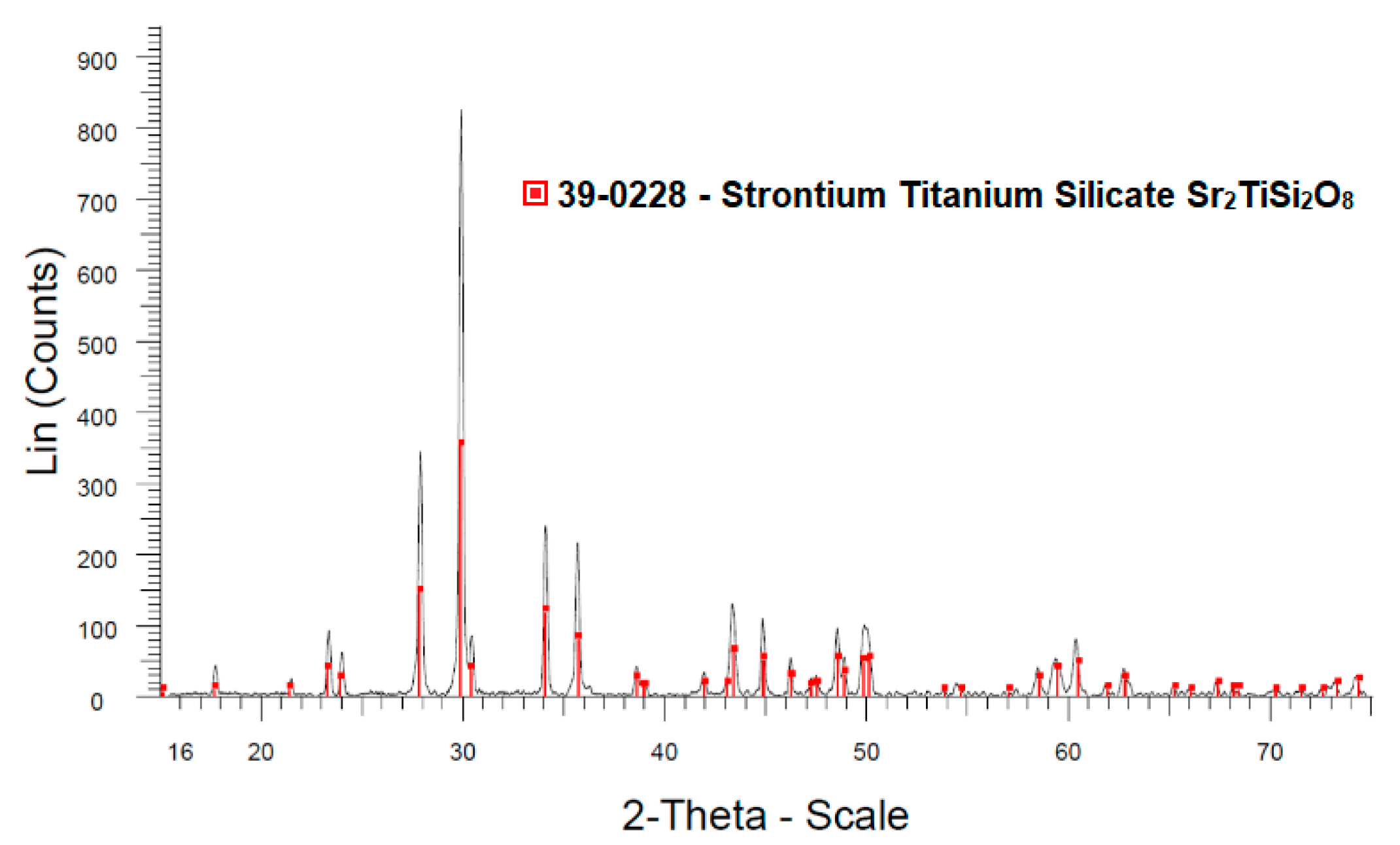
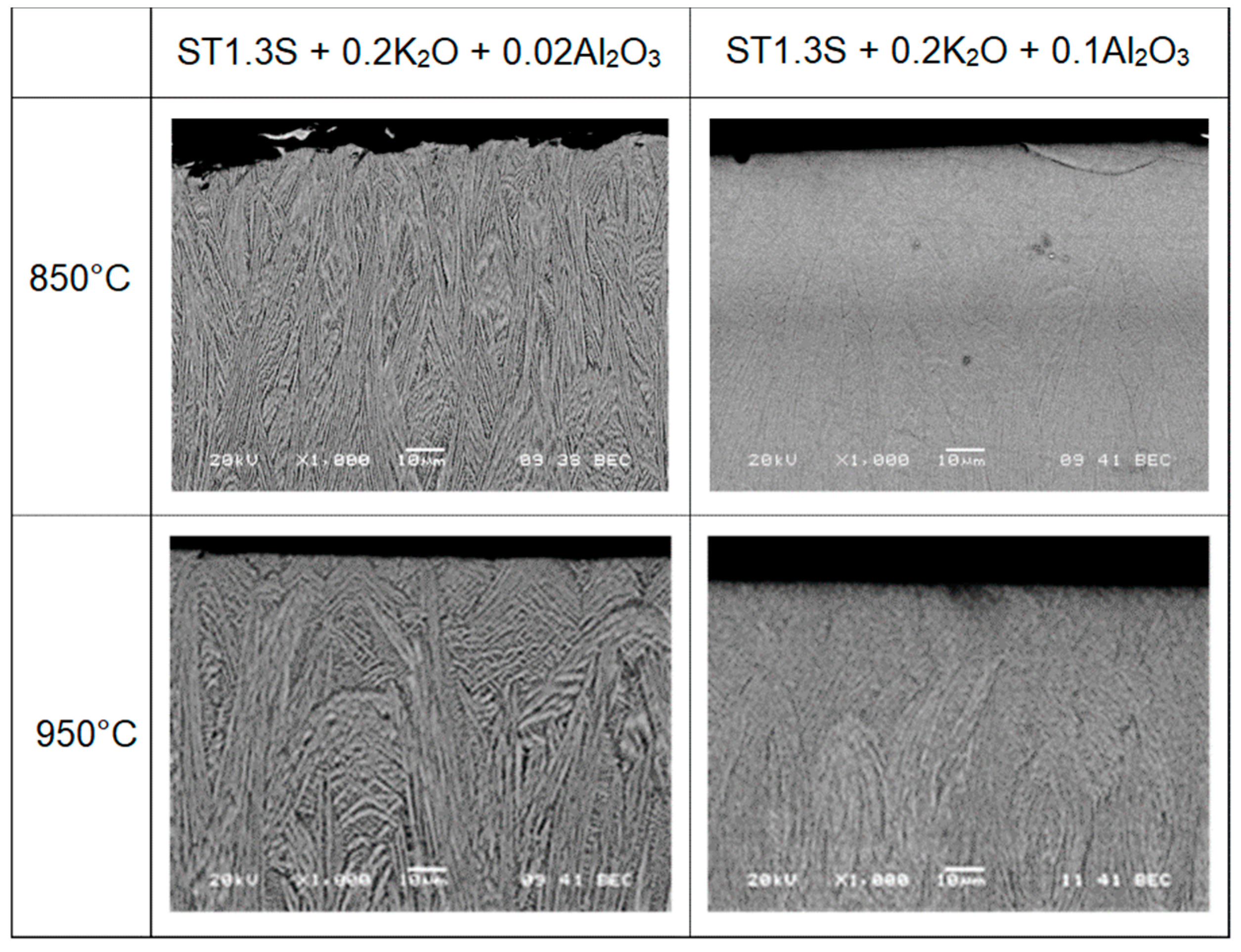
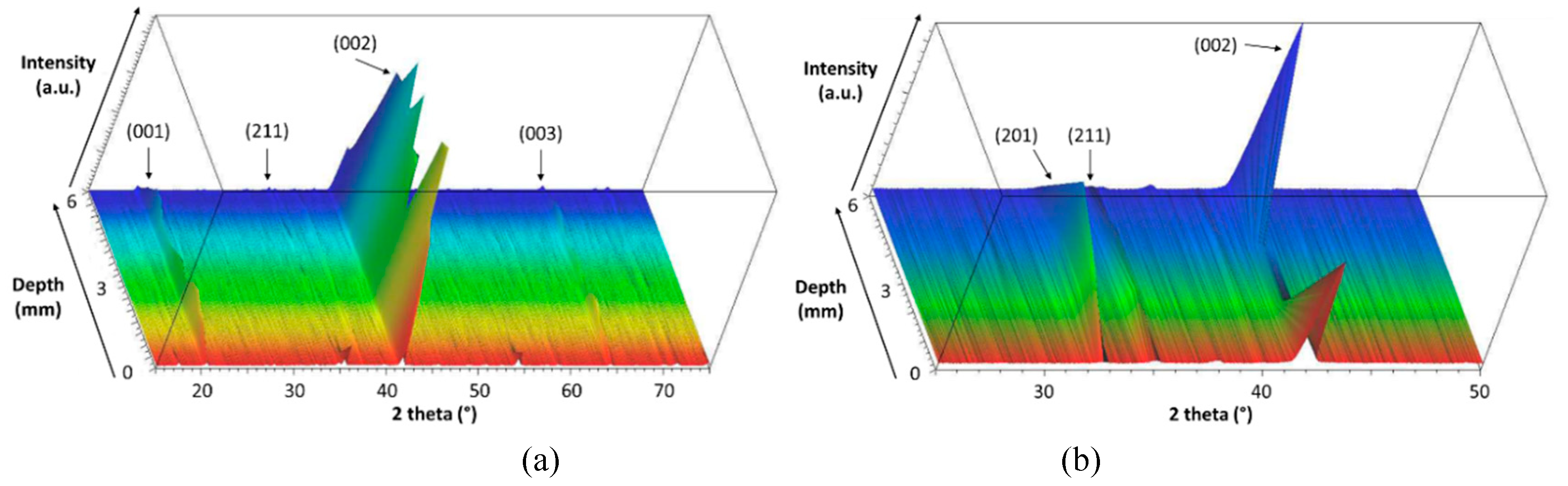
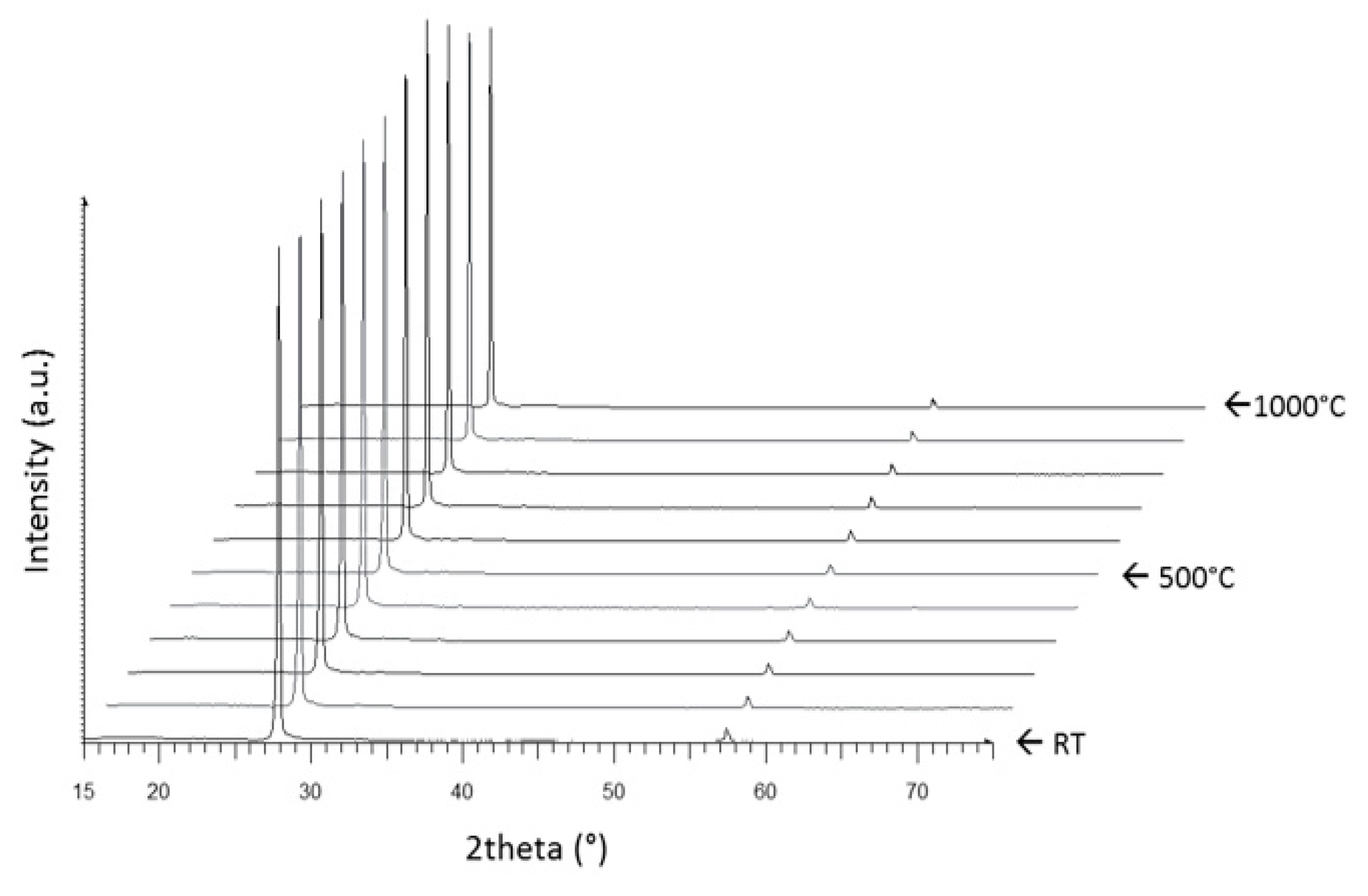
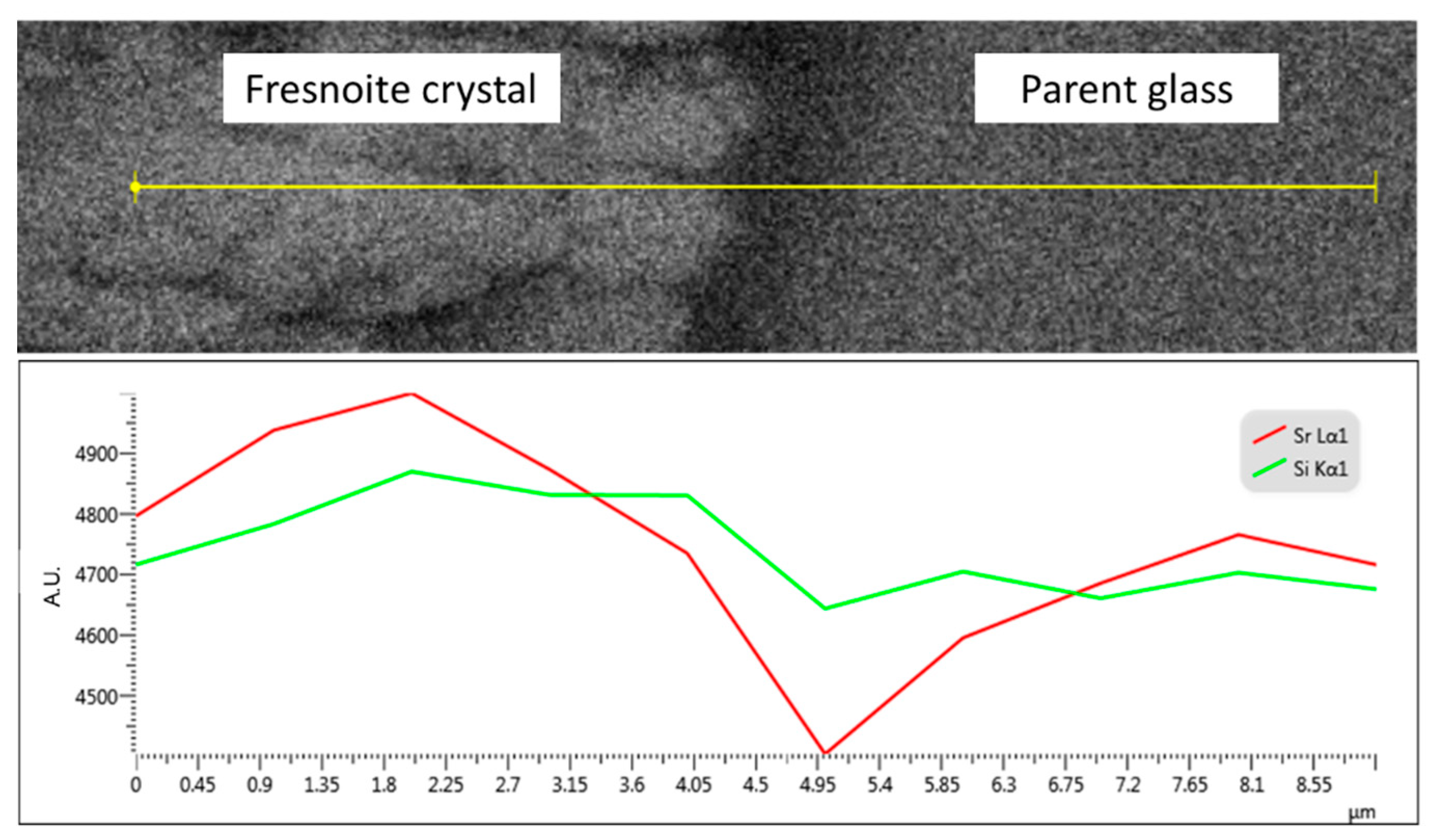
3.1. Crystallization
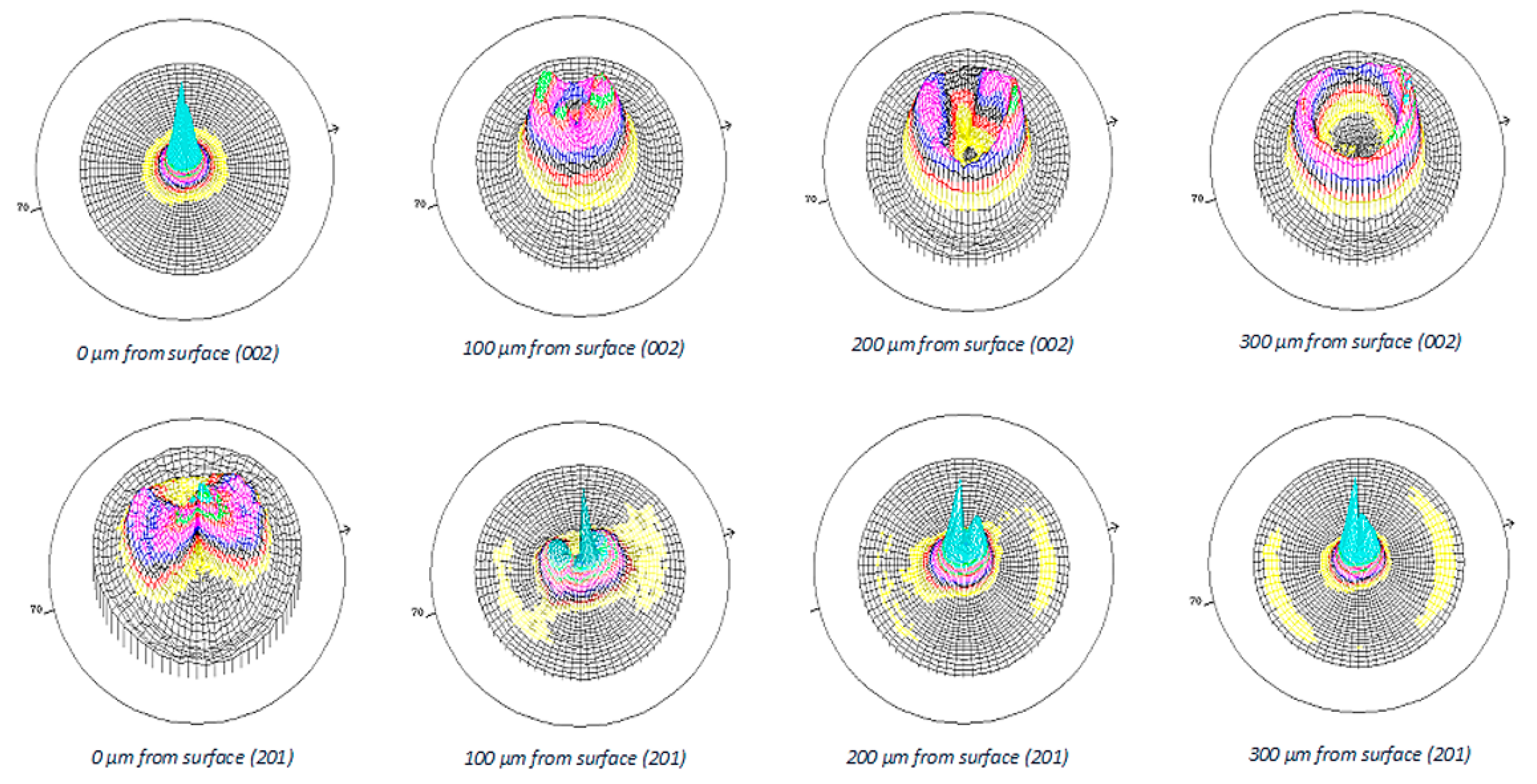
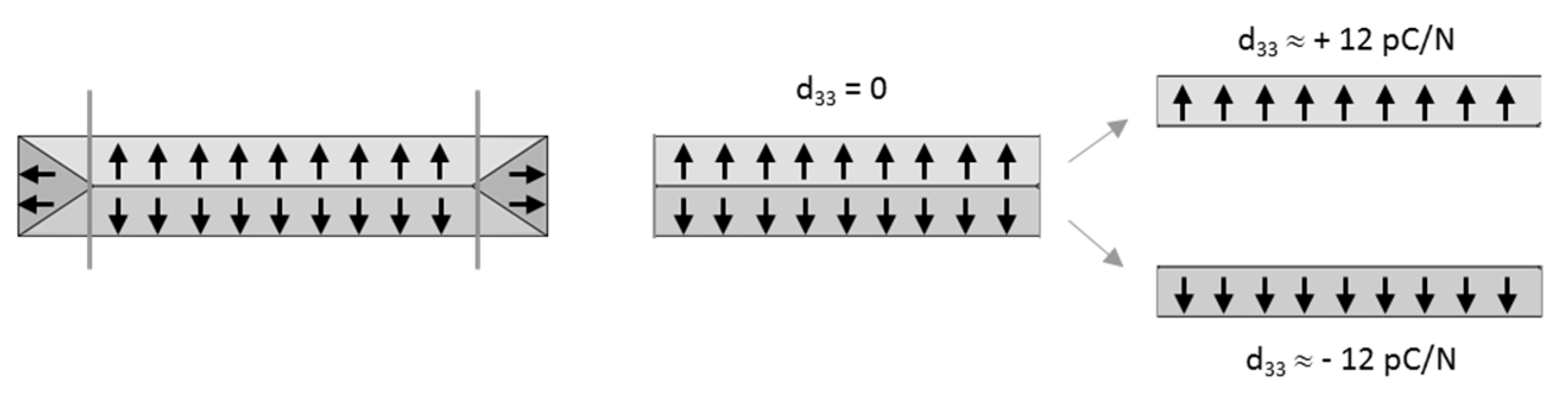
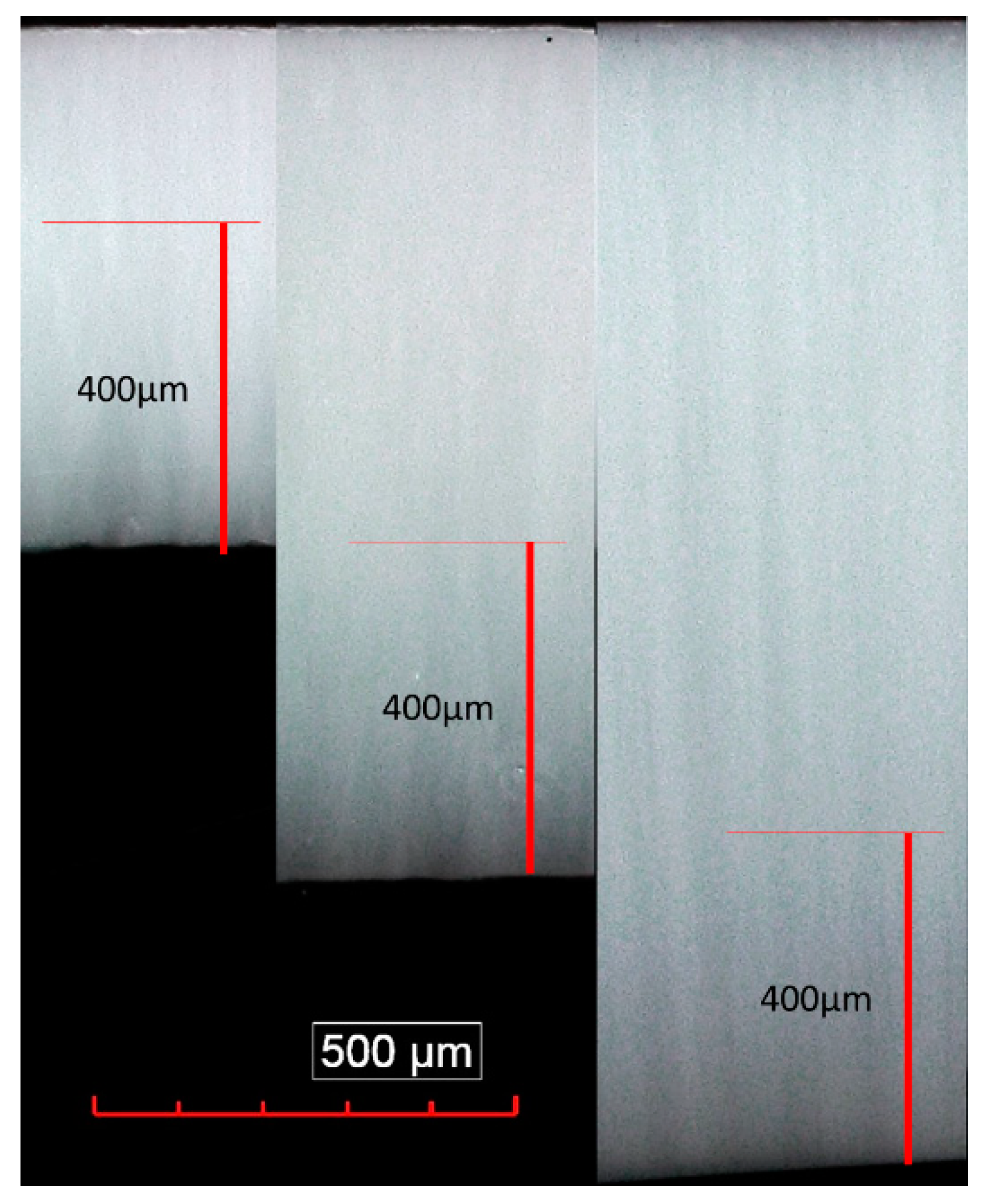
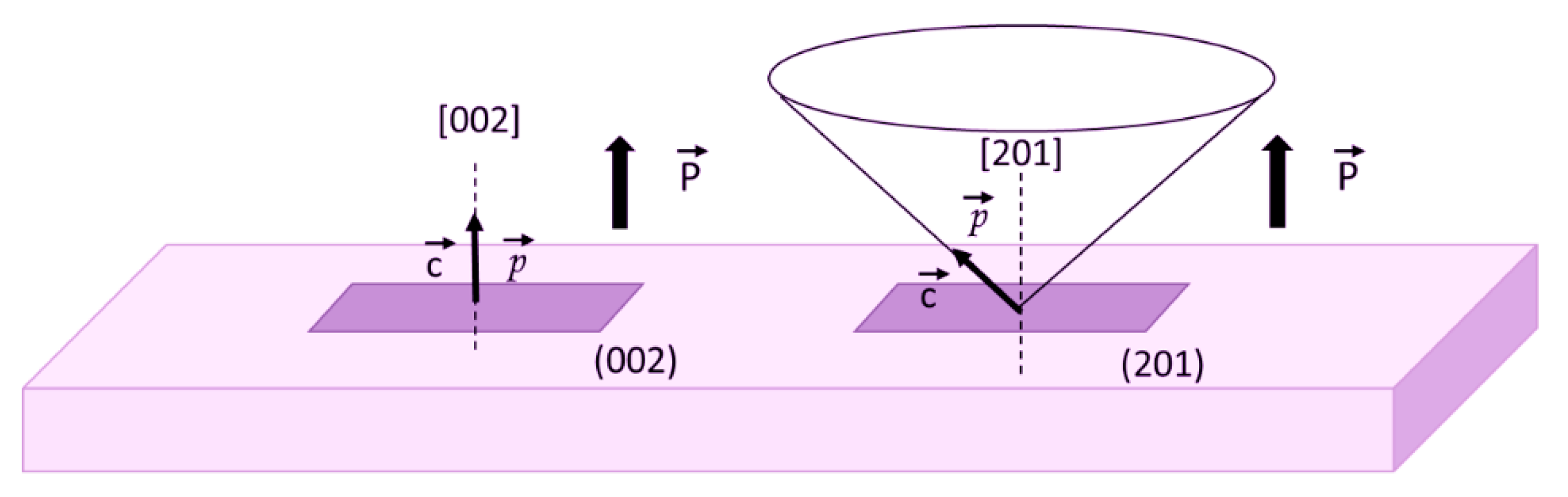
3.2. Preferential Orientation
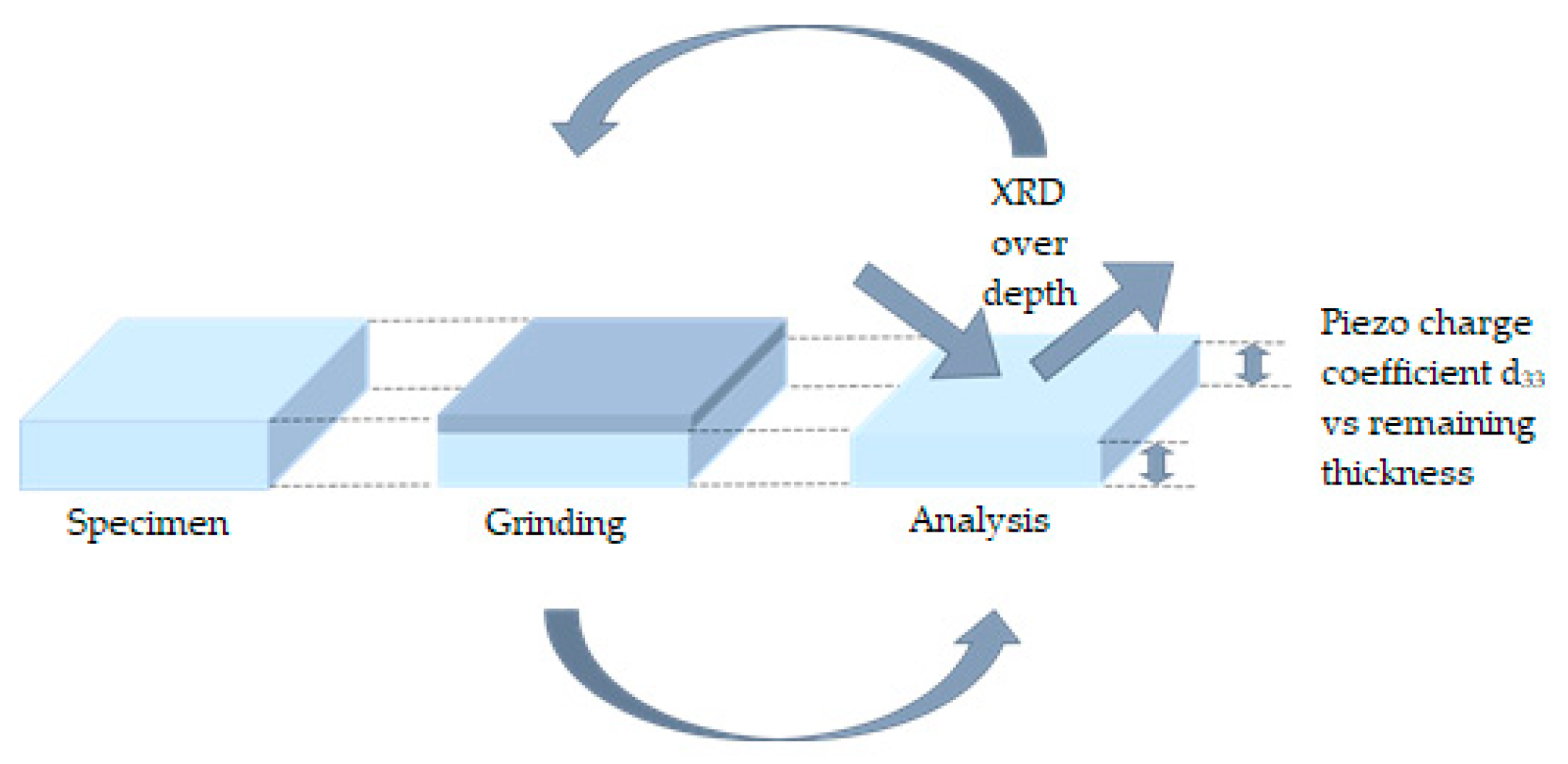
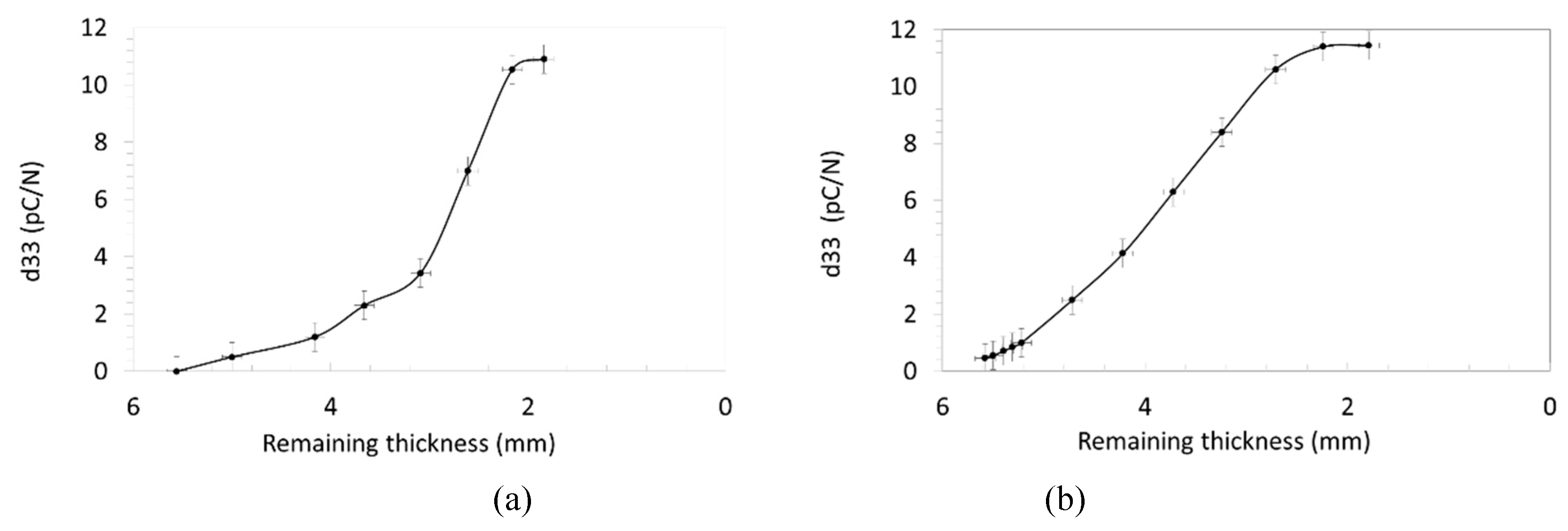
3.3. Piezoelectric Properties
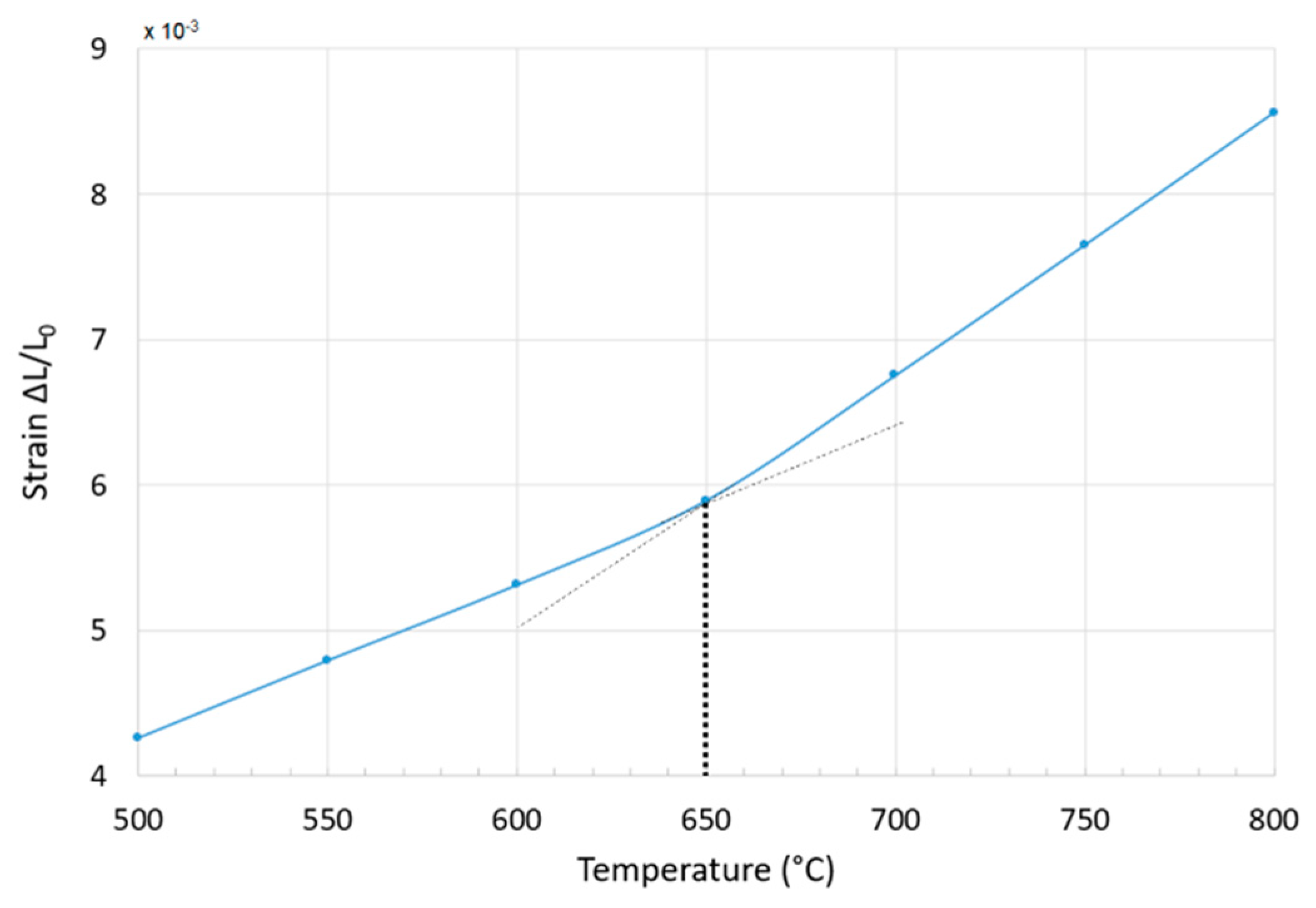
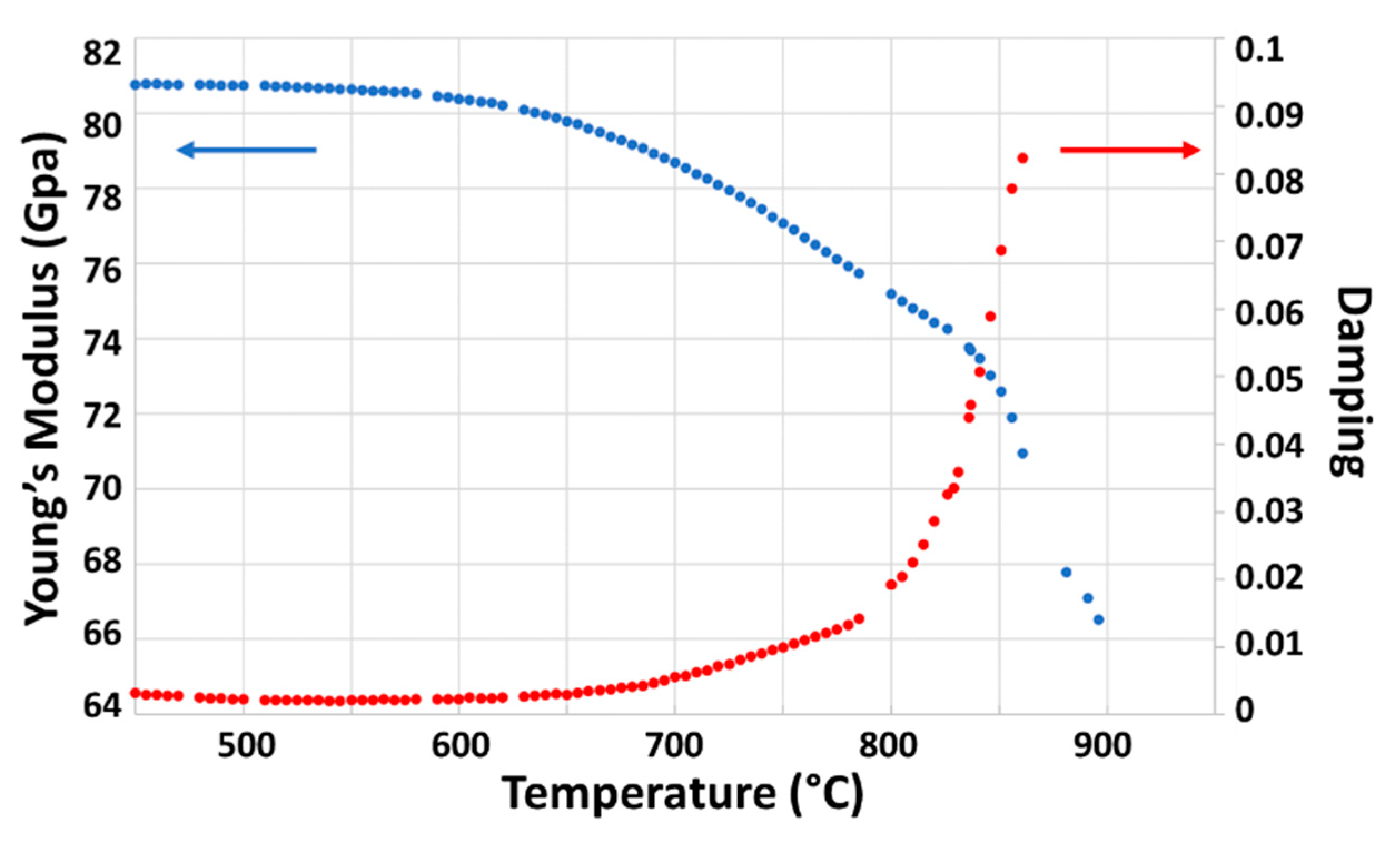
3.4. Stability at High Temperatures of ST1.3S + 0.2K2O + 0.1Al2O3 Glass-Ceramic
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabral, A.A.; Fokin, V.M.; Zanotto, E.D.; Chinaglia, C.R. Nanocrystallization of fresnoite glass. I. Nucleation and growth kinetics. J. Non-Cryst. Solids 2003, 330, 174–186. [Google Scholar] [CrossRef]
- Höche, T.; Neumann, W.; Esmaeilzadeh, S.; Uecker, R.; Lentzen, M.; Rüssel, C. The Crystal Structure of Sr2TiSi2O8. J. Solid State Chem. 2002, 166, 15–23. [Google Scholar] [CrossRef]
- Ochi, Y. Fresnoite crystal structure in glass-ceramics. Mater. Res. Bull. 2006, 41, 740–750. [Google Scholar] [CrossRef]
- Halliyal, A.; Bhalla, A.S.; Cross, L.E.; Newnham, R.E. Dielectric, piezoelectric and pyroelectric properties of Sr2TiSi2O8 polar glass-ceramic: A new polar material. J. Mater. Sci. 1985, 20, 3745–3749. [Google Scholar] [CrossRef]
- Schneider, M.; Richter, W.; Keding, R.; Rüssel, C. XPS investigations on coordination and valency of Ti in fresnoite glasses and glass ceramics. J. Non-Cryst. Solids 1998, 226, 273–280. [Google Scholar] [CrossRef]
- Tsuzuku, K.; Taruta, S.; Takusagawa, N.; Kishi, H. Crystallization of 2(Ca,Sr,Ba)O–TiO2–2SiO2 composition glasses. J. Non-Cryst. Solids 2002, 306, 50–57. [Google Scholar] [CrossRef]
- Höche, T.; Keding, R.; Rüssel, C.; Hergt, R. Microstructural Characterization of Grain Oriented Glass Ceramics in the System Ba2TiSiO8SiO2. J. Mater. Sci. 1999, 34, 195–208. [Google Scholar] [CrossRef]
- Ochi, Y.; Meguro, T.; Kakegawa, K. Orientated crystallization of fresnoite glass-ceramics by using a thermal gradient. J. Eur. Ceram. Soc. 2006, 26, 627–630. [Google Scholar] [CrossRef]
- Keding, R.; Rüssel, C. Electrochemical nucleation for the preparation of oriented glass ceramics. J. Non-Cryst. Solids 1997, 219, 136–141. [Google Scholar] [CrossRef]
- Keding, R.; Rüssel, C. Oriented strontium fresnoite glass-ceramics prepared by electrochemically induced nucleation. J. Mater. Sci. 2004, 39, 1433–1435. [Google Scholar] [CrossRef]
- Keding, R.; Rüssel, C. The mechanism of electrochemically induced nucleation in glasses with the composition 2BaO · TiO2 · 2.75SiO2. J. Non-Cryst. Solids 2005, 351, 1441–1446. [Google Scholar] [CrossRef]
- Ding, Y.; Masuda, N.; Miura, Y.; Osaka, A. Preparation of polar oriented Sr2TiSi2O8 films by surface crystallization of glass and second harmonic generation. J. Non-Cryst. Solids 1996, 203, 88–95. [Google Scholar] [CrossRef]
- Rüssel, C. Oriented crystallization of glass. A review. J. Non-Cryst. Solids 1997, 219, 212–218. [Google Scholar] [CrossRef]
- Wisniewski, W.; Thieme, K.; Rüssel, C. Fresnoite glass-ceramics—A review. Progress Mater. Sci. 2018, 98, 68–107. [Google Scholar] [CrossRef]
- Basile, N.; Gonon, M.; Petit, F.; Cambier, F. Processing of a glass ceramic surface by selective focused beam laser treatment. Ceram. Int. 2016, 42, 1720–1727. [Google Scholar] [CrossRef]
- Maury, N.; Cambier, F.; Gonon, M. Bulk crystallisation of (00l) oriented fresnoite Sr2TiSi2O8 in glass-ceramics of the Sr–Ti–Si–K–B–O system. J. Non-Cryst. Solids 2011, 357, 1079–1084. [Google Scholar] [CrossRef]
- Maury, N.; Gonon, M.; Erauw, J.P.; Simons, J.; Cambier, F. Effect of K2O addition on the crystallization of Sr2TiSi2O8 in glass-ceramics of the Sr-Ti-Si-O system. In Proceedings of the 10th European Ceramic Society Conference, Berlin, Germany, 17–21 June 2007; pp. 621–625. [Google Scholar]
- Maury, N.; Cambier, F.; Gonon, M. Influence of the viscosity of the residual glass in glass ceramics containing fresnoite crystals. In Proceedings of the 13th European Inter-Regional Conference on Ceramics, Barcelona, Spain, 12–14 September 2012; pp. 21–24. [Google Scholar]
- Roebben, G.; Bollen, B.; Brebels, A.; van Humbeeck, J.; van der Biest, O. Impulse excitation apparatus to measure resonant frequencies, elastic moduli, and internal friction at room and high temperature. Rev. Sci. Instrum. 1997, 68, 4511–4515. [Google Scholar] [CrossRef]
- Fluegel, A. Global Model for Calculating Room-Temperature Glass Density from the Composition. J. Am. Ceram. Soc. 2008, 90, 2622–2625. [Google Scholar] [CrossRef]
- Wisniewski, W.; Takano, K.; Takahashi, Y.; Fujiwara, T.; Rüssel, C. Microstructure of Transparent Strontium Fresnoite Glass-Ceramics. Sci. Rep. 2015, 5, 9069. [Google Scholar] [CrossRef]
- Wisniewski, W.; Dimitrijevic, J.; Rüssel, C. Oriented Nucleation and Crystal Growth of Sr-Fresnoite (Sr2TiSi2O8) in 2SrO·TiO2·2SiO2 glasses with additional SiO2. CrystEngComm 2018, 20, 3234–3245. [Google Scholar] [CrossRef]
- Patschger, M.; Wisniewski, W.; Rüssel, C. Piezoelectric glass-ceramics produced via oriented growth of Sr2TiSi2O8 fresnoite: Thermal annealing of surface modified quenched glasses. CrystEngComm 2012, 14, 7368–7373. [Google Scholar] [CrossRef]
- Halliyal, A.; Bhalla, A.S.; Newnham, R.E. Polar glass ceramics—A new family of electroceramic materials: Tailoring the piezoelectric and pyroelectric properties. Mater. Res. Bull. 1983, 18, 1007–1019. [Google Scholar] [CrossRef]
- W Wisniewski, M.P.; Rüssel, C. Sr-fresnoite surface crystallization in a 2SrO·TiO2·2.75 SiO2 glass studied by EBSD. CrystEngComm 2012, 14, 5425–5433. [Google Scholar] [CrossRef]
- Davis, M.J.; Vullo, P.; Kocher, M.; Hovhannisyan, M.; Letz, M. Piezoelectric glass-ceramic for high-temperature applications. J. Non-Cryst. Solids 2018, 501, 159–166. [Google Scholar] [CrossRef]

| ST1.3S | Al/Si | ρpg (Archi.) ± 0.01 g/cm3 | Tg ± 2 °C |
|---|---|---|---|
| + 0.2K2O | 0.0000 | 3.41 | 712 |
| + 0.2K2O + 0.02Al2O3 | 0.0061 | 3.41 | 716 |
| + 0.2K2O + 0.05Al2O3 | 0.0152 | 3.41 | 713 |
| + 0.2K2O + 0.10Al2O3 | 0.0303 | 3.40 | 712 |
| + 0.2K2O + 0.15Al2O3 | 0.0455 | 3.40 | 720 |
| ST1.3S | Crystallization Speed (mm/h) | Crystallization Speed (µ/s) | ||||
|---|---|---|---|---|---|---|
| 850 °C | 900 °C | 950 °C | 850 °C | 900 °C | 950 °C | |
| + 0.2K2O | 0.06 | 0.36 | 2.6 | 0.017 | 0.010 | 0.72 |
| + 0.2K2O + 0.02Al2O3 | 0.05 | 0.33 | 2.1 | 0.014 | 0.092 | 0.58 |
| + 0.2K2O + 0.05Al2O3 | 0.06 | 0.30 | 1.4 | 0.017 | 0.083 | 0.39 |
| + 0.2K2O + 0.10Al2O3 | 0.04 | 0.23 | 1.3 | 0.011 | 0.064 | 0.36 |
| + 0.2K2O + 0.15Al2O3 | 0.04 | 0.19 | 1.2 | 0.011 | 0.053 | 0.33 |
| Residual Glass 1.3SiO2-0.2K2O-yAl2O3 | Glass-Ceramic | |||||||
|---|---|---|---|---|---|---|---|---|
| ST1.3S | Al/Si | mrg wt% | ρrg (Fluegel) ±0.02 g/cm3 | ρrg (Archi.) ±0.01 g/cm3 | vrg ±0.3 vol% | Tg ±2 °C | ρth ±0.02 g/cm3 | ρgc (Archi.) ±0.01 g/cm3 |
| + 0.2K2O | 0 | 19.2 | 2.33 | - | 28.4 | 605 | 3.44 | 3.43 |
| + 0.2K2O + 0.02Al2O3 | 0.0154 | 19.6 | 2.33 | - | 28.8 | 610 | 3.43 | 3.43 |
| + 0.2K2O + 0.05Al2O3 | 0.0385 | 20.0 | 2.34 | - | 29.4 | 624 | 3.43 | 3.42 |
| + 0.2K2O + 0.10Al2O3 | 0.0769 | 20.8 | 2.35 | 2.34 | 30.3 | 640 | 3.42 | 3.40 |
| + 0.2K2O + 0.15Al2O3 | 0.1154 | 21.6 | 2.37 | - | 31.1 | 652 | 3.41 | 3.39 |
| 850 °C | 900 °C | 950 °C | ||||
|---|---|---|---|---|---|---|
| ST1.3S | Surf | −0.3 mm | Surf | −0.3 mm | Surf | −0.3 mm |
| + 0.2K2O | 0.97 | 0.05 | 1 | 0.02 | 0.99 | 0 |
| + 0.2K2O + 0.02 Al2O3 | 0.98 | 0.1 | 1 | 0.01 | 0.98 | 0.01 |
| + 0.2K2O + 0.05 Al2O3 | 1 | 0.1 | 0.99 | 0.02 | 1 | 0.05 |
| + 0.2K2O + 0.10 Al2O3 | 1 | 1 | 0.99 | 0.96 | 0.99 | 0.3 |
| + 0.2K2O + 0.15 Al2O3 | 1 | 1 | 0.97 | 1 | 1 | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renoirt, M.-S.; Maury, N.; Dupla, F.; Gonon, M. Structure and Properties of Piezoelectric Strontium Fresnoite Glass-Ceramics Belonging to the Sr–Ti–Si–Al–K–O System. Ceramics 2019, 2, 86-97. https://doi.org/10.3390/ceramics2010008
Renoirt M-S, Maury N, Dupla F, Gonon M. Structure and Properties of Piezoelectric Strontium Fresnoite Glass-Ceramics Belonging to the Sr–Ti–Si–Al–K–O System. Ceramics. 2019; 2(1):86-97. https://doi.org/10.3390/ceramics2010008
Chicago/Turabian StyleRenoirt, Marie-Sophie, Nathalie Maury, Florian Dupla, and Maurice Gonon. 2019. "Structure and Properties of Piezoelectric Strontium Fresnoite Glass-Ceramics Belonging to the Sr–Ti–Si–Al–K–O System" Ceramics 2, no. 1: 86-97. https://doi.org/10.3390/ceramics2010008
APA StyleRenoirt, M.-S., Maury, N., Dupla, F., & Gonon, M. (2019). Structure and Properties of Piezoelectric Strontium Fresnoite Glass-Ceramics Belonging to the Sr–Ti–Si–Al–K–O System. Ceramics, 2(1), 86-97. https://doi.org/10.3390/ceramics2010008






