Preparation of Electrically Conductive Calcium Phosphate Composite Foams by Particle-Stabilized Emulsion Route
Abstract
:1. Introduction
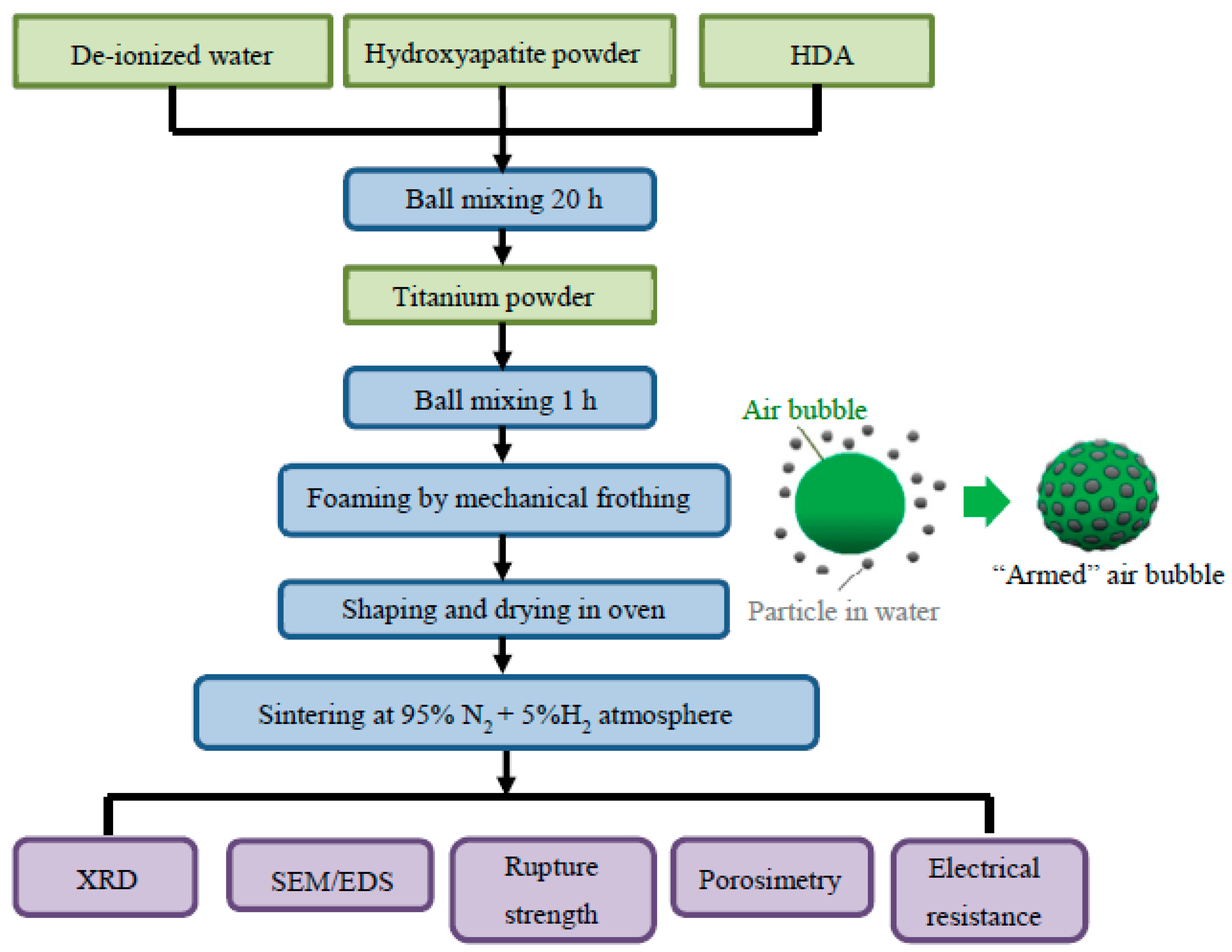
2. Materials and Methods
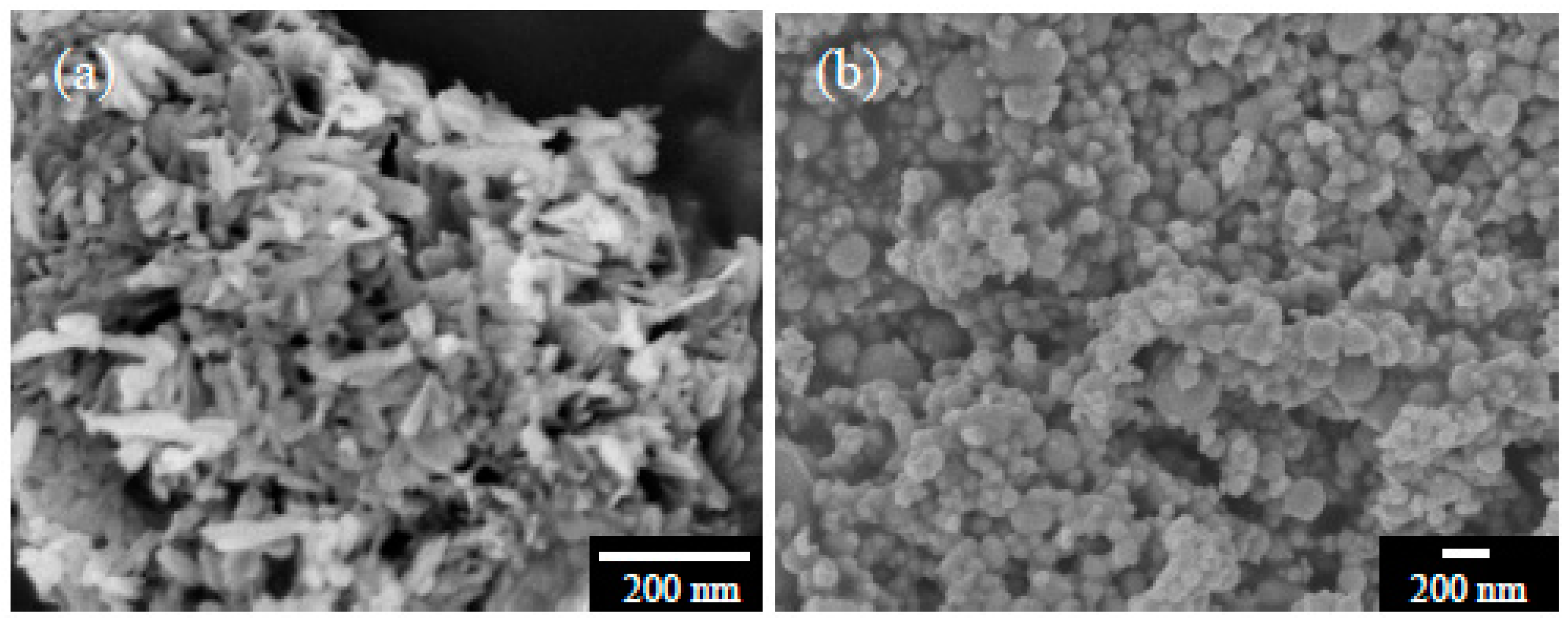
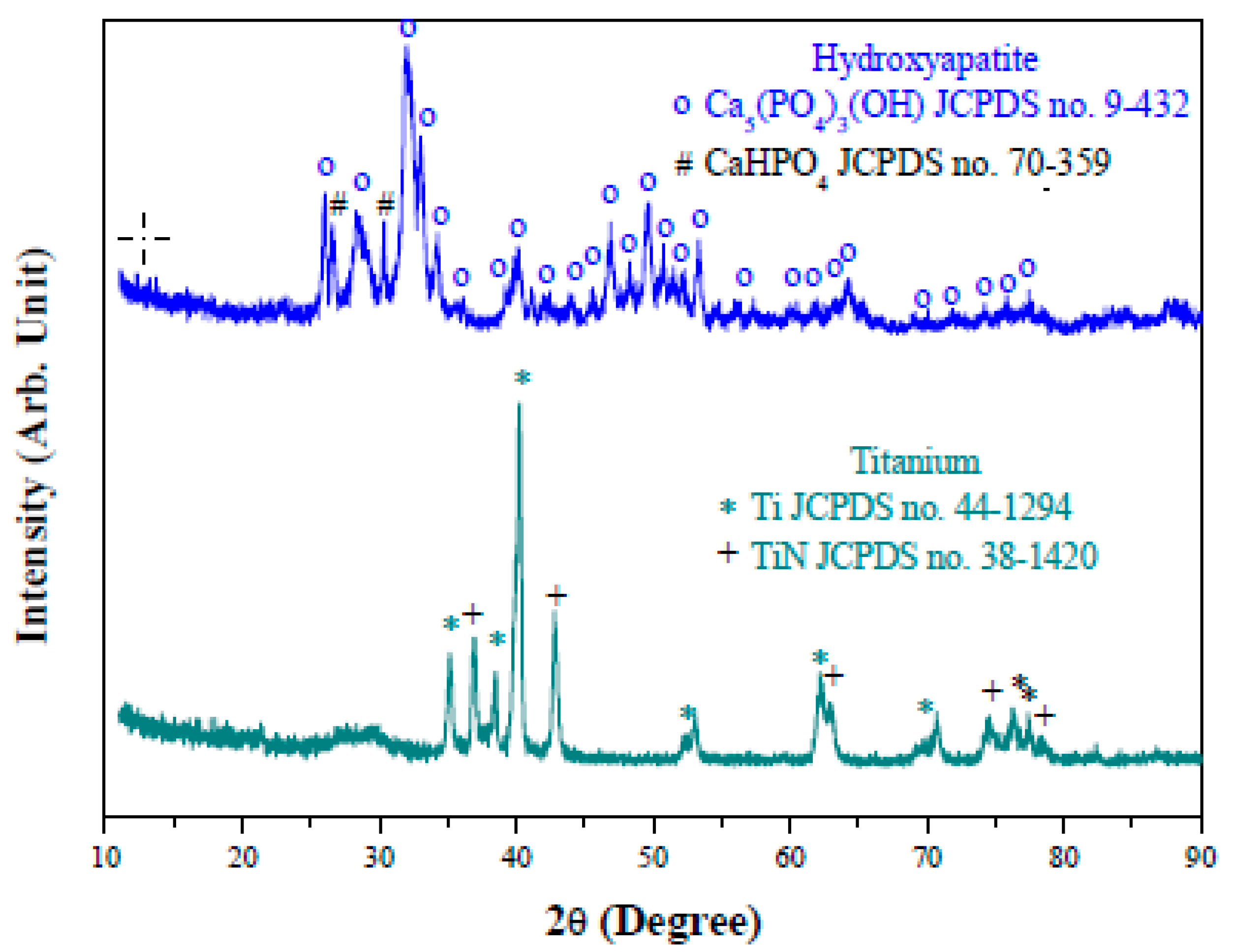
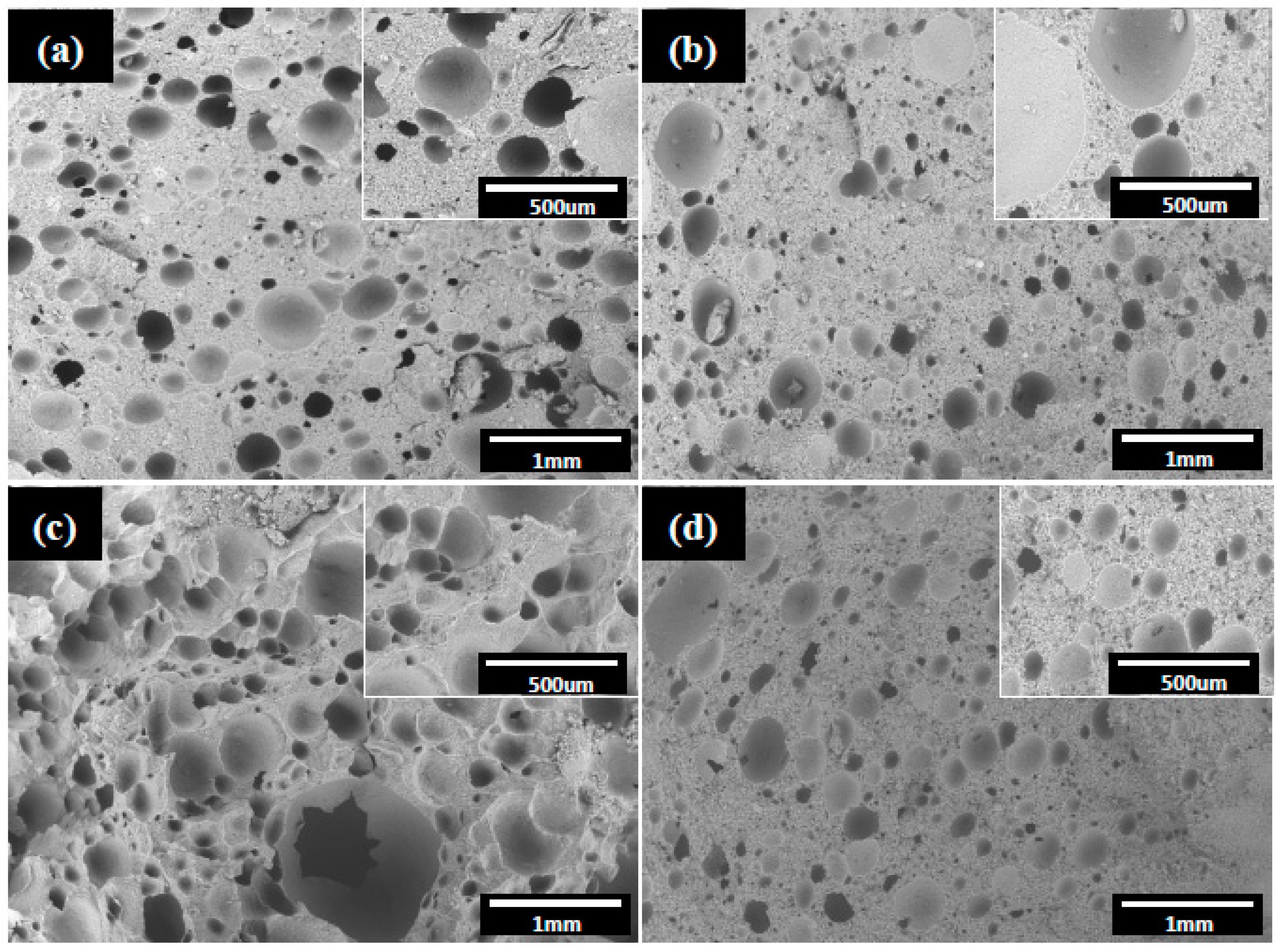
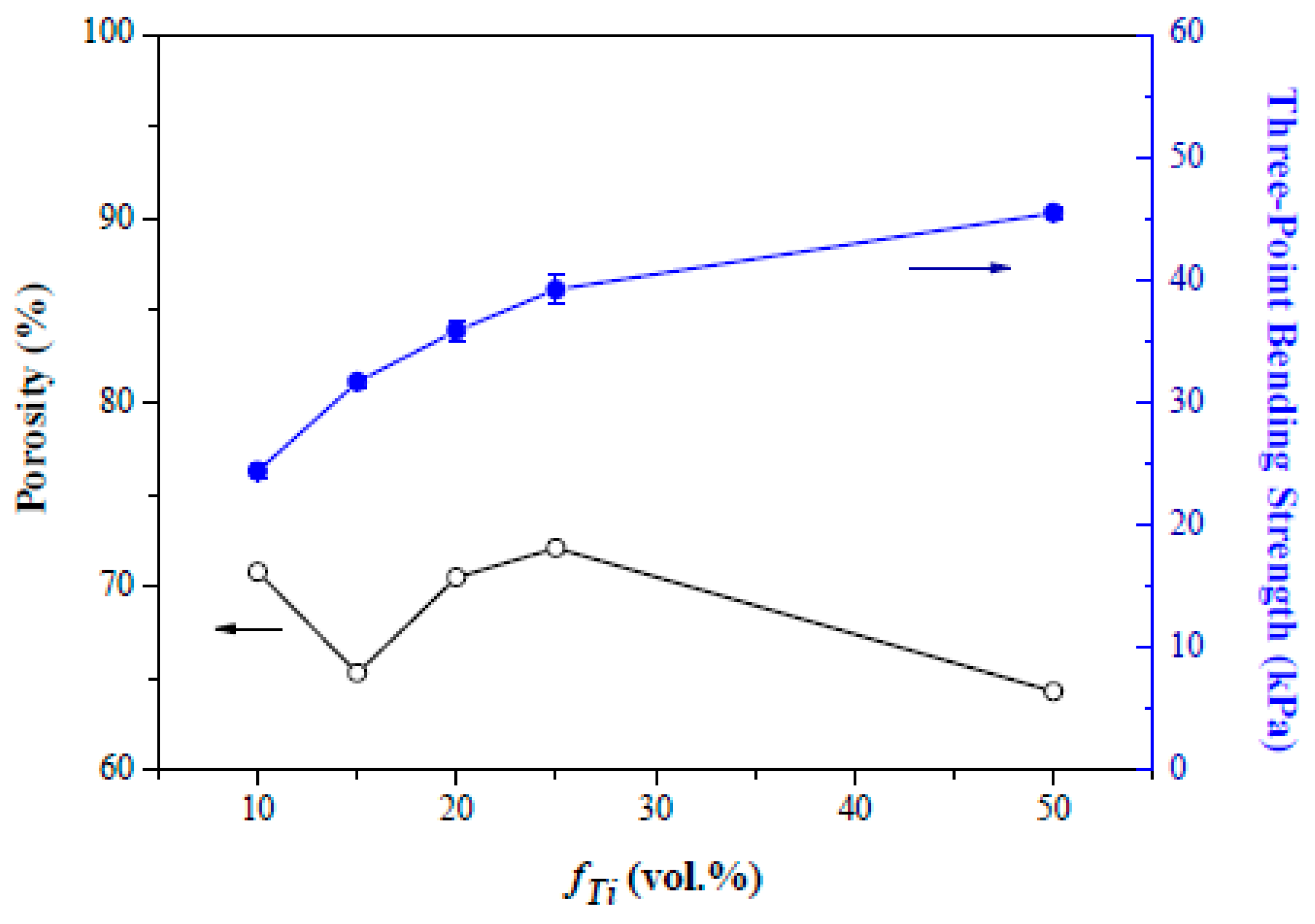
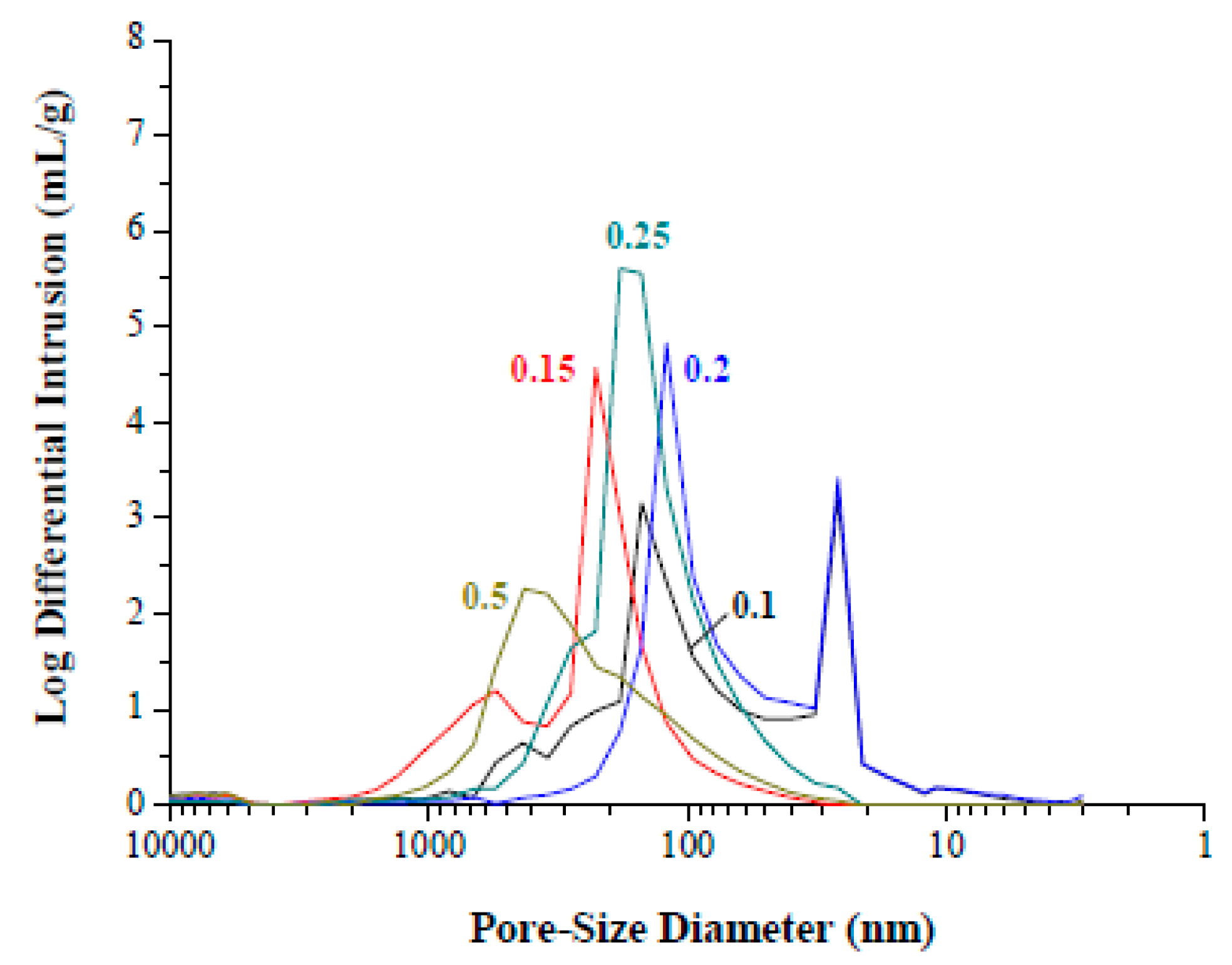
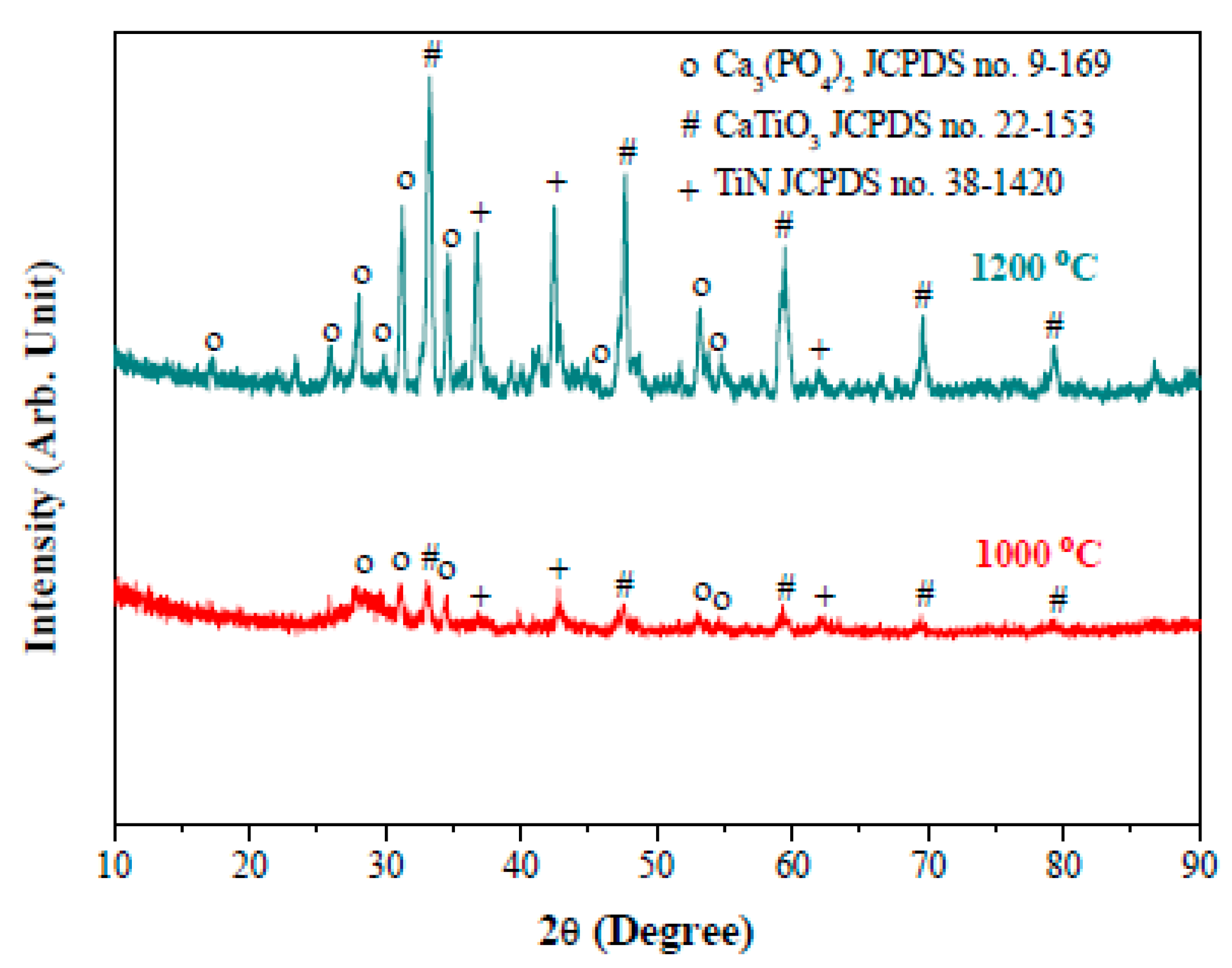
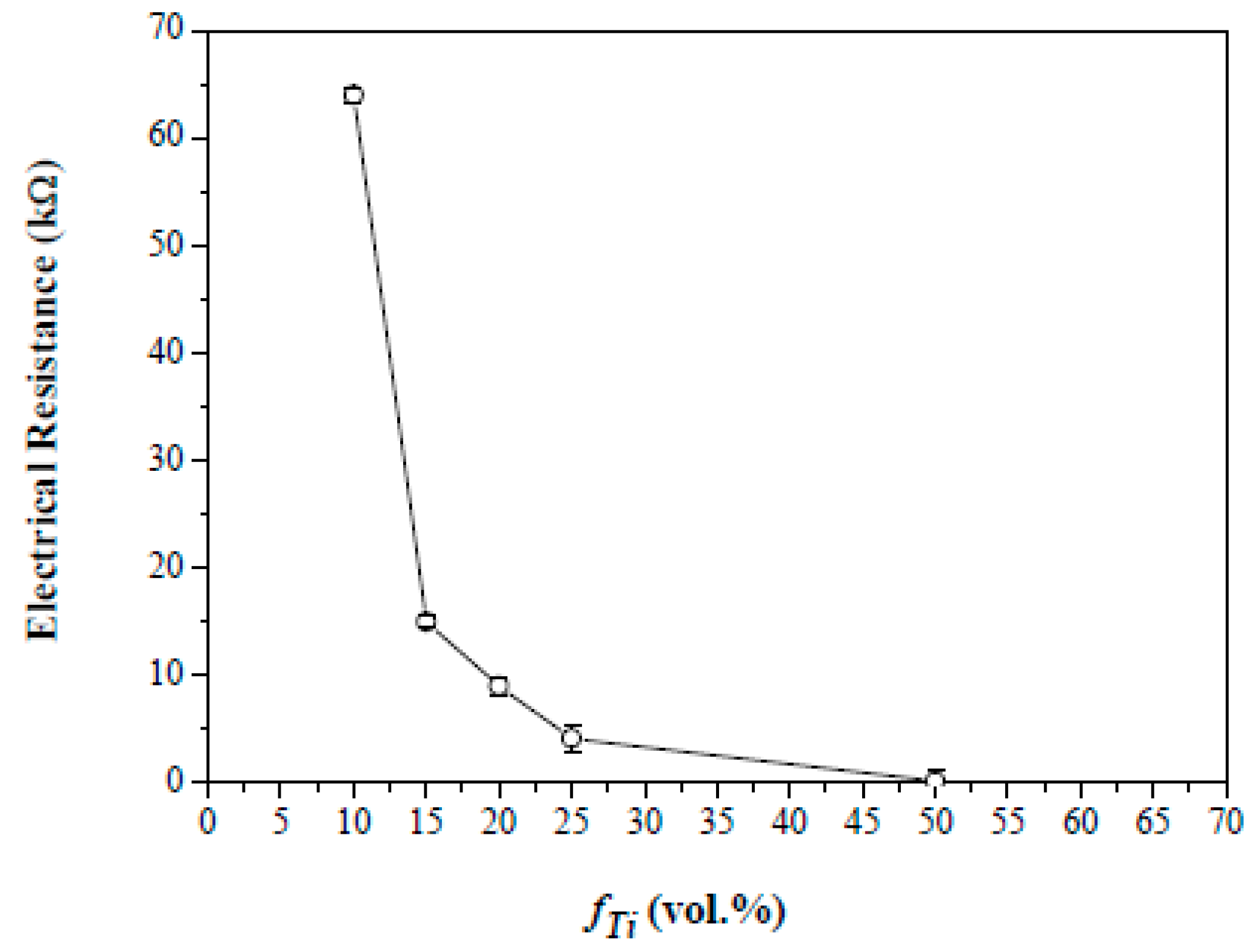
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Gauthier, O.; Sourice, S.; Pilet, P.; Rethore, G.; Khairoun, K.; Bouler, J.-M.; Tancret, F.; Weiss, P. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: Syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2016, 31, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Traykova, T.; Planell, J.A. Calcium phosphate cements: Competitive drug carriers for the musculoskeletal system? Biomaterials 2006, 27, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanchenko, L.A.; Pinchuk, N.D. Making calcium phosphate biomaterials. Powder Metall. Met. Ceram. 2003, 42, 357–371. [Google Scholar] [CrossRef]
- Varma, H.K.; Yokogawa, Y.; Espinosa, F.F.; Kawamoto, Y.; Nishizawa, K.; Nagata, F.; Kameyama, T. Porous calcium phosphate coating over phosphorylated chitosan film by a biomimetic method. Biomaterials 1999, 20, 879–884. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; De Wijn, J.R.; Li, J.; Layrolle, P.; De Groot, K. Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Eng. 2003, 9, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Ramay, H.R.R.; Zhang, M. Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials 2004, 25, 5171–5180. [Google Scholar] [CrossRef] [PubMed]
- Sarin, P.; Lee, S.-J.; Apostolov, Z.D.; Kriven, W.M. Porous biphasic calcium phosphate scaffolds from cuttlefish bone. J. Am. Ceram. Soc. 2011, 94, 2362–2370. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Simon, C.G., Jr. Fast setting calcium phosphate–chitosan scaffold: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, D.; Lu, B.; Tang, Y.; Wang, C.; Wang, Z. Fabrication of a calcium phosphate scaffold with a three dimensional channel network and its application to perfusion culture of stem cells. Rapid Prototyping J. 2007, 13, 99–106. [Google Scholar] [CrossRef]
- Wilson, C.E.; van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J.A.; de Bruijn, J.D. Scaffolds with a standardized macro-architecture fabricated from several calcium phosphate ceramics using an indirect rapid prototyping technique. J. Mater. Sci. Mater. Med. 2011, 22, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, E.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, C.F.; Perera, F.H.; Marote, A.; Ferreira, S.; Vieira, S.I.; Olhero, S.; Miranda, P.; Ferreira, J.M.F. Biphasic calcium phosphate scaffolds fabricated by direct write assembly: Mechanical, anti-microbial and osteoblastic properties. J. Eur. Ceram. Soc. 2017, 37, 359–368. [Google Scholar] [CrossRef]
- Choi, W.-Y.; Kim, H.-E.; Moon, Y.-W.; Shin, K.-H.; Koh, Y.-H. Production of porous calcium phosphate (CaP) ceramics with aligned pores using ceramic/camphene-based co-extrusion. Biomater. Res. 2015, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-K.; Moon, Y.-W.; Maeng, W.-Y.; Koh, Y.-H.; Kim, H.-E. Design and production of continuously gradient macro/microporous calcium phosphate (CaP) scaffolds using ceramic/camphene-based 3D extrusion. Materials 2017, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Saiz, E.; Tomsia, A.P. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 5480–5489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interf. Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Liu, X.; Okada, M.; Maeda, H.; Fujii, S.; Furuzono, T. Hydroxyapatite/biodegradable poly(L-lactide–co-e-caprolactone) composite microparticles as injectable scaffolds by a Pickering emulsion route. Acta Biomater. 2011, 7, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gu, X.; Yang, Y.; Huang, J.; Hu, M.; Chen, W.; Tong, Z.; Wang, C. Facile fabrication of poly(L-lactic acid)-grafted hydroxyapatite/poly(lactic-co-glycolic acid) scaffolds by Pickering high internal phase emulsion templates. ACS Appl. Mater. Interf. 2014, 6, 17166–17175. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hu, Y.; Wang, C.; Binks, B.P. Fabrication of hierarchical macroporous biocompatible scaffolds by combining Pickering high internal phase emulsion templates with three-dimensional printing. ACS Appl. Mater. Interf. 2017, 9, 22950–22958. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.J.; Hsu, K.-T. Macroporous ZrO2/Ti composite foams by aqueous gelcasting of particle-stabilized emulsions. Adv. Powder Technol. 2016, 27, 839–844. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Chen, C.-W.; Hsieh, J.-H.; Tseng, W.J. Preparation of Ag/TiO2 composite foams via Pickering emulsion for bactericide and photocatalysis. Ceram. Int. 2017, 43, S797–S801. [Google Scholar] [CrossRef]
- Tseng, W.J.; Lin, P.-T. Effect of solids concentration on TiO2/Ni composite foams prepared by aqueous gelcasting of particle-stabilized emulsions. J. Eur. Ceram. Soc. 2017, 37, 5265–5272. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Narkevica, I.; Stipniece, L.; Jakobsons, E.; Cakstina, I.; Ozolins, J. Electrically active and 3D porous TiO2-x ceramic scaffolds for bone tissue regeneration. J. Eur. Ceram. Soc. 2017, 37, 833–840. [Google Scholar] [CrossRef]
- Jhao, J.-J.; Yang, M.-H.; Tseng, W.J. Effect of solids concentration on pore structure of ZnO-foams prepared by particle-stabilized foaming route. Ceram. Int. 2014, 40, 4649–4654. [Google Scholar] [CrossRef]
- Tseng, W.J.; Wu, P.-S. Effect of sodium dodecyl sulfate on stability of gibbsite platelet stabilized emulsion foams. Ceram. Int. 2012, 38, 2711–2718. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, W.J.; Kao, W.-H. Preparation of Electrically Conductive Calcium Phosphate Composite Foams by Particle-Stabilized Emulsion Route. Ceramics 2018, 1, 319-328. https://doi.org/10.3390/ceramics1020025
Tseng WJ, Kao W-H. Preparation of Electrically Conductive Calcium Phosphate Composite Foams by Particle-Stabilized Emulsion Route. Ceramics. 2018; 1(2):319-328. https://doi.org/10.3390/ceramics1020025
Chicago/Turabian StyleTseng, Wenjea J., and Wuei-Hung Kao. 2018. "Preparation of Electrically Conductive Calcium Phosphate Composite Foams by Particle-Stabilized Emulsion Route" Ceramics 1, no. 2: 319-328. https://doi.org/10.3390/ceramics1020025
APA StyleTseng, W. J., & Kao, W.-H. (2018). Preparation of Electrically Conductive Calcium Phosphate Composite Foams by Particle-Stabilized Emulsion Route. Ceramics, 1(2), 319-328. https://doi.org/10.3390/ceramics1020025




