Decision Support System in Dynamic Pricing of Horticultural Products Based on the Quality Decline Due to Bacterial Growth
Abstract
:1. Introduction
2. Brief Literature Review on Predictive Microbiology
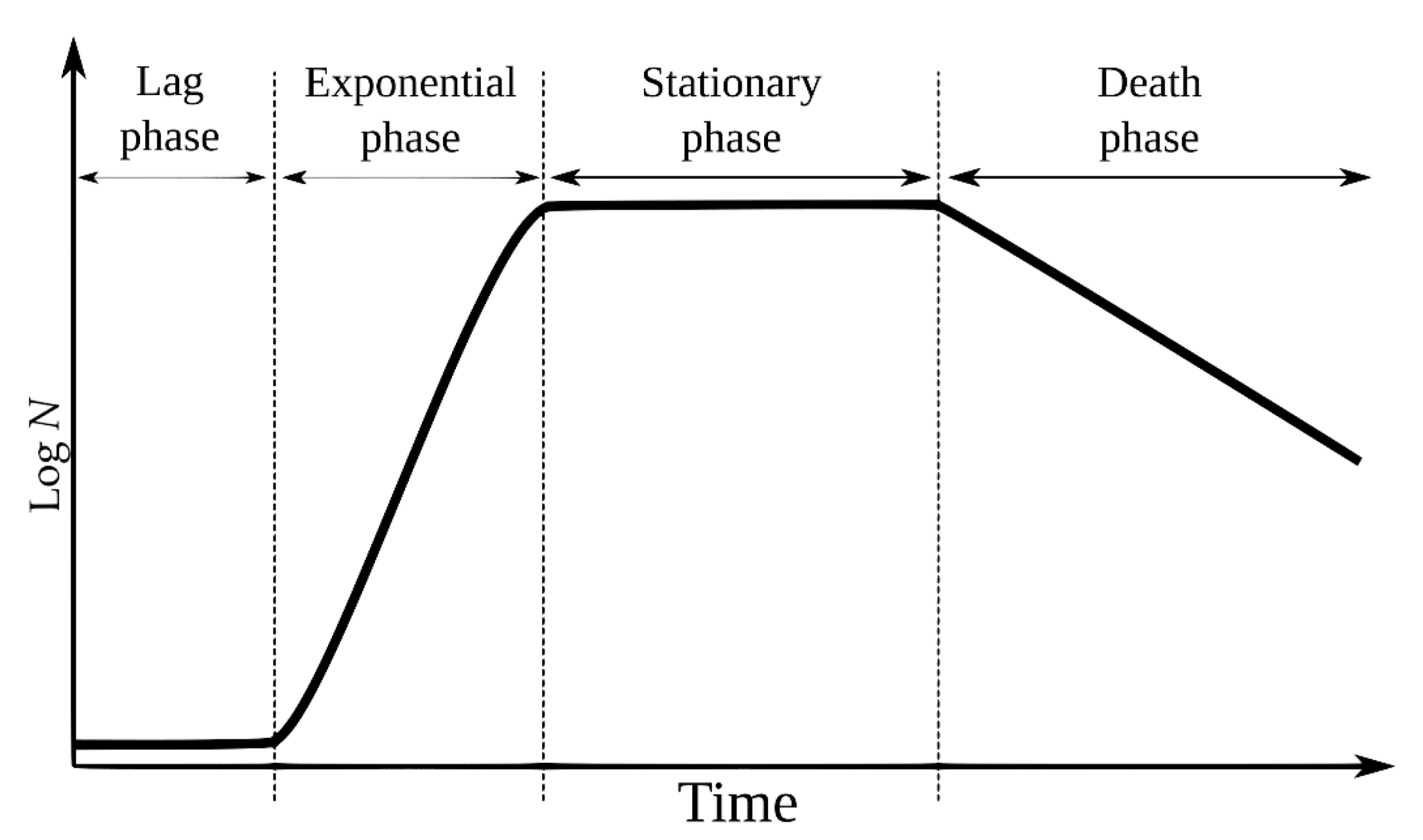
- 1.
- Lag or latency phase, characterized by the absence of microbial replication. The cells adapt to the environment, rich in nutrients, in order to initiate replication;
- 2.
- Logarithmic or exponential phase, characterized by accelerated cell division;
- 3.
- Stationary phase, characterized by the decline of the metabolic rate due to the depletion of nutrients in the environment;
- 4.
- Death or logarithmic decline phase, characterized by the exponential decline of living cells and their growth.
- Primary models, in which variations of environmental factors are not considered. They only describe the concentration of microorganisms as a function of time;
- Secondary models, which consider variations in environmental factors, such as temperature (T), pH (potential of hydrogen) or water activity (aw);
- Tertiary models: integrated models enhanced through computational modeling.
3. Methods and Materials
3.1. Shelf Life Prediction Method
3.1.1. Intrinsic and Extrinsic Factors
- Water activity (aw): one of the intrinsic factors of products that are likely to promote microbiological growth, given that bacteria normally grow in environments where water is available. Water activity is defined as the ratio between the vapor pressure of water (pv) in the food product and the saturated vapor pressure of water (psv) at the same temperature, as shown in Equation (4) [11]. Its value ranges from 0 (dry bone) and 1 (saturated water).
- Potential of Hydrogen (pH): The level of acidity or basicity of a food product is measured by using a pH scale, defined as the inverse logarithm of the hydrogen ion activity in a solution given by Equation (6).
- Temperature (T): The extrinsic temperature of a food item, i.e., the temperature of the environment in which it is stored, determines the ability of microbial organisms to multiply. As with pH, these organisms have an ideal temperature range that favors their multiplication. Both the increase and the decrease of the temperature in relation to its optimal value makes the microbial propagation slower [14]. Foodstuffs have their own specific optimal storage temperature, for which deterioration is minimal, including microbial growth, and the state of fullness is maximized. However, retailers must balance the extension of shelf life with the energy demand and the environmental impact of refrigeration systems and procedures, techniques and methods to improve both thermal performance and energy efficiency [17,18,19,20,21]. According to [22], the recommended storage temperature range for the vegetables analyzed is between 0 °C and 4 °C.
3.1.2. Bacterial Growth Prediction Method
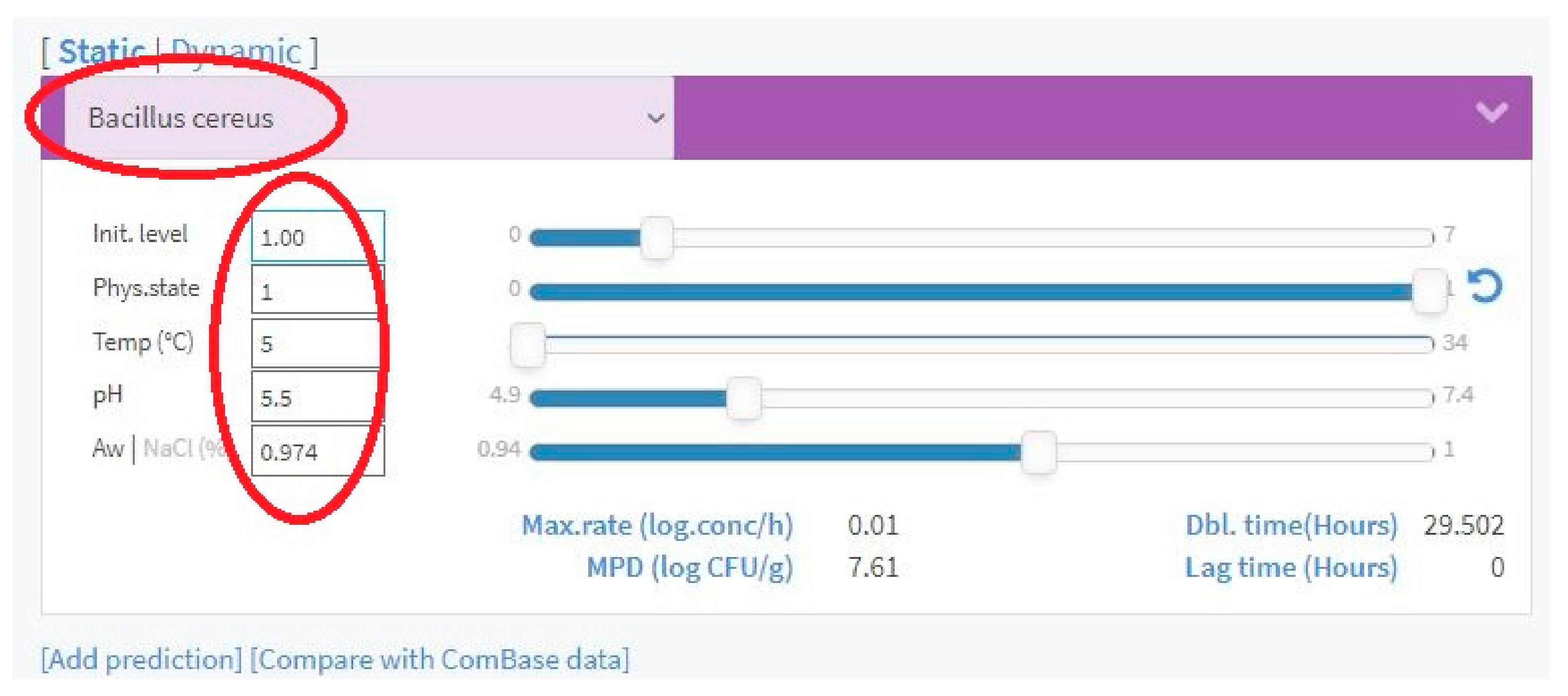
- Three different water activity scenarios, to simulate the effects of the relative humidity of the environment in the increasing bacterial doses. The adopted scenarios relate to the minimum, average, and maximum aw values specific to each vegetable, as shown in Table 1. It is expected that the retailer, as the user of the DSS, would collect the aw value that the horticultural product from a certain batch has when arriving at the final warehouse before being sold. Usually, it is impossible for the retailer to directly collect the product’s aw value. Assuming that equilibrium conditions are reached within a short period [26], the air relative humidity must be collected or measured, making use of the relation between φ and aw shown in Equation (5). Although not direct, this method for aw value estimation is more intuitive, as it does not imply the need for any specific measuring instruments, nor the use of the complex measurement processes that come with it. Thus, it just becomes necessary to have an indicator or a meter that measures the relative humidity of the air in the storage environment. As horticultural products are highly perishable and, as such, stored under modified temperature (and, sometimes, air humidity) conditions due to a mechanical refrigeration system by vapor compression, it is common for these systems to have indicators for air temperature and relative humidity. These should be the values collected by the DSS users. Thus, this method becomes, more desirable to be adopted by the targeted users of the DSS. Since it is aimed at small retailers, it is not expected for them to have significant technical knowledge in the area.
- In a specific vegetable, for each aw scenario (minimum, medium or maximum), the effects, on bacterial growth of combining various temperatures with the range of the intrinsic pH values of each horticultural product are studied. Thus, for every value of storage temperature, separately combined with the individual values of the complete pH range characteristic to the studied vegetable (as shown in Table 2), the number of hours necessary to reach the respective infective doses of the various bacteria in growth according to the prediction simulated in [23] are collected. For a given storage temperature value combined with a specific intrinsic pH value, the registered hourly intervals inherent to the different bacterial species under study are compared. The shortest value is defined as the remaining shelf life of that vegetable for the temperature and pH values and, ultimately, for aw under study. This assumption is made that after a food item reaches the infective dose of a certain bacteria, its safety is compromised, even if the infective doses of the remaining growing bacteria still take a considerable time to be reached. The temperature range under study starts at the minimum value for which bacterial growth is already verified and goes up to the temperature value at which, for a particular bacterium under study, the registered time intervals, for the full range of inherent pH, are all less than 24 h. Thus, from vegetable to vegetable, the temperature range under study may differ at its beginning and/or at its end.
- The adopted method performs a temporal prediction until bacterial contamination in a vegetable under analysis is reached, at which its safety is compromised. However, bacterial doses of food can be reduced through heat treatment processes applied when cooking the food item. In those cases, food security is once again represented, meaning that food may be consumed. This limitation becomes especially relevant when evaluating a batch of potatoes or cabbage that are horticultural products typically consumed in a cooked state. Therefore, it is considered that the time period calculated by the DSS taken as the remaining shelf life of a product represents a maximum commercialization period instead of a maximum consumption period;
- The simulation of the effects of the distribution chain on bacterial growth is given by disregarding lag phase. Thus, it is assumed that bacterial proliferation (the logarithmic phase) begins at the moment that the vegetable is stored at the retailer’s premises (the final stage before being marketed to its final consumer), defined as moment zero;
- Static analysis regarding the environment where vegetables are stored. In other words, there are no exchanges, in matters of extrinsic factors, with the surrounding environment. As a result, there is no values fluctuation in temperature and relative humidity values of the storage atmosphere. Therefore, these extrinsic aspects assume a constant value over time;
3.2. Dynamic Pricing Method
- SP(0): initial selling price;
- SP(tf): cost price;
- SL: linear decrease in price as a function of the studied vegetable estimated shelf life.
3.3. Decision Support System
- Database is constituted by the compilation of the data collected in [23], referring to the time frame until the infective dose of a certain bacterium is reached. The number of remaining hours until the infective dose of the considered bacteria is achieved is successively collected for each of the possible combinations set between aw values, temperature and pH values under study for the vegetable under analysis;
- Search Engine includes all the mechanisms necessary to the processes of database searching, processing, and returning the respective treated information to the user, as a function of the inputs given, in terms of the intrinsic and extrinsic conditions of storage and dynamic pricing parameters. All processes developed in this functional area make use of search, information processing and calculation functions inherent to Microsoft Excel: “VLOOKUP”, “MIN”, “INDEX”, “MATCH” or “SEQUENCE”.
- User Interface (UI) serves as the DSS´s mean of interaction and communication with its user. This UI will be the only functional area of the DSS to which user will have access. The remaining components are inaccessible. Here, the necessary inputs in terms of shelf life prediction and price calculation are inserted, and the respective results are displayed.
4. Case Studies
4.1. Case Study 1—Lettuce
4.2. Case Study 2—Onion
4.3. Case Study 3—Carrot
4.4. Case Study 4—Cabbage
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Food and Agriculture—Statistical Yearbook 2020; FAO-Food & Agriculture Organization of the United Nation: Rome, Italy, 2020. [Google Scholar]
- Ross, T.; McMeekin, T.A. Predictive microbiology. Int. J. Food Microbiol. 1994, 23, 241–264. [Google Scholar] [CrossRef]
- McMeekin, T.A.; Bowman, J.; McQuestin, O.; Mellefont, L.; Ross, T.; Tamplin, M. The future of predictive microbiology: Strategic research, innovative applications and great expectations. Int. J. Food Microbiol. 2008, 128, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Esty, J.R.; Meyer, K.F. The heat resistance of the spores of B. botulinus and allied anaerobes. XI. J. Infect. Dis. 1922, 31, 650–663. [Google Scholar] [CrossRef]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Bren, U. Generation times of E. coli prolong with increasing tannin concentration while the lag phase extends exponentially. Plants 2020, 9, 1680. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, I.A.; Bernaerts, K.; Dens, E.J.; Geeraerd, A.H.; Van Impe, J.F. Predictive modelling of the microbial lag phase: A review. Int. J. Food Microbiol. 2004, 94, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Whiting, R.C.; Buchanan, R.L. A classification of models for predictive microbiology. Food Microbiol. 1993, 10, 175–177. [Google Scholar]
- Baranyi, J. Modelling and Parameter Estimation of Bacterial Growth with Distributed Lag Time. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2010. [Google Scholar]
- Barany, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Subramaniam, P.; Wareing, P. The Stability and Shelf Life of Food, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Gaspar, P.D.; Domingues, C.; Gonçalves, L.C.; Andrade, L.P. Avaliação da qualidade e segurança alimentar pela previsão do crescimento microbiano em diferentes condições de conservação. In Proceedings of the V Congreso Ibérico y III Congreso Iberoamericano de Ciencias y Técnicas del Frío, Castellón, Spain, 23–25 September 2009; Sociedad Española de Ciencias y Técnicas del Frío: Murcia, Spain, 2009. [Google Scholar]
- Rahman, M. Handbook of Food Preservation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Chirife, J.; Fontan, C.F. Water activity of fresh foods. J. Food Sci. 1982, 47, 661–663. [Google Scholar] [CrossRef]
- FDA. Bad Bug Book, Foodborne Pathogenic Microorganisms and Natural Toxins, 2nd ed.; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2012.
- Lund, B.; Baird-Parker, A.C.; Gould, G.W. Microbiological Safety and Quality of Food, 1st ed.; Springer: New York, NY, USA, 2000. [Google Scholar]
- Bridges, M.A.; Mattice, M.R. Over two thousand estimations of the pH of representative foods. Dig. Dis. Sci. 1939, 6, 440–449. [Google Scholar] [CrossRef]
- Gaspar, P.D.; Silva, P.D.; Nunes, J.; Andrade, L.P. Characterization of the specific electrical energy consumption of agrifood industries in the central region of Portugal. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2014; Volume 590, pp. 878–882. [Google Scholar]
- Nunes, J.; Silva, P.D.; Andrade, L.P.; Gaspar, P.D. Characterization of the specific energy consumption of electricity in the Portuguese sausage industry. WIT Trans. Ecol. Environ. 2014, 186, 763–774. [Google Scholar]
- Silva, P.D.; Gaspar, P.D.; Nunes, J.; Andrade, L.P. Specific electrical energy consumption and CO2 emissions assessment of agrifood industries in the central region of Portugal. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2014; Volume 675–677, pp. 1880–1886. [Google Scholar]
- Gaspar, J.P.; Gaspar, P.D.; Silva, P.D.; Simões, M.P.; Santo, C.E. Energy Life-Cycle Assessment of Fruit Products—Case Study of Beira Interior’s Peach (Portugal). Sustainability 2018, 10, 3530. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, P.D.; Gonçalves, L.C.C.; Pitarma, R.A. CFD parametric studies for global performance improvement of open refrigerated display cabinets. Model. Simul. Eng. 2012, 2012, 54. [Google Scholar] [CrossRef] [Green Version]
- Mercantila, F. Guide to Food Transport: Fruit and Vegetables; Mercantila Publishers: Copenhagen, Denmark, 1989. [Google Scholar]
- ComBase—Combined Database for Predictive Microbiology. Available online: https://www.combase.cc (accessed on 14 March 2020).
- Gaspar, P.D.; Alves, J.; Pinto, P. Simplified approach to predict food safety through the maximum specific bacterial growth rate as function of extrinsic and intrinsic parameters. ChemEngineering 2021, 5, 22. [Google Scholar] [CrossRef]
- Baptista, P.; Venâncio, A. Os Perigos para a Segurança Alimentar no Processamento de Alimentos; Forvisão-Consultoria em Formação Integrada: Guimarães, Portugal, 2003. [Google Scholar]
- Imre, L. The Measurement of Equilibrium Relative Humidity, Part II. Period. Polytech.-Bp. Univ. Technol. Econ. 1964, 8, 227–242. [Google Scholar]
- Liu, X.; Tang, O.; Huang, P. Dynamic pricing and ordering decision for the perishable food of the supermarket using RFID technology. Asia Pac. J. Mark. Logist. 2008, 20, 7–22. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, Y.S. Optimal dynamic pricing for perishable assets with nonhomogeneous demand. Manag. Sci. 2000, 46, 375–388. [Google Scholar] [CrossRef]
- Rabbani, M.; Zia, N.P.; Rafiei, H. Joint optimal dynamic pricing and replenishment policies for items with simultaneous quality and physical quantity deterioration. Appl. Math. Comput. 2016, 287, 149–160. [Google Scholar] [CrossRef]
- FAO. Prevention of Post-Harvest Food Losses: Fruits, Vegetables and Root Crops—A Training Manual; Food and Agriculture Organization of the United Nations: Rome, Italy, 1989. [Google Scholar]
- Engineering ToolBox—Fruits and Vegetables Optimal Storage Conditions. Available online: https://www.engineeringtoolbox.com/fruits-vegetables-storage-conditions-d_710.html (accessed on 15 March 2020).
- USDA—Commercial Item Description: Onions, Bulb, Ready-to-Use. Available online: https://www.ams.usda.gov/sites/default/files/media/A-A-20193D_Onions_Bulb_RTU.pdf (accessed on 22 April 2021).


| Product | Minimum aw Value | Average aw Value | Maximum aw Value |
|---|---|---|---|
| Lettuce | - | 0.996 | - |
| Onion | 0.974 | 0.982 | 0.990 |
| Carrot | 0.983 | 0.988 | 0.993 |
| Cabbage | 0.990 | 0.991 | 0.992 |
| Product | Minimum pH Value | Average pH Value |
|---|---|---|
| Lettuce | 5.8 | 6.0 |
| Onion | 5.3 | 5.9 |
| Carrot | 4.9 | 6.4 |
| Cabbage | 5.2 | 6.9 |
| Bacteria | Initial Dose (CFU/g) | Infective Dose (CFU/g) |
|---|---|---|
| Aeromonas hydrophila | 103 | 105 |
| Bacillus cereus | 101 | 105 |
| Listeria monocytogenes | 101 | 102 |
| Salmonella | 102 | 105 |
| Shigella flexneri | 100 | 102 |
| Staphylococcus aureus | 101 | 105 |
| Bacteria | Lettuce | Onion | Carrot | Cabbage |
|---|---|---|---|---|
| Aeromonas hydrophila | X | X | X | X |
| Bacillus cereus | X | X | X | X |
| Listeria monocytogenes | X | |||
| Salmonella | X | X | X | X |
| Shigella flexneri | X | X | ||
| Staphylococcus aureus |
| Bacteria | Min. T (°C) | Opt. T (°C) | Max. T (°C) | Min. pH | Opt. pH | Max. pH | Min. aw | Opt. aw | Max. aw |
|---|---|---|---|---|---|---|---|---|---|
| Aeromonas hydrophila | 2 | 27 | 37 | 4.6 | 6.7 | 7.5 | 0.974 | 0.998 | 1 |
| Bacillus cereus | 5 | 34 | 34 | 4.9 | 7.4 | 7.4 | 0.940 | 0.999 | 1 |
| Listeria monocytogenes | 1 | 40 | 40 | 4.4 | 6.9 | 7.5 | 0.934 | 0.994 | 1 |
| Salmonella | 7 | 37.5 | 40 | 3.9 | 6.4 | 7.4 | 0.973 | 0.997 | 1 |
| Shigella flexneri | 15 | 37 | 37 | 5.5 | 7.3 | 7.5 | 0.971 | 0.993 | 1 |
| Staphylococcus aureus | 7.5 | 30 | 30 | 4.4 | 6.5 | 7.5 | 0.907 | 0.990 | 1 |
| T (°C) | pH | aw | Remaining Shelf Life (t, in Hours) | Contamination by |
|---|---|---|---|---|
| 1 | 5.8 | 0.996 | 155.20 | Listeria monocytogenes |
| 5.9 | 0.996 | 146.00 | Listeria monocytogenes | |
| 4 | 5.8 | 0.996 | 79.60 | Listeria monocytogenes |
| 5.9 | 0.996 | 73.60 | Aeromonas hydrophila | |
| 10 | 5.9 | 0.996 | 22.10 | Aeromonas hydrophila |
| 6.0 | 0.996 | 20.50 | Aeromonas hydrophila |
| T (°C) | pH | aw | Remaining Shelf Life (t, in Hours) | Contamination by |
|---|---|---|---|---|
| 2 | 5.3 | 0.974 | 1580.4 | Aeromonas hydrophila |
| 0.982 | 728.8 | Aeromonas hydrophila | ||
| 0.990 | 354.6 | Aeromonas hydrophila | ||
| 5.4 | 0.974 | 1359.0 | Aeromonas hydrophila | |
| 0.982 | 628.8 | Aeromonas hydrophila | ||
| 0.990 | 307.8 | Aeromonas hydrophila | ||
| 10 | 5.5 | 0.974 | 179.7 | Bacillus cereus |
| 0.982 | 102.4 | Aeromonas hydrophila | ||
| 0.990 | 50.6 | Aeromonas hydrophila | ||
| 5.6 | 0.974 | 173.4 | Bacillus cereus | |
| 0.982 | 90.2 | Aeromonas hydrophila | ||
| 0.990 | 44.8 | Aeromonas hydrophila | ||
| 18 | 5.7 | 0.974 | 35.8 | Salmonella |
| 0.982 | 23.9 | Aeromonas hydrophila | ||
| 0.990 | 12.0 | Aeromonas hydrophila | ||
| 5.8 | 0.974 | 34.9 | Salmonella | |
| 0.982 | 21.7 | Aeromonas hydrophila | ||
| 0.990 | 11.0 | Aeromonas hydrophila |
| T (°C) | pH | aw | Remaining Shelf Life (t, in Hours) | Contamination by |
|---|---|---|---|---|
| 2 | 4.9 | 0.983 | 1321.6 | Aeromonas hydrophila |
| 0.988 | 829.0 | Aeromonas hydrophila | ||
| 0.993 | 541.2 | Aeromonas hydrophila | ||
| 5.0 | 0.983 | 1096.2 | Aeromonas hydrophila | |
| 0.988 | 689.0 | Aeromonas hydrophila | ||
| 0.993 | 451.8 | Aeromonas hydrophila | ||
| 10 | 4.9 | 0.983 | 152.4 | Bacillus cereus |
| 0.988 | 126.4 | Bacillus cereus | ||
| 0.993 | 101.4 | Aeromonas hydrophila | ||
| 5.0 | 0.983 | 148.0 | Bacillus cereus | |
| 0.988 | 123.0 | Bacillus cereus | ||
| 0.993 | 84.6 | Aeromonas hydrophila | ||
| 19 | 6.3 | 0.983 | 13.6 | Aeromonas hydrophila |
| 0.988 | 9.0 | Aeromonas hydrophila | ||
| 0.993 | 6.2 | Aeromonas hydrophila | ||
| 6.4 | 0.983 | 13.1 | Aeromonas hydrophila | |
| 0.988 | 8.6 | Aeromonas hydrophila | ||
| 0.993 | 6.0 | Aeromonas hydrophila |
| T (°C) | pH | aw | Remaining Shelf Life (t, in Hours) | Contamination by |
|---|---|---|---|---|
| 2 | 5.2 | 0.990 | 413.4 | Aeromonas hydrophila |
| 0.991 | 380.4 | Aeromonas hydrophila | ||
| 0.992 | 350.4 | Aeromonas hydrophila | ||
| 5.3 | 0.990 | 354.6 | Aeromonas hydrophila | |
| 0.991 | 327.6 | Aeromonas hydrophila | ||
| 0.992 | 301.8 | Aeromonas hydrophila | ||
| 9 | 6.0 | 0.990 | 36.6 | Aeromonas hydrophila |
| 0.991 | 34.0 | Aeromonas hydrophila | ||
| 0.992 | 31.6 | Aeromonas hydrophila | ||
| 6.1 | 0.990 | 34.2 | Aeromonas hydrophila | |
| 0.991 | 31.7 | Aeromonas hydrophila | ||
| 0.992 | 29.4 | Aeromonas hydrophila | ||
| 17 | 6.8 | 0.990 | 8.7 | Aeromonas hydrophila |
| 0.991 | 8.1 | Aeromonas hydrophila | ||
| 0.992 | 7.6 | Aeromonas hydrophila | ||
| 6.9 | 0.990 | 8.8 | Aeromonas hydrophila | |
| 0.991 | 8.2 | Aeromonas hydrophila | ||
| 0.992 | 7.7 | Aeromonas hydrophila |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pina, M.; Gaspar, P.D.; Lima, T.M. Decision Support System in Dynamic Pricing of Horticultural Products Based on the Quality Decline Due to Bacterial Growth. Appl. Syst. Innov. 2021, 4, 80. https://doi.org/10.3390/asi4040080
Pina M, Gaspar PD, Lima TM. Decision Support System in Dynamic Pricing of Horticultural Products Based on the Quality Decline Due to Bacterial Growth. Applied System Innovation. 2021; 4(4):80. https://doi.org/10.3390/asi4040080
Chicago/Turabian StylePina, Miguel, Pedro Dinis Gaspar, and Tânia Miranda Lima. 2021. "Decision Support System in Dynamic Pricing of Horticultural Products Based on the Quality Decline Due to Bacterial Growth" Applied System Innovation 4, no. 4: 80. https://doi.org/10.3390/asi4040080
APA StylePina, M., Gaspar, P. D., & Lima, T. M. (2021). Decision Support System in Dynamic Pricing of Horticultural Products Based on the Quality Decline Due to Bacterial Growth. Applied System Innovation, 4(4), 80. https://doi.org/10.3390/asi4040080








