Classification of Alzheimer’s Disease Patients Using Texture Analysis and Machine Learning
Abstract
:1. Introduction
- To use texture analysis to examine the minor changes in the hippocampus caused by AD.
- To extract features by implementing texture analysis technique on MRI.
- To classify subjects into AD and cognitively normal (CN) groups by providing the information extracted as input for training machine learning models.
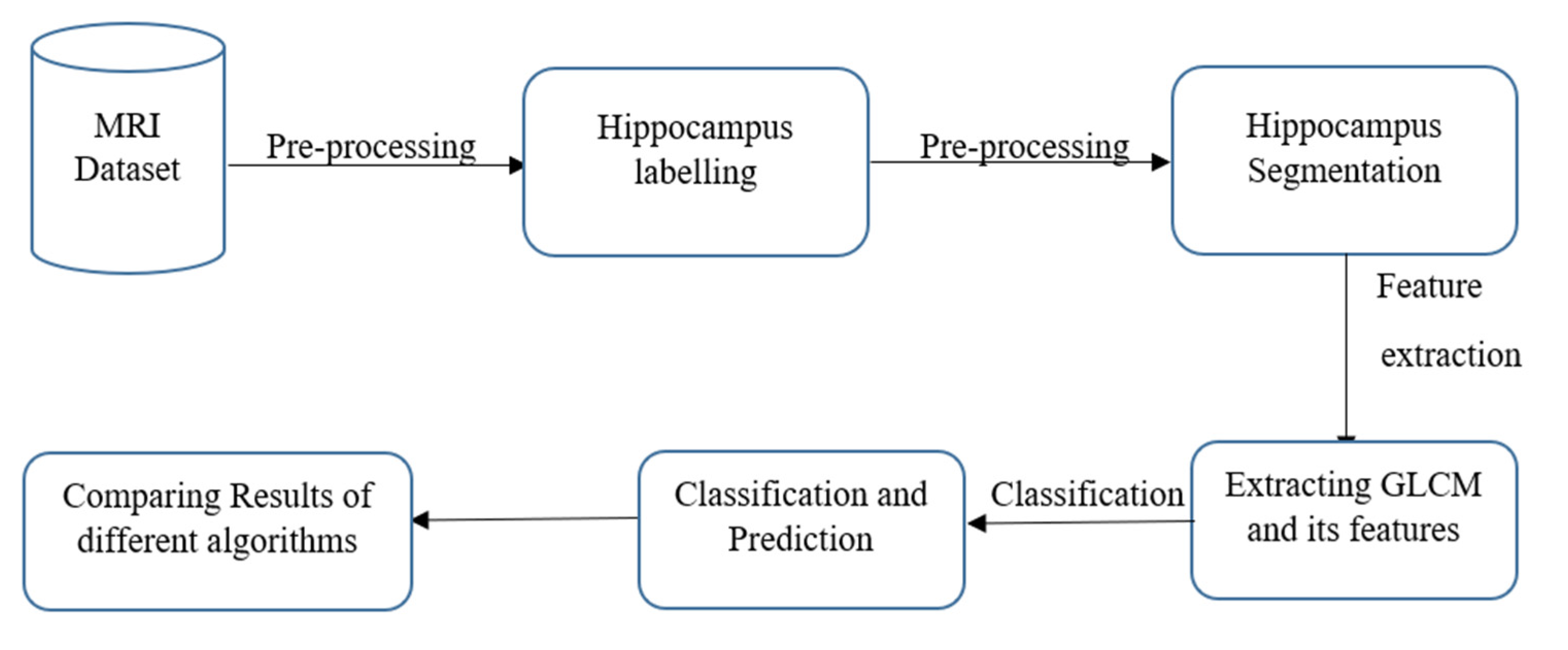
2. Materials and Methods
2.1. Alzheimer’s Disease Neuroimaging Initiative
2.2. MRI Data and Subjects
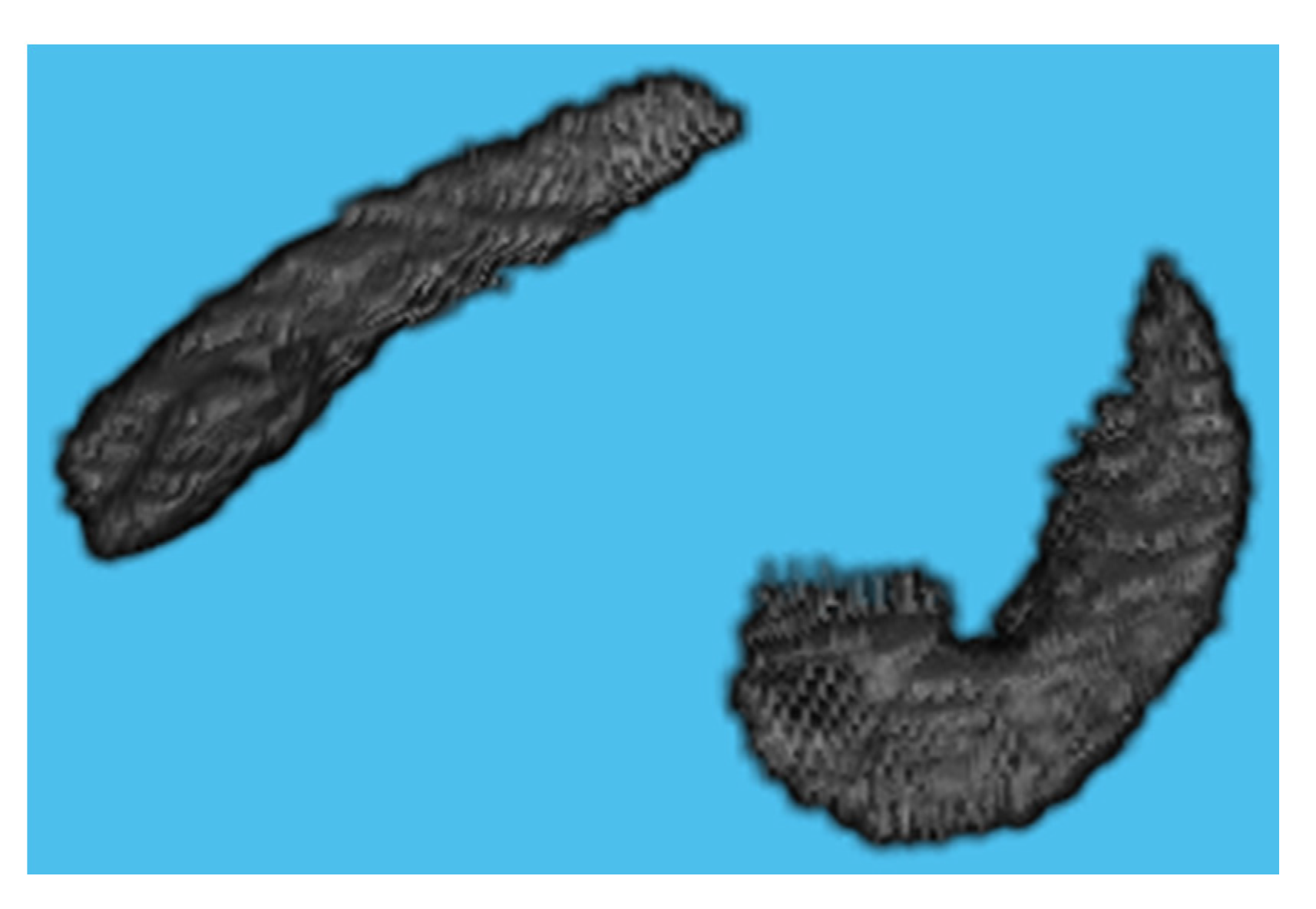
2.3. ROI Selection
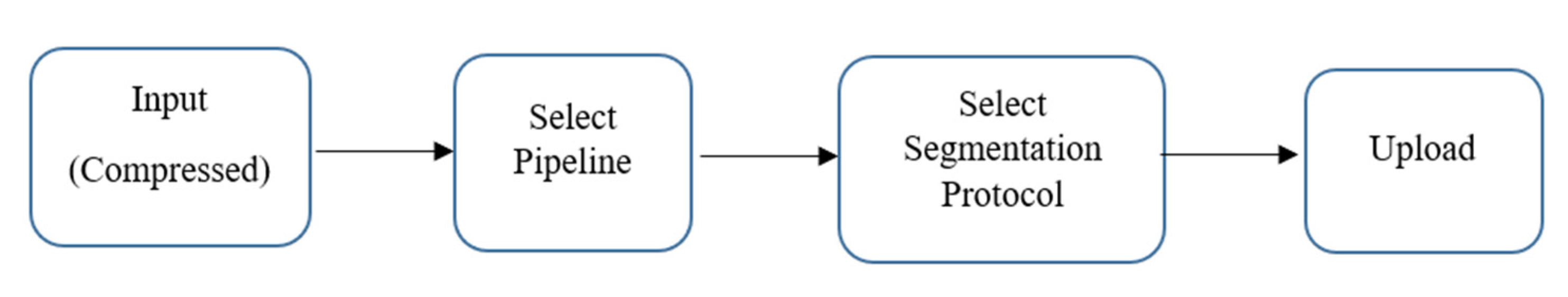
2.4. Hippocampus Segmentation
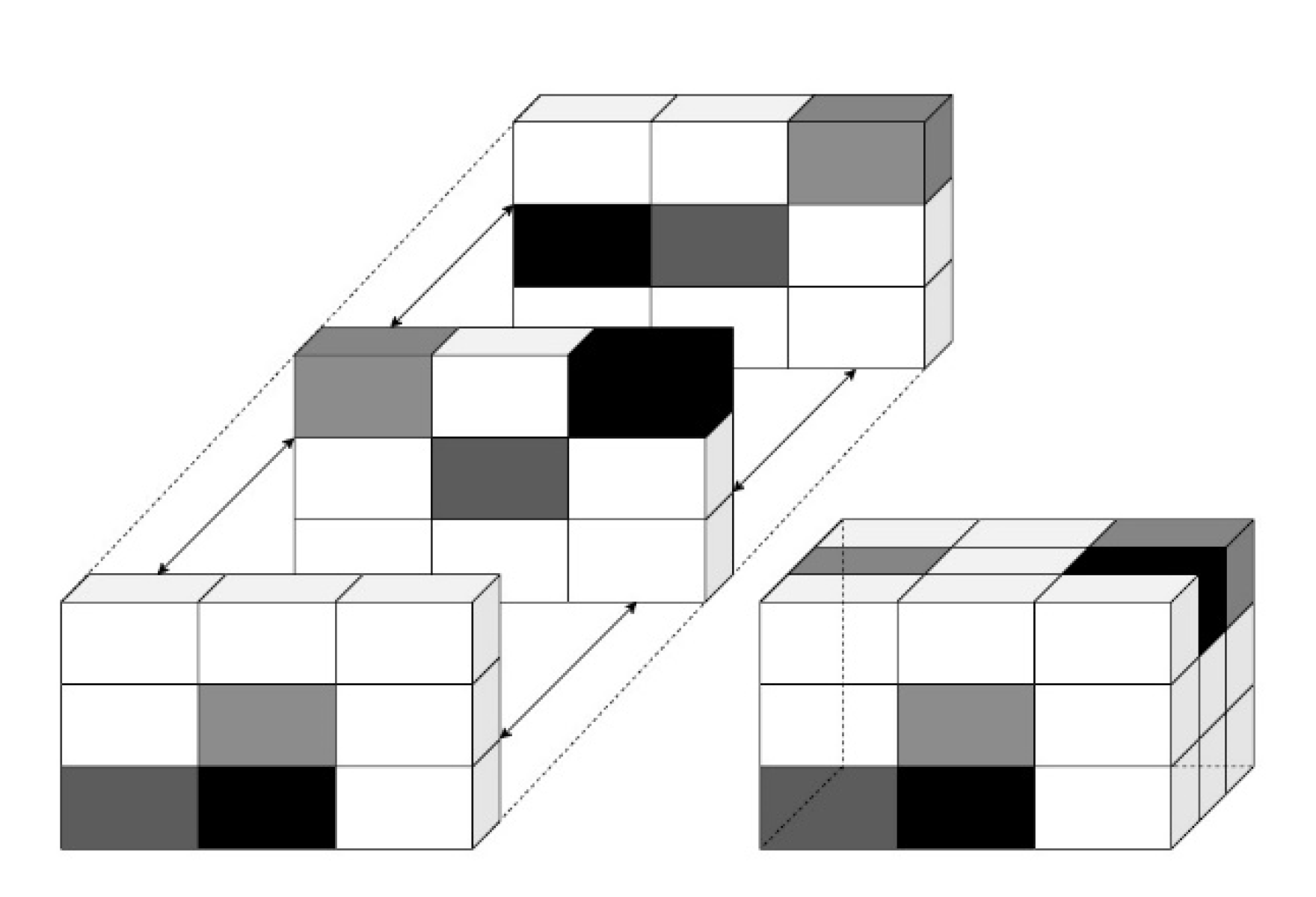
2.5. Texture Analysis
2.6. Classification Models
2.6.1. Support Vector Machine (SVM)
2.6.2. Decision Trees
2.6.3. Random Forest
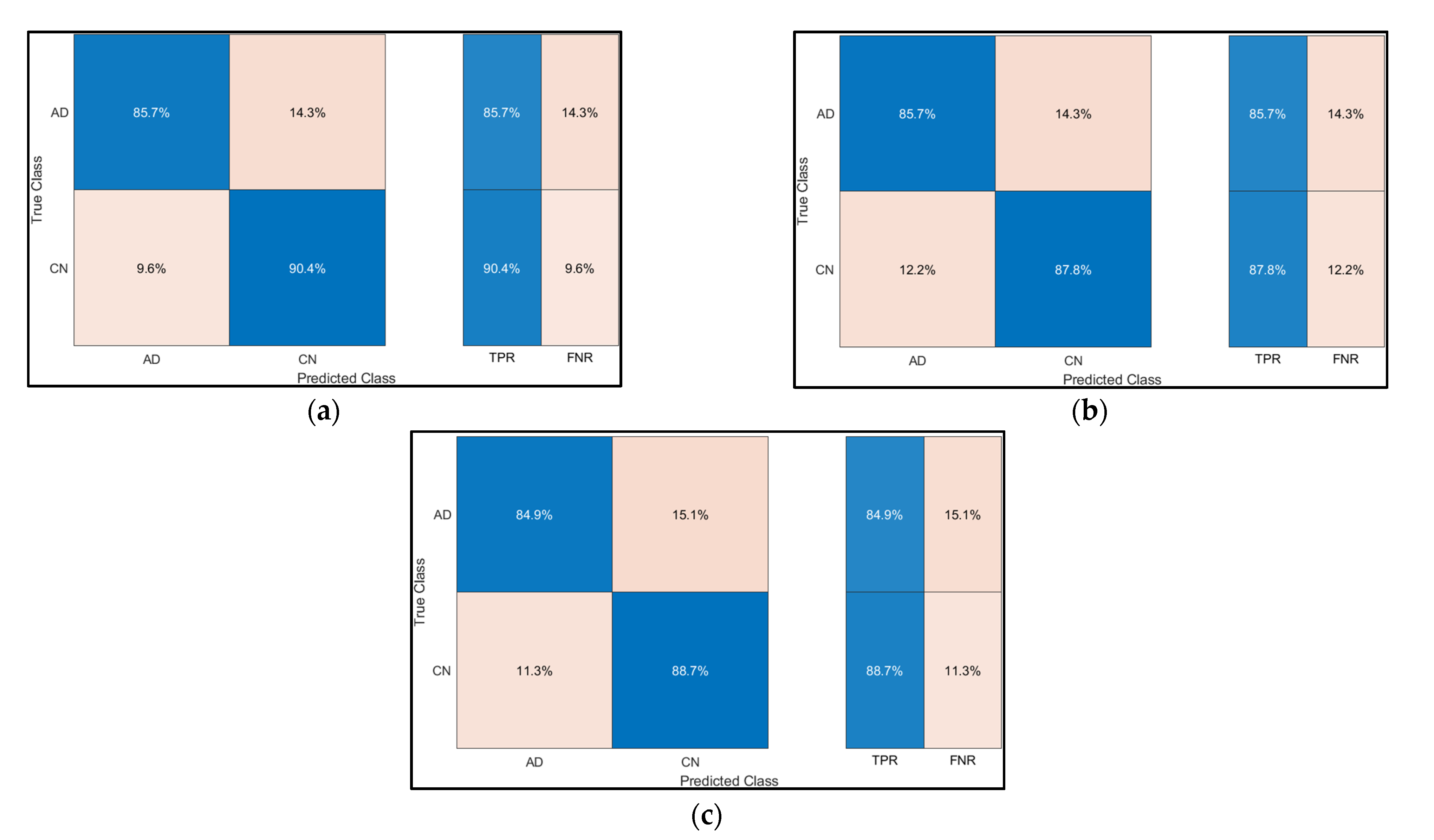
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, F.; Liu, M. Alzheimer′s Disease Neuroimaging Initiative. A hybrid convolutional and recurrent neural network for hippocampus analysis in Alzheimer’s disease. J. Neurosci. Methods 2019, 323, 108–118. [Google Scholar] [CrossRef]
- Dhikav, V.; Anand, K.S. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, C.; Jiang, G.; Liu, W.; Tong, L. 3D texture analysis on MRI images of Alzheimer’s disease. Brain Imaging Behav. 2011, 6, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sivapriya, T.R.; Saravanan, V.; Thangaiah, P.R.J. Texture Analysis of Brain MRI and Classification with BPN for the Diagnosis of Dementia; Springer: Berlin/Heidelberg, Germany, 2011; pp. 553–563. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Tong, L.; Zhou, X.; Zhang, J.; Zhou, Z.; Liu, W. Texture Analysis and Volumetry of Hippocampus and Medial Temporal Lobe in Patients with Alzheimer’s Disease. In Proceedings of the 2012 International Conference on Biomedical Engineering and Biotechnology, Macau, Macao, 28–30 May 2012; pp. 905–908. [Google Scholar] [CrossRef]
- Mohanaiah, P.; Sathyanarayana, P.; Gurukumar, L. Image Texture Feature Extraction Using GLCM Approach. Int. J. Sci. Res. Publ. 2013, 3, 1–5. [Google Scholar]
- Kusiak, J.W.; Izzo, J.A.; Zhao, B. Neurodegeneration in Alzheimer disease. Mol. Chem. Neuropathol. 1996, 28, 153–162. [Google Scholar] [CrossRef] [PubMed]
- ADNI. Alzheimer’s Disease Neuroimaging Initiative. Available online: http://adni.loni.usc.edu/ (accessed on 9 July 2021).
- Salunkhe, S.D.; Bachute, M.R. A Bibliometric Analysis on Recent Classification Techniques for Alzheimer’s Disease Publication: Library Philosophy and Practice; Digital Commons@University of Nebraska: Lincoln, NE, USA, 2021; Volume 5658. [Google Scholar]
- Leandrou, S.; Lamnisos, D.; Kyriacou, P.A.; Constanti, S.; Pattichis, C.S. Comparison of 1.5 T and 3 T MRI hippocampus texture features in the assessment of Alzheimer’s disease. Biomed. Signal Process. Control 2020, 62, 102098. [Google Scholar] [CrossRef]
- Larobina, M.; Murino, L. Medical Image File Formats. J. Digit. Imaging 2013, 27, 200–206. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Lan, T.; Qin, Z.; Zhang, X.; Wang, W. Classification of Alzheimer’s disease based on the combination of morphometric feature and texture feature. In Proceedings of the 2015 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Washington, DC, USA, 9–12 November 2015; pp. 409–412. [Google Scholar]
- Manjón, J.V.; Coupe, P. volBrain: An Online MRI Brain Volumetry System. Front. Aging Neurosci. 2016, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Frisoni, G.B.; Jack, C.R., Jr.; Bocchetta, M.; Bauer, C.; Frederiksen, K.S.; Liu, Y.; Preboske, G.; Swihart, T.; Blair, M.; Cavedo, E.; et al. The EADC-ADNI harmonized protocol for manual hippocampal segmentation on magnetic resonance: Evidence of validity. Alzheimer’s Dement. 2015, 11, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Romero, J.E.; Coupe, P.; Manjón, J.V. HIPS: A new hippocampus subfield segmentation method. NeuroImage 2017, 163, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avinbash Uppuluri. GLCM_Features4.m: Vectorized Version of GLCM_Features1.m [With Code Changes]. MATLAB Central File Exchange. Available online: https://www.mathworks.com/matlabcentral/fileexchange/22354-glcm_features4-m-vectorized-version-of-glcm_features1-m-with-code-changes (accessed on 29 May 2021).
- NITRC: MRIcron: Tool/Resource Info. Available online: https://www.nitrc.org/projects/mricron (accessed on 9 July 2021).
- Caballero, D. Feature extraction algorithms from MRI to evaluate quality parameters on meat products by using data mining. ELCVIA Electron. Lett. Comput. Vis. Image Anal. 2018, 16, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Lahmiri, S.; Shmuel, A. Performance of machine learning methods applied to structural MRI and ADAS cognitive scores in diagnosing Alzheimer’s disease. Biomed. Signal Process. Control 2019, 52, 414–419. [Google Scholar] [CrossRef]
- Barburiceanu, S.; Terebes, R.; Meza, S. 3D Texture Feature Extraction and Classification Using GLCM and LBP-Based Descriptors. Appl. Sci. 2021, 11, 2332. [Google Scholar] [CrossRef]
- Soh, L.-K.; Tsatsoulis, C. Texture analysis of SAR sea ice imagery using gray level co-occurrence matrices. IEEE Trans. Geosci. Remote. Sens. 1999, 37, 780–795. [Google Scholar] [CrossRef] [Green Version]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Wu, X.; Kumar, V.; Quinlan, J.R.; Ghosh, J.; Yang, Q.; Motoda, H.; McLachlan, G.J.; Ng, S.K.; Liu, B.; Yu, P.S.; et al. Top 10 algorithms in data mining. Knowl. Inf. Syst. 2007, 14, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Nagawa, K.; Suzuki, M.; Yamamoto, Y.; Inoue, K.; Kozawa, E.; Mimura, T.; Nakamura, K.; Nagata, M.; Niitsu, M. Texture analysis of muscle MRI: Machine learning-based classifications in idiopathic inflammatory myopathies. Sci. Rep. 2021, 11, 9821. [Google Scholar] [CrossRef]
- Ho, T.K. Random decision forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995. [Google Scholar] [CrossRef]
- Bachute, M.; Vyas, N.; Modi, K.; Khanna, S.; Katpatal, C. Bibliometric Review on Classification of Alzheimer’s Disease Library Philosophy and Practice; Digital Commons@University of Nebraska: Lincoln, NE, USA, 2020; Volume 4843. [Google Scholar]
- Raut, A.; Dalal, V. A machine learning based approach for detection of alzheimer’s disease using analysis of hippocampus region from MRI scan. In Proceedings of the 2017 International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 18–19 July 2017; pp. 236–242. [Google Scholar]
- Fan, Z.; Xu, F.; Qi, X.; Li, C.; Yao, L. Classification of Alzheimer’s disease based on brain MRI and machine learning. Neural Comput. Appl. 2019, 32, 1927–1936. [Google Scholar] [CrossRef]
- Stanzione, A.; Cuocolo, R.; Cocozza, S.; Romeo, V.; Persico, F.; Fusco, F.; Longo, N.; Brunetti, A.; Imbriaco, M. Detection of Extraprostatic Extension of Cancer on Biparametric MRI Combining Texture Analysis and Machine Learning: Preliminary Results. Acad. Radiol. 2019, 26, 1338–1344. [Google Scholar] [CrossRef]
- Yasar, H.; Ceylan, M. A novel comparative study for detection of Covid-19 on CT lung images using texture analysis, machine learning, and deep learning methods. Multimed. Tools Appl. 2020, 80, 5423–5447. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Dwyer, A.; Summers, R.M.; Mollura, D.J. Computer-aided Diagnosis of Pulmonary Infections Using Texture Analysis and Support Vector Machine Classification. Acad. Radiol. 2011, 18, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Fuse, H.; Oishi, K.; Maikusa, N.; Fukami, T. Japanese Alzheimer’s Disease Neuroimaging Initiative. Detection of alzheimer’s disease with shape analysis of MRI images. In Proceedings of the 2018 Joint 10th International Conference on Soft Computing and Intelligent Systems (SCIS) and 19th International Symposium on Advanced Intelligent Systems (ISIS), Toyama, Japan, 5–8 December 2018; pp. 1031–1034. [Google Scholar] [CrossRef]
- Kavin Kumar, K.; Devi, M.; Maheswaran, S. An efficient method for brain tumor detection using texture features and SVM classifier in MR images. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2789. [Google Scholar]
- Luk, C.C.; Ishaque, A.; Khan, M.; Ta, D.; Chenji, S.; Yang, Y.-H.; Eurich, D.; Kalra, S. Alzheimer’s disease: 3-Dimensional MRI texture for prediction of conversion from mild cognitive impairment. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Chaddad, A.; Zinn, P.O.; Colen, R.R. Radiomics texture feature extraction for characterizing GBM phenotypes using GLCM. In Proceedings of the 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI), Brooklyn, NY, USA, 16–19 April 2015; pp. 84–87. [Google Scholar] [CrossRef]
- Madusanka, N.; Choi, Y.Y.; Choi, K.Y.; Lee, K.H.; Choi, H.K. Hippocampus Segmentation and Classification in Alzheimer’s Disease and Mild Cognitive Impairment Applied on MR Images. J. Korea Multimed. Soc. 2017, 20, 205–215. [Google Scholar] [CrossRef]
- Ranjbar, S.; Velgos, S.N.; Dueck, A.C.; Geda, Y.E.; Mitchell, J.R.; Alzheimer’s Disease Neuroimaging Initiative. Brain MR radiomics to differentiate cognitive disorders. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 210–219. [Google Scholar] [CrossRef]
- Karim, R.; Shahrior, A.; Rahman, M.M. Machine learning-based tri-stage classification of Alzheimer’s progressive neurodegenerative disease using PCA and mRMR administered textural, orientational, and spatial features. Int. J. Imaging Syst. Technol. 2021. [Google Scholar] [CrossRef]



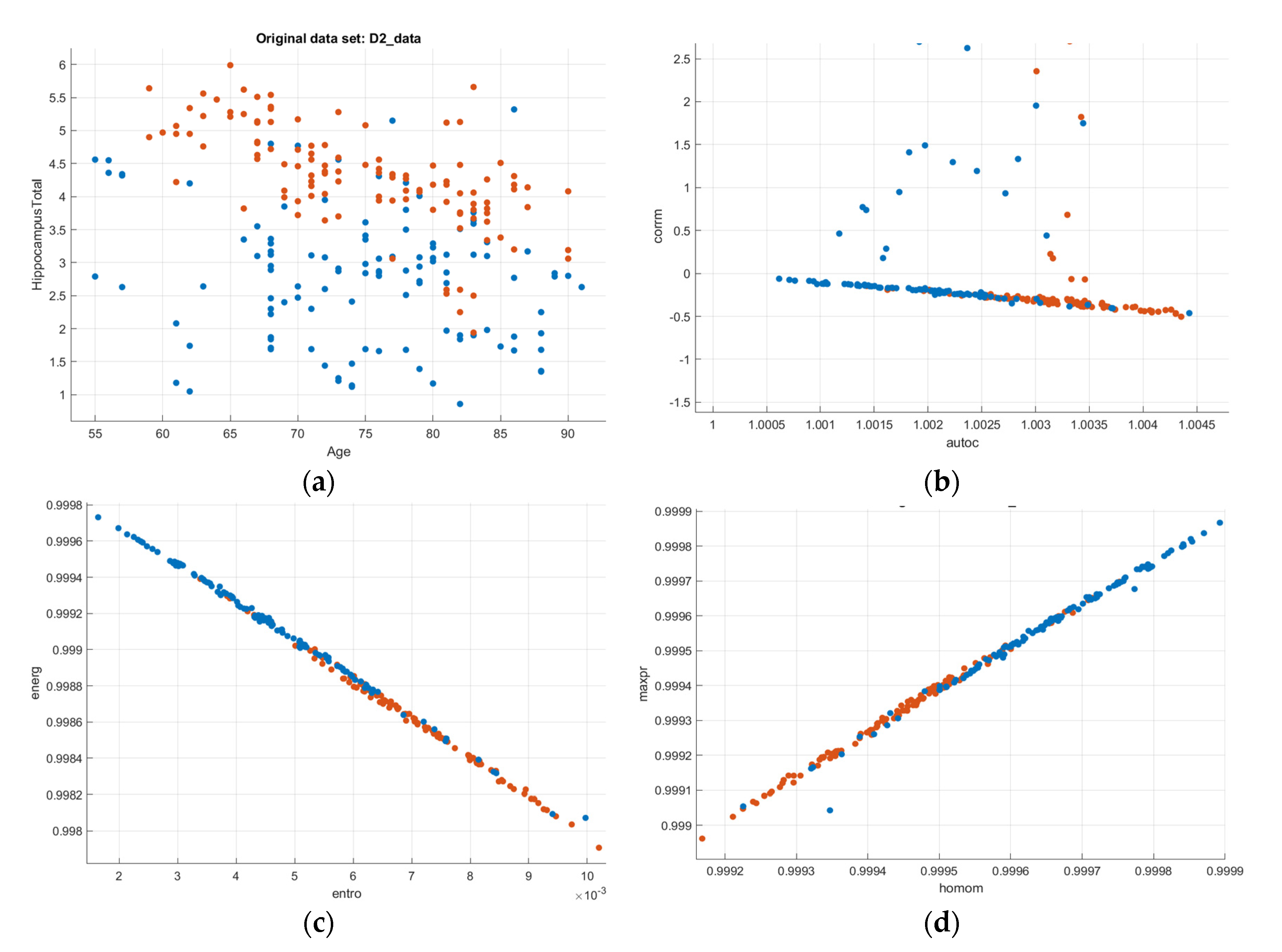


| Group | Description | Subject Age |
|---|---|---|
| AD | Number of Cases- 119 | - |
| Minimum | 55 | |
| Maximum | 91 | |
| Mean (Standard Deviation) | 75.10 (±8.60) | |
| CN | Number of Cases- 115 | - |
| Minimum | 59 | |
| Maximum | 90 | |
| Mean (Standard Deviation) | 74.80 (±7.95) |
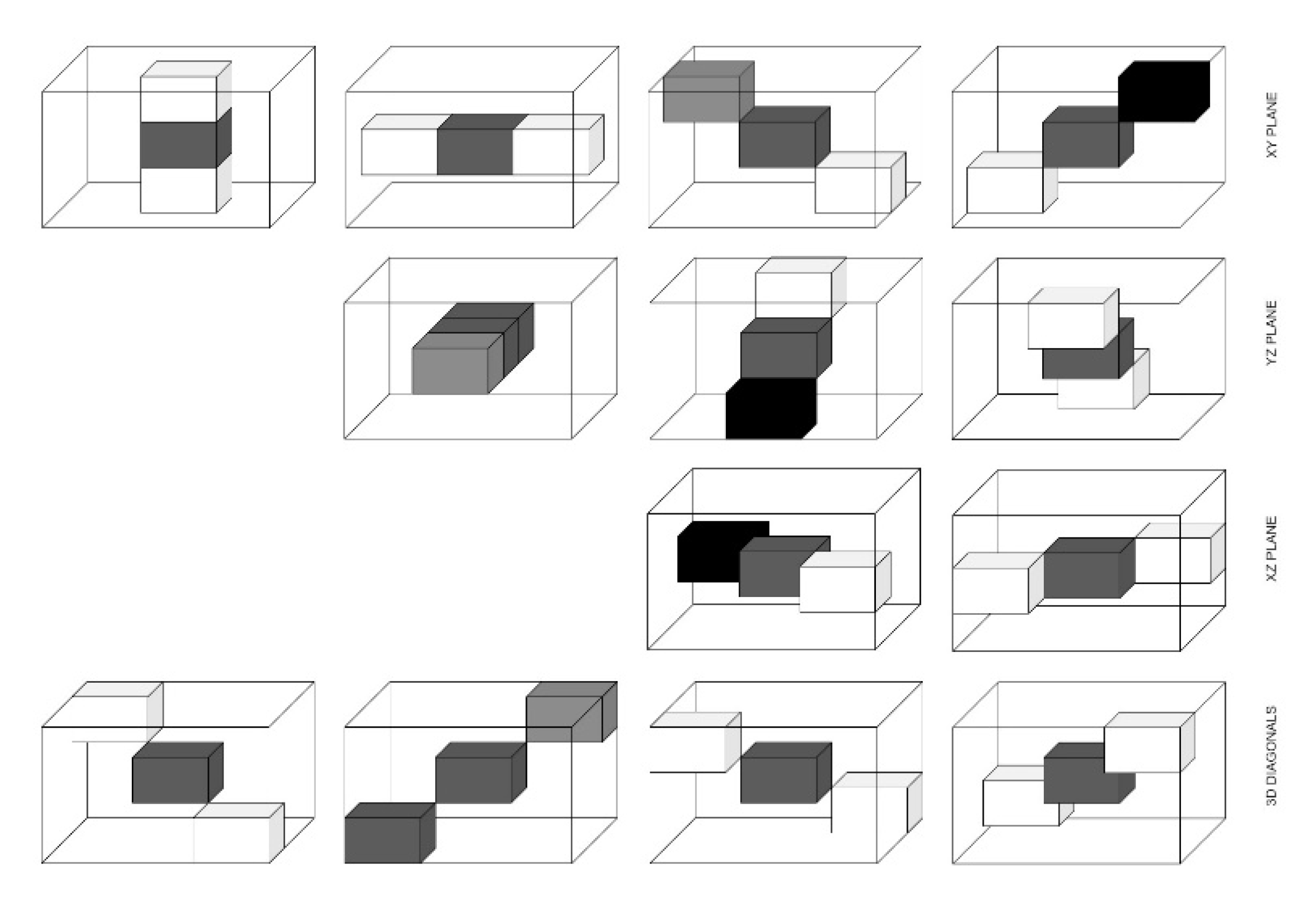
| Sr. No. | Directions |
|---|---|
| 1 | (0, 0, d) |
| 2 | (0, d, −d) |
| 3 | (0, d, 0) |
| 4 | (d, 0, −d) |
| 5 | (0, d, d) |
| 6 | (d, d, −d) |
| 7 | (−d, d, −d) |
| 8 | (d, 0, 0) |
| 9 | (d, 0, d) |
| 10 | (d, d, 0) |
| 11 | (−d, d, 0) |
| 12 | (d, d, d) |
| 13 | (−d, d, d) |
| Sr. No. | Feature | Formula | Notations |
|---|---|---|---|
| 1 | Autocorrelation | –number of grey levels. i–row number j–column number | |
| 2 | Contrast | –Element i,j of the normalized symmetrical GLCM –number of paired data | |
| 3 | Correlation | –the GLCM mean (being an estimate of the intensity of all pixels in the relationships that contributed to the GLCM) –the variance of the intensities of all reference pixels in the relationships that contributed to the GLCM (symmetric) | |
| 4 | Cluster Prominence | ||
| 5 | Cluster Shade | ||
| 6 | Dissimilarity | ||
| 7 | Energy | ||
| 8 | Entropy | ||
| 9 | Homogeneity | ||
| 10 | Maximum Probability | ||
| 11 | Variance | ||
| 12 | Sum average | ||
| 13 | Sum variance | ||
| 14 | Sum entropy | ||
| 15 | Difference variance | ||
| 16 | Difference entropy | ||
| 17 | Information measure of correlation1 | and are entropies of and | |
| 18 | Information measure of correlation2 | ||
| 19 | Inverse difference | ||
| 20 | Inverse difference moment normalized |
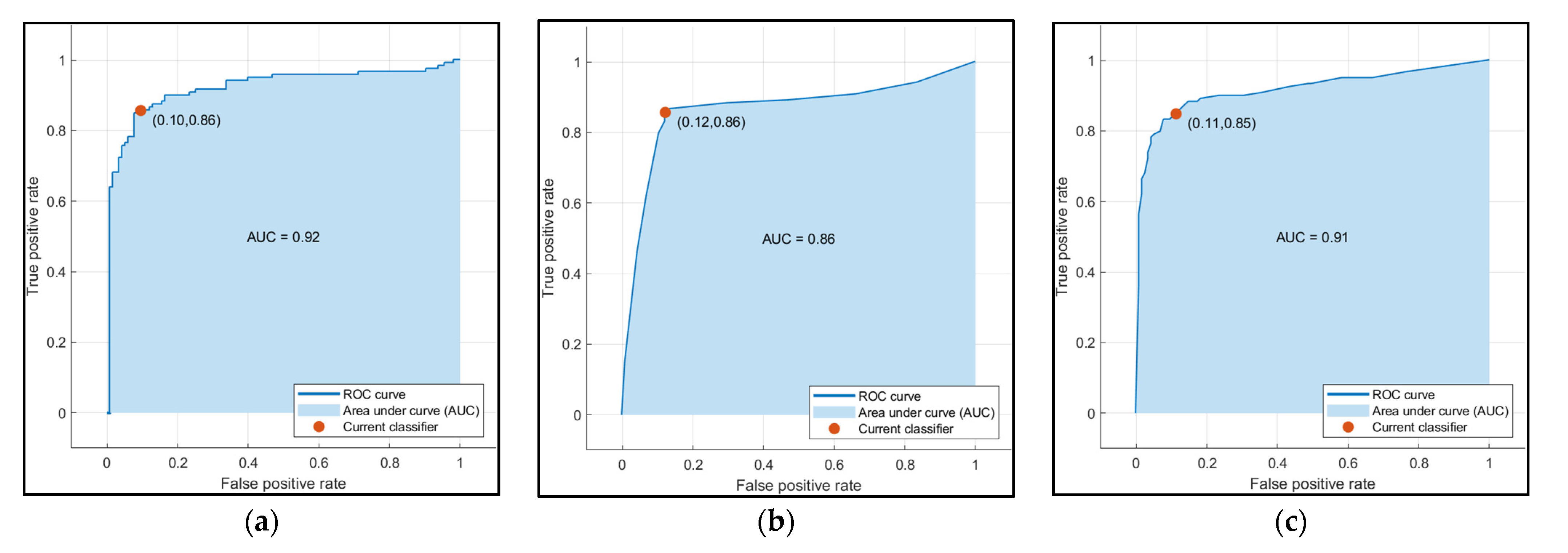
| Sr. No | Model | Classification Accuracy (%) |
|---|---|---|
| 1 | Decision Trees | 86.8 |
| 2 | SVM | 87.2 |
| 3 | Ensemble | 86.3 |
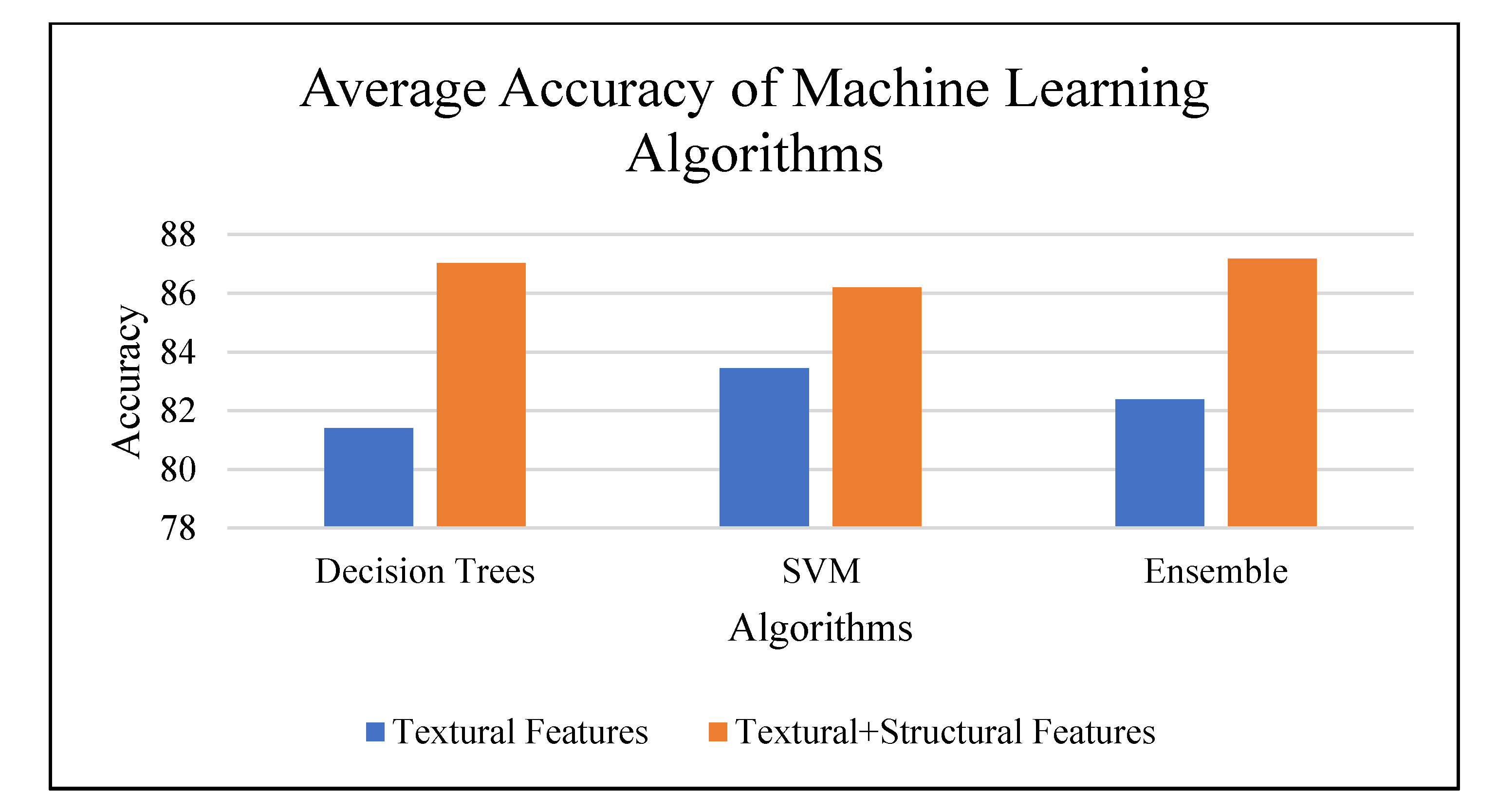
| Distance and Directions | Classification Model Accuracy | ||||||
|---|---|---|---|---|---|---|---|
| With Textural Data Only | With Textural and Structural Data | ||||||
| Decision Trees | SVM | Ensemble | Decision Trees | SVM | Ensemble | ||
| d = 1 | 011 | 81.6 | 82.5 | 82.1 | 86.8 | 85.9 | 84.6 |
| 100 | 81.2 | 84.6 | 82.9 | 88 | 86.8 | 88.5 | |
| 101 | 82.9 | 82.9 | 83.8 | 86.3 | 85.5 | 86.8 | |
| 110 | 82.5 | 84.2 | 85 | 85.9 | 87.2 | 87.6 | |
| 111 | 79.5 | 83.3 | 82.1 | 86.3 | 87.2 | 86.8 | |
| 11–1 | 79.9 | 82.1 | 80.3 | 88 | 85.5 | 90.2 | |
| 001 | 82.5 | 83.3 | 82 | 88.5 | 86.6 | 86.3 | |
| 01–1 | 82.9 | 83.3 | 82.9 | 88 | 86.8 | 86.3 | |
| 010 | 80.8 | 84.2 | 83.8 | 85.9 | 85.9 | 88 | |
| 10–1 | 81.2 | 83.8 | 81.6 | 88.5 | 85.9 | 88.9 | |
| −11–1 | 82.1 | 84.2 | 82.9 | 86.3 | 86.3 | 86.8 | |
| −110 | 82.9 | 84.2 | 82.5 | 88 | 86.3 | 87.6 | |
| −111 | 81.6 | 84.6 | 80.8 | 88.9 | 85.9 | 86.8 | |
| d = 2 | 022 | 81.6 | 81.2 | 83.8 | 85.9 | 85.9 | 84.6 |
| 200 | 80.3 | 85.9 | 81.6 | 85 | 85.5 | 86.3 | |
| 202 | 80.3 | 83.3 | 84.2 | 87.2 | 86.8 | 86.8 | |
| 220 | 81.6 | 84.2 | 81.2 | 88 | 86.8 | 88 | |
| 222 | 79.1 | 82.9 | 80.3 | 87.2 | 85.9 | 88.5 | |
| 22–2 | 79.9 | 82.1 | 82.9 | 88 | 86.8 | 86.3 | |
| 002 | 80.8 | 83.3 | 81.6 | 88.5 | 85 | 87.2 | |
| 02–2 | 82.5 | 82.5 | 79.9 | 85.9 | 87.2 | 86.3 | |
| 020 | 82.5 | 83.8 | 83.8 | 88.9 | 86.3 | 87.6 | |
| 20–2 | 80.8 | 84.2 | 80.8 | 84.2 | 85.9 | 87.6 | |
| −22–2 | 83.3 | 84.2 | 84.2 | 85 | 85.5 | 88 | |
| −220 | 80.3 | 84.2 | 82.9 | 86.3 | 86.3 | 88.5 | |
| −222 | 82.1 | 80.8 | 82.1 | 87.2 | 85.5 | 85.5 | |
| Author | Structure | Texture Analysis Method (Features) | Machine Learning Technique | Accuracy (%) |
|---|---|---|---|---|
| Xiao, Z. et al. [6] | Brain | GLCM, Gabor filter | SVM | For AD-CN data: 85.71 |
| Oishi, K. et al. [33] | Gray Matter, White Matter, Cerebral Spinal Fluid and Background | Coefficient of Probability Changes | SVM | Maximum: 95 Average: 70 |
| Kumar, K. et al. [34] | Brain | GLCM | k-NN | 74.73 |
| Luk, C.C. et al. [35] | Brain | 3D GLCM | - | Maximum: 90.5 Average: 76 |
| Chaddad, A. et al. [36] | Brain | 3D GLCM | Ensemble | 74.19 |
| Madusanka, N. et al. [37] | Hippocampus | 3D GLCM | SVM | 86.61 |
| Ranjbar, S. et al. [38] | Hippocampus | Hippocampal Volume | Diagonal Quadratic Discriminant Analysis (Naïve Bayes) | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salunkhe, S.; Bachute, M.; Gite, S.; Vyas, N.; Khanna, S.; Modi, K.; Katpatal, C.; Kotecha, K. Classification of Alzheimer’s Disease Patients Using Texture Analysis and Machine Learning. Appl. Syst. Innov. 2021, 4, 49. https://doi.org/10.3390/asi4030049
Salunkhe S, Bachute M, Gite S, Vyas N, Khanna S, Modi K, Katpatal C, Kotecha K. Classification of Alzheimer’s Disease Patients Using Texture Analysis and Machine Learning. Applied System Innovation. 2021; 4(3):49. https://doi.org/10.3390/asi4030049
Chicago/Turabian StyleSalunkhe, Sumit, Mrinal Bachute, Shilpa Gite, Nishad Vyas, Saanil Khanna, Keta Modi, Chinmay Katpatal, and Ketan Kotecha. 2021. "Classification of Alzheimer’s Disease Patients Using Texture Analysis and Machine Learning" Applied System Innovation 4, no. 3: 49. https://doi.org/10.3390/asi4030049
APA StyleSalunkhe, S., Bachute, M., Gite, S., Vyas, N., Khanna, S., Modi, K., Katpatal, C., & Kotecha, K. (2021). Classification of Alzheimer’s Disease Patients Using Texture Analysis and Machine Learning. Applied System Innovation, 4(3), 49. https://doi.org/10.3390/asi4030049







