Artificial Pancreas Control Strategies Used for Type 1 Diabetes Control and Treatment: A Comprehensive Analysis
Abstract
:1. Introduction
1.1. What Is Diabetes?
- ▪
- Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease in which the human body does not produce enough insulin while insulin inoculations are required on a daily basis. T1DM was further classified into two subgroups: immune mediated and idiopathic by the American Diabetes Association (ADA) in 2007. Meanwhile, the idiopathic type-1 diabetes is considered to be type-2 diabetes by several researchers and clinicians. Patients of T1DM become entirely dependent on externally administered insulin, and it is the only treatment available in medicine. However, the daily dose of insulin varies and depends heavily on a range of other factors including age, gender, daily exercise, and physique. However, an average daily dose is about 1-unit of insulin per kg weight per day [2].
- ▪
- Type 2 diabetes mellitus (T2DM) also known as non-insulin dependent diabetes mellitus (NIDDM), it is characterized by the defect in both insulin secretion and insulin resistance. High levels of BG are managed with the reduced food intake, improved physical activity, and ultimately oral medications or insulin [3].
- ▪
- Gestational diabetes (GD) can occur temporarily during pregnancy, and recent findings suggest that it can occur in 2~10% of the all pregnancies. During pregnancy, significant hormonal changes can lead to the blood sugar elevation in genetically predisposed individuals which is known as gestational diabetes (GD).
1.2. What Causes Diabetes in the Human Body?
- ▪
- T1DM diabetes causes are not as well documented compared to T2DM. Family history is a known risk factor for the T1DM. Other risk factors can include having certain infections or diseases of the pancreas. T1DM is primarily characterized as an autoimmune disease resulting in damage of the insulin-producing β-cells in the pancreas by T-cells (CD4+ and CD8+), and macrophages penetrating the islets. Both genetic as well as environmental factors as yet unclear trigger autoimmune responses against β-cells and destroy them, thus significantly proliferating the disease in humans [4]. According to the latest studies, genetic factors are becoming more evident in causing T1DM disease [5,6,7].
- ▪
- T2DM develops when the body becomes resistant to insulin or when the pancreas is unable to produce enough insulin. The main cause of this is as yet unknown, although genetics and environmental factors, such as being inactive, and overweight seem to be main causes for the T2DM disease.
- ▪
- GD can occur due to the significant hormonal changes during the pregnancy period, and blood sugar elevation in genetically predisposed individuals.
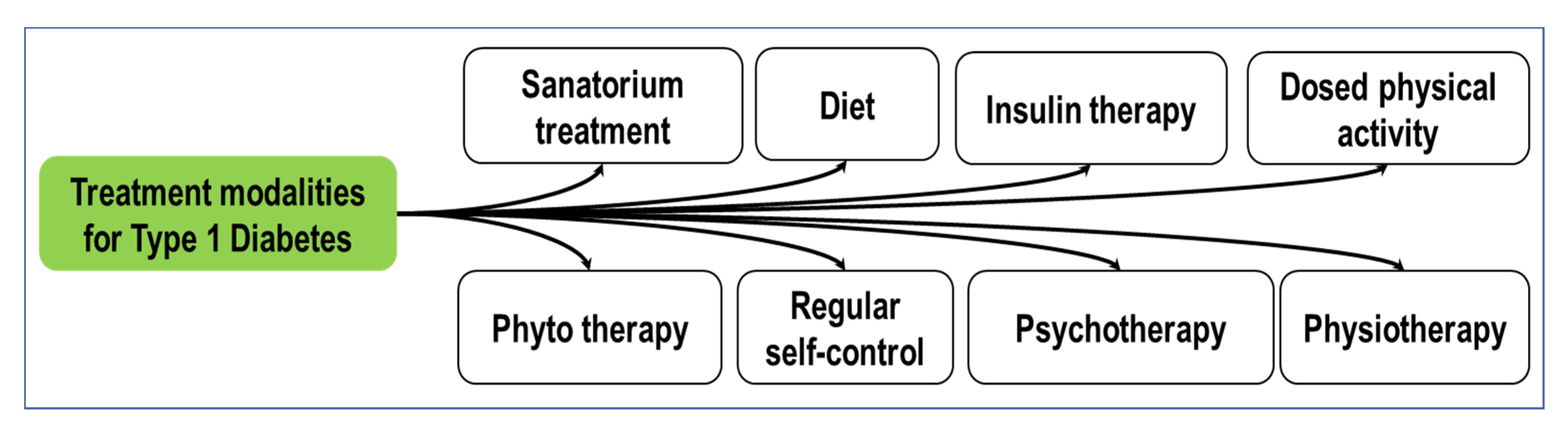
1.3. Current Treatment Modalities Available for Diabetes in Medicine
1.4. Manuscript Contribution in the Field of Study
1.5. Manuscript Organization
2. Physiological Methods of Insulin Delivery
2.1. Significance of First and Second Phase Insulin Secretion in Human Body
2.2. Hyperglycemia and Hypoglycemia
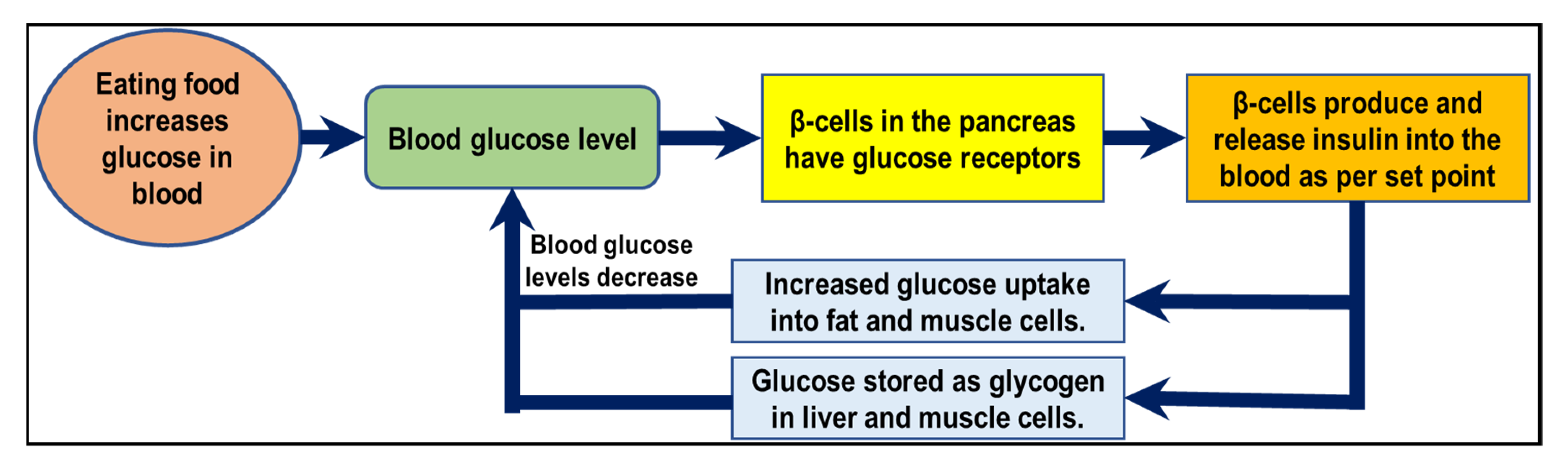
2.3. Biological Perspective on How β-Cell Achieves Glucose Control and Energy Metabolism in Type 1 Diabetes Mellitus (T1DM)
3. Open Loop Administration of an Insulin
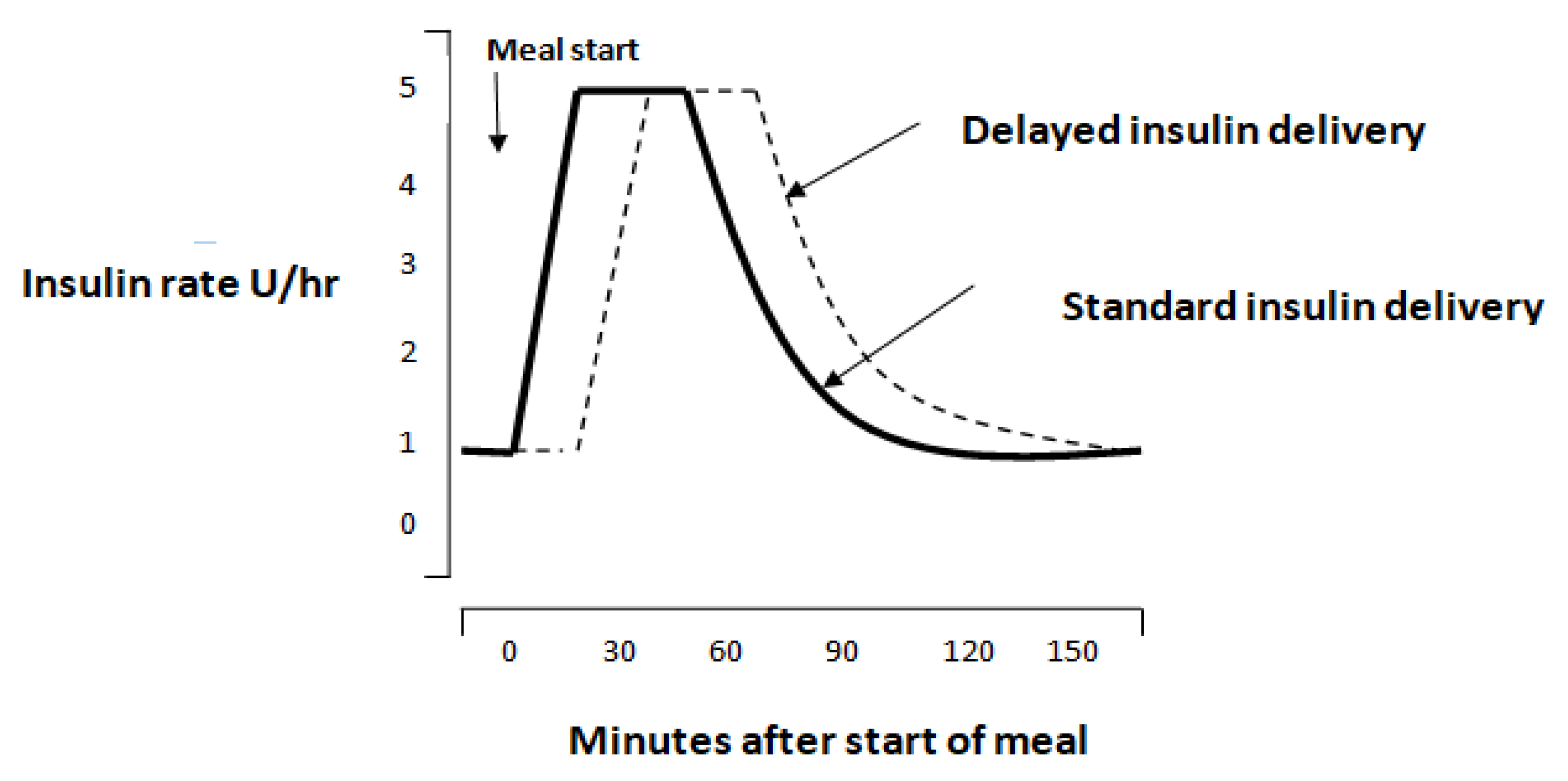
3.1. Timing of Insulin Delivery
3.2. Manual Administration of the Insulin
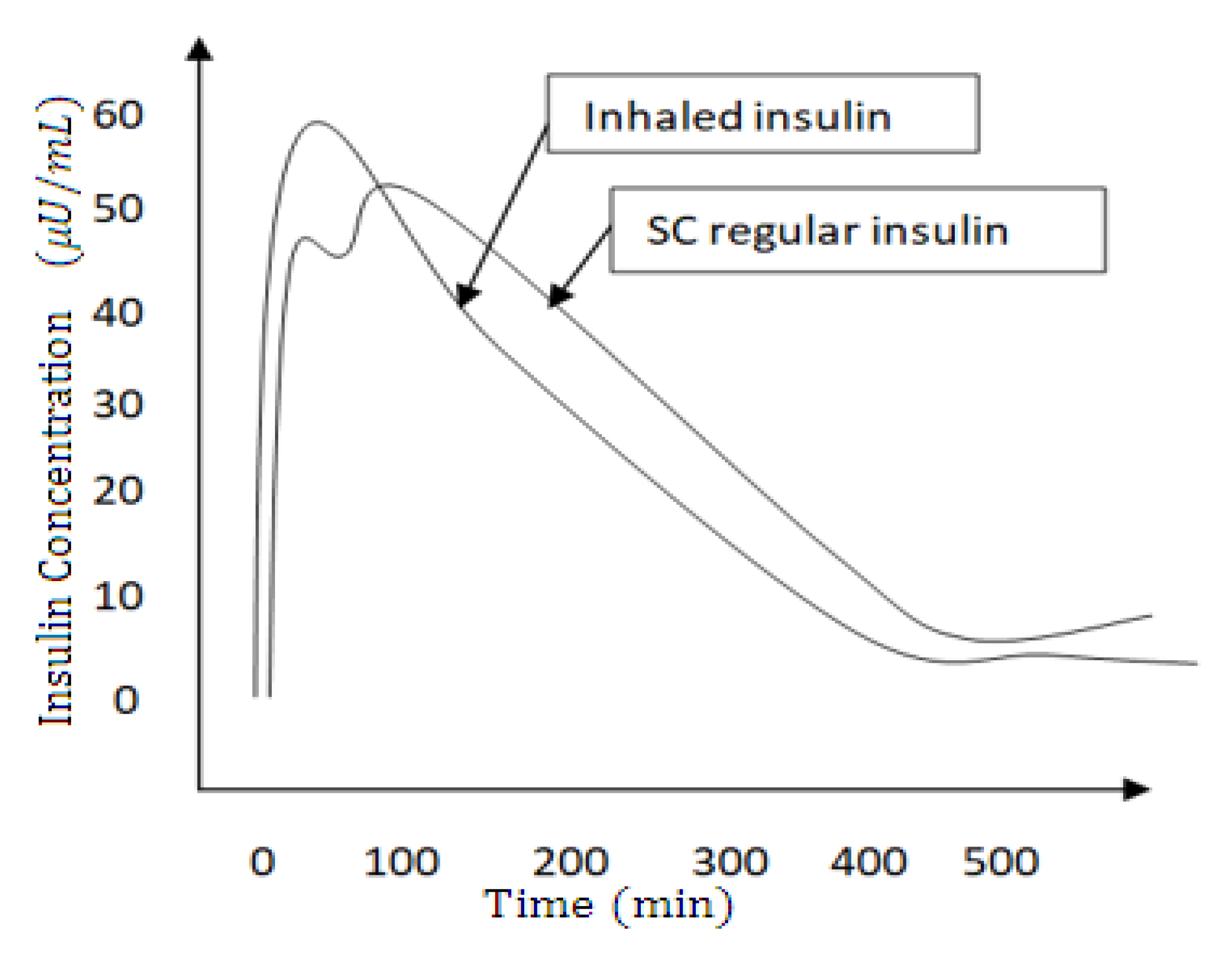
3.3. Subcutaneous Versus Inhaled Insulin
3.4. Multiple Daily Insulin Therapy
3.5. Continuous Subcutaneous Insulin Therapy
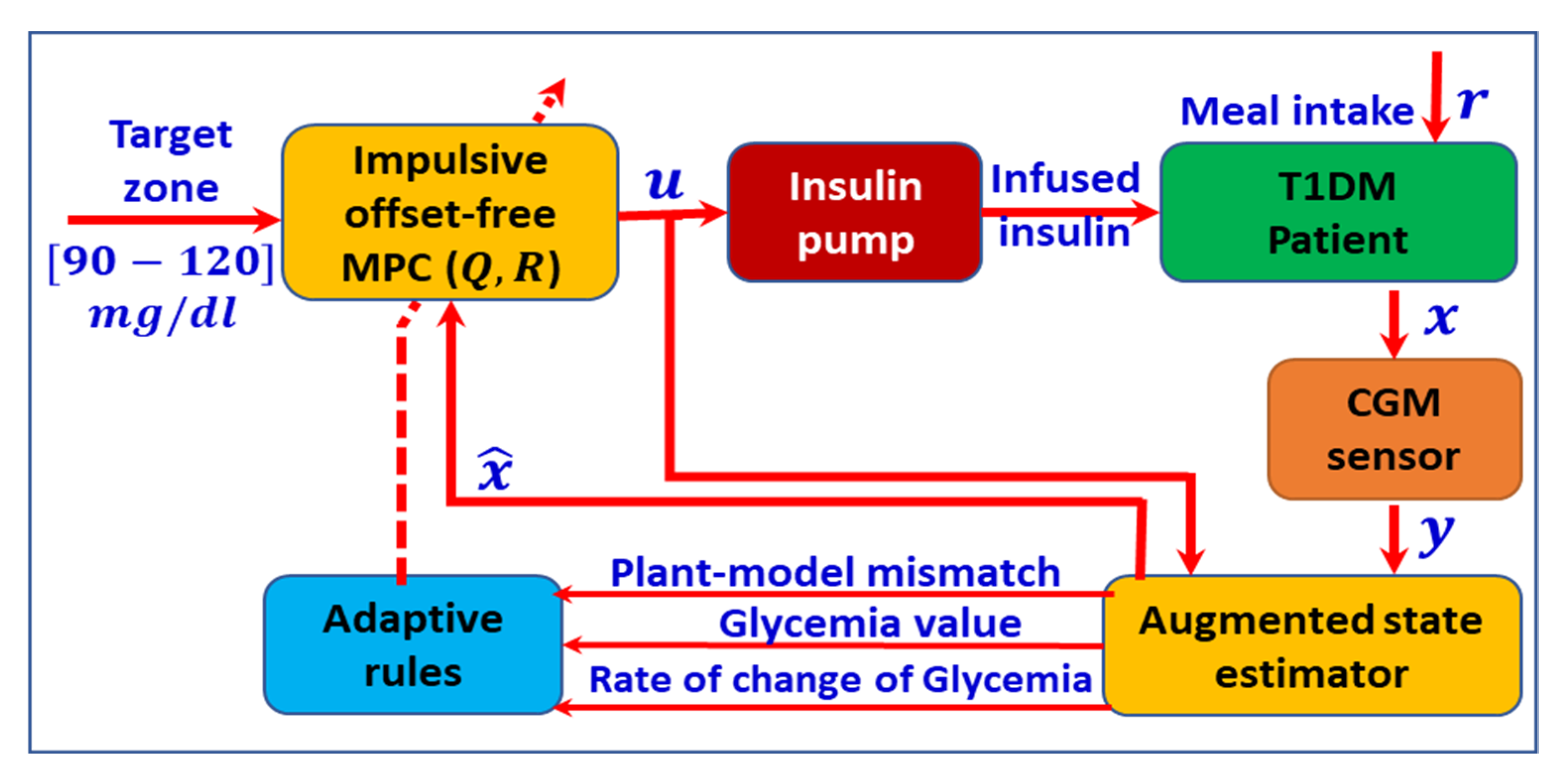
4. Closed Loop Administration of Insulin
5. Proportional Integral Derivative (PID) Controller
6. Linear and Non-Linear Insulin Infusion Control Schemes
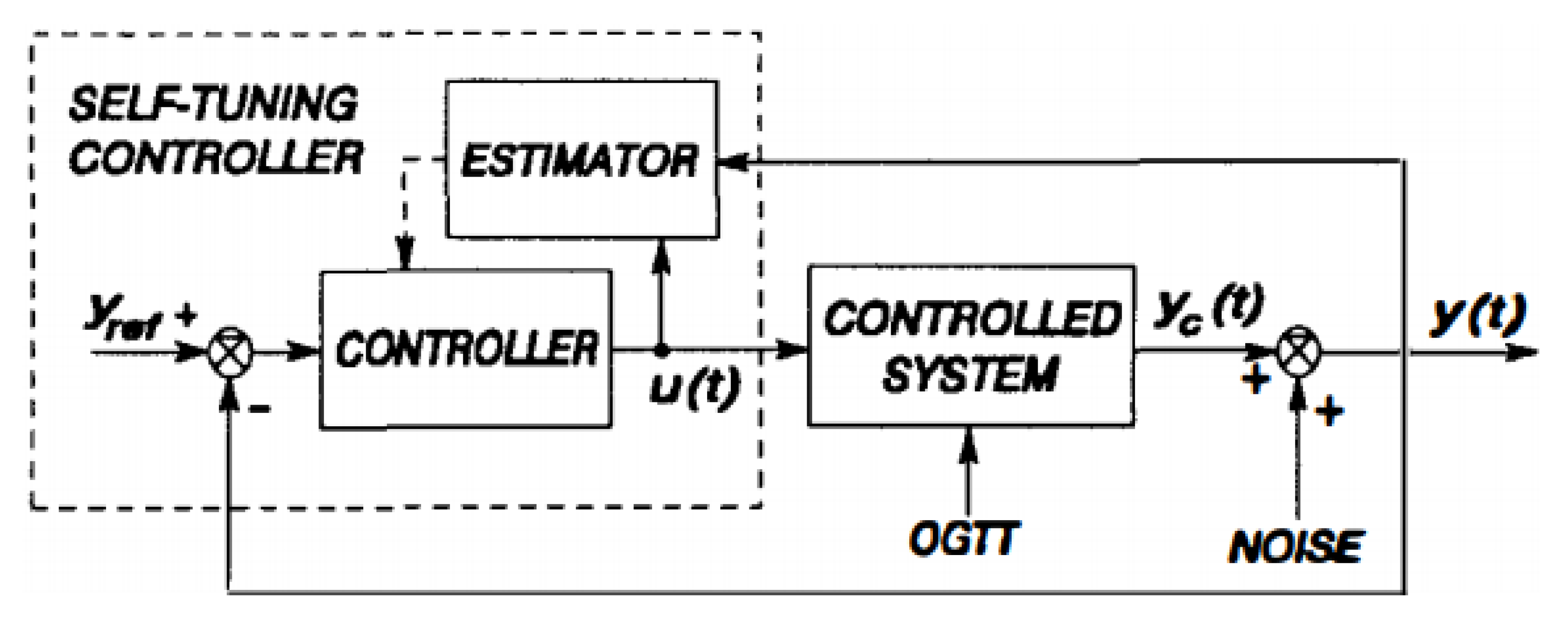
6.1. Self-Tuning Control
6.2. Adaptive Control
6.3. Sliding Mode Control (SMC)
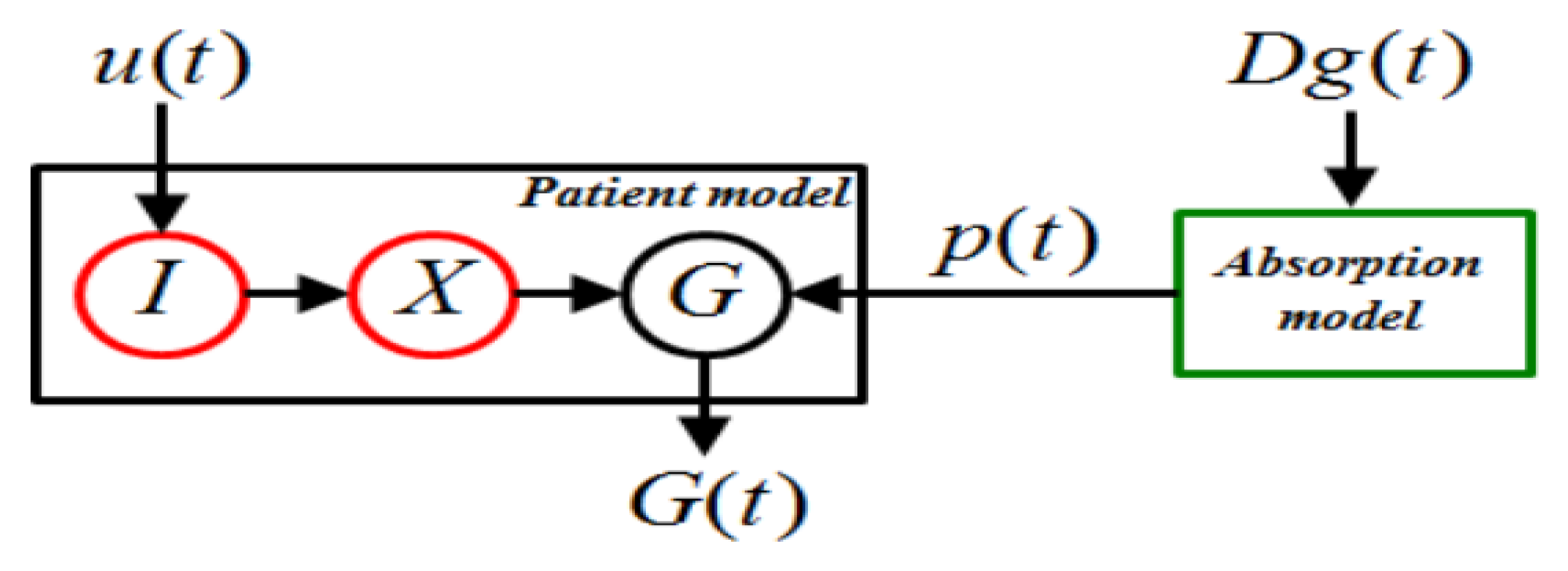
6.4. Model Predictive Control (MPC)
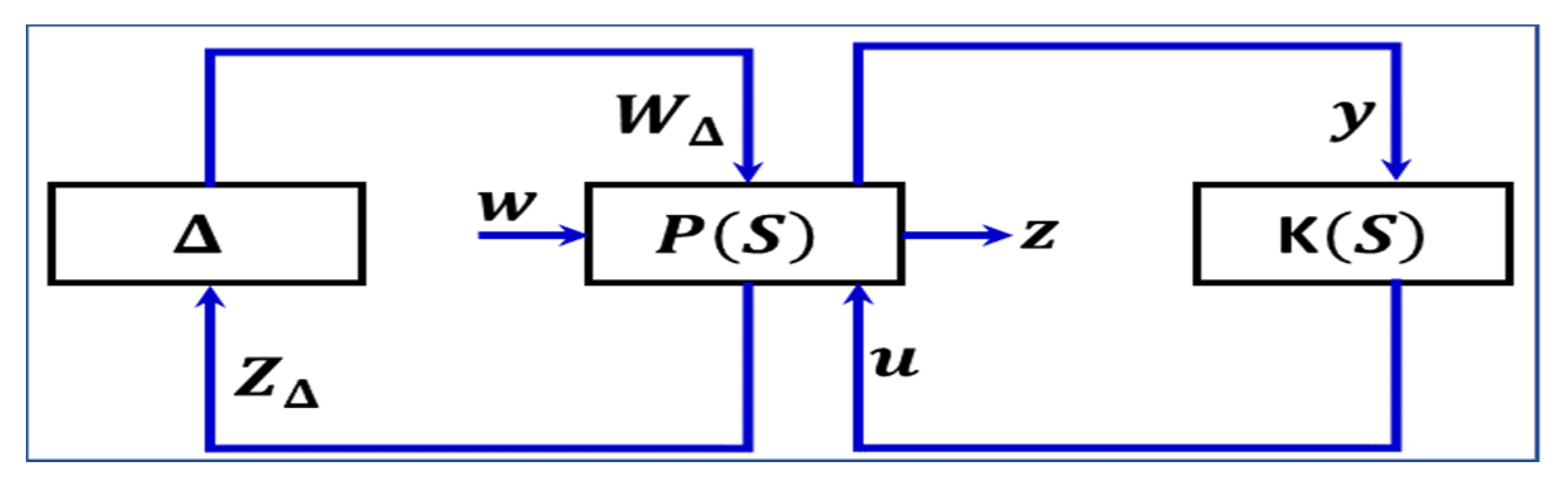
6.5. H∞ Control
6.6. State-Dependent Riccati Equation (SDRE)
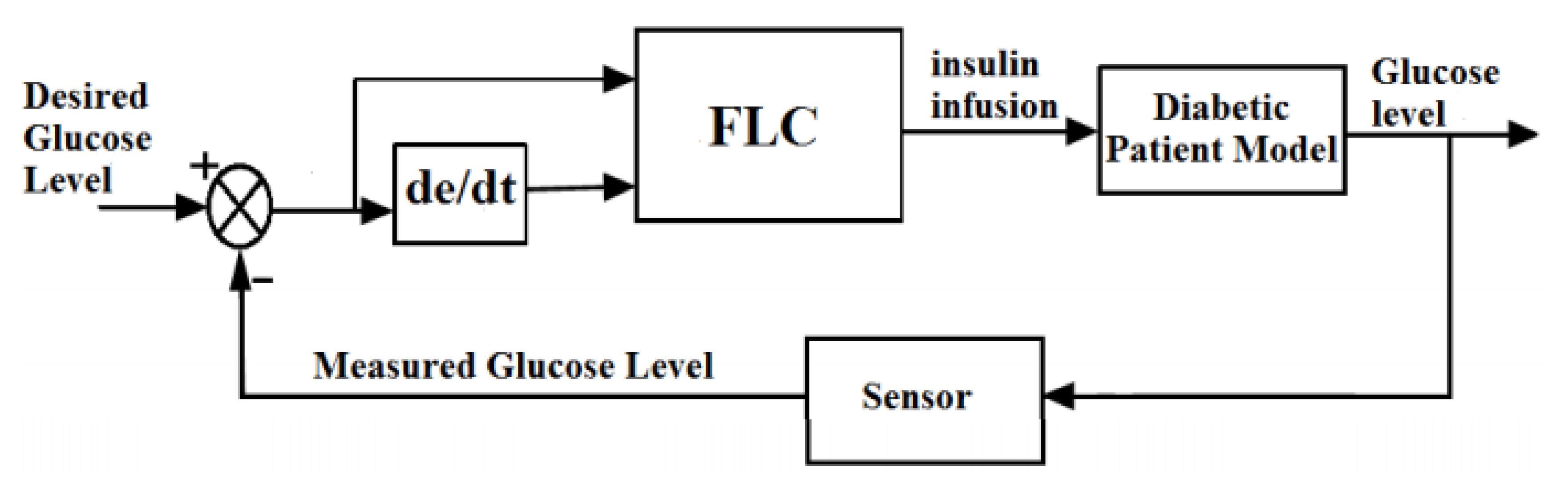
6.7. Fuzzy Logic Control
7. Model Predictive Control (MPC) Strategy Used in T1DM Therapy
- MPC’s prediction property makes it suitable for anticipatory and measured insulin delivery in a human body.
- MPC can exceed the physiological delays associated with the subcutaneous flow.
- MPC can resolve the compensation of the dead time, commonly seen in the glucose concentration problem.
- The efficient feed-forward control technique embedded in the MPC can handle the known disturbances such as meal intake or metabolic changes.
- MPC can easily handle the constraints on the system inputs and outputs.
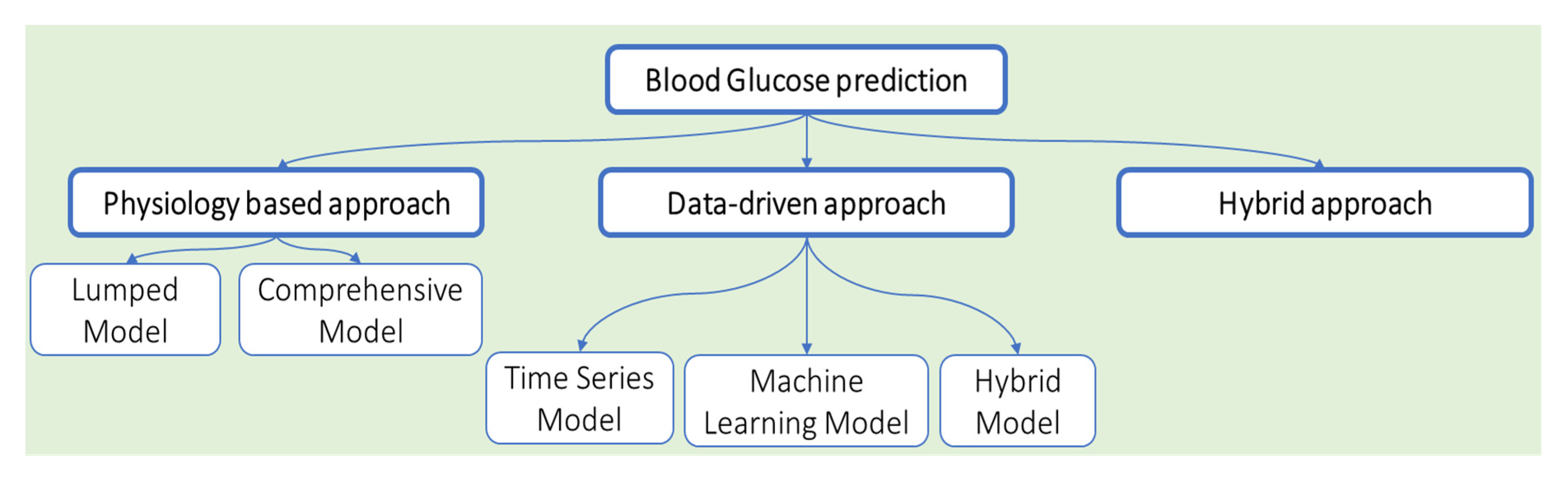
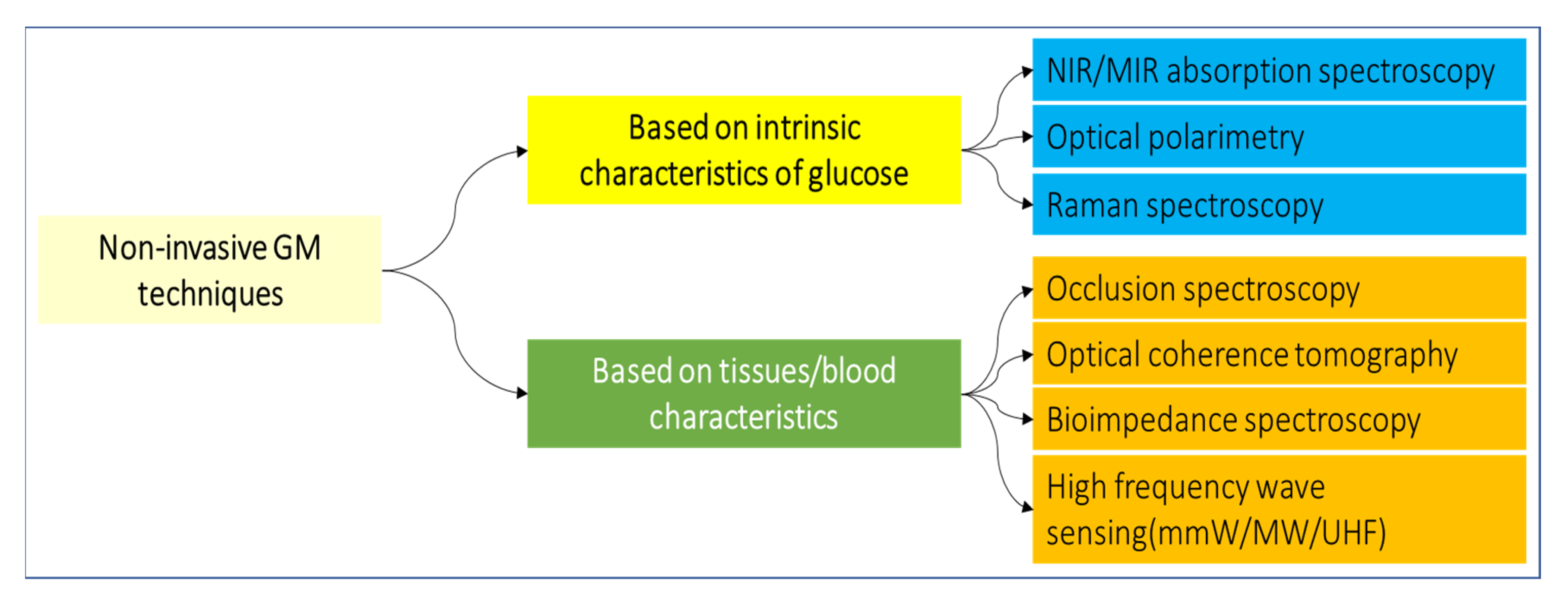
8. Glucose Measurement
9. Lesson Learned and Discussion
10. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loghmani, E. Diabetes mellitus: Type 1 and type 2. Guidelines for adolescent nutrition services. In Diabetes Mellitus in 21st Century; Springer: New York, NY, USA, 2005; pp. 167–182. [Google Scholar]
- Aschner, P.; Horton, E.; Leiter, L.A.; Munro, N.; Skyler, J.S. Global partnership for effective diabetes management. Practical steps to improving the management of type 1 diabetes: Recommendations from the Global Partnership for effective diabetes management. Int. J. Clin. Pract. 2010, 64, 305–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magliano, D.J.; Zimmet, P.; Shaw, J.E. Classification of diabetes mellitus and other categories of glucose intolerance. In International Textbook of Diabetes Mellitus; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 1–16. [Google Scholar]
- Gillespie, K.M. Type 1 diabetes: Pathogenesis and prevention. Cmaj 2006, 175, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Nyaga, D.M.; Vickers, M.H.; Jefferies, C.; Perry, J.K.; O’Sullivan, J.M. Type 1 diabetes mellitus-associated genetic variants contribute to overlapping immune regulatory networks. Front. Genet. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Pociot, F. Type 1 diabetes genome-wide association studies: Not to be lost in translation. Clin. Transl. Immunol. 2017, 6, e162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, A.; Go, M.J.; Zhang, W.; Below, J.E.; Gaulton, K.J.; Ferreira, T.; Horikoshi, M.; Johnson, A.D.; Ng, M.C.; Prokopenko, I.; et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat. Genet. 2014, 46, 234. [Google Scholar] [CrossRef]
- IDF 2019 Data. Available online: https://www.diabetesatlas.org/en/ (accessed on 10 June 2020).
- Szalay, P.; Eigner, G.; Kovács, L.A. Linear matrix inequality-based robust controller design for type-1 diabetes model. IFAC Proc. Vol. 2014, 47, 9247–9252. [Google Scholar] [CrossRef] [Green Version]
- Mullins, P.; Sharplin, P. Negative binomial meta-regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven Phase III and IV studies of insulin glargine compared with neutral protamine Hagedorn insulin in type 1 and type 2 diabetes mellitus. Clin. Therap. 2007, 29, 1607–1619. [Google Scholar] [CrossRef]
- Plank, J.; Siebenhofer Aand Berghold, A. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch. Intern. Med. 2005, 165, 1337–1344. [Google Scholar] [CrossRef] [Green Version]
- Warsi, G.G.; Saini, S.; Khatri, K. Ensemble learning on diabetes data set and early diabetes prediction. In Proceedings of the 2019 International Conference on Computing, Power and Communication Technologies (GUCON), Greater Nioda, UP, India, 27–28 September 2019; pp. 182–187. [Google Scholar]
- Ali, H.A.; Boutayeb, W.; Boutayeb, A.; Merabet, N. A mathematical model for type 1 diabetes, on the effect of growth hormone. In Proceedings of the 2019 8th International Conference on Modeling Simulation and Applied Optimization (ICMSAO), Zallaq, Bahrain, 15–17 April 2019. [Google Scholar]
- Farias, A.F.S.; Mendizabal, A.; Gonzalez-Garrido, A.A.; Romo-Vazquez, R.; Morales, A. Long short-term memory neutral networks for identifying type 1 diabetes patients with functional magnetic resonance imaging. In Proceedings of the 2018 IEEE Latin American Conference on Computational Intelligence (LA-CCI), Guadalajara, Jalisco, Mexico, 7–9 November 2019. [Google Scholar]
- Mao, S.; Feng Sand Que, L. Detection of autoantibodies for type 1 diabetes using label-free optical sensors. In Proceedings of the Transducers 2019—EUROSENSORS XXXIII, Berlin, Germany, 23–27 June 2019; pp. 578–581. [Google Scholar]
- Mertz, L. Automated insulin delivery. IEEE Pulse 2018, 9, 2154–2287. [Google Scholar] [CrossRef]
- Juniastuti, S.; Ghifari, H.M.A.; Nugroho SMSand Purnama, I.K.E. Development of casual game on android devices for children with diabetes type 1 treatment. In Proceedings of the 2019 International Conference of Computer Engineering, Network, and Intelligent Multimedia (CENIM), Surabaya, Indonesia, 19–20 November 2019. [Google Scholar]
- Migliorelli, L.; Moccia, S.; Avenllino, I.; Fiorentino, M.C.; Fronton, E. MyDi application: Towards automatic activity annotation of young patients with type 1 diabetes. In Proceedings of the 2019 IEEE 23rd International Symposium on Consumer Tchnologies (ISCT), Ancona, Italy, 19–21 June 2019; pp. 220–224. [Google Scholar]
- Zhang, P.; Schmidt, D.C.; White, J.; Mulvaney, S.A. Towards precision behavioral medicine with IoT: Iterative design and optimization of a self-management tool for type 1 diabetes. In Proceedings of the 2018 IEEE International Conference on Healthcare Informatics, New York, NY, USA, 4–7 June 2018; pp. 64–74. [Google Scholar]
- Pinsker, J.E.; Lee, J.B.; Dassau, E.; Seborg, D.E.; Bradley, P.K.; Gondhalekar, R.; Bevier, W.C.; Huyett, L.; Zisser, H.C.; Doyle, F.J. Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care 2016, 39, 1135–1142. [Google Scholar]
- Gondhalekar, R.; Dassau, E.; Doyle, F.J. Periodic zone-MPC with asymmetric costs for outpatient-ready safety of an artificial pancreas to treat type 1 diabetes. Automatica 2016, 71, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Mayne, D.Q. Model predictive control: Recent developments and future promise. Automatica 2014, 50, 2967–2986. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Dunning, T.; Dhatariya, K.; Sheu, W.H.H.; Lin, S.Y.; Marfella, R.; An International Group of Experts. Clinical guidelines for type 1 diabetes mellitus with an emphasis on older adults: An executive summary. Diabetic Med. 2020, 37, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Dhatariya, K.; James, J.; Kong, M.F.; Berrington, R.; Joint British Diabetes Society (JBDS) for Inpatient Care Group and Guidelines Writing Group. Diabetes at the front door. A guideline for dealing with glucose related emergencies at the time of acute hospital admission from the Joint British Diabetes Society (JBDS) for Inpatient Care Group. Diabetic Med. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Shields, B.M.; Dennis, J.M.; Hattersley, A.T.; McDonald, T.J.; Thomas, N.J. The challenge of diagnosing type 1 diabetes in older adults. Diabetic Med. 2020. [Google Scholar] [CrossRef]
- Janež, A.; Guja, C.; Mitrakou, A.; Lalic, N.; Tankova, T.; Czupryniak, L.; Tabák, A.G.; Prazny, M.; Martinka, E.; Smircic-Duvnjak, L. Insulin therapy in adults with type 1 diabetes mellitus: A narrative review. Diabetes Ther. 2020, 11, 387–409. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.C.; Ahmed, M.; Zaid, M.; Hasnain, S. Biochemical, serological, and genetic aspects related to gene HLA-DQB1 and its association with type 1 diabetes mellitus (T1DM). Mol. Genet. Genom. Med. 2020, 8, e1147. [Google Scholar] [CrossRef]
- Cobo Vuilleumier, N.; Gauthier, B.R. Time for a paradigm shift in treating type 1 diabetes mellitus: Coupling inflammation to islet regeneration. Metabolism 2020, 104, 154137. [Google Scholar] [CrossRef] [Green Version]
- Buzzetti, R.; Zampetti, S.; Pozzilli, P. Impact of obesity on the increasing incidence of type 1 diabetes. Diabetes Obesity Metab. 2020, 22, 1009–1013. [Google Scholar] [CrossRef]
- Dayal, D. COVID-19: Considerations for Children and Adolescents with Diabetes. Preprints 2020, 2020040225. [Google Scholar] [CrossRef]
- Björk, A.; Mandalenakis, Z.; Giang, K.W.; Rosengren, A.; Eriksson, P.; Dellborg, M. Incidence of Type 1 diabetes mellitus and effect on mortality in young patients with congenital heart defect–A nationwide cohort study. Int. J. Cardiol. 2020, 310, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Cobelli, C.; Renard, E.; Kovatchev, B. Artificial pancreas: Past, present, future. Diabetes 2011, 60, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steil, G.; Panteleon, A.; Rebrin, K. Closed-loop insulin delivery—The path to physiological glucose control. Adv. Drug Deliv. Rev. 2004, 56, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Cherrington, A.D.; Sindela, D.; Edgerton, D.; Steine, K.; McGuinness, O.P. Physiological consequences of phasic insulin release in the normal animal. Diabetes 2002, 51, S103–S108. [Google Scholar] [CrossRef] [Green Version]
- Bergman, R.N.; Finegood, D.T.; Ade, M. Assessment of insulin sensitivity in vivo. Endocr. Rev. 1985, 6, 45–86. [Google Scholar] [CrossRef]
- Pratley, R.E.; Foley, J.E.; Dunning, B.E. Rapid acting insulinotropic agents: Restoration of early insulin secretion as a physiologic approach to improve glucose control. Curr. Pharmaceut. Design 2001, 7, 1375–1397. [Google Scholar] [CrossRef]
- Thurmond, D.C.; Herbert, Y.G. Recent insights into beta-cell exocytosis in Type 2 diabetes. J. Mol. Biol. 2020, 432, 1310–1325. [Google Scholar] [CrossRef]
- Van den Berghe, G. First do no harm… Hypoglycemia or hyperglycemia? Crit. Care Med. 2006, 34, 2843–2844. [Google Scholar] [CrossRef]
- Krinsley, J.S. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin. Proc. 2003, 78, 1471–1478. [Google Scholar] [CrossRef] [Green Version]
- Kraegen, E.; Chisholm, D.; McNamara, M.E. Timing of insulin delivery with meals. Horm. Metab. Res. 1981, 13, 365–367. [Google Scholar] [CrossRef]
- Klec, C.; Ziomek, G.; Pichler, M.; Malli, R.; Graier, W.F. Calcium signaling in ß-cell Physiology and Pathology: A Revisit. Int. J. Mol. Sci. 2019, 20, 6110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamborlane, W.V.; Beck, R.W.; Bode, B.W.; Buckingham, B.; Chase, H.P.; Clemons, R.; Fiallo-Scharer, R.; Fox, L.A.; Gilliam, L.K.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N. Engl. J. Med. 2008, 359, 1464–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolinder, J.; Antuna, R.; Geelhoed-Duijvestijn, P.; Kröger, J.; Weitgasser, R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: A multicentre, non-masked, randomised controlled trial. Lancet 2016, 388, 2254–2263. [Google Scholar] [CrossRef]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. J. Am. Med. Assoc. 2017, 317, 371–378. [Google Scholar] [CrossRef]
- Pickup, J.C.; Ford, H.M.; Samsi, K. Real-time continuous glucose monitoring in type 1 diabetes: A qualitative framework analysis of patient narratives. Diabetes Care 2015, 38, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Ritholz, M.D.; Henn, O.; Castillo, A.A.; Wolpert, H.; Edwards, S.; Fisher, L.; Toschi, E. Experiences of adults with type 1 diabetes using glucose sensor–based mobile technology for glycemic variability: Qualitative study. JMIR Diabetes 2019, 4, e14032. [Google Scholar] [CrossRef]
- White, N.D.; Knezevich, E. Flash glucose monitoring technology impact on diabetes self-care behavior. Am. J. Lifestyle Med. 2020, 14, 130–132. [Google Scholar] [CrossRef]
- Kubiak, T.; Priesterroth, L.; Barnard-Kelly, K.D. Psychosocial aspects of diabetes technology. Diabetic Med. 2020, 37, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Alfa, R.W.; Park, S.; Skelly, K.R.; Poffenberger, G.; Jain, N.; Gu, X.; Kockel, L.; Wang, J.; Liu, Y.; Powers, A.C.; et al. Suppression of insulin production and secretion by a decretin hormone. Cell Metab. 2015, 21, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Renstrom, E.; Ding, W.G.; Bokvist, K.; Rorsman, P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron 1996, 17, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Eaton, R.P.; Allen, R.C.; Schade, D.S.; Standefer, J.C. Normal insulin secretion: The goal of artificial insulin delivery systems? Diabetes Care 1980, 3, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.D.; Gerich, J.E. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care 1983, 6, 374–377. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Insulin administration. Diabetes Care 2003, 26, s121–s124. [Google Scholar] [CrossRef] [Green Version]
- Furler, S.M.; Kraegen, E.W.; Smallwood, R.H.; Chisholm, D.J. Blood glucose control by intermittent loop closure in the basal mode: Computer simulation studies with a diabetic model. Diabetes Care 1985, 8, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S.; Bukar, J.; Nagarajan, S. Inhaled insulin. Adv. Drug Deliv. Rev. 1999, 35, 235–247. [Google Scholar] [CrossRef]
- Boss, A.H.; Petrucci, R.; Lorber, D. Coverage of prandial insulin requirements by means of an ultra-rapid-acting inhaled insulin. J. Diabetes Sci. Technol. 2012, 6, 773–779. [Google Scholar] [CrossRef]
- Gajewska, K.A.; Biesma, R.; Bennett, K.; Sreenan, S. Availability of and access to continuous subcutaneous insulin infusion therapy for adults with type 1 diabetes in Ireland. Acta Diabetol. 2020, 57, 875–882. [Google Scholar] [CrossRef]
- Danne, T.; Bangstad, H.J.; Deeb, L.; Jarosz-Chobot, P.; Mungaie, L.; Saboo, B.; Urakami, T.; Battelino, T.; Hanas, R. Insulin treatment in children and adolescents with diabetes. Pediatr. Diabetes 2014, 15, 115–134. [Google Scholar] [CrossRef]
- Phillip, M.; Battelino, T.; Rodriguez, H.; Danne, T.; Kaufman, F. Use of insulin pump therapy in the pediatric age-group: Consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2007, 30, 1653–1662. [Google Scholar]
- Qin, Y.; Yang, L.H.; Huang, X.L.; Chen, X.H.; Yao, H. Efcacy and safety of continuous subcutaneous insulin infusion vs. multiple daily injections on type 1 diabetes children: A meta-analysis of randomized control trials. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 316–323. [Google Scholar]
- Cummins, E.; Royle, P.; Snaith, A.; Greene, A.; Robertson, L.; McIntyre, L.; Waugh, N. Clinical efectiveness and cost-efectiveness of continuous subcutaneous insulin infusion for diabetes: Systematic review and economic evaluation. Health Technol. Assess. 2010, 14, 1. [Google Scholar] [CrossRef]
- Šoupal, J.; Petruželková, L.; Grunberger, G.; Hásková, A.; Flekač, M.; Matoulek, M.; Parkin, C.G. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care 2019, 43, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Roze, S.; Cook, M.; Jethwa, M.; de Portu, S. Projection of long term health-economic benefts of sensor augmented pump (SAP) versus Pump Therapy Alone (CSII) in Type 1 Diabetes, a UK Perspective. Value Health 2014, 17, A348. [Google Scholar]
- Dufus, S.H.; Ta’ani, Z.A.; Slaughter, J.C.; Niswender, K.D.; Gregory, J.M. Increased proportion of time in hybrid closed-loop “Auto Mode” is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes. Metab. 2019, 22, 688–693. [Google Scholar] [CrossRef]
- Jendle, J.; Pohlmann, J.; de Portu, S.; Smith-Palmer, J.; Roze, S. Cost-efectiveness analysis of the MiniMed 670G hybrid closedloop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol. Ther. 2019, 21, 110–118. [Google Scholar] [CrossRef]
- Diabetes Technology Network UK. Best Practice Guide: Continuous Subcutaneous Insulin Infusion (CSII). A Clinical Guide for Adult Diabetes Services; Association of British Clinical Diabetologists: Knowle, UK, 2018. [Google Scholar]
- Neumiller, J. Pharmacologist. Ann. Pharmacother. 2010, 44, 1231–1239. [Google Scholar] [CrossRef]
- McGill, J.B.; Ahn, D.; Edelman, S.V.; Kilpatrick, C.R.; Cavaiola, T.S. Making insulin accessible: Does inhaled insulin fill an unmet need? Adv. Ther. 2016, 33, 1267–1278. [Google Scholar] [CrossRef]
- Siekmeier Rand Scheuch, G. Inhaled insulin–does it become reality? J. Physiol. Pharmacol. 2008, 59 (Suppl. 6), 81–113. [Google Scholar]
- Rave, K.; Bott, S.; Heinemann, L.; Sha, S.; Becker, R.H.; Willavize, S.A.; Heise, T. Time-action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulin. Diabetes Care 2005, 28, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J.; Barnett, A.H. Why is Exubera being withdrawn? BMJ 2007, 335, 1156. [Google Scholar] [CrossRef] [Green Version]
- Santos Cavaiola, T.; Edelman, S. Inhaled insulin: A breath of fresh air? A review of inhaled insulin. Clin. Therapeut. 2014, 36, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Heise, T. Review: Current status of the development of inhaled insulin. Br. J. Diabetes Vasc. Dis. 2004, 4, 295–301. [Google Scholar] [CrossRef]
- Heinemann, L.; Parkin, C.G. Rethinking the viability and utility of inhaled insulin in clinical practice. J. Diabetes Res. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Easa, N.; Alany, R.G.; Carew, M.; Vangala, A. A review of non-invasive insulin delivery systems for diabetes therapy in clinical trials over the past decade. Drug Discov. Today 2019, 24, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Pettus, J.; Santos Cavaiola, T.; Edelman, S.V. Recommendations for initiating use of Afrezza inhaled insulin in individuals with type 1 diabetes. Diabetes Technol. Therapeut. 2018, 20, 448–451. [Google Scholar] [CrossRef]
- Liu, H.; Shan, X.; Yu, J.; Li, X.; Hu, L. Recent advances in inhaled formulations and pulmonary insulin delivery systems. Curr. Pharmaceut. Biotechnol. 2019, 21, 180–193. [Google Scholar] [CrossRef]
- Mohanty, R.R.; Das, S. Inhaled insulin-current direction of insulin research. J. Clin. Diagn. Res. 2017, 11, OE01–OE02. [Google Scholar] [CrossRef]
- Wilson, L.M.; Castle, J.R. Recent advances in insulin therapy. Diabetes Technol. Therapeut. 2020, 10, 379–384. [Google Scholar] [CrossRef]
- Ehlbeck, K.; Moonesinghe, K. Is inhaled insulin as effective as SC insulin in the management of diabetes mellitus? Evid. Based Pract. 2020, 23, 37–38. [Google Scholar] [CrossRef]
- Binder, C.; Lauritzen, T.; Faber, O.; Pramming, S. Insulin pharmacokinetics. Diabetes Care 1984, 7, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Patton, J.S.; Bukar, J.G.; Eldon, M.A. Clinical pharmacokinetics and pharmacodynamics of inhaled insulin. Clin. Pharmacokinet. 2004, 43, 781–801. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.H.; Frick, A.D. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin. Pharmacokinet. 2008, 47, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, M.J.; Candelino, N.; Mehrvarz, A.; Jalili, N. Physiological closed-loop control (PCLC) systems: Review of a modern frontier in automation. arXiv 2019, arXiv:1910.03768. [Google Scholar] [CrossRef]
- American Diabetes Association. 8. pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41 (Suppl. 1), S73–S85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, L.E. Insulin. In Diabetes in Pregnancy; Springer: New York, NY, USA, 2018; pp. 87–101. [Google Scholar]
- Medtronic. Multiple Daily Injections Insulin Therapy. Available online: https://www.medtronic.com/ca-en/diabetes/home/what-is-diabetes/insulin-therapy/mdi.html (accessed on 15 June 2020).
- Joshi, M.; Choudhary, P. Multiple daily injections or insulin pump therapy: Choosing the best option for your patient—An evidence-based approach. Curr. Diabetes Rep. 2015, 15, 81. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, U.; Kristensen, P.L.; Beck-Nielsen, H.; Nørgaard, K.; Perrild, H.; Christiansen, J.S.; Jensen, T.; Hougaard, P.; Parving, H.H.; Thorsteinsson, B.; et al. Effect of insulin analogues on risk of severe hypoglycaemia in patients with type 1 diabetes prone to recurrent severe hypoglycaemia (HypoAna trial): A prospective, randomised, open-label, blinded-endpoint crossover trial. Lancet Diabetes Endocrinol. 2014, 2, 553–561. [Google Scholar] [CrossRef]
- Cavan, D.A.; Ziegler, R.; Cranston, I.; Barnard, K.; Ryder, J.; Vogel, C.; Parkin, C.G.; Koehler, W.; Vesper, I.; Petersen, B.; et al. Automated bolus advisor control and usability study (ABACUS): Does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? BMC Fam. Pract. 2012, 13, 102. [Google Scholar] [CrossRef] [Green Version]
- Parkin, C.G.; Barnard, K.; Hinnen, D.A. Safe and efficacious use of automated bolus advisors in individuals treated with multiple daily insulin injection (MDI) therapy: Lessons learned from the automated bolus advisor control and usability study (ABACUS). J. Diabetes Sci. Technol. 2015, 9, 1138–1142. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Meldgaard, M.; Serifovski, N.; Storm, C.; Christensen, T.M.; Gade-Rasmussen, B.; Nørgaard, K. Use of an automated bolus calculator in MDI-treated type 1 diabetes: The BolusCal study, a randomized controlled pilot study. Diabetes Care 2012, 35, 984–990. [Google Scholar] [CrossRef] [Green Version]
- Pathak, V.; Pathak, N.M.; O’Neill, C.L.; Guduric-Fuchs, J.; Medina, R.J. Therapies for Type 1 Diabetes: Current scenario and future perspectives. Clin. Med. Insights Endocrinol. Diabetes 2019, 12. [Google Scholar] [CrossRef]
- Pozzilli, P.; Battelino, T.; Danne, T.; Hovorka, R.; Jarosz-Chobot, P.; Renard, E. Continuous subcutaneous insulin infusion in diabetes: Patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab. Res. Rev. 2016, 32, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.; Keen, H.; Parsons, J.; Alberti, K. Continuous subcutaneous insulin infusion: An approach to achieving normoglycaemia. BMJ 1978, 1, 204–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baru, A.; Amir, S.; Ekelund, M.; Montagnoli, R.; Da Rocha Fernandes, J.D. A survey of physician experience and treatment satisfaction using fast-acting insulin aspart in people with type 1 or type 2 diabetes. Postgrad. Med. 2020, 132, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickup, J. Insulin pumps. Int. J. Clin. Pract. Suppl. 2011, 170, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C. Insulin-pump therapy for type 1 diabetes mellitus. N. Engl. J. Med. 2012, 366, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.B.; Bode, B.W.; Garg, S.; Lane, W.S.; Sussman, A.; Hu, P.; Santiago, O.M.; Kolaczynski, J.W. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005, 28, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.B.; Patel, M.; Maahs, D.M.; Shah, V.N. Insulin delivery methods: Past, present and future. Int. J. Pharmaceut. Investig. 2016, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Parker, R.S.; Doyle, F.J.; Peppas, N.A. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans. Biomed. Eng. 1999, 46, 148–157. [Google Scholar] [CrossRef]
- Candas, B.; Radziuk, J. An adaptive plasma glucose controller based on a nonlinear insulin/glucose model. IEEE Trans. Biomed. Eng. 1994, 41, 116–124. [Google Scholar] [CrossRef]
- Galadanci, J.; Shafik, R.A.; Mathew, J.; Acharyya, A.; Pradhan, D.K. A closed-loop control strategy for glucose control in artificial pancreas systems. In Proceedings of the 2012 International Symposium on Electronic System Design (ISED), Kolkata, India, 19–22 December 2012; pp. 295–299. [Google Scholar]
- Sun, L.; Kwok, E.; Gopaluni, B.; Vahidi, O. A feedback glucose control strategy for type II diabetes mellitus. In Proceedings of the ADNOCIP 2011: International Symposium on Advanced Control of Industrial Processes, Hangzhou, Zhejiang, China, 23–27 May 2011; pp. 349–352. [Google Scholar]
- Elleri, D.; Dunger, D.B.; Hovorka, R. Closed-loop insulin delivery for treatment of type 1 diabetes. BMC Med. 2011, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- El Hachimi, M.; Ballouk, A.; Lebbar, H. Overcoming control challenges in the artificial pancreas. In Proceedings of the 2016 11th International Conference on Intelligent Systems: Theories and Applications (SITA 2016), Mohammedia, Morocco, 19–20 October 2016; pp. 1–6. [Google Scholar]
- Palerm, C.C. Physiologic insulin delivery with insulin feedback: A control systems perspective. IFAC Proc. 2009, 42, 31–36. [Google Scholar] [CrossRef]
- El Youssef, J.; Castle, J.; Ward, W.K. A review of closed-loop algorithms for glycemic control in the treatment of type 1 diabetes. Algorithms 2009, 2, 518–532. [Google Scholar] [CrossRef] [Green Version]
- Steil, G.M.; Rebrin, K.; Darwin, C.; Hariri, F.; Saad, M.F. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006, 55, 3344–3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, V.; Awais, Q. Diabetes Millitus Control Exogenous Insulin Infusion: A Review. Pak. J. Eng. Technol. 2020, 3, 18–23. [Google Scholar]
- Steil, G.M.; Palerm, C.C.; Kurtz, N.; Voskanyan, G.; Roy, A.; Paz, S.; Kandeel, F.R. The effect of insulin feedback on closed loop glucose control. J. Clin. Endocrinol. Metab. 2011, 96, 1402–1408. [Google Scholar] [CrossRef] [Green Version]
- Chee, F.; Fernando, T.L.; Savkin, A.V.; Van Heeden, V. Expert PID control system for blood glucose control in critically ill patients. IEEE Trans. Inf. Technol. Biomed. 2003, 7, 419–425. [Google Scholar] [CrossRef]
- Fuchs, J.; Hovorka, R. Closed-loop control in insulin pumps for type-1 diabetes mellitus: Safety and efficacy. Expert Rev. Med Devices 2020. [Google Scholar] [CrossRef]
- Yadav, J.; Rani, A.; Singh, V. Performance analysis of fuzzy-PID controller for blood glucose regulation in type- 1 diabetic patients. J. Med. Syst. 2016, 40, 254. [Google Scholar] [CrossRef]
- Ramprasad, Y.; Rangaiah, G.P.; Lakshminarayanan, S. Robust PID controller for blood glucose regulation in type I diabetics. Ind. Eng. Chem. Res. 2004, 43, 8257–8268. [Google Scholar] [CrossRef]
- Shijo, J.K.; Palani, T.K.; Kumar, S.S. Design of controllers for T1DM blood glucose insulin dynamics based on constrained firefly algorithm. In Proceedings of the 2018 4th International Conference on Electrical Energy Systems (ICEES), Chennai, India, 7–9 February 2018; pp. 116–120. [Google Scholar]
- Huyett, L.M.; Dassau, E.; Zisser, H.C.; Doyle, F.J., III. Design and evaluation of a robust PID controller for a fully implantable artificial pancreas. Ind. Eng. Chem. Res. 2015, 54, 10311–10321. [Google Scholar] [CrossRef]
- Lee, J.J.; Dassau, E.; Zisser, H.; Tamborlane, W.; Weinzimer, S.; Doyle, F.J. The impact of insulin pharmacokinetics and pharmacodynamics on the closedloop artificial pancreas. In Proceedings of the IEEE Conference on Decision and Control, Florence, Italy, 10–13 December 2013; pp. 127–132. [Google Scholar]
- Huyett, L.M.; Dassau, E.; Zisser, H.C.; Doyle, F.J., III. Glucose sensor dynamics and the artificial pancreas: The impact of lag on sensor measurement and controller performance. IEEE Control Syst. Mag. 2018, 38, 30–46. [Google Scholar] [CrossRef]
- Turksoy, K.; Cinar, A. Adaptive control of artificial pancreas systems-a review. J. Healthcare Eng. 2014, 5, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovc, K.; Battelino, T. Closed-loop insulin delivery systems in children and adolescents with type 1 diabetes. Expert Opin. Drug Deliv. 2020, 17, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.G.; Mekhiel, N. New microprocessor-based insulin controller. IEEE Trans. Biomed. Eng. 1983, 30, 11. [Google Scholar] [CrossRef]
- Shainer, G.; Inbar, G.F. Model development and controller desing for artificial pancreas. In Proceedings of the European Control Conference (ECC), Porto, Portugal, 4–7 September 2001. [Google Scholar]
- Ionescu, C.; De Keyser, R. EPSAC Predictive control of blood glucose level in type i diabetic patients. In Proceedings of the 44th IEEE Conference on Decision and Control, and the European Control Conference 2005, Seville, Spain, 12–15 December 2005. [Google Scholar]
- Bequette, B.W. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu. Rev. Control 2012, 36, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Haque, S.; Paul, P.S.; Ahmed, M.S.; Zaman, M.A.U.; Mannan, M.A. Performance studies of different closed loop glucose controllers for treating type 1 diabetes mellitus. In Proceedings of the 2015 3rd International Conference on Advances in Electrical Engineering, Dhaka, Bangladesh, 17–19 December 2015. [Google Scholar]
- Campos-Delgado, D.U.; Femat, R.; Gordillo-Moscoso, A. Fuzzy-based controller for glucose regulation in type-1 diabetic patients by subcutaneous route. IEEE Trans. Biomed. Eng. 2006, 53, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, R. Simulation Study on Blood Glucose Control in Diabetics; Institute of Biomedical Engineering; Yan Shan University: Qinhuangdao, China, 2007; pp. 1103–1106. [Google Scholar]
- Maleki, A.; Geramipour, A. Continuous control of blood glucose in tidm using fuzzy logic controller in insulin pump: A simulation study. In Proceedings of the 2011 2nd International Conference on Control, Instrumentation and Automation (ICCIA), Shiraz, Iran, 27–29 December 2011. [Google Scholar]
- Soylu, S.; Danışman, K.; Saçu, İ.E.; Alçı, M. Closed-Loop Control of Blood Glucose Level in Type-1 Diabetics: A Simulation Study; Erciyes University, Department of Electrical and Electronics Engineering: Kayseri, Turkey, 2013. [Google Scholar]
- Sawsan, M.; Gharghory, D.; El-Dib, A.; Mahmoud, M. Low power fuzzy control system for adjusting the blood glucose level. In Proceedings of the 2016 28th International Conference on Microelectronics, Giza, Egypt, 17–20 December 2016; pp. 333–336. [Google Scholar]
- Marchetti, G.; Barolo, M.; Jovanovic, L.; Zisser, H.; Seborg, D.E. An improved PID switching control strategy for type 1 diabetes. IEEE Trans. Biomed. Eng. 2008, 55, 3. [Google Scholar] [CrossRef] [PubMed]
- Soylu, S.; Danisman, K.; Alçı, M. Closed-loop control of blood glucose level in type-1 diabetics: A simulation study. In Proceedings of the 2013 8th International Conference on Electrical and Electronics Engineering (ELECO), Bursa, Turkey, 28–30 November 2013; pp. 371–375. [Google Scholar]
- Sarti, E.; Cruciani, P. Self-tuning control algorithm for wearable artificial pancreas. In Proceedings of the 14th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Paris, France, 29 October–1 November 1992; pp. 2267–2269. [Google Scholar]
- Eigner, G.; Tar, J.K. Adaptive control solution for T1DM control. In Proceedings of the 2015 IEEE 10th Jubilee International Symposium on Applied Computational Intelligence and Informatics, Timisoara, Romania, 21–23 May 2015; pp. 215–220. [Google Scholar]
- Bhitre, N.; Padhi, R. An adaptive insulin infusion approach for customized blood glucose regulation of type i diabetic patients. In Proceedings of the 2011 IEEE International Conference on Control Applications (CCA), Denver, CO, USA, 28–30 September 2011; pp. 127–132. [Google Scholar]
- Kaveh, P.; Shtessel, Y.B. Blood glucose regulation using higher-order sliding mode control. Int. J. Robust. Nonlinear Control 2008, 18, 557–569. [Google Scholar] [CrossRef]
- Abu-Rmileh, A.; Garcia-Gabin, W.; Zambrano, D. Internal model sliding mode control approach for glucose regulation in type 1 diabetes. Biomed. Signal Process. Control 2010, 5, 94–102. [Google Scholar] [CrossRef]
- Zavitsanou, S.; Mantalaris, A. In silico closed-loop control validation studies for optimal insulin delivery in type 1 diabetes. IEEE Trans. Biomed. Eng. 2015, 62, 2369–2378. [Google Scholar] [CrossRef]
- Aicha, H.; Mourad, A. H-infinity controller design for blood glucose regulation in diabetes patients in the presence of uncertain parameters. In Proceedings of the 2015 3rd International Conference on Control, Engineering & Information Technology (CEIT), Tlemcen, Algeria, 25–27 May 2015; pp. 1–6. [Google Scholar]
- Batmani, Y. Blood glucose concentration control for type 1 diabetic patients: A nonlinear suboptimal approach. IET Syst. Biol. 2017, 11, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.C.; Kamath, S.; Vidyasagar, S. Blood glucose regulation and control of insulin and glucagon infusion using single model predictive control for type 1 diabetes mellitus. IET Syst. Biol. 2020, 14, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Patek, S.D.; Magni, L.; Dassau, E.; Karvetski, C.; Toffanin, C.; De Nicolao, G.; Del Favero, S.; Breton, M.; Dalla Man, C.; Renard, E.; et al. Modular closed-loop control of diabetes. IEEE Trans. Biomed. Eng. 2012, 59, 2986–2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, F.J.; Huyett, L.M.; Lee, J.B.; Zisser, H.C.; Dassau, E. Closed-loop artificial pancreas systems: Engineering the algorithms. Diabetes Care 2014, 37, 1191–1197. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.C.; Mastrototaro, J.J.; Moberg, S.B.; Mueller, J.C., Jr.; Clark, H.B.; Tolle, M.C.V.; Williams, G.L.; Wu, B.; Steil, G.M. Algorithm Sensoraugmented Bolus Estimator for Semi-Closed-Loop Infusion System. U.S. Patent 9.320.471, 26 April 2016. [Google Scholar]
- Jacobs, P.G.; Youssef, J.E.; Castle, J.R.; Engle, J.M.; Branigan, D.L.; Johnson, P.; Massoud, R.; Kamath, A.; Ward, W.K. Development of a fully automated closed-loop artificial pancreas control system with dual pump delivery of insulin and glucagon. In Proceedings of the 2011 Annual International Conference IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 397–400. [Google Scholar]
- Boughton, C.K.; Hovorka, R. Is an artificial pancreas (closed-loop system) for type 1 diabetes effective? Diabetes Med. 2019, 36, 279–286. [Google Scholar] [CrossRef]
- Wang, L. Model Predictive Control System Design and Implementation Using MATLAB®; Springer: London, UK, 2009. [Google Scholar]
- Dougherty, D.; Cooper, D. A practical multiple model adaptive strategy for multivariable model predictive control. Control Eng. Pract. 2003, 11, 649–664. [Google Scholar] [CrossRef]
- Villa-Tamayo, M.F.; Rivadeneira, P.S. Adaptive impulsive offset-free MPC to handle parameter variations for type 1 diabetes treatment. Ind. Eng. Chem. Res. 2020, 59, 5865–5876. [Google Scholar] [CrossRef]
- Nath, A.; Biradar, S.; Balan, A.; Dey, R.; Padhi, R. Physiological models and control for type 1 diabetes mellitus: A brief review. IFAC PapersOnLine 2018, 51, 289–294. [Google Scholar] [CrossRef]
- Schaller, S.; Lippert, J.; Schaupp, L.; Pieber, T.R.; Schuppert, A.; Eissing, T. Robust pbpk/pdbased model predictive control of blood glucose. IEEE Trans. Biomed. Eng. 2016, 63, 1492–1504. [Google Scholar] [CrossRef]
- Aradóttir, T.B.; Boiroux, D.; Bengtsson, H.; Kildegaard, J.; Jensen, M.L.; Jørgensen, J.B.; Poulsen, N.K. Model predictive control for dose guidance in long acting insulin treatment of type 2 diabetes. IFAC J. Syst. Control 2019, 9, 100067. [Google Scholar] [CrossRef]
- Aradóttir, T.B.; Boiroux, D.; Bengtsson, H.; Jørgensen, J.B.; Poulsen, N.K. Model predictive control with sub-frequency actuation for long acting insulin treatment in type 2 diabetes. In Proceedings of the 2019 IEEE Conference on Control Technology and Applications (CCTA), Hong Kong, China, 19–21 August 2019. [Google Scholar]
- Incremona, G.P.; Messori, M.; Toffanin, C.; Cobelli, C.; Magni, L. Model predictive control with integral action for artificial pancreas. Control Eng. Practice 2018, 77, 86–94. [Google Scholar] [CrossRef]
- Boiroux, D.; Bátora, V.; Hagdrup, M.; Wendt, S.L.; Poulsen, N.K.; Madsen, H.; Jørgensen, J.B. Adaptive model predictive control for a dual-hormone artificial pancreas. J. Process Control 2018, 68, 105–117. [Google Scholar] [CrossRef]
- Soru, P.; De Nicolao, G.; Toffanin, C.; Dalla Man, C.; Cobelli, C.; Magni, L.; AP@ Home Consortium. MPC based artificial pancreas: Strategies for individualization and meal compensation. Annu. Rev. Control 2012, 36, 118–128. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Kumbasar, T. A new fractional-order general type-2 fuzzy predictive control system and its application for glucose level regulation. Appl. Soft Comput. 2020, 91, 106241. [Google Scholar] [CrossRef]
- Bianchi, F.D.; Moscoso-Vásquez, M.; Colmegna, P.; Sánchez-Pena, R.S. Invalidation and low-order model set for artificial pancreas robust control design. J. Process Control 2019, 76, 133–140. [Google Scholar] [CrossRef]
- Moscoso-Vásquez, M.; Colmegna, P.; Rosales, N.; Garelli, F.; Sanchez-Pena, R. Control-oriented model with intra-patient variations for an artificial pancreas. IEEE J. Biomed. Health Inform. 2020. [Google Scholar] [CrossRef]
- Masuda, K.; Uchiyama, K. Simply Robust Control Strategy Based on Model Predictive Control. In Proceedings of the IEEE 2020 SICE International Symposium on Control Systems (SICE ISCS), Tokushima, Japan, 3–5 March 2020; pp. 99–106. [Google Scholar]
- Kovatchev, B.P.; Breton, M.; Dalla Man, C.; Cobelli, C. In silico preclinical trials: A proof of concept in closed-loop control of type 1 diabetes. J. Diabetes Sci. Technol. 2009, 3, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Man, C.D.; Micheletto, F.; Lv, D.; Breton, M.; Kovatchev, B.; Cobelli, C. The UVA/PADOVA type 1 diabetes simulator: New features. J. Diabetes Sci. Technol. 2014, 8, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Visentin, R.; Dalla Man, C.; Kovatchev, B.; Cobelli, C. The university of Virginia/Padova type 1 diabetes simulator matches the glucose traces of a clinical trial. Diabetes Technol. Therapeut. 2014, 16, 428–434. [Google Scholar] [CrossRef]
- Toffanin, C.; Visentin, R.; Messori, M.; Di Palma, F.; Magni, L.; Cobelli, C. Toward a run-to-run adaptive artificial pancreas: In silico results. IEEE Trans. Biomed. Eng. 2017, 65, 479–488. [Google Scholar] [CrossRef]
- Visentin, R.; Campos-Náñez, E.; Schiavon, M.; Lv, D.; Vettoretti, M.; Breton, M.; Kovatchev, B.P.; Dalla Man, C.; Cobelli, C. The UVA/Padova type 1 diabetes simulator goes from single meal to single day. J. Diabetes Sci. Technol. 2018, 12, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.; Park, S.W.; Jin, S.M.; Park, S.M. Toward a fully automated artificial pancreas system using a bioinspired reinforcement learning design: In silico validation. IEEE J. Biomed. Health Inform. 2020. [Google Scholar] [CrossRef]
- Visentin, R.; Schiavon, M.; Giegerich, C.; Klabunde, T.; Dalla Man, C.; Cobelli, C. Incorporating long-acting insulin glargine into the UVA/padova Type 1 diabetes simulator for in silico testing of MDI therapies. IEEE Trans. Biomed. Eng. 2019, 66, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Deb, D.; Dey, R. An augmented subcutaneous type 1 diabetic patient modelling and design of adaptive glucose control. J. Process Control 2020, 86, 94–105. [Google Scholar] [CrossRef]
- Hajizadeh, I.; Samadi, S.; Sevil, M.; Rashid, M.; Cinar, A. Performance assessment and modification of an adaptive model predictive control for automated insulin delivery by a multivariable artificial pancreas. Ind. Eng. Chem. Res. 2019, 58, 11506–11520. [Google Scholar] [CrossRef]
- Calupiña, D.; García, A.; Camacho, O.; Rosales, A.; Rivadeneira, P. Non-linear PID and Dynamic SMC for the Artificial Pancreas control in the treatment of Type 1 Diabetes. In Proceedings of the 2018 IEEE Third Ecuador Technical Chapters Meeting (ETCM), Cuenca, Ecuador, 15–19 October 2018; pp. 1–6. [Google Scholar]
- Karimi-Maleh, H.; Cellat, K.; Arıkan, K.; Savk, A.; Karimi, F.; Şen, F. Palladium–nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater. Chem. Phys. 2020, 250, 123042. [Google Scholar] [CrossRef]
- Ramirez-Vargas, M.A.; Flores-Alfaro, E.; Uriostegui-Acosta, M.; Alvarez-Fitz, P.; Parra-Rojas, I.; Moreno-Godinez, M.E. Effects of exposure to malathion on blood glucose concentration: A meta-analysis. Environ. Sci. Pollut. Res. 2018, 25, 3233–3242. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [Green Version]
- Makaram, P.; Owens, D.; Aceros, J. Trends in nanomaterial-based non-invasive diabetes sensing technologies. Diagnostics 2014, 4, 27–46. [Google Scholar] [CrossRef]
- Klonoff, D.C. Fog computing and edge computing architectures for processing data from diabetes devices connected to the medical internet of things. J. Diabetes Sci. Technol. 2017, 11, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Pickering, D.; Marsden, J. How to measure blood glucose. Commun. Eye Health 2014, 27, 56–57. [Google Scholar]
- Patton, S.R.; Clements, M.A. Continuous glucose monitoring versus self-monitoring of blood glucose in children with type 1 diabetes-are there pros and cons for both? US Endocrinol. 2012, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardacci, E.A.; Bode, B.W.; Hirsch, I.B. Individualizing care for the many: The evolving role of professional continuous glucose monitoring systems in clinical practice. Diabetes Educ. 2010, 36 (Suppl. 1), 4S–19S, quiz 20S–21S. [Google Scholar] [CrossRef]
- Wadwa, R.P.; Fiallo-Scharer, R.; VanderWel, B.; Laurel, H.; Messer, E.C.; Chase, H.P. Continuous glucose monitoring in youth with type 1 diabetes. Diabetes Technol. Therapeut. 2009, 11, S83. [Google Scholar] [CrossRef]
- Berg, A.K.; Olsen, B.S.; Thyssen, J.P.; Zachariae, C.; Simonsen, A.B.; Pilgaard, K.; Svensson, J. High frequencies of dermatological complications in children using insulin pumps or sensors. Pediatr. Diabetes 2018, 19, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, M.E.; Levy, W.; Anderson, B.J.; Whitehouse, A.L.; Commissariat, P.V.; Harrington, K.R.; Laffel, L.M.; Miller, K.M.; Van Name, M.; Tamborlane, W.V.; et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol. Therapeut. 2019, 21, 493–498. [Google Scholar] [CrossRef]
- Ajjan, R.; Slattery, D.; Wright, E. Continuous glucose monitoring: A brief review for primary care practitioners. Adv. Ther. 2019, 36, 579–596. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.Y.; Bailey, T.S. Benefits and limitations of continuous glucose monitoring in type 1 diabetes. Exp. Rev. Endocrinol. Metab. 2020, 15, 41–49. [Google Scholar] [CrossRef]
- Klemen, D.; Battelino, T. Evolution of diabetes technology. Endocrinol. Metab. Clin. 2020, 49, 1–18. [Google Scholar]
- Boucher, S.E.; Aum, S.H.; Crocket, H.R.; Wiltshire, E.J.; Tomlinson, P.A.; de Bock, M.I.; Wheeler, B.J. Exploring parental perspectives after commencement of flash glucose monitoring for type 1 diabetes in adolescents and young adults not meeting glycaemic targets: A qualitative study. Diabet. Med. 2020, 37, 657–664. [Google Scholar] [CrossRef]
- Saiti, K.; Macaš, M.; Lhotská, L.; Štechová, K.; Pithová, P. Ensemble methods in combination with compartment models for blood glucose level prediction in type 1 diabetes mellitus. Comput. Methods Programs Biomed. 2020, 196, 105628. [Google Scholar] [CrossRef] [PubMed]
- Woldaregay, A.Z.; Årsand, E.; Walderhaug, S.; Albers, D.; Mamykina, L.; Botsis, T.; Hartvigsen, G. Data-driven modeling and prediction of blood glucose dynamics: Machine learning applications in type 1 diabetes. Artif. Intell. Med. 2019, 98, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Shokrekhodaei, M.; Quinones, S. Review of non-invasive glucose sensing techniques: Optical, electrical and breath acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westphal, P.; Kaltenbach, J.M.; Wicker, K. Corneal birefringence measured by spectrally resolved Mueller matrix ellipsometry and implications for non-invasive glucose monitoring. Biomed. Optics Express 2016, 7, 1160–1174. [Google Scholar] [CrossRef] [Green Version]
- Velasco Cabo, J.M.; Hidalgo Pérez, J.I. Identification of blood glucose patterns in patients with type1 diabetes using continuous glucose monitoring and clustering techniques. Endocrinolog. Diabetes Nutr. 2020. [Google Scholar] [CrossRef]
- Haidar, A. The artificial pancreas: How closed-loop control is revolutionizing diabetes. IEEE Control Syst. Mag. 2016, 36, 28–47. [Google Scholar]
- Miller, S.; Nimri, R.; Atlas, E.; Grunberg, E.A.; Phillip, M. Grunberg, and Moshe Phillip. Automatic learning algorithm for the MD-logic artificial pancreas system. Diabetes Technol. Ther. 2011, 13, 983–990. [Google Scholar] [CrossRef]
- Ossai, C.I.; Wickramasinghe, N. Intelligent Therapeutic Decision Support for 30 days Readmission of Diabetic Patients with Different Comorbidities. J. Biomed. Inform. 2020, 107, 103486. [Google Scholar] [CrossRef]
- Quiroz, G. The evolution of control algorithms in artificial pancreas: A historical perspective. Annu. Rev. Control 2019, 48, 222–232. [Google Scholar] [CrossRef]
- Tyler, N.S.; Jacobs, P.G. Artificial Intelligence in Decision Support Systems for Type 1 Diabetes. Sensors 2020, 20, 3214. [Google Scholar] [CrossRef]
- Zhu, T.; Li, K.; Herrero, P.; Georgiou, P. Basal glucose control in type 1 diabetes using deep reinforcement learning: An in silico validation. arXiv 2020, arXiv:2005.09059. [Google Scholar]
- Knebel, T.; Neumiller, J.J. Medtronic MiniMed 670G hybrid closed-loop system. Clin. Diabetes 2019, 37, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Elshimy, G.; Correa, R. Updates on technology for diabetes mellitus. Curr. Emergency Hospital Med. Rep. 2020, 8, 35–39. [Google Scholar] [CrossRef]
- Hjerde, S.T.N. Evaluating Deep Q-Learning Techniques for Controlling Type 1 Diabetes. Master’s Thesis, UiT Norges arktiske universitet, Tromsø, Norway, 2020. [Google Scholar]
- Neinstein, A.; Wong, J.; Look, H.; Arbiter, B.; Quirk, K.; McCanne, S.; Sun, Y.; Blum, M.; Adi, S. A case study in open source innovation: Developing the Tidepool Platform for interoperability in type 1 diabetes management. J. Am. Med. Inform. Assoc. 2016, 23, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.C.; Izadi, Z.; Schroeder, S.; Nader, M.; Min, J.; Neinstein, A.B.; Adi, S. A pilot study of use of a software platform for the collection, integration, and visualization of diabetes device data by health care providers in a multidisciplinary pediatric setting. Diabetes Technol. Therapeut. 2018, 20, 806–816. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diabetes technology: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43 (Suppl. 1), S77–S88. [Google Scholar] [CrossRef] [Green Version]
- Grosman, B.; Ilany, J.; Roy, A.; Kurtz, N.; Wu, D.; Parikh, N.; Voskanyan, G.; Konvalina, N.; Mylonas, C.; Gottlieb, R.; et al. Hybrid closed-loop insulin delivery in type 1 diabetes during supervised outpatient conditions. J. Diabetes Sci. Technol. 2016, 10, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Bergenstal, R.M.; Garg, S.; Weinzimer, S.A.; Buckingham, B.A.; Bode, B.W.; Tamborlane, W.V.; Kaufman, F.R. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. Jama 2016, 316, 1407–1408. [Google Scholar] [CrossRef]
- De Bock, M.; Dart, J.; Roy, A.; Davey, R.; Soon, W.; Berthold, C.; Retterath, A.; Grosman, B.; Kurtz, N.; Davis, E.; et al. Exploration of the performance of a hybrid closed loop insulin delivery algorithm that includes insulin delivery limits designed to protect against hypoglycemia. J. Diabetes Sci. Technol. 2017, 11, 68–73. [Google Scholar] [CrossRef]
- Tagougui, S.; Taleb, N.; Molvau, J.; Nguyen, É.; Raffray, M.; Rabasa-Lhoret, R. Artificial pancreas systems and physical activity in patients with type 1 diabetes: Challenges, adopted approaches, and future perspectives. J. Diabetes Sci. Technol. 2019, 13, 1077–1090. [Google Scholar] [CrossRef]
- Riddell, M.C.; Sam, N.; Scott, P.A.; Fournier, S.R.; Colberg, I.W.; Othmar Moser, G.; Stettler, C. The competitive athlete with type 1 diabetes. Diabetologia 2020, 63, 1475–1490. [Google Scholar] [CrossRef]
- Kushner, T.; Bequette, B.W.; Cameron, F.; Forlenza, G.; Maahs, D.; Sankaranarayanan, S. Models, devices, properties, and verification of artificial pancreas systems. In Automated Reasoning for Systems Biology and Medicine; Springer: Cham, Switzerland, 2019; pp. 93–131. [Google Scholar]
- Weaver, K.W.; Hirsch, I.B. The hybrid closed-loop system: Evolution and practical applications. Diabetes Technol. Therapeut. 2018, 20, S2–S16. [Google Scholar] [CrossRef]
- Kovatchev, B. Automated closed-loop control of diabetes: The artificial pancreas. Bioelectron. Med. 2018, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Zavitsanou, S.; Chakrabarty, A.; Dassau, E.; Doyle, F.J. Embedded control in wearable medical devices: Application to the artificial pancreas. Processes 2016, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Bleris, L.G.; Kothare, M.V. Real-time implementation of model predictive control. In Proceedings of the American Control Conference (ACC), Portland, OR, USA, 8–10 June 2005; pp. 4166–4171. [Google Scholar]
- Garg, S.K.; Rodbard, D.; Hirsch, I.B.; Forlenza, G.P. Managing new-onset type 1 diabetes during the COVID-19 pandemic: Challenges and opportunities. Diabetes Technol. Therapeut. 2020. [Google Scholar] [CrossRef]
- Welsh, J.B.; Hu, G.; Walker, T.C.; Sharma, N.; Cherñavvsky, D. Glucose monitoring and diabetes management in the time of Coronavirus disease 2019. J. Diabetes Sci. Technol. 2020. [Google Scholar] [CrossRef]

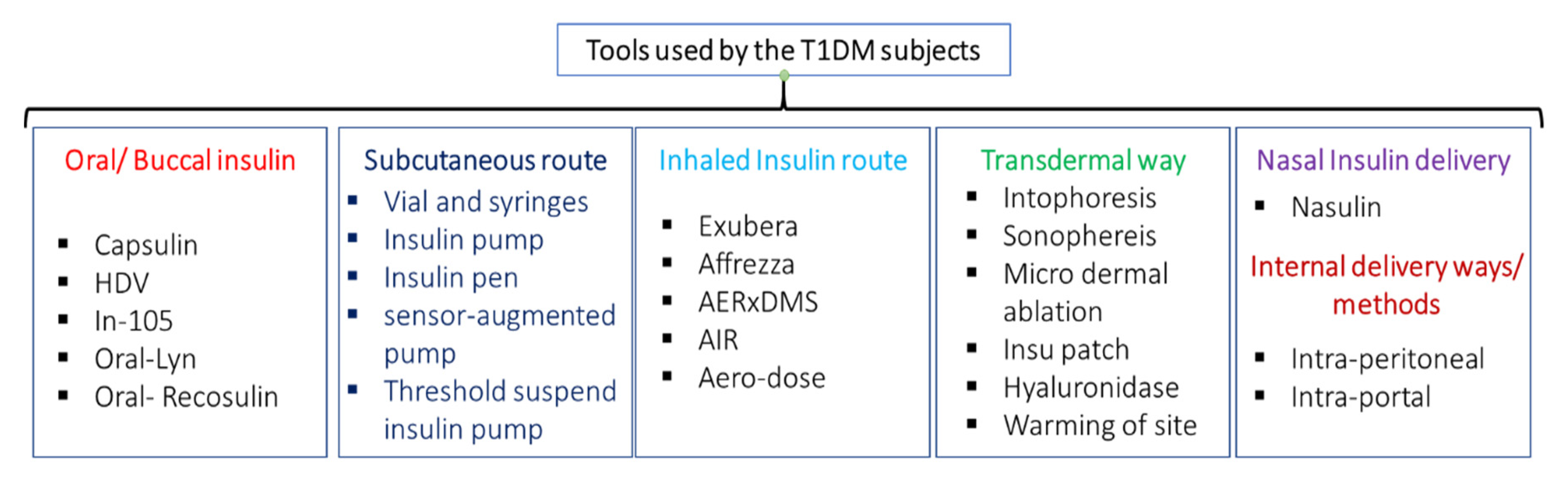
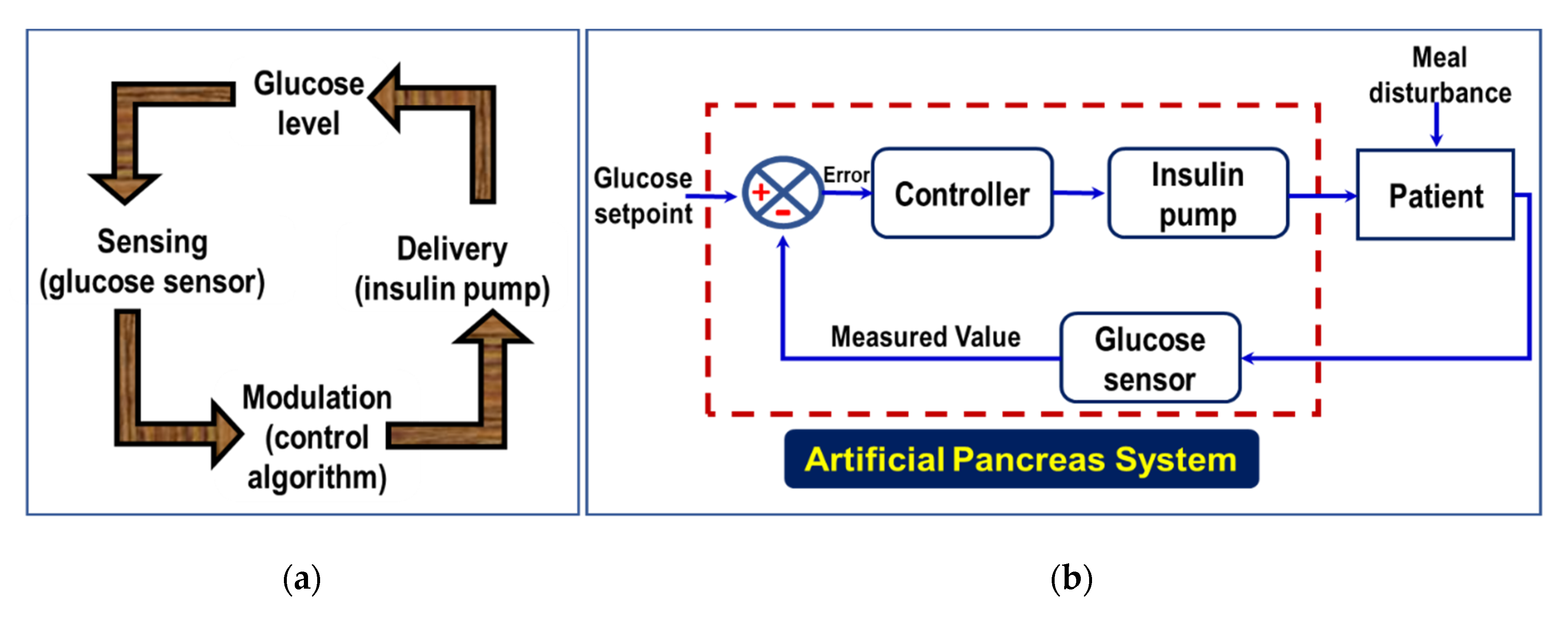
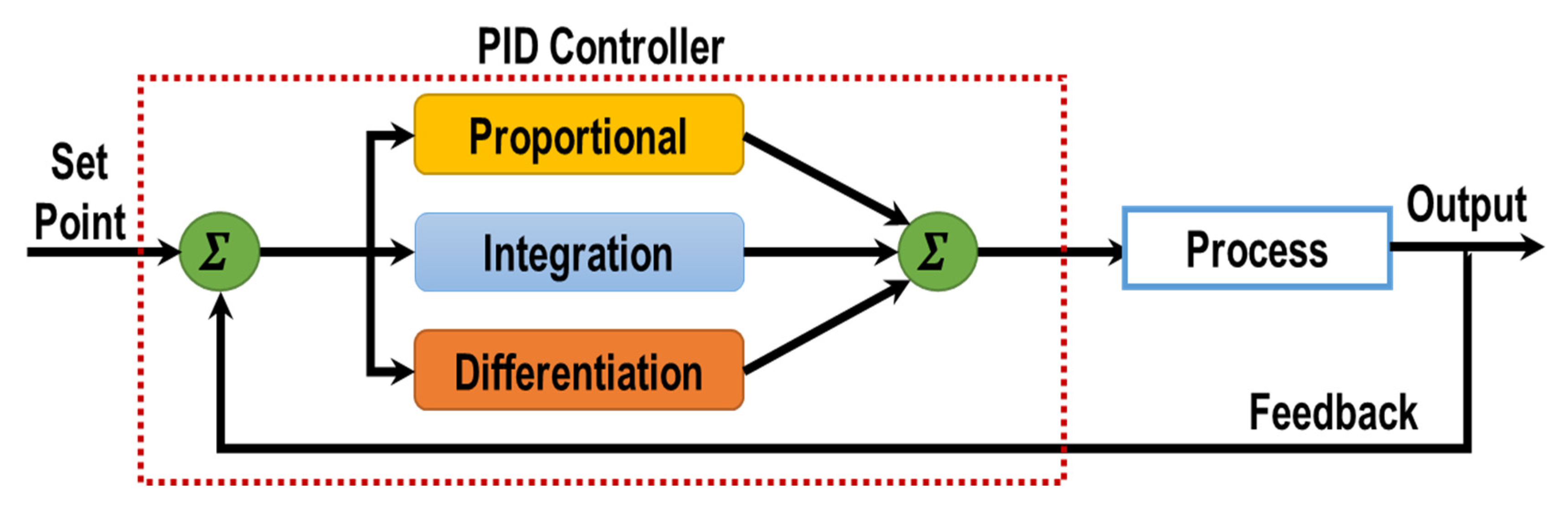
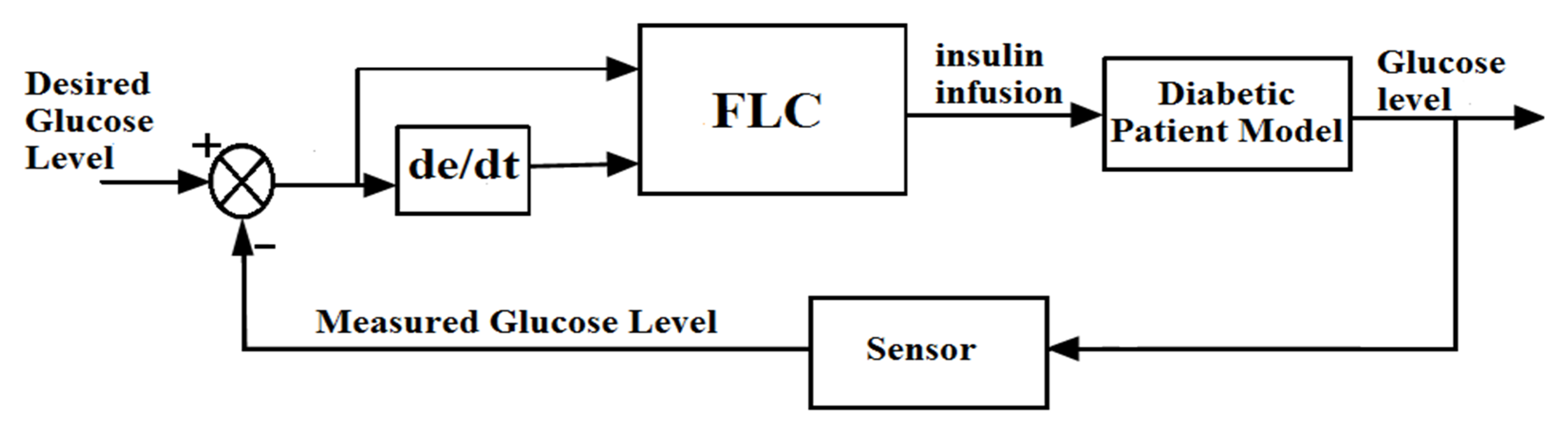
| Type of Insulin | Time Action Profile | Dose |
|---|---|---|
| Short acting | Begins from the 30-min after the subcutaneous with reaching peak action in 2–4 h | 3 times in a day, 30 min before taking a meal |
| Long acting | Beyond 24 h and up to 36 h | Once daily subcutaneous, at the same time with at least 8h interval between consecutive doses |
| Rapid acting | Generally, 4–20 min after subcutaneous injection with peak at 20–30 min. | Three times a day up to 15 min before food intake |
| Intermediate acting | Peak onset from 4–6 h, with the duration of action until 14–16 h | 1 or 2 time daily subcutaneous |
| GM Method | Advantages | Disadvantage |
|---|---|---|
| SMBG |
|
|
| CGM |
|
|
| Controller | Advantages | Disadvantages | References |
|---|---|---|---|
| Self-Tuning | Accurate physiological response with short time. | Parameter optimization and manual tuning is required. | [134] |
| Sliding Mode Control | Better robustness and insensitivity to the inter-patient variability/diversity in metabolic conditions. | SMC has some intrinsic problems such as discontinuous control that suffers from the chattering. SMC is only applicable for the degree one systems otherwise higher order sliding mode ( HOSM )is used. | [137,138] |
| Adaptive Control | It can react promptly during the large and rapid variations in insulin action. | In the presence/entrance of the unknown parameters in the process model, it becomes relatively difficult to construct a continuously parameterized controller. | [118,119] |
| Model Predictive Control | It can be tuned for personalized insulin delivery. It has feed-forward insulin action for delayed insulin effect. | There is no compensation for the unknown disturbances, and the metabolic uncertainty is not considered in the MPC. | [122] |
| H∞ | H∞ controller works well in the presence of uncertain parameters. | Unable to effectively resolve the tradeoff between the strength of control action and the tracking error. | [123] |
| SDRE | Can tackle any non-linear terms, and effectively maintains the non-linear characteristics of the system. It exhibits robustness against parametric uncertainties. | It involves very complex mathematical calculations especially when a system is of higher order. | [124] |
| PID | It is the best controller with situation awareness. It may be proportional (P), proportional-integtative(PI), proportional derivative (PD), and/or PID. | Its physical implementation for the clinical trials is relatively difficult. | [105] |
| Fuzzy logic | Fuzzy logic is opposite to that of binary logic. It is helpful at any point between 0 to 10. | Simulations wise developed but its validity in clinics has not been rigorously verified. | [116] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, S.; Ahmad, I.; Arif, H.; Ammara, U.E.; Majeed, A. Artificial Pancreas Control Strategies Used for Type 1 Diabetes Control and Treatment: A Comprehensive Analysis. Appl. Syst. Innov. 2020, 3, 31. https://doi.org/10.3390/asi3030031
Mehmood S, Ahmad I, Arif H, Ammara UE, Majeed A. Artificial Pancreas Control Strategies Used for Type 1 Diabetes Control and Treatment: A Comprehensive Analysis. Applied System Innovation. 2020; 3(3):31. https://doi.org/10.3390/asi3030031
Chicago/Turabian StyleMehmood, Sohaib, Imran Ahmad, Hadeeqa Arif, Umm E Ammara, and Abdul Majeed. 2020. "Artificial Pancreas Control Strategies Used for Type 1 Diabetes Control and Treatment: A Comprehensive Analysis" Applied System Innovation 3, no. 3: 31. https://doi.org/10.3390/asi3030031






