Abstract
Nowadays, wild grapevine populations are quite limited and sporadic mainly due to habitat destruction, land-use change, and the spread of pathogens that have reduced their distribution range. Palaeoecological, archaeobotanical, and genetic studies indicate that modern cultivars of Vitis vinifera are the results of the domestication of the dioecious, and sometimes hermaphrodite, wild species standing in riparian zones and wet environments. Wild grapevine populations have declined as a consequence of various forms of anthropogenic disturbance and were assigned by the IUCN Red List of Threatened Species to the Least Concern category. The River Crati Natural Reserve (Riserva Naturale Foce del Crati), located in southern Italy, hosts a population of Vitis vinifera subsp. sylvestris in a rewilding wet forest close to the Ionian Sea. These protected areas are of high scientific, biogeographic, and conservation interest in terms of Mediterranean biodiversity. Dendroecological and pollen morpho-biometric analyses of the wild grapevine are presented in this study. Palaeoecological perspectives for a landscape management strategy aimed at conserving and restoring the relic grapevine population are discussed.
1. Introduction
The common grapevine species, Vitis vinifera L., belongs to the family of Vitaceae and includes two subspecies: V. vinifera subsp. sylvestris (C.C. Gmel.) Hegi and V. vinifera subsp. vinifera. The wild vine—V. vinifera subsp. sylvestris—grows in moist colluvial and alluvial forests and wetlands. It is widely distributed from eastern to western Europe and in the Mediterranean up to Asia Minor, including northwest Africa and East Asia [1,2,3,4,5,6,7,8]. It is the only endemic taxon of the family Vitaceae in Europe and Maghreb [9]. Arnold et al. [10] reported that wild grapevine populations grow in Italy, Spain, France, Switzerland, Romania, Bulgaria, Hungary, Austria, and the formerly Yugoslavian countries. The largest number in terms of population seems to grow in Italy and Spain [11,12,13] and in the eastern Mediterranean, whereas thousands of grape varieties are considered native in Georgia [14].
The history of this plant is described by finds of Vitis pollen in archaeological sites and off-site anthropogenic contexts, from which a close relationship between this plant and human settlements emerges in the Mediterranean basin [14,15,16,17]. Studies suggest that the three Mediterranean peninsulas provided refugia to wild grapevines during the last Ice Age, about 12,000 years ago [5,18,19].
In 2007, Vitis vinifera subsp. sylvestris was assigned by the IUCN Red List of Threatened Species to the Least Concern category, corresponding to the lowest level of Extinction Risk [20]. However, this assessment could rely on the scarcity of information about the actual consistency of European sub-populations, as they seemed to be not well quantified. In fact, the IUCN Assessment Information indicates that the status of threat for this species needs updating (IUCN website accessed on 1 March 2023). Wild grapevine populations have declined because of various forms of anthropogenic disturbance, including land-use change and the degradation/exploitation of wetlands and moist forests, climate change, and the spread of exotic pathogens such as powdery mildew, phylloxera, and downy mildew [2,7,21]. Italy features an unequal distribution of V. vinifera subsp. sylvestris [22]. Populations are concentrated in the central and southern regions and the highest number of individuals occurs in forests and wetlands habitats with low anthropogenic disturbances (e.g., protected areas) [22]. The in situ conservation of V. vinifera relic populations is thus carried out in protected areas, whose regulatory protection allows their safeguard.
1.1. Conservation of Wild Grapevine in Southern Italy
The Regional Nature Reserves of River Crati and Lake Tarsia (Riserve Naturali Regionali Foce del Crati e Lago di Tarsia) are among the few sites in Italy where wild grapevines populations are protected in a rewilding wet forest. The conservation measures of these reserves include the protection of the genetic heritage with the goal of conserving wild grapevine in situ and creating an ex situ collection to preserve the germplasm (Con.Vi.vi. project, implemented by the Reserves managing body—Amici della Terra Italia, in scientific partnership with the University of Calabria). The monitoring of the specimens in the protected areas is being carried out with studies on the conservation status and the genetic structure of the Vitis population for a possible productive use in this territory boasting an agricultural history starting since the Greek colony of Sybaris (ca. 2670 years BP).
For limiting the erosion of the genetic resources of the local wild grapevine, an experimental field located in the municipality of Tarsia was carried out. The ex situ activities mainly consisted in the constitution of a germplasm bank, with the setting up of greenhouses and an open-field collection. As part of the Con.Vi.vi. project, the investigation conducted by the University of Calabria concerned the genetic characterisation of the wild-grape varieties present in the study area. These vines, in addition to representing the wild form of Vitis vinifera, constitute a heritage variety of great value, both for the conservation of grapevine germplasm and for the genetic breeding programs of the many cultivated grape varieties [23,24]. Previous studies carried out on more than 660 individuals emphasised a high genetic diversity and a low inbreeding level in Italian wild grapevine [22]. Maintaining the genetic variability of wild grapevine populations is thus a conservation priority [21].
1.2. Wild vs. Domestic Grapevine
There are substantial differences between the two subspecies of Vitis vinifera related to morphological and ecophysiological features. Differences include the morpho-physiology of flowers and pollen and the size and morphology of various vegetative and reproductive parts (e.g., berries and seeds) [25]. The wild grapevine is dioecious, with morphologically hermaphroditic but functionally unisexual flowers, while domestic grapevine has both functionally and morphologically true hermaphroditic flowers [23]. In wild grapevine, male flowers have well-developed erect stamens that produce a large amount of fertile pollen and an aborted pistil (with a small ovary, without a style and stigma). Female flowers have well-developed pistils but reflexed filaments and anthers that release sterile pollen ([26,27], see Figure 1 in [25]). Stamens are released by the petals breaking away from their base by curving upward while the caliptra is pushed away. Berries are acidic and not very juicy. These contain small, robust, rounded-shaped seeds called pips, that are an excellent source of food for many bird species, and allow the propagation of the species. The dispersal of pips is endozoan zoocora, and particularly ornithozoan dispersal. Grapevines are heliophilous and can reach considerable heights, sometimes more than 20 m [28,29]. Interestingly, wild grapevine prefers wet soils, while domestic grapevine also grows in more arid soils [21,30].
1.3. A Multidisciplinary Study of Grapevine Populations: Why Does It Matter?
The present paper aims at characterising the wild grapevine population of River Crati Natural Reserve by describing the age structure of natural populations, growth trends, pollen morphology, and the morphometric traits of male or female flowers. Shading light on growth trends and some aspects of reproductive biology could help defining management strategies to conserve and restore relic grapevine populations, both in terms of population size and genetic diversity. Morphopalynological analyses were carried out to test the presence of pollen dimorphism, a phenomenon often observed in dioecious species (e.g., [26] and the references therein). Vitis vinifera is an important taxon in palaeo-palynological records. However, its presence in past pollen rain can hardly be directly connected to human activities due to its underrepresentation and ubiquity. Defining the morphological differences in the dimorphic pollen of wild grapevines can aid in the discrimination of wild and domestic populations and increase Vitis occurrences in pollen records.
2. Materials and Methods
2.1. Study Area
The River Crati (39°43′24″ N; 16°31′44″ E) Natural Reserve is located in the municipalities of Cassano allo Ionio and Corigliano Calabro-Rossano (Cosenza), in the Ionian side of the Calabria region (Figure 1). In 2018, the reserve was designated as Special Area of Conservation (SAC) for the Natura 2000 Network under the Habitats Directive (92/42/EC). It is also included in the official list of protected natural areas maintained by the Italian Ministry of the Environment and Protection of Land and Sea under Law 394/91. The River Crati Natural Reserve represents a biotope of great naturalistic interest extending over an area of 208 hectares and an altitude of between 0 and 12 m a.s.l. Along the riverbank, the pedological features consist of incoherent sediments (coarse to moderately fine sands) transported and reshaped by the river. In the nearby floodplain area, moderately deep-to-deep, alkaline-reacting, and moderately calcareous soils are present with a fine texture characterised by sand and silt subject to autumn–winter alluvial events [31].
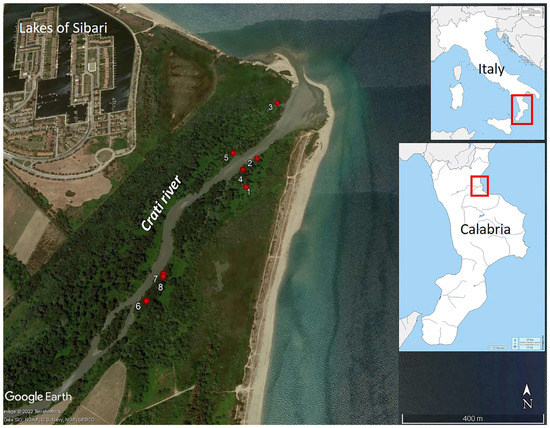
Figure 1.
The River Crati Natural Reserve, Italy: the location of the eight pollen samples was taken from different specimens of Vitis vinifera subsp. sylvestris (red dots). The basemap is a modified satellite image provided by GoogleEarth™. The geographical maps are downloaded from: https://d-maps.com (accessed on 3 March 2023).
The area is a wetland whose vegetation mainly consists of tamarisks, willows, poplars, and marsh reeds. The flora, with the dominance of Mediterranean elements, consists of 482 taxa, including 15 endemic species to Calabria and 18 species included in the regional Red Lists [32].
Together with the Lake Tarsia Natural Reserve, the two reserves extend on a wetland area of approximately 600 hectares along the course of the River Crati, the longest in the Calabria region (81 km). River Crati originates from Mount Timpone Bruno (1742 m a.s.l.) and runs with an average annual flow of 26.19 m3 s−1. The basin and valley of the River Crati cover about 2400 km2; the area is characterised by a Mediterranean-type climate with average temperatures between 15 °C and 18 °C, mild winters, and hot summers (http://www.riservetarsiacrati.it/territorio/, accessed on 10 March 2023). Pluviometric data of the last 30 years (1991–2020) show an average annual precipitation of 818 mm. The wettest months are December and January, and the driest months are July and August. The distribution of rainfall throughout the year is typical of the Mediterranean regime, with a main maximum in winter, and a secondary maximum in autumn [33]. Regarding the hydrological regime, the average flow of the River Crati at its mouth is 36 m3/s, with stable values also in summer; this allows the surface water table to be kept constant.
2.2. Dendroecological Analysis
During the summer of 2019, nine individuals of Vitis vinifera subsp. sylvestris growing in the River Crati Natural Reserve were sampled for dendroecological analyses. Wooden samples were taken 1.30 m above the ground level and analysed according to dendrochronological standard methods. Ring-width was also measured along the main stem at intervals of 2 m in one individual. This multi-sample analysis had the purpose of assessing the growth in diameter and the wood anatomy in the different heights of a single individual. Wooden samples were cut and polished, and annual rings were measured through the Computer Controlled Tree Ring Measurement Device (CCTRMD) and processed through the Computer Aided Tree Ring Analysis (CATRAS) software [34].
2.3. Pollen Analysis
Inflorescences were collected in June and August 2019 and in June 2022. The sampling was carried out by the staff of the River Crati Natural Reserve. Eight samples were taken from different specimens of Vitis vinifera subsp. sylvestris in the reserve (Table 1; Figure 1 and Figure 2). The plants are adult specimens, located within the wet forest, corresponding to Habitat 92A0 (Salix alba and Populus alba gallery forests). They are climbing lianas growing on living supports, represented by white poplar trees with an average height of 20 m.

Table 1.
List of samples of flowers taken from wild grapevines of the River Crati Natural Reserve. * = this sample was not suitable for pollen analysis because pollen was not present in the anthers and the female flowers have already started the fruit development.

Figure 2.
The River Crati Natural Reserve, Italy: examples of Vitis vinifera subsp. sylvestris selected for pollen sampling.
Based on the floral morphology observed, two samples were taken from female plants (with well-developed pistils and the presence of berries) and six samples were taken from male plants (with small and not fully developed pistils) (Table 1).
Pollen samples were subjected to acetolysis and permanent slides were prepared for morpho-biometrical observations. For each sample from which pollen was obtained, a count of 500 pollen grains was carried out at 400× magnification, in order to assess the presence, number, and position of pollen apertures. In addition, in each sample, 30 pollen grains were measured and photographed at 1000× magnification in equatorial view, which is the prevalent one, and in polar view, which is less frequent in permanent slides (Figure 3).
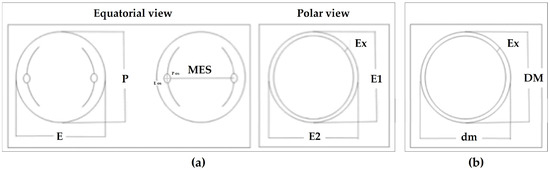
Figure 3.
Pollen grain morphometry: (a) morphological parameters of trizonocolporate pollen (equatorial and polar views): P, polar axis; E, equatorial diameter; MES, maximum distance between colpi in mesocolpium; P os, polar axis porus; E os, equatorial axis porus; Ex, exine thickness; and (b) morphological parameters of the inaperturate pollen: DM, equatorial diameter maximum value; dm, equatorial diameter minimum value; Ex, exine thickness.
3. Results
3.1. Dendroecology and Age of Wild Grapevines
The dendroecological analysis highlighted an uneven ages structure in the analysed population (age range: 4–35 years). In the wild grapevines of Crati Reserve, the relationship between age and diameter follows a statistically significant linear regression (Figure 4). The maximum age was 35 years (diameter 18 cm) while the minimum was 4 years (diameter 4 cm). The tree-ring analysis was made difficult by the anatomy of the grapevines wood and the absence of a clear latewood layer, which often made it hard to distinguish the border between two adjacent annual rings [35]. Of note, some individuals showed an unclear pattern of anatomical parts (e.g., semiporous ring in Figure 5a), while others had clear ring-porous wood (Figure 5b). Differences in the wood anatomy also stood out along the stem of a single individual. As the distance from the root collar increased, the organisation of rings’ wood anatomy became less clear. The multi-sample analysis performed at an interval of 2 m highlighted 12 rings at the root collar (0 m), 11 rings at 2 and 4 m above the ground, 10 rings at 6 m, and unexpectedly, 12 rings at 8 m below the ground, suggesting the presence of false or missing rings (Figure 6). This analysis highlights the heterogeneous organisation of wood porosity along the stem in wild grapevines (Figure 5).
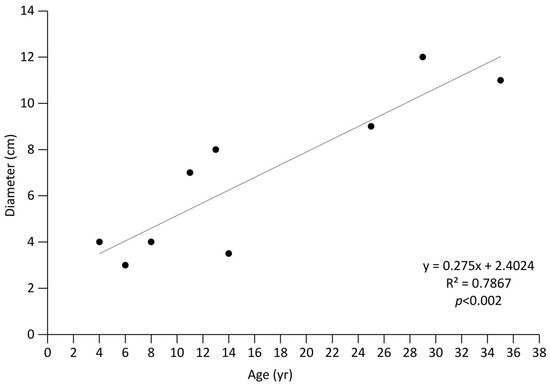
Figure 4.
Age–diameter relationship of nine individuals of wild grapevines sampled in the River Crati Natural Reserve. The main statistics of the linear regression are shown.
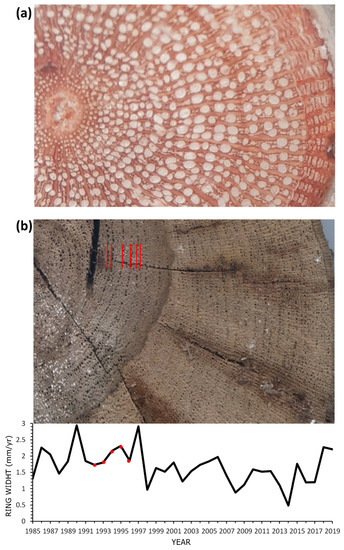
Figure 5.
Examples of heterogeneous porosity in wild grapevines. (a) Wild grapevine featuring a distribution of vessels (diffuse porosity) that does not allow for a clear identification of annual rings; (b) Wild grapevine showing ring-porous wood. In the graphic below, the chronology of the individual in panel (b); and red dots indicate the annual rings marked with red bars in panel (b).
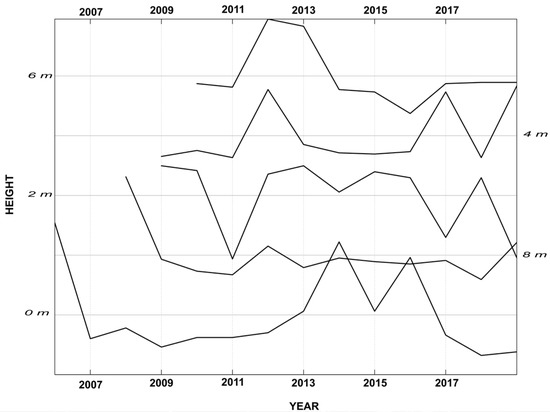
Figure 6.
Spaghetti plot of a dendrochronological series of a grapevine individual. The five series come from samples taken at intervals of 2 m (from 0 to 8 m). Series are ordered from the longest to the shortest.
Because of the short annual tree-ring series and the wide growth differences between the nine individuals, visual and statistical synchronisations gave no clear match. Indeed, it was not possible to apply dendrochronological methods for studying climatic signals. A strong growth in individuality was identified during the dendrochronological analysis. The individuality of growth emerges both yearly, i.e., by considering yearly seasonality changes as a driver of growth trends (Figure 7a), and on the cambial age of individuals (i.e., by considering the ontogenetic cycle as the main factor determining the ring width and growth trends, as shown in Figure 7b).
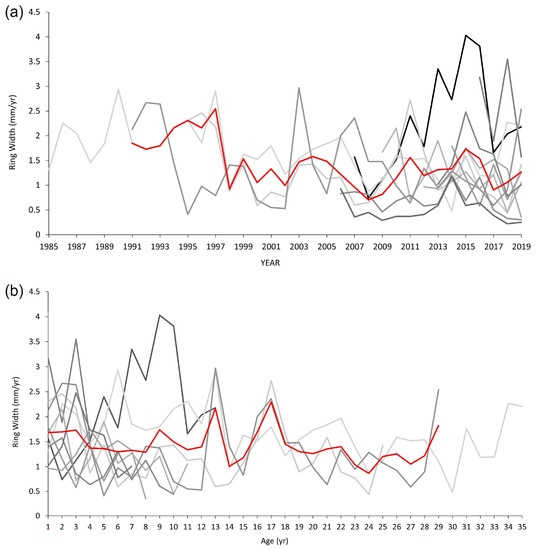
Figure 7.
Individual tree ring-width series of wild grapevines. (a) Ring-width series plotted on the basis of the calendar year, which displays visual synchronisation—i.e., the growth of the grapevine linked to climate/environmental variation. (b) Ring-width series organised on the basis of the plant age (ontogenetic cycle)—i.e., how individuals have grown in their first, second, third, etc. year. Red lines in both panels are the average curves.
3.2. Pollen Analysis
Seven plants had pollen useful for morpho-biometric analysis, while in one female specimen, the allegation phase was already occurring, and no anther had pollen yet. The inaperturate pollen of the female specimen, no. 3, is roughly spheroidal in shape, with the polar P axis of 22.4 ± 2.3 μm and an equatorial E axis of 23.8 ± 1.8 μm. The inaperturate grains have about 10% medium-sized grains. This contrasts with the trizonocolporate grains that all fall into the small size class (<25 μm). Exine is thin, with average values of 1.6 ± 0.3 μm.
Functionally male flowers show isopolar, radiosymmetric, and mostly trizonocolporate pollen. Interestingly, in some of these samples, the presence of inaperturate and anomalous pollen grains (= with undefined apertures or with hits without pores) was observed (Table 2; Figure 8).

Table 2.
Pollen morphology of Vitis samples taken from wild grapevine plants growing in the River Crati Natural Reserve.
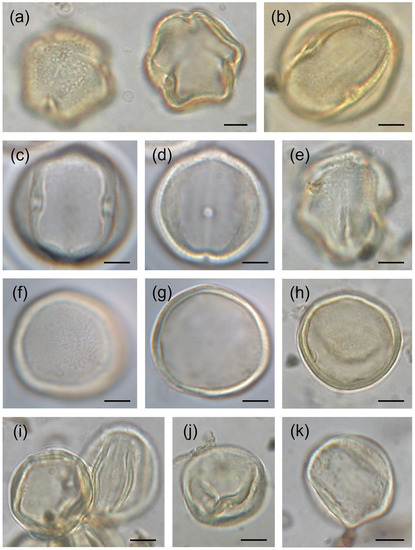
Figure 8.
Pollen of Vitis vinifera subsp. vinifera, light micrographs: (a) polar view (sample 8); (b) equatorial view (sample 8); (c,d) equatorial view (sample 4); (e) polar view (sample 6); (f,g) inaperturate pollen (sample 3); (h) inaperturate pollen (sample 6); (i,j) anomalous pollen (sample 6); and (k) anomalous pollen (sample 8). Scale bars = 5 µm.
In this study, trizonocolporate pollen was observed in all male specimens (>99%), with a very small percentage of inaperturate (0.2–0.4% in three samples) and anomalous pollen grains (0.2–0.6% in four samples). Inaperturate pollen (99.2%) was mostly present in the female specimen no. 3, with a small percentage of trizonocolporate (0.8%) and no anomalous pollen.
The trizonocolporate pollen presents a narrow groove-shaped eso-aperture with a pore-shaped endo-aperture in the centre. The three composed apertures are located at the equator (zono-aperture). The trizonocolporate pollen is isopolar and radially symmetrical, ranging in shape from spheroidal (27.8%), oblate-spheroidal (9.5%), prolate-spheroidal (40.5%), and subprolate (22.2%). The polar P axis has an average value around 22 μm ranging from 19 μm to 24.2 μm. The equatorial E axis has an average value of 20.5 μm, from 19.7 μm to 21.4 μm. These values agree with those reported by Punt et al. [36]. In an equatorial view, the pores are clearly visible with a polar axis of 2.2 μm and an equatorial axis of 2.2 μm, on average. Exine is very thin, with average values of 1 μm in polar view and 1.1 μm in equatorial view (Table 3). The sculptures of the exine appear to be micro-reticulated under the light microscope. In the literature, the exine is described from psilate (smooth) to micro-rugulate (elongated elements of ornamentation > 1 μm arranged to form an irregular pattern) by Punt et al. [36], and from scabrate (elements < 1 μm)/verrucate to foveolate (rounded depressions or lumina > 1 μm in diameter) by Gallardo et al. [27].

Table 3.
Morphometric data of Vitis pollen grains (measurements were taken with light microscopy in equatorial and polar views). Equatorial view: each value is the average of 30 individual measurements ± standard deviation. P, polar axis; E, equatorial diameter; MES, maximum distance between colpi in mesocolpium; Ex, exine thickness; P os, polar axis porus; E os, equatorial axis porus. Polar view: each value is the average of 10 individual measurements ± standard deviation. D max, equatorial diameter maximum value; d min, equatorial diameter minimum value; Ex, exine thickness.
4. Discussion
The interdisciplinary approach used to study wild grapevine populations in the River Crati Natural Reserve allowed to improve the knowledge on the biology and ecology (wood anatomy, dendroecology, and palynology) of Vitis vinifera subsp. sylvestris.
4.1. Dendroecology and the Strong Individuality of Wild Grapevines
Dendroecological analyses showed the distinctive growth features and properties of wild grapevines. Differences in the organisation of wood porosity between individuals and from the root collar to the top of each plant emerged as a distinctive functional trait of wild grapevines ecology in a wet-warm temperate forest. This strong individuality of growth likely generates the heterogeneous organisation of vessels in different individuals (Figure 5), while anatomical differences along an individual’s stem are a biological phenomenon that deserves further investigation. The strong individuality of growth indicates that the grapevine population is poorly influenced by climate variations (i.e., precipitation and temperatures), suggesting no important water deficit because of the stable water table. As mentioned above, due to the absence of synchronisation between individual ring-width series, it was not possible to perform dendroclimatic analysis. The dendrochronological analysis showed no limiting factors influencing the population’s growth trends and responses. The strong individuality of the ring-width series indicates that growth develops independently to the yearly weather variation and ontogenetic cycle. This means that (i) in the wet forests of southern Italy, wild grapevine grows under optimum ecological conditions; (ii) grapevines growth trends may be affected by micro-site environmental conditions such as soil properties, microtopography, and local competition; and (iii) wild grapevines tree-ring growth has no clear age-driven growth trends (at least for the studied ages, up to 35 years).
Generally, growth is strongly correlated to climate in the ecological border of the species, where positive or adverse climatic events generate a positive or negative growth response [37]. In the River Crati Natural Reserve, the wet soil and the humid air, coupled with an average yearly warm-mid temperatures and the lack of extreme winter cold events, makes the wild grapevines growth subject to much more influence by ecological factors at the microsite scale. Therefore, the River Crati Natural reserve is a strategic habitat to ensure the biological conservation of wild grapevines within a highly functional natural wet forest ecosystem in a biodiversity hotspot for Europe and Mediterranean basin.
4.2. Palynology and the Pollen Dimorphism with Some Anomalies of Dioecious Plants
The wild grapevines studied show pollen dimorphism, which means that two pollen types, namely trizonocolporate and inaperturate pollen, are present in the same species. Pollen dimorphism is known in several spermatophytes and can have different origins. For example, an abnormal meiotic division during the microsporogenesis leads to the formation of pollen grains of different size and cytoplasmic content in tobacco (Nicotiana tabacum L.) [38]. Moreover, in functional dioecious species, the male plant produces fertile pollen grains while the female plant produces pollen that is unable to germinate but can act as an attractant for pollinating animals [39]. This is the case of the genus Vitis that shows functional dimorphism in several species, such as V. riparia Michx, V. aestivalis Michx, and V. coignetiae Pulliat [40,41,42].
Despite the presence of stamens, female flowers seem to produce sterile pollen grains, which lack apertures. The term ‘inaperturate’ is used to describe pollen that has a morphology excluding apertures, i.e., those areas with a thinner exine that have the function of facilitating the growing of the pollen tube. Germination has never been observed in the inaperturate pollen of dioecious V. vinifera, despite the fact that its viability has been demonstrated in several studies [25,26,43]. In contrast, the pollen produced by the male specimens, which have three apertures, is normally able to germinate. Recently, the recovery of inaperturate pollen in archaeological samples has been reported as an indication of the local presence of wild grapevine (Middle Bronze Age, Italy; [25]). The record is rare, and this could be since its pollen falls down with the female flowers, close to the source plant.
In the samples obtained from the wild plants growing in the River Crati Natural Reserve, as expected, the pollen produced by the male flowers is mostly trizonocolporate, with three narrow furrows and a pore positioned in the centre of each narrow one, on the equatorial line and an elongated (prolate) shape. The pollen produced by the female flower is mostly inaperturate and spheroidal in shape. The spheroidal to prolate the shape of the trizonocolporate pollen and the thickness of the exine are in agreement with Punt et al. [36] and Gallardo et al. [27]. Contrary to the measurements reported by Gallardo et al. [28], but like Mercuri et al. [25], the pollen of functionally female flowers is slightly larger than that of functionally male flowers. The exine thickness of the inaperturate grains shows a higher average value than that observed by Mercuri et al. [25] in modern and archaeological samples.
Interestingly, a small number of trizonocolporate pollen grains in four samples (1,5,6,8) show some abnormal morphology including the presence of colpi without pores or an anomalous shape (Table 2; Figure 8). In some other cases, although pores are present, they are not evident and recognisable at low magnification (up to 400×), while they can be visible only at 1000× magnification. Although these anomalies are not common, they may be morphological evidence of some genetic diversity in the studied plants. Moreover, the slight differences with other data in the literature suggest the great variability of Vitis pollen and the special nature of the wild grapevine population of the River Crati Natural Reserve.
4.3. Past and Present Role of the Wild Grapevine
As far as the past and present use of the plant is concerned, both the domesticated and wild subspecies may be searched for fruit collection or the preparation of beverages. Wine is traditionally made from wild plants in the Caucasus [44]. In Italy, a traditional wine exclusively made with wild plants is known from Sardinia. Archaeobotanical studies have demonstrated that grapevine care/cultivation can be traced back to the Bronze Age [45,46,47,48], and then its spread was due to the expansion of Greek colonies in southern Italy [15]. Cultural exchanges improved the competence of the plant use and the spread of cultivation led to the development of techniques and tools for wine production. However, the issue of the transition between the grape collection in the wild and the introduction of the domesticated subspecies in Italy and East Mediterranean is still highly debated.
The discovery of tartaric acid residues [49] and grape seeds, morphologically identified as domestic in the Terramara of Montale (N Italy, 3550–3200 years BP), would confirm that human communities in Italy already began the cultivation of domesticated grapevine during the Recent Bronze Age [45,47]. This finding is also confirmed by the study of grape seeds found within wells at the Sa Osa site in Sardinia, where most of the archaeological grape seeds from the Recent Bronze Age have been attributed to domestic varieties [46]. Subsequently, archaeobotanical records increasingly include grape pips as a major part of plant macroremains from the Roman era onwards, demonstrating the key role of domesticated plants in the diet of the peninsula (e.g., [50,51]). However, Vitis pollen retrieved from the Roman amphorae of San Felice Circeo was interpreted by Chassouant et al. [52] as a possible ‘intermediary stage of domestication, being characterised by thick exine, absence of germinative pores, and presence of colpi’.
The evidence from the wild grapevines of River Crati suggests that the Vitis pollen of San Felice Circeo could have also been produced by wild plants with a large genotype diversity, in historical times. Palynological and archaeobotanical data support the coexistence of wild and domesticated subspecies for a long time, with the human protection mainly of domesticated grapevines and the restriction of wetlands as a risk for wild plants.
The conservation of wild grapevines today plays a strategic role of three-fold importance: (i) being the ancestor of domesticated grapevines, wild plants have an important genepool for viticulture and agronomy industry; (ii) they could provide genetic variations for the adaptation of cultivated vines to global changes [53]; (iii) the preservation of wild plants may be an important vector to preserve the endangered habitats in which they grow. The peculiarities of the local population are evidence that the preservation of the diversity of the Vitis genepool from the River Crati Natural Reserve plays a key role in the conservation of biodiversity in this Mediterranean region.
5. Conclusions
In this study, dendroecology has revealed a good growth performance of wild grapevine plants in the rewilding wet forest. Wood growth curves showed a strong individuality, probably due to microsite ecological conditions in a natural forest environment that is optimal for the existence, development, growth, and reproduction (ecological optimum) of wild grapevine. Palynology suggests the existence of large variability besides the pollen dimorphism in wild grapevines, linked to the genetic diversity and reproductive strategy of these plants.
The results obtained from the dendroecological and palynological study support the molecular evidence of a viable population. Within the Con.Vi.vi. project, in fact, molecular studies of the SSR markers of the plants growing in the River Crati Natural Reserve have highlighted intra-specific differences in the Vitis genus, and intra-varietal differences, distinguishing individuals with different genetic profiles within an apparently and phenotypically homogeneous population [54]. The first genetic characterisation of the wild grapevine population in the reserve represents a useful contribution, both to the knowledge of the genetic resources of this species and to the understanding and reconstruction of grapevine domestication processes. In addition, the intra-varietal differences strongly support the hypothesis that the genotypes and ecotypes of peculiar conservation value may be present within this population.
Wild grapevines in the River Crati Natural Reserve are a relic population. Here, viable individuals survived under favourable environmental conditions, and today, they are not much impacted by the ongoing climate changes such as drought stress, increased air temperatures, extreme cold events, and other climate factors often influencing growth trends in trees. The reduction in natural populations could be due to habitat destruction and other impacts of anthropogenic nature—e.g., the introduction of phylloxera [55]. During the past, wild grapevine populations were likely more widespread in the Mediterranean area, as evidenced by the pollen records of wild Vitis that are unconnected to indicators of human activities [56]. In this sense, wild grapevines can represent an indicator of functionality and the ecological integrity of wet forests. Vitis pollen associated with high arboreal pollen percentages and the absence/low amount of anthropogenic pollen indicators could indicate the ecological integrity of wet forests and riparian areas in palaeoecological reconstructions. Wild grapevine populations are sensitive to human impacts, and the case study of River Crati Natural Reserve testifies to the role that the conservative management of the wet forest and the riparian zone (strict protection) can play in restoring a viable population of wild grapevine in the Mediterranean.
Author Contributions
Conceptualisation, A.M.M. and G.P.; methodology, P.T., M.B. and A.M.M.; formal analysis, P.T., E.C., M.B. and J.P.; investigation, A.B., R.M., G.P. and A.F.; data curation, M.B., J.P., P.T., E.C. and A.F.; writing—original draft preparation, A.M.M., A.F., E.C., G.P., J.P. and M.B.; writing—review and editing, E.C., P.T., M.B., A.B., R.M., E.S., J.P., A.M.M., G.P. and A.F.; visualisation, E.C., J.P. and M.B.; supervision, A.M.M. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially financed by “Riserve naturali Lago di Tarsia e della Foce del Crati”. The position of E. Clò was funded by the project “National Biodiversity Future Center–NBFC”.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Acknowledgments
We are grateful to Carlo Pelizzola, who contributed to the description and measurements of Vitis pollen during the analytical phase. The research project was implemented under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree No. 3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—Next Generation EU. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP J83C22000860007 and CUP E93C22001090001, Project title “National Biodiversity Future Center—NBFC”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marinval, P. Vigne sauvage et vigne cultivée dans le Bassin méditerranéen: Émergence de la viticulture, contribution archéobotanique. In L’histoire du Vin, une Histoire de Rites; Office International de la Vigne et du Vin: Paris, France, 1997; pp. 137–172. [Google Scholar]
- Ocete, R.; Lopez, M.A.; Lara, M.; Del Tío, R. The sanitary state of a phytogenetic resource: The Spanish wild grapevine, Vitis vinifera sylvestris Gmelin (Hegi), populations. Plant Genet. Resour. Newsl. 1997, 110, 5–12. [Google Scholar]
- Lacombe, T.; Laucou, V.; Di Vecchi, M.; Bordenave, L.; Bourse, T.; Siret, R.; David, J.; Boursiquot, J.M.; Bronner, A.; Merdinoglu, D.; et al. Inventory and characterization of Vitis vinifera L. ssp. silvestris in France. In Proceedings of the VIII International Conference on Grape Genetics and Breeding, Kecskemét, Hungary, 26–31 August 2002; Hajdu, E., Borbás, E., Eds.; Acta Horticulturae: Leuven, Belgium, 2003; pp. 553–557. [Google Scholar]
- Schneider, A.; Boccacci, P.; Ruffa, P.; Torello Marinoni, D.; Cavallo, L.; Festari, I.; Rotti, G.; Raimondi, S. Identification and characterization of Vitis vinifera subsp. sylvestris populations in north-western Italy. Vitis 2015, 54, 223–225. [Google Scholar] [CrossRef]
- Butorac, L.; Hančević, K.; Lukšić, K.; Škvorc, Ž.; Leko, M.; Maul, E.; Zdunić, G. Assessment of wild grapevine (Vitis vinifera ssp. sylvestris) chlorotypes and accompanying woody species in the Eastern Adriatic region. PLoS ONE 2018, 13, e0199495. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; Rutishauser, S.; Aguilar, S. Supplemental protocol for liana censuses. For. Ecol. Manag. 2008, 255, 1044–1049. [Google Scholar] [CrossRef]
- Ocete, R.; Armendáriz, I.; Cantos, M.; Álvarez, D.; Azcón, R. Ecological characterization of wild grapevine habitats focused on arbuscular mycorrhizal symbiosis. Vitis 2015, 54, 207–211. [Google Scholar]
- André, G.; André, M.; Ferrez, Y.; Lacombe, T. Les vignes sauvages colluviales Vitis vinifera subsp. sylvestris (Gmelin) Hegi dans le massif jurassien, nouvelles données. Les Nouv. Arch. Flore Jurassienne Nord.-Est Fr. 2017, 15, 113–145. [Google Scholar]
- Arroyo García, R.A.; Revilla, E. The current status of wild grapevine populations (Vitis vinifera ssp. sylvestris) in the mediterranean basin. In The Mediterranean Genetic Code—Grapevine and Olive; Poljuha, D., Sladonja, B., Eds.; InTech: Rijeka, Croatia, 2013; pp. 51–72. [Google Scholar]
- Arnold, C.; Gillet, F.; Gobat, J.M. Situation de la vigne sauvage Vitis vinifera ssp. silvestris en Europe. Vitis 1998, 37, 159–170. [Google Scholar] [CrossRef]
- Anzani, R.; Favilla, O.; Scienza, A.; Campostrini, F. Wild grapevine (Vitis vinifera ssp. silvestris) in Italy: Distribution, characteristics and germoplast preservation—1989 report. Vitis 1990, 29, 97–113. [Google Scholar] [CrossRef]
- Ocete, R.; López, M.A.; Pérez, M.A.; Del Tío, R.; Lara, M. Las poblaciones españolas de vid silvestre. Monografías INIA. Agrícola 1999, 3, 1–52. [Google Scholar]
- Cantos, M.; Arroyo-García, R.; García, J.L.; Lara, M.; Morales, R.; López, M.Á.; Gallardo, A.; Ocete, C.A.; Rodríguez, Á.; Valle, J.M.; et al. Current distribution and characterization of the wild grapevine populations in Andalusia (Spain). C. R. Biol. 2017, 340, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Bouby, L.; Wales, N.; Jalabadze, M.; Rusishvili, N.; Bonhomme, V.; Ramos-Madrigal, J.; Evin, A.; Ivorra, S.; Lacombe, T.; Pagnoux, C.; et al. Tracking the history of grapevine cultivation in Georgia by combining geometric morphometrics and ancient DNA. Veg. Hist. Archaeobot. 2021, 30, 63–76. [Google Scholar] [CrossRef]
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early Neolithic wine of Georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk, A. Vitis vinifera sylvestris i Vitis vinifera sativa. Udomowienie i upowszechnienie się uprawy winorośli w starym świecie na podstawie badań archeologicznych i paleobotanicznych. Folia Praehist. Posnan 2016, XXI, 123–148. [Google Scholar] [CrossRef]
- Naqinezhad, A.; Ramezani, E.; Djamali, M.; Schnitzler, A.; Arnold, C. Wild grapevine (Vitis vinifera subsp. sylvestris) in the Hyrcanian relict forests of northern Iran: An overview of current taxonomy, ecology and palaeorecords. J. For. Res. 2018, 29, 1757–1768. [Google Scholar] [CrossRef]
- Grassi, F.; De Mattia, F.; Zecca, G.; Sala, F.; Labra, M. Historical isolation and Quaternary range expansion of divergent lineages in wild grapevine. Biol. J. Linn. 2008, 95, 611–619. [Google Scholar] [CrossRef]
- Levadoux, L. Les Populations Sauvages et Cultivées des Vitis vinifera L; Institut National de la Recherche Agronomique: Villeurbanne, France, 1956; Volume 1, pp. 59–117. [Google Scholar]
- Kyratzis, A.; Vörösváry, G.; Magos Brehm, J.; Eliáš, P.; Vögel, R.; Duarte, M.C.; Tavares, M.; Holubec, V. Vitis vinifera (Europe Assessment). The IUCN Red List of Threatened Species 2011: E.T63537A12687780. Available online: https://www.iucnredlist.org/species/63537/12687780 (accessed on 1 March 2023).
- Grassi, F.; Labra, M.; Imazio, S.; Ocete Rubio, R.; Failla, O.; Scienza, A.; Sala, F. Phylogeographical structure and conservation genetics of wild grapevine. Conserv. Genet. 2006, 7, 837–845. [Google Scholar] [CrossRef]
- Biagini, B.; De Lorenzis, G.; Imazio, S.; Failla, O.; Scienza, A. Italian wild grapevine (Vitis vinifera L. subsp. sylvestris) population: Insights into eco-geographical aspects and genetic structure. Tree Genet. Genomes 2014, 10, 1369–1385. [Google Scholar] [CrossRef]
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef]
- De Mattia, F.; Imazio, S.; Grassi, F.; Lovicu, G.; Tardaguila, J.; Failla, O.; Maitt, C.; Scienza, A.; Labra, M. Genetic characterization of Sardinia grapevine cultivars by SSR markers analysis. J. Int. Sci. Vigne Vin. 2008, 41, 175–184. [Google Scholar] [CrossRef]
- Mercuri, A.M.; Torri, P.; Florenzano, A.; Clò, E.; Mariotti Lippi, M.; Sgarbi, E.; Bignami, C. Sharing the Agrarian Knowledge with Archaeology: First Evidence of the Dimorphism of Vitis Pollen from the Middle Bronze Age of N Italy (Terramara Santa Rosa di Poviglio). Sustainability 2021, 13, 2287. [Google Scholar] [CrossRef]
- Caporali, E.; Spada, A.; Marziani, G.; Failla, O.; Scienza, A. The arrest of development of abortive reproductive organs in the unisexual flower of Vitis vinifera ssp. silvestris. Sex. Plant Reprod. 2003, 15, 291–300. [Google Scholar] [CrossRef]
- Gallardo, A.; Ocete, R.; López, M.A.; Lara, M.; Rivera, D. Assessment of pollen dimorphism of Vitis vinifera L. subspecies sylvestris (Gmelin) Hegi in Spain. Vitis 2009, 48, 59–62. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017; Volume 2. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2019; Volume 4. [Google Scholar]
- Grassi, F.; Labra, M.; Imazio, S.; Spada, A.; Sgorbati, S.; Scienza, A.; Sala, F. Evidence of secondary grapevine domestication centre detected by SSR analysis. Theor. Appl. Genet. 2003, 107, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Caridi, D.; Aramini, G.; Colloca, C. I suoli della Calabria. Programma Interregionale Agricoltura-Qualità, Misura 5; ARSSA Agenzia Regionale per lo Sviluppo e per i Servizi in Agricoltura: Cosenza, Italy, 2003. [Google Scholar]
- Maiorca, G.; Spampinato, G.; Crisafulli, A.; Cameriere, P. The vascular flora and vegetation of “Foce del Crati” Regional Nature Reserve (Calabria, South Italy). Webbia 2007, 62, 121–174. [Google Scholar] [CrossRef]
- Marsico, L.; Rotundo, R. Meteo-Clima. In Edizione 2022—Annuario dei dati ambientali ARPACAL; ARPACal: Catanzaro, Italy, 2022; pp. 40–44. [Google Scholar]
- Aniol, R.W. Tree-ring analysis using CATRAS. Dendrochronologia 1983, 1, 45–53. [Google Scholar]
- Schweingruber, F.H.; Poschlod, P. Growth Rings in Herbs and Shrubs: Life span, age determination and stem anatomy. For. Snow Landsc. Res. 2005, 79, 195–415. [Google Scholar]
- Punt, W.; Marks, A.; Hoen, P.P. Vitaceae. In The Northwest European Pollen Flora VIII; Punt, W., Blackmore, S., Hoen, P.P., Stafford, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 67–70. [Google Scholar]
- Fritts, H.C.; Swetnam, T.W. Dendroecology: A tool for evaluating variations in past and present forest environments. Adv. Ecol. Res. 1989, 19, 111–188. [Google Scholar]
- Horner, M.; Street, H.E. Pollen dimorphism—Origin and significance in pollen plant formation by another culture. Ann. Bot. 1978, 42, 763–777. [Google Scholar] [CrossRef]
- Anderson, G.J.; Symon, D.E. Functional dioecy and andromonoecy in Solanum. Evolution 1989, 43, 204–219. [Google Scholar] [CrossRef]
- Kevan, P.G.; Longair, R.W.; Gadawski, R.M. Dioecy and pollen dimorphism in Vitis riparia (Vitaceae). Can. J. Bot. 1985, 63, 2263–2267. [Google Scholar] [CrossRef]
- Kevan, P.G.; Blades, D.C.A.; Posluszny, U.; Ambrose, J.D. Pollen dimorphism and dioecy in Vitis aestivalis. Vitis 1988, 27, 143–146. [Google Scholar]
- Kimura, P.H.; Okamoto, G.; Hirano, K. The mode of pollination and stigma receptivity in Vitis coignetiae Pulliat. Am. J. Enol. Vitic. 1998, 49, 1–5. [Google Scholar] [CrossRef]
- Abreu, I.; Costa, I.; Oliveira, M.; Cunha, M.; De Castro, R. Ultrastructure and germination of Vitis vinifera cv. Loureiro pollen. Protoplasma 2006, 228, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Maghradze, D.; Melyan, G.; Salimov, V.; Chipashvili, R.; Íñiguez, M.; Puras, P.; Melendez, E.; Vaca, R.; Ocete, C.; Rivera, D.; et al. Wild grapevine (Vitis sylvestris C.C.Gmel.) wines from the Southern Caucasus region. OENO One 2020, 54, 849–862. [Google Scholar] [CrossRef]
- Cardarelli, A.; Bosi, G.; Rinaldi, R.; Ucchesu, M.; Bacchetta, G. Vino o non vino. Nuovi dati sui vinaccioli della Terramara di Montale (Modena) tra la fine della media età del Bronzo e il Bronzo Recente. In Proceedings of the 50th Riunione Preistoria del Cibo. L’Alimentazione nella Preistoria e nella Protostoria, Rome, Italy, 5–8 October 2015; Available online: http://preistoriadelcibo.iipp.it/contributi/3_31.pdf (accessed on 7 July 2020).
- Ucchesu, M.; Orrù, M.; Grillo, O.; Venora, G.; Usai, A.; Serreli, P.F.; Bacchetta, G. Earliest evidence of a primitive cultivar of Vitis vinifera L. during the Bronze Age in Sardinia (Italy). Veg. Hist. Archaeobot. 2015, 24, 587–600. [Google Scholar] [CrossRef]
- Cremaschi, M.; Mercuri, A.M.; Torri, P.; Florenzano, A.; Pizzi, C.; Marchesini, M.; Zerboni, A. Climate change versus land management in the Po Plain (Northern Italy) during the Bronze Age: New insights from the VP/VG sequence of the Terramara Santa Rosa di Poviglio. Quat. Sci. Rev. 2016, 136, 153–172. [Google Scholar] [CrossRef]
- Valamoti, S.M.; Pagnoux, C.; Ntinou, M.; Bouby, L.; Bonhomme, V.; Terral, J.-F. More than meets the eye: New archaeobotanical evidence on Bronze Age viticulture and wine making in the Peloponnese, Greece. Veg. Hist. Archaeobot. 2020, 29, 35–50. [Google Scholar] [CrossRef]
- Pecci, A.; Borgna, E.; Mileto, S.; Dalla Longa, E.; Bosi, G.; Florenzano, A.; Mercuri, A.M.; Corazza, S.; Marchesini, M.; Vidale, M. Wine consumption in Bronze Age Italy: Combining organic residue analysis, botanical data and ceramic variability. J. Archaeol. Sci. 2020, 123, 105256. [Google Scholar] [CrossRef]
- Bosi, G.; Castiglioni, E.; Rinaldi, R.; Mazzanti, M.; Marchesini, M.; Rottoli, M. Archaeobotanical evidence of food plants in Northern Italy during the Roman period. Veg. Hist. Archaeobot. 2020, 29, 681–697. [Google Scholar] [CrossRef]
- Bosi, G.; Mercuri, A.M.; Guarnieri, C.; Bandini Mazzanti, M. Luxury food and ornamental plants at the 15th century AD Renaissance court of the Este family (Ferrara, Northern Italy). Veg. Hist. Archaeobot. 2009, 18, 389–402. [Google Scholar] [CrossRef]
- Chassouant, L.; Celant, A.; Delpino, C.; Di Rita, F.; Vieillescazes, C.; Mathe, C.; Magri, D. Archaeobotanical and chemical investigations on wine amphorae from San Felice Circeo (Italy) shed light on grape beverages at the Roman time. PLoS ONE 2022, 17, e0267129. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Duan, S.; Xia, Q.; Liang, Z.; Dong, X.; Margaryan, K.; Musayev, M.; Goryslavets, S.; Zdunić, G.; Bert, P.-F.; et al. Dual domestications and origin of traits in grapevine evolution. Science 2023, 379, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Brusco, A.; Marchianò, R.; Misasi, G.; Puntillo, M.; Puntillo, D. Conservazione della Vite Selvatica (Vitis vinifera ssp. sylvestris) nelle Riserve Naturali del Lago di Tarsia e della Foce del Crati; Edizione Amici della Terra Italia/Gestore Riserve Tarsia-Crati: Tarsia, Italy, 2015. [Google Scholar]
- Tello, J.; Mammerler, R.; Čajić, M.; Forneck, A. Major Outbreaks in the Nineteenth Century Shaped Grape Phylloxera Contemporary Genetic Structure in Europe. Sci. Rep. 2019, 9, 17540. [Google Scholar] [CrossRef]
- Arroyo-García, R.; Cantos, M.; Lara, M.; López, M.-Á.; Gallardo, A.; Ocete, C.A.; Pérez, Á.; Bánáti, H.; García, J.L.; Ocete, R. Characterization of the largest relic Eurasian wild grapevine reservoir in Southern Iberian Peninsula—Spanish. J. Agric. Res. 2016, 14, e0708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).