Late Pleistocene to Holocene Palaeohydrological History of the Thermal-Spring-Fed Lake Pețea (NW Romania) Revealed by Radiocarbon Dating and Complex Sedimentological Investigations
Abstract
1. Introduction
2. Materials and Methods
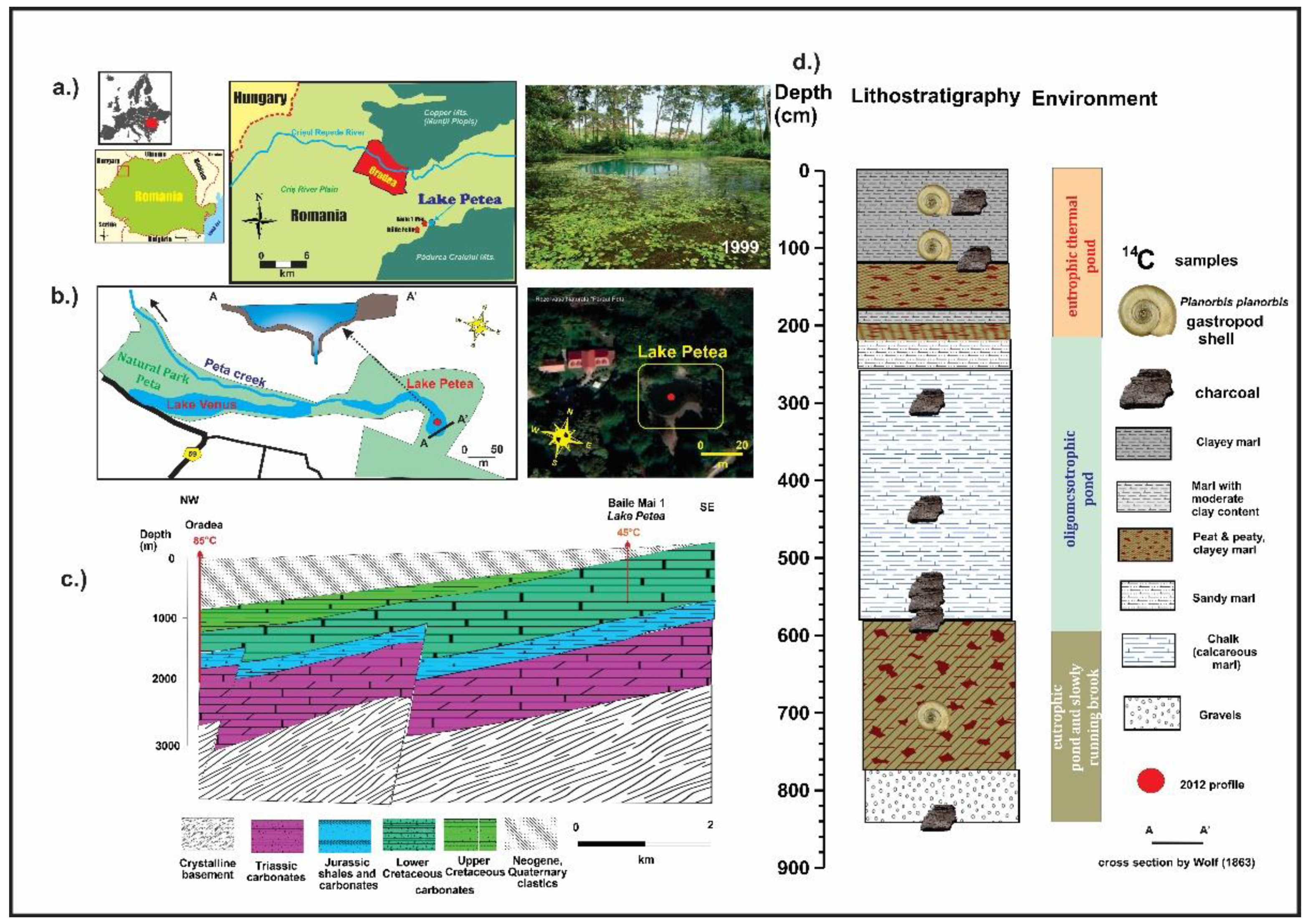
2.1. Study Area
2.2. Stratigraphy and Sampling
2.3. 14C Dating
2.4. Age–Depth Modeling and Sedimentation Time Estimation
2.5. Environmental Magnetism
2.6. Grain-Size Distribution and Concentrations of Small Carbonate Concretions and Charcoal
2.7. Statistical Data Analysis
3. Results
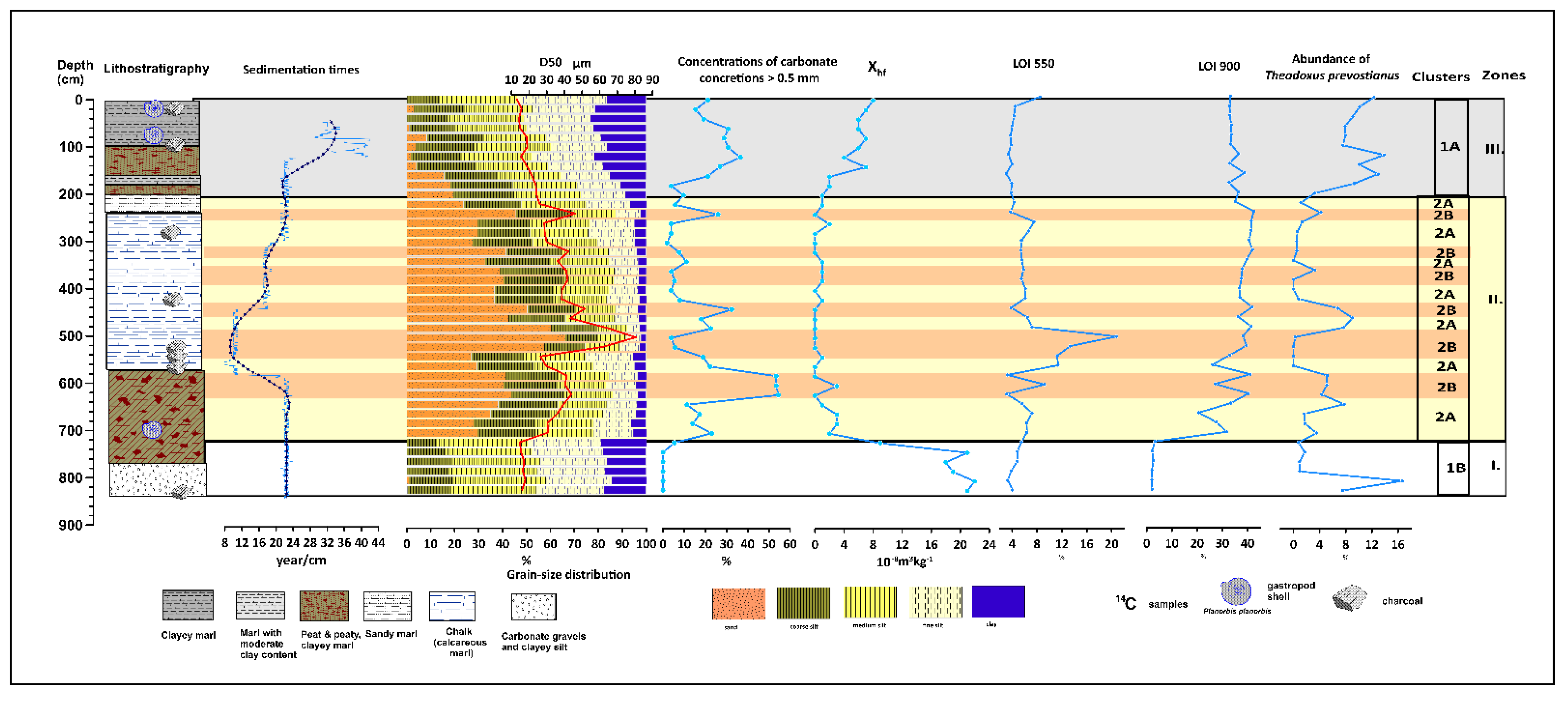
3.1. Radiocarbon Ages and Chronology
3.2. Grain Size, Magnetic Susceptibility, LOI Data, and Sediment Accumulation
3.3. Factor Analysis
4. Discussion

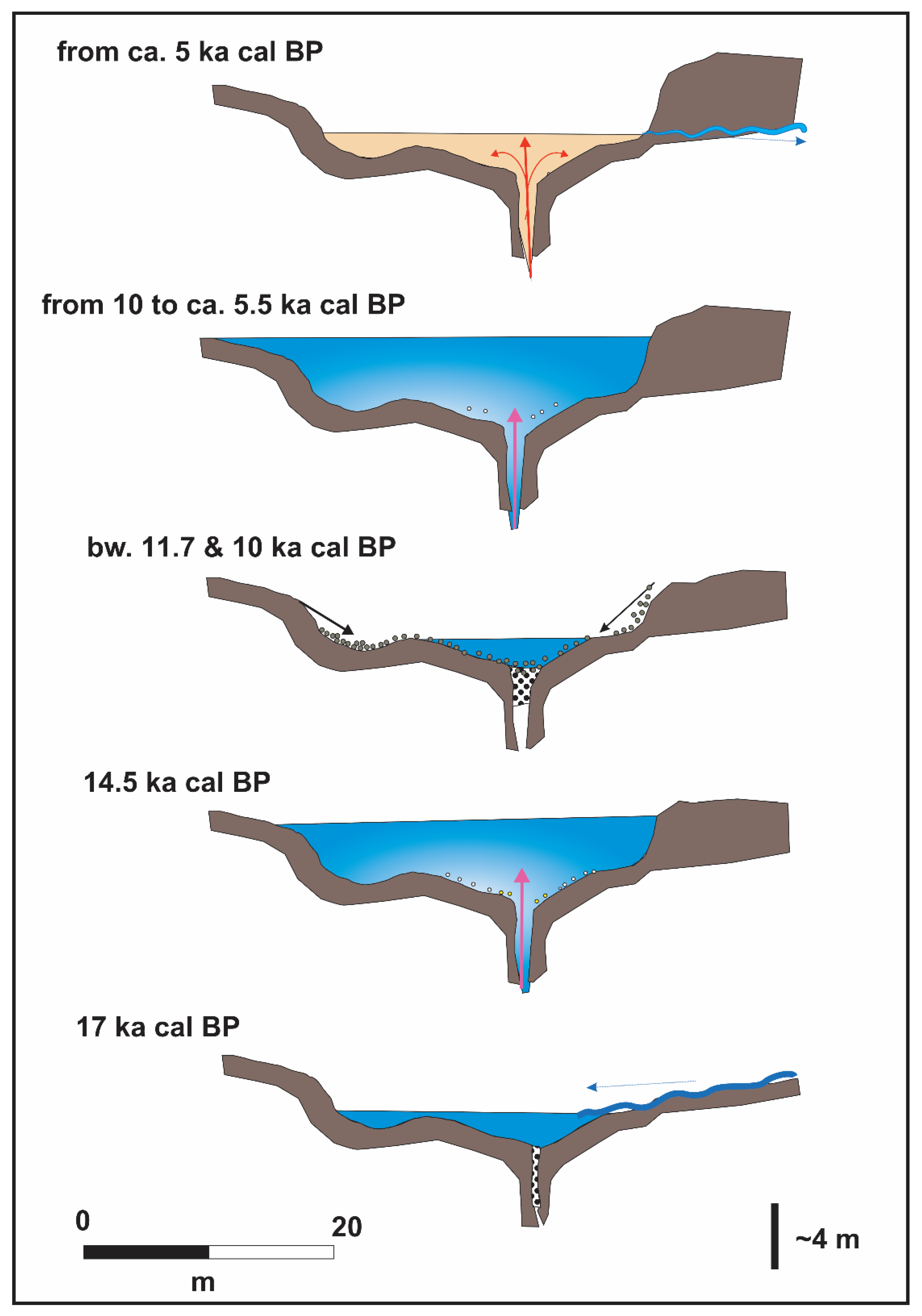
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulyás, S.; Sümegi, P.; Müller, T.; Geary, D.H.; Magyar, I.; Nagy, B.; Benyó-Korcsmáros, R. Assessing phenotypic variation and plasticity of endemic gastropods from thermal water refugia using complex morphometric techniques: A case study of Lake Pețea melanopsids. Palaeontology, submitted.
- Neubauer, T.A.; Harzhauser, M.; Georgopoulou, E.; Wrozyna, C. Population bottleneck triggering millennial-scale morphospace shifts in endemic thermal-spring melanopsids. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 414, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Sümegi, P.; Molnár, D.; Sávai, S.; Gulyás, S. Malacofauna evolution of the Lake Pețea (Püspökfürdő), Oradea region, Romania. Nymphaea Folia Nat. Bihariae 2012, 34, 5–29. [Google Scholar]
- Sümegi, P.; Molnár, D.; Sávai, S.; Töviskes, R.J. Preliminary radiocarbon dated paleontological and geological data for the Quaternary malacofauna at Püspökfürdő (Băile 1 Mai, Oradea region, Romania). Malakol. Táj. 2012, 30, 31–37. [Google Scholar]
- Sümegi, P.; Gulyás, S.; Molnár, D.; Náfrádi, K.; Törőcsik, T.; Sümegi, B.P.; Müller, T.; Szilágyi, G.; Varga, Z. Ice Age Terrestrial and Freshwater Gastropod Refugia in the Carpathian Basin, Central Europe. In Biological Resources of Water; Ray, S., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Kormos, T. Beitrage zur kenntnis der Melanopsis-arten aus den Thermen von Püspökfürdő bei Nagyvárad. Földtani Közlöny 1903, 33, 496–503. [Google Scholar]
- Kormos, T. Új adatok a Püspökfürdő élő csigáinak ismeretéhez. Állatani Közlöny 1904, 3, 102–111. [Google Scholar]
- Kormos, T. A Melanopsis hungarica, Korm. alkalmazkodásáról. Állatani Közlöny 1905, 4, 155–156. [Google Scholar]
- Kormos, T. Über den ursprung der thermenfauna von Püspökfürdő. Földtani Közlöny 1905, 35, 421–450. [Google Scholar]
- Paucă, M. Les mollusques pléistocènes de Băile Episcopeşti. Bull. Soc. Roum. Géol. 1937, 3, 130–142. [Google Scholar]
- Sîrbu, I.; Gagiu, A.; Benedek, A.M. On the brink of extinction: Fate of the Pețea thermal lake (Romania) and its endemic species. Tentacle 2013, 21, 34–37. [Google Scholar]
- Sîrbu, I.; Sárkány-Kiss, A. Endangered freshwater mollusc species from the eastern tributaries of the Tisa river (Romanian territory). Tisc. Monogr 2002, 6, 71–80. [Google Scholar]
- Tóth, M. Data about Diluvial formations of Nagyvárad region. Magy. Orv. És Természetvizsgálók 25. Vándorgyűlése Munkálatai 1891, 25, 474–479. [Google Scholar]
- Covaciu-Marcov, S.D.; Ghira, I.; Ardeleanu, A.; Cogalniceanu, D. Studies on the influence of thermal water from Western Romania upon Amphibians. Biota 2003, 4, 9–20. [Google Scholar]
- Müller, T.; Várkonyi, L.; Gagiu, A.; Kovács, B.; Búza, E.; Majoros, G.; Staszny, Á.; Horváth, Á.; Bernáth, G.; Tóth, G.; et al. Attempts on ex situ conservation of Melanopsis parreyssii and Scardinius racovitzai. In II. Sustainable Development in the Carpathian Basin" International Conference—Book of Abstracts; Zimmermann, Z., Szabó, G., Eds.; GATE University: Gödöllő, Hungary, 2014; pp. 35–38. [Google Scholar]
- Sîrbu, I.; Benedek, A.M. Requiem for Melanopsis parreyssii or the anatomy of a new extinction in Romania. Tentacle 2016, 24, 26–28. [Google Scholar]
- Telcean, I.C.; Cupşa, D. Threatened and rare fishes from Upper Tisa valley and its Romanian left shore tributaries (North-Western Romania). Pisces Hung. 2012, 6, 87–94. [Google Scholar]
- Telcean, I.C.; Cupşa, D. The drastic decline of fish fauna in the thermal lake of “Baile 1 Mai” (Baile Episcopale, Bihor County, Romania). Pisces Hung. 2013, 7, 141–142. [Google Scholar]
- Mintaş, O.; Vicaş, G.; Mintaş, I. The influence of the natural factors on the evolution of the Pețea thermal hydro-geo-ecosystem in the period 2009–2012. Ann. Univ. Oradea Fasc. Prot. Med. 2012, 19, 733–738. [Google Scholar]
- Telcean, I.C.; Cupșa, D. Püspökfürdő endemikus hala a Racovitza kele (Scardinius racovitzai). Halászat 2006, 99, 135. [Google Scholar]
- Brusina, S. Eine subtropische Oasis in Ungarn. Mitt. Naturwiss. Ver. Steiermark 1903, 39, 101–121. [Google Scholar]
- Wolf, H. Bericht über die geologischen Verhältnisse im Körösthale in Ungarn, nach den Aufnahmen im Jahre 1860. Jahrbruh Kais. Königlich Geol. Keischsanstalt 1863, 13, 265–292. [Google Scholar]
- Gheorghe, A.l.; Grǎciun, P. Thermal aquifers in Romania. J. Hydrol. 1993, 145, 111–123. [Google Scholar] [CrossRef]
- Hertelendi, E.; Csongor, É.; Záborszky, L.; Molnár, I.; Gál, I.; Győrffy, M.; Nagy, S. Counting system for high precision C-14 dating. Radiocarbon 1989, 32, 399–408. [Google Scholar] [CrossRef]
- Hertelendi, E.; Sümegi, P.; Szöőr, G. Geochronologic and paleoclimatic characterization of Quaternary sediments in the Great Hungarian Plain. Radiocarbon 1992, 34, 833–839. [Google Scholar] [CrossRef]
- Molnár, M.; Janovics, R.; Major, I.; Orsovszki, J.; Gönczi, R.; Veres, M.; Leonard, A.G.; Castle, S.M.; Lange, T.E.; Wacker, L.; et al. Status Report of the New AMS 14C Sample Preparation Lab of the Hertelendi Laboratory of Environmental Studies (Debrecen, Hungary). Radiocarbon 2013, 55, 665–676. [Google Scholar] [CrossRef]
- Jull, A.J.T.; Burr, G.S.; Beck, J.W.; Hodgins, G.W.L.; Biddulph, D.L.; Gann, J.; Hatheway, A.L.; Lange, T.E.; Lifton, N.A. Application of accelerator mass spectrometry to environmental and paleoclimate studies at the University of Arizona. Radioact. Environ. 2006, 8, 3–23. [Google Scholar]
- Synal, H.A.; Stocker, M.; Suter, M. MICADAS: A new compact radiocarbon AMS system. Nucl. Instrum. Methods Phys. Res. 2007, 259, 7–13. [Google Scholar] [CrossRef]
- Wacker, L.; Bonani, G.; Friedrich, M.; Hajdas, I.; Kromer, B.; Némec, M.; Ruff, M.; Suter, M.; Synal, H.A.; Vockenhuber, C. MICADAS: Routine and high-precision radiocarbon dating. Radiocarbon 2010, 52, 252–262. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, J.A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 2011, 3, 457–474. [Google Scholar] [CrossRef]
- Reimer, P.; Austin, W.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M. TheIntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, J.A.; Benett, K.D.; Reimer, P.J. Double the dates and go for Bayes-Impacts of model choice, dating density and quality of chronologies. Quat. Sci. Rev. 2018, 188, 58–66. [Google Scholar] [CrossRef]
- Dearing, J.A.; Dann, R.J.L.; Hay, K.; Lees, J.A.; Loveland, P.J.; Maher, B.A.; O’Grady, K. Frequency-dependent susceptibility measuremements of environmental materials. Geophys. J. Int. 1996, 124, 228–240. [Google Scholar] [CrossRef]
- Munsell Soil Color Charts; Munsell Color Company, Pantone LLC: Ashford, UK, 2002.
- Dean, W.E. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. J. Sed. Petrol. 1974, 44, 242–248. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; Freeman: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Riggs, A.C. Major carbon-14 deficiency in modern snail shells from Southern Nevada Springs. Science 1984, 224, 58–61. [Google Scholar]
- McConnaughey, T.A.; Gillikin, D.P. Carbon isotopes in mollusk shell carbonates. Geo Mar. Lett. 2008, 28, 287–299. [Google Scholar] [CrossRef]
- Brennan, R.; Quade, J. Reliable Late-Pleistocene stratigraphic ages and shorter groundwater travel times from 14C in fossil snails from the Southern Great Basin. Quat. Res. 1997, 47, 329–335. [Google Scholar] [CrossRef]
- Björck, S.; Walker, M.J.C.; Cwynar, L.; Johnsen, S.J.; Knudsen, K.L.; Lowe, J.J.; Wohlfarth, B. INTIMATE Members. An event stratigraphy for the last termination in the North Atlantic region based on the Greenland Ice Core record: A proposal by the INTIMATE group. J. Quat. Sci. 1998, 13, 283–292. [Google Scholar] [CrossRef]
- Blockley, S.P.E.; Lane, C.S.; Turney, C.S.M.; Ramsey, C.B. The INTegration of Ice core, MArine and TErrestrial records of the last termination (INTIMATE) 60,000 to 8000 BP. Quat. Sci. Rev. 2012, 36, 1. [Google Scholar]
- Blockley, S.P.E.; Lane, C.S.; Hardiman, M.; Rasmussen, S.O.; Seierstad, I.K.; Steffensen, J.P.; Svensson, A.; Lotter, A.F.; Turney, C.S.M.; Ramsey, C.B. Synchronisation of palaeoenvironmental records over the last 60,000 years, and an extended INTIMATE event stratigraphy to 48,000 b2k. Quat. Sci. Rev. 2012, 36, 2–10. [Google Scholar] [CrossRef]
- Rasmussen, S.O.; Andersen, K.K.; Svensson, A.M.; Steffensen, J.P.; Vinther, B.M.; Clausen, H.B.; Siggaard-Andersen, M.L.; Johnsen, S.J.; Larsen, L.B.; Dahl-Jensen, D.; et al. A new Greenland ice core chronology for the last glacial termination. J. Geophys. Res. 2006, 111, D6. [Google Scholar]
- Walker, M.J.C.; Björck, S.; Lowe, J.J.; Cwynar, L.; Johnsen, S.; Knudsen, K.L.; Wohlfarth, B. INTIMATE group. Isotopic ‘events’ in the GRIP ice core: A stratotype for the Late Pleistocene. Quat. Sci. Rev. 1999, 18, 1143–1150. [Google Scholar]
- Tămaş, T.; Onac, B.P.; Bojar, A.V. Lateglacial-Middle Holocene stable isotope records in two coeval stalagmites from the Bihor Mountains, NW Romania. Geol. Q. 2005, 49, 125–136. [Google Scholar]
- Onac, B.P. Mineralogical studies and Uranium-series dating of speleothems from Scarisoara Glacier Cave (Bihor Mountains, Romania). Theor. Appl. Karstol. 2001, 13–14, 33–38. [Google Scholar]
- Feurdean, A.; Perşoiu, A.; Tanţău, I.; Stevens, T.; Magyari, E.K.; Onac, B.P.; Marković, S.; Andrič, M.; Connor, S.; Fărcaş, S.; et al. Climate variability and associated vegetation response throughout Central and Eastern Europe (CEE) between 60 and 8 ka. Quat. Sci. Rev. 2014, 106, 206–224. [Google Scholar] [CrossRef]
- Feurdean, A.; Klotz, S.; Mosbrugger, V.; Wohlfarth, B. Pollen-based quantitative reconstruction of Holocene climate variability in NW Romania. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 260, 494–504. [Google Scholar] [CrossRef]
- Tóth, M.; Magyari, E.K.; Brooks, S.J.; Braun, M.; Buczkó, K.; Bálint, M.; Heiri, O. A chironomid-based reconstruction of late glacial summer temperatures in the southern Carpathians (Romania). Quat. Res. 2012, 77, 122–131. [Google Scholar] [CrossRef]
- Benyó-Korcsmáros, R.; Torma, A.; Gulyás, S.; Sümegi, P. Lotus from Nagyvárad: The question of the origin of the thermal water lily from Püspökfürdő. In Magyarok a Kárpát-Medencében 4. Régmúlt és Jelen: Tudományos Nemzetközi Konferencia Szeged, Magyarország: Egyesület Közép-Európa Kutatására; Szónokyné, A.G., Ed.; Innovariant Kft: Szeged, Hungary, 2020; pp. 97–103. [Google Scholar]
- Benyó-Korcsmáros, R.; Gulyás, S.; Bóni, Z.; Nagy, B.; Törőcsik, T.; Sümegi, P. Identification and environmental background of Chara remains in the Holocene sequence of Lake Pețea NW Romania. Archeometriai Műhely, 2023; in press. [Google Scholar]
- Benyó-Korcsmáros, R.; Gulyás, S.; Sebők, D.; Benyó, D.; Cseh, P.; Sümegi, P. Methodological add-ons to the non-destructive, micro-CT-based taxonomic-morphometric analysis of Chara remains. Geologos, 2023; in press. [Google Scholar]
- Bóni, Z. Kagylósrákok Taxonómiai, Paleoökológiai Vizsgálata a Püspökfürdői Szent László-tó Üledékeiből (Taxonomic and Paleoecological Analysis of Ostracod Remains from Lake Pețea, Püspökfürdő). Master’s Thesis, University of Szeged, Szeged, Hungary, 2022. (Unpublished work). [Google Scholar]
- Björkman, L.; Feurdean, A.; Wohlfarth, B. Late Glacial and Holocene vegetation development at Steregoiu in the Gutaiului Mountains, NW Romania. Rev. Palaeobot. Palynol. 2003, 124, 79–85. [Google Scholar]
- Feurdean, A.; Spessa, A.; Magyari, E.K.; Willis, K.J.; Veres, D.; Hickler, T. Trends in biomass burning in the Carpathian region over the last 15,000 years. Quat. Sci. Rev. 2012, 45, 111–123. [Google Scholar] [CrossRef]
- Feurdean, A.; Liakka, J.; Vannière, B.; Marinova, E.; Hutchinson, S.M.; Mosburgger, V.; Hickler, T. 12,000-Years of fire regime drivers in the lowlands of Transylvania (Central-Eastern Europe): A data-model approach. Quat. Sci. Rev. 2013, 81, 48–52. [Google Scholar] [CrossRef]
- Feurdean, A.; Gałka, M.; Tanţău, I.; Geantă, A.; Hutchinson, S.M.; Hickler, T. Tree and timberline shifts in the northern Romanian Carpathians during the Holocene and the responses to environmental changes. Quat. Sci. Rev. 2016, 134, 206–223. [Google Scholar]
- Constantin, S.; Bojar, A.V.; Lauritzen, S.E.; Lundberg, J. Holocene and Late Pleistocene climate in the sub-Mediterranean continental environment: A speleothem record from Poleva Cave (Southern Carpathians, Romania). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 243, 322–338. [Google Scholar] [CrossRef]
- Magyari, E.K.; Buczkó, K.; Jakab, G.; Braun, M.; Pál, Z.; Karátson, D.; Papp, P. Palaeolimnology of the last crater lake in the Eastern Carpathian Mountains—A multiproxy study of Holocene hydrological changes. Hydrobiologia 2009, 631, 29–63. [Google Scholar] [CrossRef]
- Buczkó, K.; Magyari, E.; Hübener, T.; Braun, M.; Bálint, M.; Tóth, M.; Lotter, A.F. Responses of diatoms to the Younger Dryas climatic reversal in a South Carpathian Mountain lake (Romania). J. Paleolimnol. 2012, 48, 417–431. [Google Scholar] [CrossRef]
- Perşoiu, I. Reconstruction of Holocene Geomorphological Evolution of Somesu Mic Valley. Ph.D. Thesis, “A. I. Cuza” University, Iasi, Romania, 2010. (Unpublished work). [Google Scholar]
- Howard, A.J.; Macklin, M.G.; Bailey, D.W.; Mills, S.; Andreescu, R. Late glacial and Holocene river development in the Teleorman Valley on the southern Romanian Plain. J. Quat. Sci. 2004, 19, 271–280. [Google Scholar] [CrossRef]
- Onac, B.P.; Constantin, S.; Lundberg, J.; Lauritzen, S.E. Isotopic climate record in a Holocene stalagmite from Ursilor Cave (Romania). J. Quat. Sci. 2002, 17, 319–327. [Google Scholar] [CrossRef]
- Mayewski, P.A.; Rohling, E.E.; Curt Stager, J.; Karlen, W.; Maasch, K.A.; Meeker, L.D.; Eric, A.; Meyerson, E.A.; Gasse, F.; Steig, E.J. Holocene climate variability. Quat. Res. 2004, 62, 243–255. [Google Scholar] [CrossRef]
- Tóth, M.; Magyari, E.K.; Buczkó, K.; Braun, M.; Panagiotopoulos, K.; Heiri, O. Chironomid-inferred Holocene temperature changes in the South Carpathians (Romania). Holocene 2015, 25, 569–582. [Google Scholar] [CrossRef]
- Grindean, R.; Feurdean, A.; Hurdu, B.; Fracas, S.; Tantau, I. Lateglacial/Holocene transition to mid-Holocene: Vegetation responses to climate changes in the Apuseni Mountains (NW Romania). Quat. Int. 2015, 388, 76–86. [Google Scholar] [CrossRef]
- Dragusin, V.; Staubwasser, M.; Hoffmann, D.L.; Ersek, V.; Onac, B.P.; Veres, D. Constraining Holocene hydrological changes in the Carpathian–Balkan region using speleothem 18O and pollen-based temperature reconstructions. Clim. Past Discuss. 2014, 10, 381–427. [Google Scholar] [CrossRef]
- Gałka, M.; Tanţău, I.; Ersek, V.; Feurdean, A. A 9000-year record of cyclic vegetation changes identified in a montane peatland deposit located in the Eastern Carpathians (Central-Eastern Europe): Autogenic succession or regional climatic influences? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 449, 1325–1336. [Google Scholar]
- van Geel, B.; van der Plicht, J.; Kilian, M.; Klaver, E.; Kouwenberg, J.; Renssen, H.; Reynaud-Farrera, I.; Waterbolk, H. The sharp rise of Delta C-14 ca, 800 cal BC: Possible causes, related climatic teleconnections and the impact on human environments. Radiocarbon 1998, 40, 535–550. [Google Scholar]
- Mauquoy, D.; van Geel, B.; Blaauw, M.; Speranza, A.; van der Plicht, J. Changes in solar activity and Holocene climatic shifts derived from 14C wiggle-match dated peat deposits. Holocene 2004, 14, 45–52. [Google Scholar]
- Gałka, M.; Miotk-Szpiganowicz, G.; Goslar, T.; Jęśko, M.; van der Knaap, O.W.; Lamentowicz, M. Palaeohydrology, fires and vegetation succession in the southern Baltic during the last 7500 years reconstructed from a raised bog based on multi-proxy data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 370, 209–221. [Google Scholar] [CrossRef]
- Swindles, G.T.; Plunkett, G.; Roe, H.M. A delayed climatic response to solar forcing at 2800 cal. BP: Multi-proxy evidence from three Irish peatlands. Holocene 2007, 17, 177–182. [Google Scholar] [CrossRef]
- Feurdean, A.; Gałka, M.; Kuske, E.; Tanţău, I.; Lamentowicz, M.; Florescu, G.; Hutchinson, S.M.; Liakka, J.; Mulch, A.; Hickler, T. Last millennium hydro-climate variability in Central Eastern Europe (Northern Carpathians, Romania). Holocene 2015, 25, 1179–1192. [Google Scholar]
- Yates, T. Studies of non-marine mollusks for the selection of shell samples for radiocarbon dating. Radiocarbon 1986, 28, 457–463. [Google Scholar] [CrossRef]
- Pigati, J.S.; Quade, J.; Shahanan, T.M.; Haynes, C.V. Radiocarbon dating of minute gastropods and new constraints on the timing of late Quaternary spring-discharge deposits in southern Arizona, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 204, 33–36. [Google Scholar]
- Rech, J.A.; Pigati, J.S.; Lehmann, S.B.; McGimpsey, C.M.; Grimley, D.A.; Nekola, J.C. Assessing open-system behavior of 14C in terrestrial gastropod shells. Radiocarbon 2011, 53, 325–335. [Google Scholar] [CrossRef]
- Evin, J.; Marechal, J.; Lyon, C.; Puissegur, J. Conditions involved in dating terrestrial shells. Radiocarbon 1980, 22, 545–556. [Google Scholar] [CrossRef]
- Goodfriend, A.G.; Stipp, J.J. Limestone and the problem of radiocarbon dating of land-snail shell carbonate. Geology 1983, 11, 575–577. [Google Scholar] [CrossRef]
- Goodfriend, G.A. Radiocarbon age anomalies in shell carbonate of land snails from semi-arid areas. Radiocarbon 1987, 29, 159–167. [Google Scholar] [CrossRef]
- Goodfriend, A.G.; Ellis, G.L.; Toolin, L.J. Radiocarbon age anomalies in land snail shells from Texas; ontogenetic, individual, and geographic patterns of variation. Radiocarbon 1999, 41, 149–156. [Google Scholar]
- Dong, J.; Cheng, P.; Eiler, J. Implications of the apparent 14C age of cultured Achatina fulica and the spatial features of 14C ages among modern land snail shells in China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 545, 109654. [Google Scholar] [CrossRef]
- Rubin, M.; Likins, R.C.; Berry, E.G. On the validity of radiocarbon dates from snail shells. J. Geol. 1961, 71, 84–89. [Google Scholar] [CrossRef]
- Tamers, M.A. Validity of radiocarbon dates on terrestrial snail shells. Am. Antiq. 1970, 35, 94–100. [Google Scholar] [CrossRef]
- Romaniello, L.; Quarta, G.; Mastronuzzi, G.; D’Elia, M.; Calcagnile, L. 14C age anomalies in modern land snails shell carbonate from Southern Italy. Quat. Geochronol. 2008, 3, 68–73. [Google Scholar] [CrossRef]
- Xu, B.; Gu, Z.; Han, J.; Liu, Z.; Pei, Y.; Lu, Y.; Wu, N.; Chen, Y. Radiocarbon and stable carbon isotope analyses of land snails from the Chinese loess plateau environmental and chronological implications. Radiocarbon 2010, 52, 149–156. [Google Scholar] [CrossRef]
- Xu, B.; Gu, Z.; Han, J.; Hao, Q.; Lu, Y.; Wang, L.; Wu, N.; Pei, Y. Radiocarbon age anomalies of land snail shells in the Chinese Loess Plateau. Quat. Geochronol. 2011, 6, 383–395. [Google Scholar] [CrossRef]
- Pigati, J.S.; Rech, J.A.; Nekola, J.C. Radiocarbon dating of small terrestrial gastropod shells in North America. Quat. Geochronol. 2010, 5, 519–530. [Google Scholar] [CrossRef]
- Pigati, J.S.; McGeehin, J.P.; Muhs, D.R.; Bettis, E.A. Radiocarbon dating late Quaternary loess deposits using small terrestrial gastropod shells. Quat. Sci. Rev. 2013, 76, 114–126. [Google Scholar] [CrossRef]
- Pigati, J.S.; McGeehin, J.P.; Muhs, D.R.; Grimley, D.A.; Nekola, J.C. Radiocarbon dating loess deposits in the Mississippi Valley using terrestrial gastropod shells (Polygyridae, Helicinidae, and Discidae). Aeolian Res. 2015, 16, 25–42. [Google Scholar] [CrossRef]


| Sample No. | Depth | Lab code | Material | 14C Ages (BP ± 1σ) | Calibrated Ages (cal BP ± 2σ) | pMC (± 1σ) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 40 cm | DeA-19820 | charcoal | 518 ± 23 | 534 ± 34 | 93.8 ± 0.268 | This study |
| 40 cm | DeA-11793 | gastropod shell | 22,156 ± 156 | 26,432 ± 530 | 6.34 ± 0.122 | This study | |
| 3 | 80 cm | DeA-11794 | charcoal | 1900 ± 25 | 1802 ± 82 | 78.9 ± 0.245 | This study |
| 4 | 100 cm | DeA-11792 | gastropod shell | 23,178 ± 94 | 27,456 ± 230 | 5.58 ± 0.065 | This study |
| 5 | 120 cm | DeA-19819 | charcoal | 3167 ± 23 | 3397 ± 58 | 67.4 ± 0.193 | This study |
| 6 | 300 cm | DeA-21049 | charcoal | 6480 ± 29 | 7373 ± 64 | 44.6 ± 0.161 | This study |
| 440 cm | DeA-21047 | charcoal | 9038 ± 33 | 10,212 ± 52 | 32.5 ± 0.133 | This study | |
| 8 | 540 cm | DeA-21045 | charcoal | 9594 ± 34 | 10,938 ± 218 | 30.3 ± 0.128 | Sümegi et al. [5] |
| 9 | 560 cm | DeA-21046 | charcoal | 9612 ± 34 | 10,957 ± 228 | 30.2 ± 0.128 | Gulyás et al. [1] |
| 10 | 580 cm | DeA-21041 | charcoal | 9746 ± 36 | 11,184 ± 102 | 29.7 ± 0.133 | This study |
| 11 | 700 cm | DeA-11790 | gastropod shell | 23,899 ± 102 | 28,030 ± 322 | 5.1 ± 0.064 | This study |
| 12 | 840 cm | DeA-11791 | charcoal | 18,624 ± 71 | 22,553 ± 250 | 9.84 ± 0.086 | This study |
| Sample No. | Depth | Lab Code | Material | Model 1 (cal BP ± 2σ) | Model 2 (cal BP ± 2σ) |
|---|---|---|---|---|---|
| 1 | 40 cm | DeA-19820 | charcoal | 2397 ± 591 | 546 ± 59 |
| 2 | 40 cm | DeA-11793 | gastropod shell | 2397 ± 591 | 546 ± 59 |
| 3 | 80 cm | DeA-11794 | charcoal | 2827 ± 514 | 1808 ± 105 |
| 4 | 100 cm | DeA-11792 | gastropod shell | 3118 ± 361 | 2608 ± 444 |
| 5 | 120 cm | DeA-19819 | charcoal | 3419 ± 222 | 3376 ± 108 |
| 6 | 300 cm | DeA-21049 | charcoal | 7364 ± 100 | 7369 ± 106 |
| 7 | 440 cm | DeA-21047 | charcoal | 9821 ± 304 | 9844 ± 306 |
| 8 | 540 cm | DeA-21045 | charcoal | 10,848 ± 136 | 10,869 ± 149 |
| 9 | 560 cm | DeA-21046 | charcoal | 11,020 ± 123 | 11,048 ± 142 |
| 10 | 580 cm | DeA-21041 | charcoal | 11,195 ± 98 | 11,255 ± 226 |
| 11 | 700 cm | DeA-11790 | gastropod shell | 12,477 ± 434 | 13,939 ± 927 |
| 12 | 840 cm | DeA-11791 | charcoal | 13,960 ± 637 | 17,081 ± 1382 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulyás, S.; Sümegi, P. Late Pleistocene to Holocene Palaeohydrological History of the Thermal-Spring-Fed Lake Pețea (NW Romania) Revealed by Radiocarbon Dating and Complex Sedimentological Investigations. Quaternary 2023, 6, 37. https://doi.org/10.3390/quat6020037
Gulyás S, Sümegi P. Late Pleistocene to Holocene Palaeohydrological History of the Thermal-Spring-Fed Lake Pețea (NW Romania) Revealed by Radiocarbon Dating and Complex Sedimentological Investigations. Quaternary. 2023; 6(2):37. https://doi.org/10.3390/quat6020037
Chicago/Turabian StyleGulyás, Sándor, and Pál Sümegi. 2023. "Late Pleistocene to Holocene Palaeohydrological History of the Thermal-Spring-Fed Lake Pețea (NW Romania) Revealed by Radiocarbon Dating and Complex Sedimentological Investigations" Quaternary 6, no. 2: 37. https://doi.org/10.3390/quat6020037
APA StyleGulyás, S., & Sümegi, P. (2023). Late Pleistocene to Holocene Palaeohydrological History of the Thermal-Spring-Fed Lake Pețea (NW Romania) Revealed by Radiocarbon Dating and Complex Sedimentological Investigations. Quaternary, 6(2), 37. https://doi.org/10.3390/quat6020037






