Multiporate Pollen of Poaceae as Bioindicator of Environmental Stress: First Archaeobotanical Evidence from the Early–Middle Holocene Site of Takarkori in the Central Sahara
Abstract
1. Introduction
1.1. Developmental Conditions of Multiporate Pollen
1.2. Aim of the Paper and the Archaeobotanical Evidence
2. Materials and Methods
- −
- Late Acacus (LA) = hunter-gatherer-fishers, ~10,170–~8180 cal BP
- −
- Early Pastoral (EP) = pastoralists, ~8300–~6890 cal BP
- −
- Middle Pastoral (MP) = pastoralists, ~7160–~5610 cal BP
- −
- Late Pastoral (LP) = pastoralists, ~5700–~4650 cal BP
3. Results
- −
- LA2, L275, 3 specimens with 3.2%, 0.5%, 0.5%
- −
- LA3, L69, 1 specimen with 0.9%
- −
- MP2, L25, 2 specimens with 0.9%, 0.8%.
4. Discussion
4.1. Polyploidy as Effect and Adaptation to Environmental or Anthropogenic Stresses
4.1.1. Multiporate Poaceae and the Climate Stress
4.1.2. Multiporate Poaceae and the Anthropogenic Stress
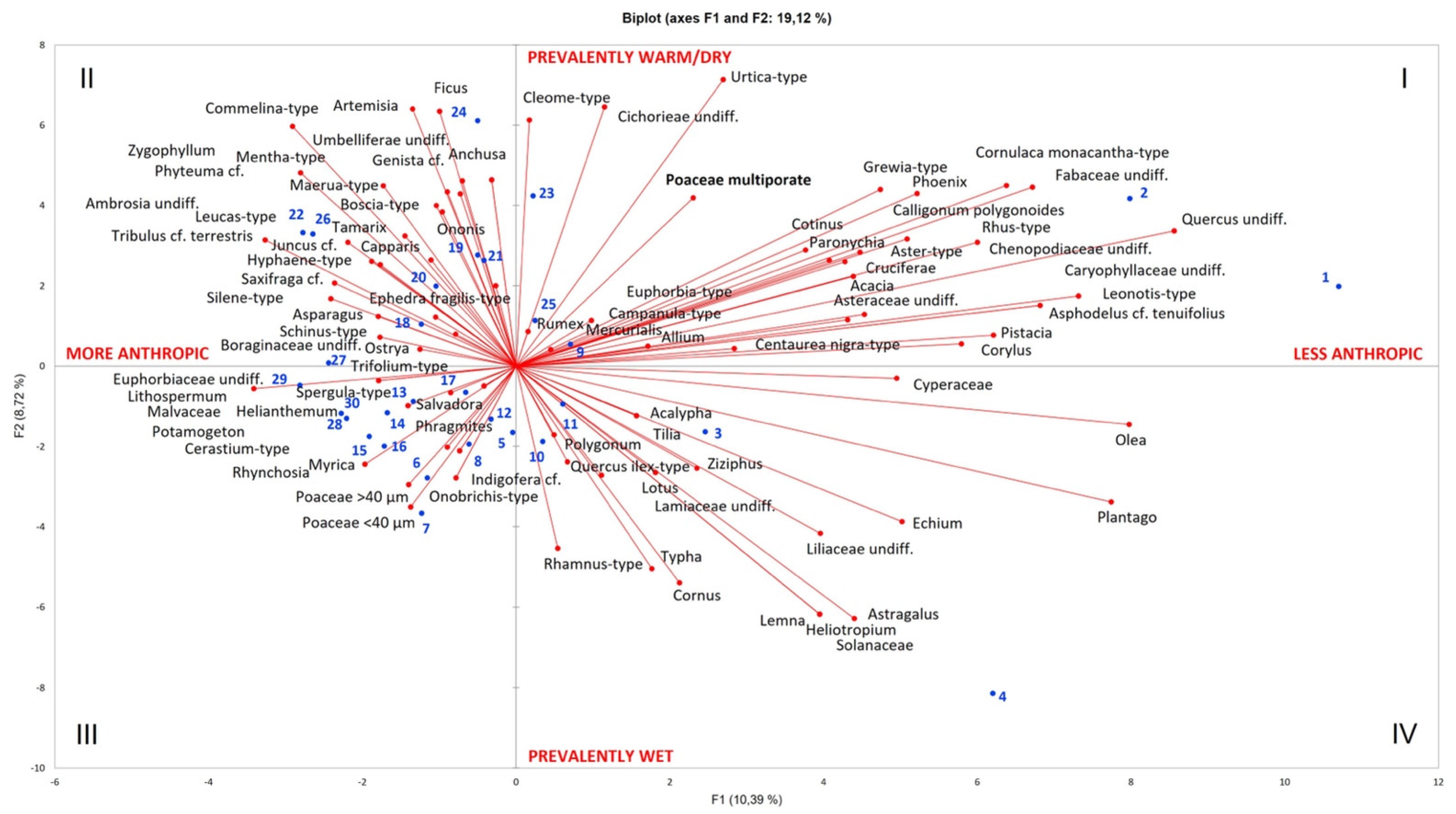
4.2. Multiporate Pollen as Palaeoecological Marker
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdtman, G. An Introduction to Pollen Analysis; Chronica Botanica Company: Waltham, MA, USA, 1944. [Google Scholar]
- Ma, G.; Huang, X.; Zhao, N.; Xu, Q. Apospory in Paspalum thunbergia. Aust. J. Bot. 2004, 52, 81–86. [Google Scholar] [CrossRef]
- Ma, G.; Xuelin, H.; Qiusheng, X.; Bunn, E. Multiporate pollen and apomixis in Panicoideae. Pak. J. Bot. 2009, 41, 2073–2082. [Google Scholar]
- Radaeski, J.N.; Bauermann, S.G. Diporate pollen grains of Poaceae species: High pollen resolution for reconstruction of grasslands vegetation. J. Agric. Res. 2017, 2, 135–137. [Google Scholar]
- Liu, Q.; Zhao, N.-X.; Hao, G. Pollen morphology of the Chloridoideae (Gramineae). Grana 2004, 43, 238–248. [Google Scholar] [CrossRef][Green Version]
- Ma, G.H.; Huang, X.L. Cytological and embryological studies on apospory in Bothriochloa ischaemum L. Acta. Biol. Hung. 2007, 58, 421–429. [Google Scholar] [CrossRef]
- Kihara, H. Wheat Studies—Retrospect and Prospects; Developments in Crop Science, 3; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Linde-Laursen, I. Cytogenetic analysis of Miscanthus ‘Giganteus’, an interspecific hybrid. Hereditas 1993, 119, 297–300. [Google Scholar] [CrossRef]
- Tomaszewska, P.; Kosina, R. Variability of pollen grains quality in oat amphiploids and their parental species. BioRxiv 2021. [Google Scholar] [CrossRef]
- Florek, M.; Kosina, R. rDNA cytogenetics and some structural variability in an Avena barbata Pott ex Link X A. sativa subsp. nuda (L.) Gillet et Magne amphiploid after 5-azaC treatment. Genet. Resour. Crop Evol. 2017, 64, 1723–1741. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Gao, J.; Lu, W.; Wang, H. Abnormal pollen development of bread wheat-Leymus mollis partial amphiploid. Euphytica 2005, 144, 247–253. [Google Scholar] [CrossRef]
- Kosina, R.; Florek, M.; Tomaszewska, P. Pollen grain morphogenesis in Triticeae and Avena amphiploids. Annu. Wheat Newsl. 2014, 60, 102–103. [Google Scholar]
- Grant, V. Plant Speciation; Columbia University Press: New York, NY, USA, 1981. [Google Scholar]
- Liu, B.; Wendel, J.F. Epigenetic Phenomena and the Evolution of Plant Allopolyploids. Mol. Phylogenet. Evol. 2003, 29, 365–379. [Google Scholar] [CrossRef]
- Soltis, D.E.; Soltis, P.S.; Tate, J.A. Advances in the study of polyploidy since plant speciation. New Phytol. 2004, 161, 173–191. [Google Scholar] [CrossRef]
- Fox, D.T.; Soltis, D.E.; Soltis, P.S.; Ashman, T.-L.; Van de Peer, Y. Polyploidy: A biological force from cells to ecosystems. Trends Cell Biol. 2020, 30, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Dwivedi, K.K.; Khurana, J.P.; Sopory, S.K. Apomixis: An enigma with potential applications. Curr. Sci. 2005, 89, 1879–1893. [Google Scholar]
- Carneiro, V.T.C.; Dusi, D.M.A.; Ortiz, J.P.A. Apomixis: Occurrence, applications and improvements. In Floriculture, Orna-mental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Science Books: London, UK, 2006; Volume 1, pp. 564–571. [Google Scholar]
- Biagetti, S.; di Lernia, S. Holocene deposits of Saharan rock shelters: The case of Takarkori and other sites from the Tadrart Acacus Mts. (SW Libya). Afr. Archaeol. Rev. 2013, 30, 305–338. [Google Scholar] [CrossRef]
- Cremaschi, M.; Zerboni, A.; Mercuri, A.M.; Olmi, L.; Biagetti, S.; di Lernia, S. Takarkori rock shelter (SW Libya): An archive of Holocene climate and environmental changes in the central Sahara. Quat. Sci. Rev. 2014, 101, 36–60. [Google Scholar] [CrossRef]
- Dunne, J.; Mercuri, A.M.; Evershed, R.P.; Bruni, S.; di Lernia, S. Earliest direct evidence of plant processing in prehistoric Saharan pottery. Nat. Plants 2016, 3, 16194. [Google Scholar] [CrossRef]
- Fornaciari, R.; Fornaciari, S.; Francia, E.; Mercuri, A.M.; Arru, L. Panicum spikelets from the Early Holocene Takarkori rockshelter (SW Libya): Archaeo-molecular and-botanical investigations. Plant Biosyst. 2016, 152, 1–13. [Google Scholar] [CrossRef]
- Mercuri, A.M.; Fornaciari, R.; Gallinaro, M.; Vanin, S.; di Lernia, S. Plant behaviour from human imprints and the cultivation of wild cereals in Holocene Sahara. Nat. Plants 2018, 4, 71–81. [Google Scholar] [CrossRef]
- di Lernia, S.; Massamba N’siala, I.; Mercuri, A.M. Saharan prehistoric basketry. Archaeological and archaeobotanical analysis of the early-middle Holocene assemblage from Takarkori (Acacus Mts., SW Libya). J. Archaeol. Sci. 2012, 39, 1837–1853. [Google Scholar] [CrossRef]
- di Lernia, S.; Bruni, S.; Cislaghi, I.; Cremaschi, M.; Gallinaro, M.; Guglielmi, V.; Mercuri, A.M.; Poggi, G.; Zerboni, A. Colour in context. Pigments and other coloured residues from the Early-Middle Holocene site of Takarkori (SW Libya). Archaeol. Anthropol. Sci. 2016, 8, 381–402. [Google Scholar] [CrossRef]
- Cherkinsky, A.; di Lernia, S. Bayesian Approach to 14C dates for estimation of long-term archaeological sequences in arid environments: The Holocene site of Takarkori Rockshelter, Southwest Libya. Radiocarbon 2013, 55, 771–782. [Google Scholar] [CrossRef]
- Rotunno, R.; Mercuri, A.M.; Florenzano, A.; Zerboni, A.; Di Lernia, S. Coprolites from Rock Shelters: Hunter-Gatherers “Herding” Barbary Sheep in the Early Holocene Sahara. J. Afr. Archaeol. 2019, 17, 76–94. [Google Scholar] [CrossRef]
- Rotunno, R.; Mercuri, A.M.; Florenzano, A.; Zerboni, A.; di Lernia, S. The visibility of mobility: Coprolites, dung and Neolithic herders in Central Saharan Rock Shelters. Environ. Archaeol. 2020, 1–16. [Google Scholar] [CrossRef]
- Ozenda, P. Flore et Végétation du Sahara; CNRS: Paris, France, 2000.
- Wasylikowa, K. Holocene flora of the Tadrart Acacus area, SW Libya, based on plant macrofossils from Uan Muhuggiag and Ti-n-Torha Two Caves archaeological sites. Origini 1992, 16, 125–152. [Google Scholar]
- Mercuri, A.M. Human influence, plant landscape, evolution and climate inferences from the archaeobotanical records of the Wadi Teshuinat area (Libyan Sahara). J. Arid Environ. 2008, 72, 1950–1967. [Google Scholar] [CrossRef]
- Brown, W.V.; Emery, W.H.P. Apomixis in the Gramineae, Tribe Andropogoneae: Themeda triandra and Bothriochloa ischaemum. Bot. Gaz. 1957, 118, 246–253. [Google Scholar] [CrossRef]
- Kaushal, P.; Dwivedi, K.K.; Radhakrishna, A.; Srivastava, M.K.; Malaviya, D.R.; Roy, A.K.; Saxena, S.; Paul, S. Development and characterization of a hexaploid Pennisetum orientale (2n = 6x = 54) cytotype recovered through BIII hybridization. Cytologia 2015, 80, 37–43. [Google Scholar] [CrossRef]
- Fall, P.L. Pollen evidence for plant introductions in a Polynesian tropical island ecosystem, Kingdom of Tonga. In Altered Ecologies: Fire, Climate and Human Influence on Terrestrial Landscapes; Haberle, S.G., Stevenson, J., Prebble, M., Eds.; ANU E-Press: Canberra, Australia, 2010. [Google Scholar]
- Cremaschi, M.; Zerboni, A.; Spötl, C.; Felletti, F. The calcareous tufa in the Tadrart Acacus Mt. (SW Fezzan, Libya): An early Holocene palaeoclimate archive in the central Sahara. Palaeogeogr. Paleoclimatol. Palaeoecol. 2010, 287, 81–94. [Google Scholar] [CrossRef]
- Dinies, M.; Schimmel, L.; Hoelzmann, P.; Kröpelin, S.; Darius, F.; Neef, R. Holocene high-altitude vegetation dynamics on Emi Koussi, Tibesti Mountains (Chad, Central Sahara). In Quaternary Vegetation Dynamics—The African Pollen Database, 1st ed.; Runge, J., Gosling, W.D., Lézine, A.-M., Scott, L., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 27–50. [Google Scholar]
- Hély, C.; Lézine, A.-M. Holocene changes in African vegetation: Tradeoff between climate and water availability. Clim. Past 2014, 10, 681–686. [Google Scholar] [CrossRef]
- Amrani, S. The Holocene Flora and Vegetation of Ti-n Hanakaten (Tassili n’Ajjer, Algerian Sahara). In Plant and People in the African Past; Mercuri, A.M., D’Andrea, A., Fornaciari, R., Höhn, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 123–145. [Google Scholar]
- Van Neer, W.; Alhaique, F.; Wouters, W.; Dierickx, K.; Gala, M.; Goffette, Q.; Mariani, G.S.; Zerboni, A.; di Lernia, S. Aquatic fauna from the Takarkori rock shelter reveals the Holocene central Saharan climate and palaeohydrography. PLoS ONE 2020, 15, e0228588. [Google Scholar] [CrossRef] [PubMed]
- Kuper, R.; Kröpelin, S. Climate-controlled Holocene occupation in the Sahara: Motor of Africa’s evolution. Science 2006, 313, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Prange, M.; Caley, T.; Gimeno, L.; Beckmann, B.; Mulitza, S.; Skonieczny, C.; Roche, D.; Schefuß, E. Rapid termination of the African Humid Period triggered by northern high-latitude cooling. Nat. Commun. 2017, 8, 1372. [Google Scholar] [CrossRef] [PubMed]
- Lézine, A.M.; Hély, C.; Grenier, C.; Braconnot, P.; Krinner, G. Sahara and Sahel vulnerability to climate changes, lessons from Holocene hydrological data. Quat. Sci. Rev. 2011, 30, 3001–3012. [Google Scholar] [CrossRef]
- Mercuri, A.M.; Sadori, L.; Uzquiano Ollero, P. Mediterranean and north-African cultural adaptations to mid-Holocene environmental and climatic changes. Holocene 2011, 21, 189–206. [Google Scholar] [CrossRef]
- Watrin, J.; Lézine, A.-M.; Hély, C. Plant migration and plant communities at the time of the “green Sahara”. CR Geosci. 2009, 341, 656–670. [Google Scholar] [CrossRef]
- Clayton, W.D.; Renvoize, S.A. Flora of Tropical East Africa. Gramineae (Part 3); A.A. Balkema: Rotterdam, The Netherlands, 1982. [Google Scholar]
- Mercuri, A.M. Preliminary analyses of fruits, seeds and other few plants macrofossils from the Early Holocene sequence. In Uan Tabu in the Settlement History of the Libyan Sahara; Garcea, E.A.A., Ed.; All’Insegna del Giglio: Firenze, Italy, 2001; pp. 161–188. [Google Scholar]
- Benca, J.; Duijnstee, I.; Looy, C. Fossilized pollen malformations as indicators of past environmental stress and meiotic disruption: Insights from modern conifers. Paleobiology 2022, 2022, 1–34. [Google Scholar] [CrossRef]
- Kürschner, W.M.; Batenburg, S.J.; Mander, L. Aberrant Classopollis pollen reveals evidence for unreduced (2n) pollen in the conifer family Cheirolepidiaceae during the Triassic–Jurassic transition. Proc. R. Soc. B 2013, 280, 20131708. [Google Scholar] [CrossRef]
- Schinkel, C.C.; Kirchheimer, B.; Dellinger, A.S.; Klatt, S.; Winkler, M.; Dullinger, S.; Hörandl, E. Correlations of polyploidy and apomixis with elevation and associated environmental gradients in an alpine plant. AoB Plants 2016, 8, plw064. [Google Scholar] [CrossRef]
- Božič, A.; Šiber, A. Mechanics of inactive swelling and bursting of porate pollen grains. Biophys. J. 2021, 121, 782–792. [Google Scholar] [CrossRef]
- Ortiz, J.P.; Quarin, C.L.; Pessino, S.C.; Acuña, C.; Martínez, E.J.; Espinoza, F.; Hojsgaard, D.H.; Sartor, M.E.; Cáceres, M.E.; Pupilli, F. Harnessing apomictic reproduction in grasses: What we have learned from Paspalum. Ann Bot. 2013, 112, 767–787. [Google Scholar] [CrossRef] [PubMed]
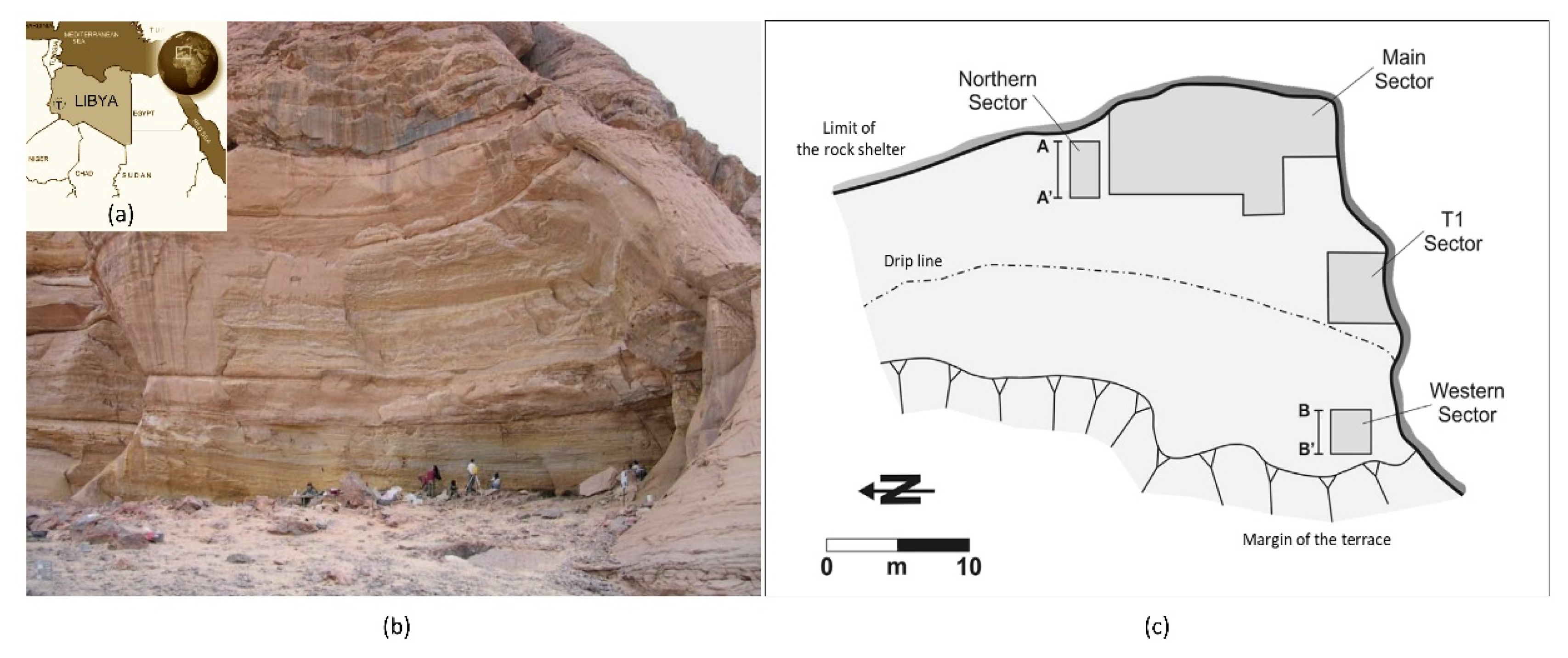


| TK-NS Pollen Sequence (Cremaschi et al., 2014: [20]) | Main Sector | |||||
|---|---|---|---|---|---|---|
| Sample No. | Depth (cm) | Unit | Description | Cultural Phase | Chronology (cal BP) | Coprolites (Number, Layer, and Square) |
| 1 | 6.0 | I | Organic loose sand, lamination; common plant remains, charcoal and coprolites; large stones from vault collapse | MP2—Middle Pastoral 2 | 6300–5750 | 10 specimens from one layer (L25 V24) |
| 2 | 11.0 | |||||
| 3 | 18.5 | |||||
| 4 | 21.5 | |||||
| 5 | 26.5 | |||||
| 6 | 32.5 | |||||
| 7 | 35.0 | |||||
| 8 | 42.5 | II | Organic sand; frequent charred and uncharred plant remains; lenses of white ash | MP1—Middle Pastoral 1 | 6950–6300 | |
| 9 | 49.0 | |||||
| 10 | 51.5 | |||||
| 11 | 53.5 | III | Loose thin laminated sand; frequent uncharred plant remains and coprolites | EP1—Early Pastoral 1 | 8250–7800 | |
| 12 | 58.5 | |||||
| 13 | 61.5 | |||||
| 14 | 66.0 | |||||
| 15 | 71.5 | IV | Organic sand, from loose to moderately hard; frequent charred and uncharred plant remains. Lenses of white ash and black charred material | LA3—Late Acacus 3 | 8950–8450 | 25 specimens from three layers (L103 S32; L69 T22; L147 V22-23) |
| 16 | 75.0 | |||||
| 17 | 81.0 | |||||
| 18 | 84.0 | |||||
| 19 | 87.5 | |||||
| 20 | 91.5 | V | Organic loose sand including two thin layers of laminated sand cemented by organic matter | LA2—Late Acacus 2 | 9450–8950 | 20 specimens from one layer (L275 R23) |
| 21 | 97.0 | |||||
| 22 | 101.0 | VI | Loose sand; scarce plant fragments, often charred | |||
| 23 | 105.0 | |||||
| 24 | 110.0 | |||||
| 25 | 115.0 | |||||
| 26 | 120.0 | VII | Loose organic sand; rare plant remains distributed in thin layers; rare weathered sandstone fragments | LA1—Late Acacus 1 | 9950–9450 | |
| 27 | 121.0 | |||||
| 28 | 122.5 | |||||
| 29 | 130.0 | |||||
| 30 | 132.0 | |||||
| TK-NS Pollen Sequence (Cremaschi et al., 2014: [20]) | Poaceae Pollen (This Paper) | |||||
|---|---|---|---|---|---|---|
| Sample No. | Poaceae Sum | Large Poaceae (X = > 40 µm; § = > 60 µm) | Monoporate | Multiporate | ||
| % On Total Pollen | p/g | Count | Count | % | ||
| 1 | 50.2 | 55,613 | X | 1866 | 0 | |
| 2 | 50.6 | 67,752 | X | 179 | 2 | 1.12 |
| 3 | 59.5 | 25,922 | X; § | 1026 | 2 | 0.19 |
| 4 | 61.3 | 103,526 | X | 935 | 2 | 0.21 |
| 5 | 74.7 | 192,008 | X | 406 | 0 | |
| 6 | 76.3 | 430,656 | X | 454 | 1 | 0.22 |
| 7 | 73.3 | 140,453 | X | 610 | 0 | |
| 8 | 87.2 | 145,972 | X; § | 188 | 0 | |
| 9 | 76.9 | 222,823 | X | 1612 | 1 | 0.06 |
| 10 | 75.3 | 77,963 | - | 3691 | 2 | 0.05 |
| 11 | 72.5 | 77,145 | - | 103 | 0 | |
| 12 | 73.8 | 78,635 | X | 362 | 0 | |
| 13 | 80.6 | 340,566 | X | 193 | 1 | 0.52 |
| 14 | 82.6 | 191,471 | X | 367 | 2 | 0.54 |
| 15 | 96.4 * | 40,956 | X | 29 | 0 | |
| 16 | 86.2 | 5373 | X | 57 | 0 | |
| 17 | 77.6 | 52,360 | X | 176 | 1 | 0.57 |
| 18 | 73.5 | 116,702 | X; § | 189 | 0 | |
| 19 | 63.4 | 35,476 | X | 208 | 0 | |
| 20 | 49.3 | 44,790 | X | 441 | 0 | |
| 21 | 37.8 | 20,713 | X | 376 | 3 | 0.80 |
| 22 | 52.0 | 13270 | X | 724 | 4 | 0.55 |
| 23 | 44.8 | 21,426 | X | 143 | 1 | 0.70 |
| 24 | 41.1 | 8877 | X | 384 | 1 | 0.26 |
| 25 | 71.6 | 5654 | X | 1587 | 0 | |
| 26 | 44.0 | 5103 | X | 1504 | 1 | 0.07 |
| 27 | 79.9 | 7901 | X; § | 902 | 1 | 0.11 |
| 28 | 75.0 | 22,123 | X | 1415 | 2 | 0.14 |
| 29 | 55.5 | 640 | X | 2108 | 0 | |
| 30 | 74.9 | 14,439 | X | 2066 | 0 | |
| TK-NS Pollen Sequence | ||||||
|---|---|---|---|---|---|---|
| Sample No. | Pollen Zones | Plant Accumulations of Caryopses and Spikelets | Regional Palaeoenvironment and Context | Climate | ||
| Label | Vegetation | Spot | Mix | |||
| 1 | Tk2c | Spread of dry savanna and xeric vegetation, reduction of grassland with significant change toward environmentally dry conditions; wet habitats became smaller | 1 spot of Echinochloa 65% and Panicum 18% | 5 mix of Paniceae chaff: Echinochloa (79%, 65%) and Panicum (34%, 15%) | Rapid regression of humid environments giving way to savanna-desert: gradual establishment of drier climate. Spread of xerophilous desert savanna and psammophilous vegetation. Pastoralists try to reorganize by abandoning cattle ranching and intensifying the raising of goats and sheep, with lower water requirements; this is to cope with the early stages of the arid crisis that transformed the Sahara into a desert | Warm/Dry |
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | Tk2b | A mosaic of xeric and freshwater habitats was present during this phase; Poaceae included a high quantity of different large pollen grasses due to increased natural availability and selection of ‘new’ wild cereals | ||||
| 7 | ||||||
| 8 | 10 spots of Echinochloa (59%, 52%, 50%), of Urochloa (82%, 75%, 65%), of Panicum (32%, 20%) | Wooded savanna with plant landscape beginning to be exploited by pastoralists from with cattle herds and small livestock that took over from hunter-gatherers; drying up the environment with oscillations. The results of palynological and macroremains analyses conducted at the Uan Muhuggiag rockshelter and Mathendush Cave suggest the spread of a Sahelian-type savanna in the area during the middle part of the middle Holocene | Warm/From dry to wet | |||
| 9 | ||||||
| 10 | ||||||
| 11 | ||||||
| 12 | ||||||
| 13 | Tk2a | Spread of grassland, wet environments and reduction of dry savanna vegetation; intensive plant accumulation | ||||
| 14 | ||||||
| 15 | Tk1c | Environmental instability, increased seasonality and changes in plant exploitation are evident; further reduction of permanent water bodies is visible at the end of the LA3 phase; notable increase of large pollen types, belonging to some Panicum, Echinochloa and Setaria | 5 spots of Paniceae and Andropogoneae: Pennisetum 15%; Sorghum 72%, 15%; Urochloa 87%, 75%, 63%; 1 spot of Polygonum 87% | 1 mix of Paniceae and Andropogoneae with chaff: Brachiaria/Urochloa 32%; Pennisetum 25% | ||
| 16 | ||||||
| 17 | ||||||
| 18 | ||||||
| 19 | ||||||
| 20 | 1 spot of Setaria 71% | 4 mix of Paniceae and Andropogoneae with chaff: Brachiaria 26%, 19%; Brachiaria/Urochloa 17%; Urochloa 45%; Pennisetum 14%, 11% | ||||
| 21 | ||||||
| 22 | Tk1b | Widening of shallow-water marginal zones or general lowering of the water level; wooded savanna is present but xerophilous plants began to expand while grassland reduces | ||||
| 23 | ||||||
| 24 | ||||||
| 25 | ||||||
| 26 | Transformation of plant cover in relation to an arid climate. There is a concomitant erosion phase with a dry interval, demonstrated, for example, by the disruption of the sedimentary sequence in Uan Afuda Cave and the ingression of windblown sand in the Ti-n- Hanakaten sequence | Cold/Dry | ||||
| 27 | ||||||
| 28 | Tk1a | Grassland and sparsely wooded savanna; local permanent freshwater habitats with Potamogeton, and wet environments with reeds, cattails | ||||
| 29 | ||||||
| 30 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercuri, A.M.; Clò, E.; Florenzano, A. Multiporate Pollen of Poaceae as Bioindicator of Environmental Stress: First Archaeobotanical Evidence from the Early–Middle Holocene Site of Takarkori in the Central Sahara. Quaternary 2022, 5, 41. https://doi.org/10.3390/quat5040041
Mercuri AM, Clò E, Florenzano A. Multiporate Pollen of Poaceae as Bioindicator of Environmental Stress: First Archaeobotanical Evidence from the Early–Middle Holocene Site of Takarkori in the Central Sahara. Quaternary. 2022; 5(4):41. https://doi.org/10.3390/quat5040041
Chicago/Turabian StyleMercuri, Anna Maria, Eleonora Clò, and Assunta Florenzano. 2022. "Multiporate Pollen of Poaceae as Bioindicator of Environmental Stress: First Archaeobotanical Evidence from the Early–Middle Holocene Site of Takarkori in the Central Sahara" Quaternary 5, no. 4: 41. https://doi.org/10.3390/quat5040041
APA StyleMercuri, A. M., Clò, E., & Florenzano, A. (2022). Multiporate Pollen of Poaceae as Bioindicator of Environmental Stress: First Archaeobotanical Evidence from the Early–Middle Holocene Site of Takarkori in the Central Sahara. Quaternary, 5(4), 41. https://doi.org/10.3390/quat5040041








