On the Use of Spores of Coprophilous Fungi Preserved in Sediments to Indicate Past Herbivore Presence
Abstract
:1. Introduction
2. A Short History of Coprophilous Fungi in Palynology
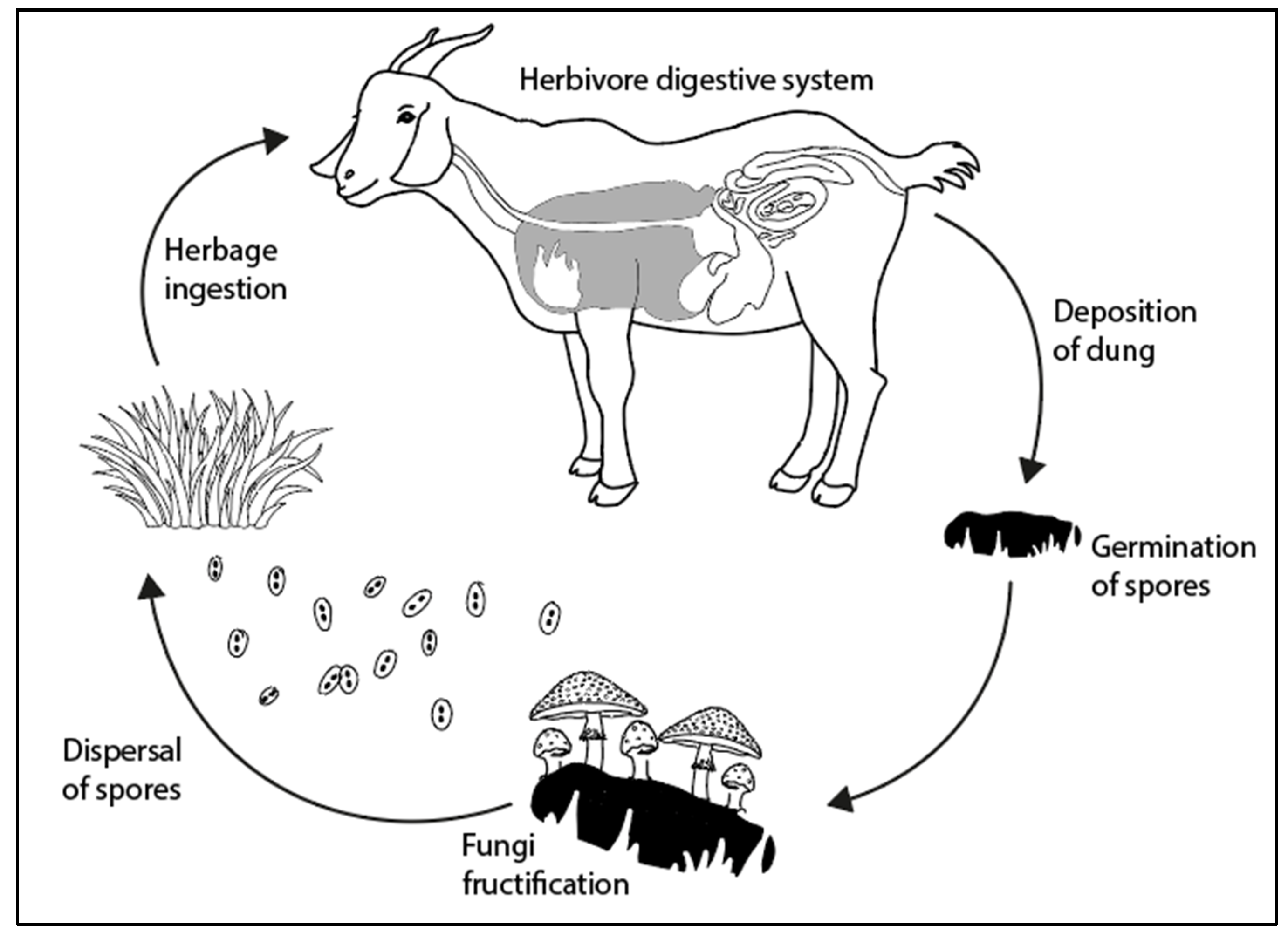
3. Fungal Spore Life Cycle
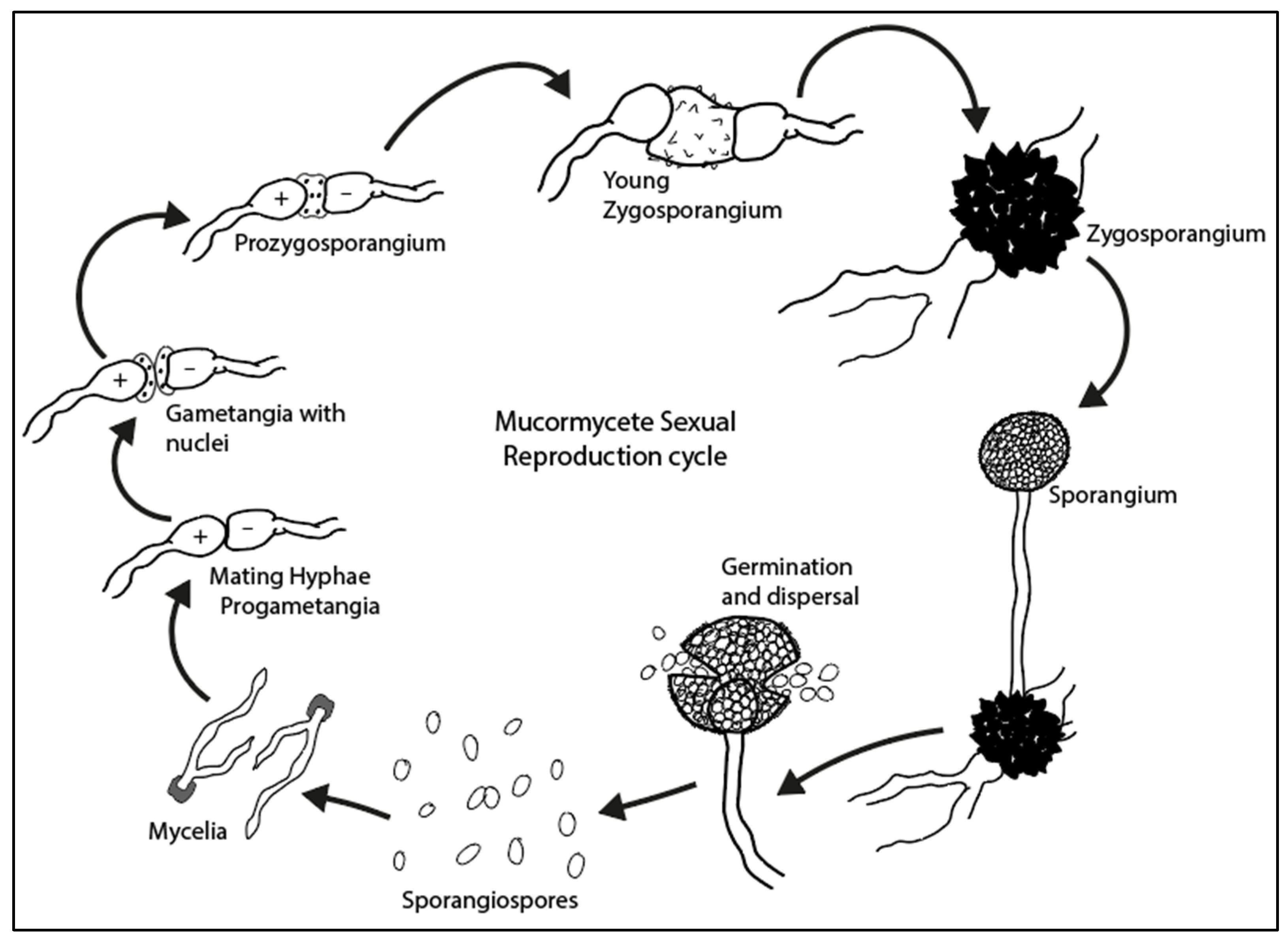
3.1. Mucormycetes
3.2. Ascomycetes
3.3. Basidiomycetes
3.4. Fungi Imperfecti
4. Constructing a Record of SCF from Sedimentary Samples
4.1. Preparation
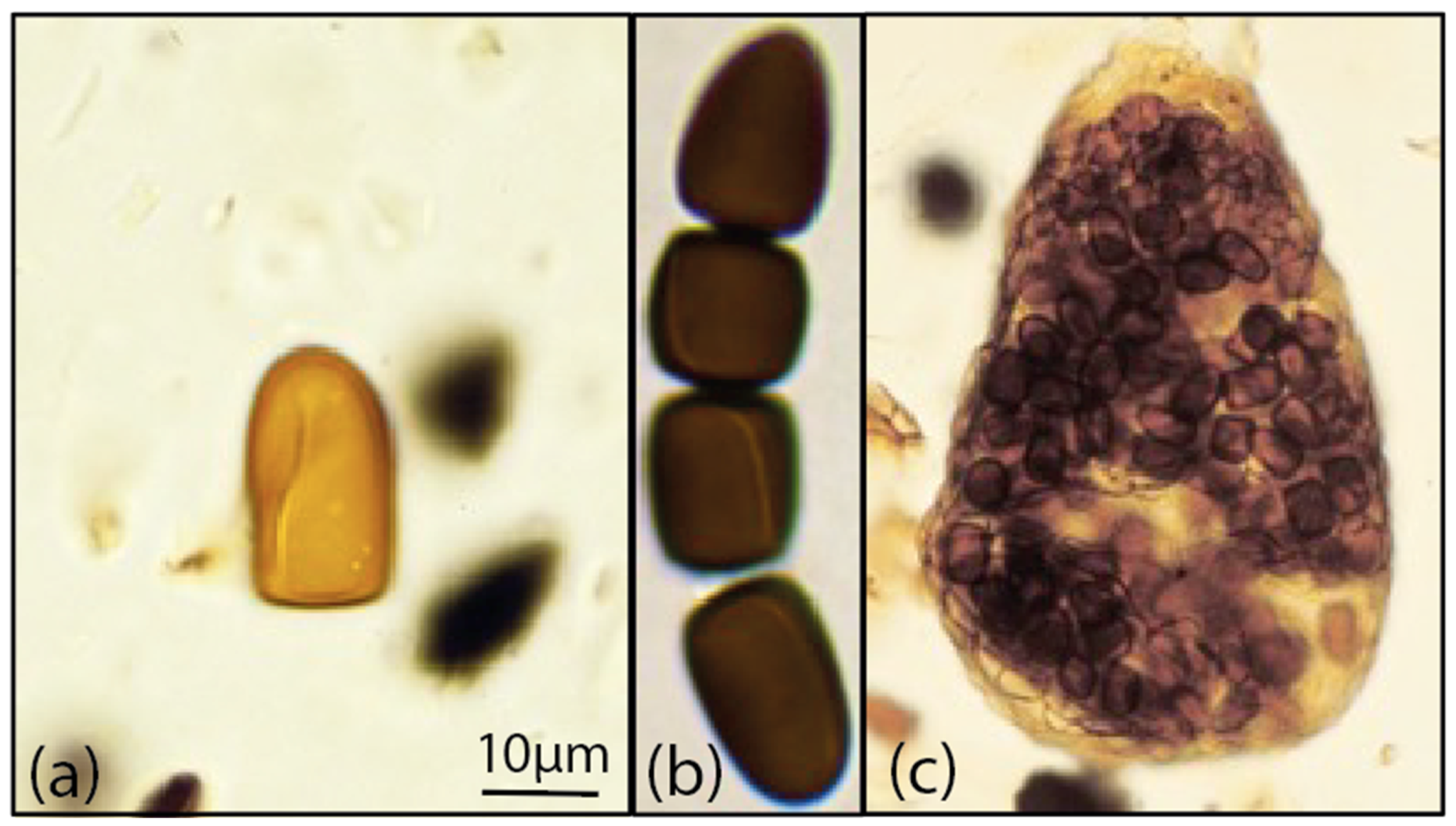
4.2. Morphological Identification
4.3. Genetic Identification
4.4. Quantification
5. Interpretation
5.1. Herbivore Density Influences
5.2. Vegetation and Landscape Influences
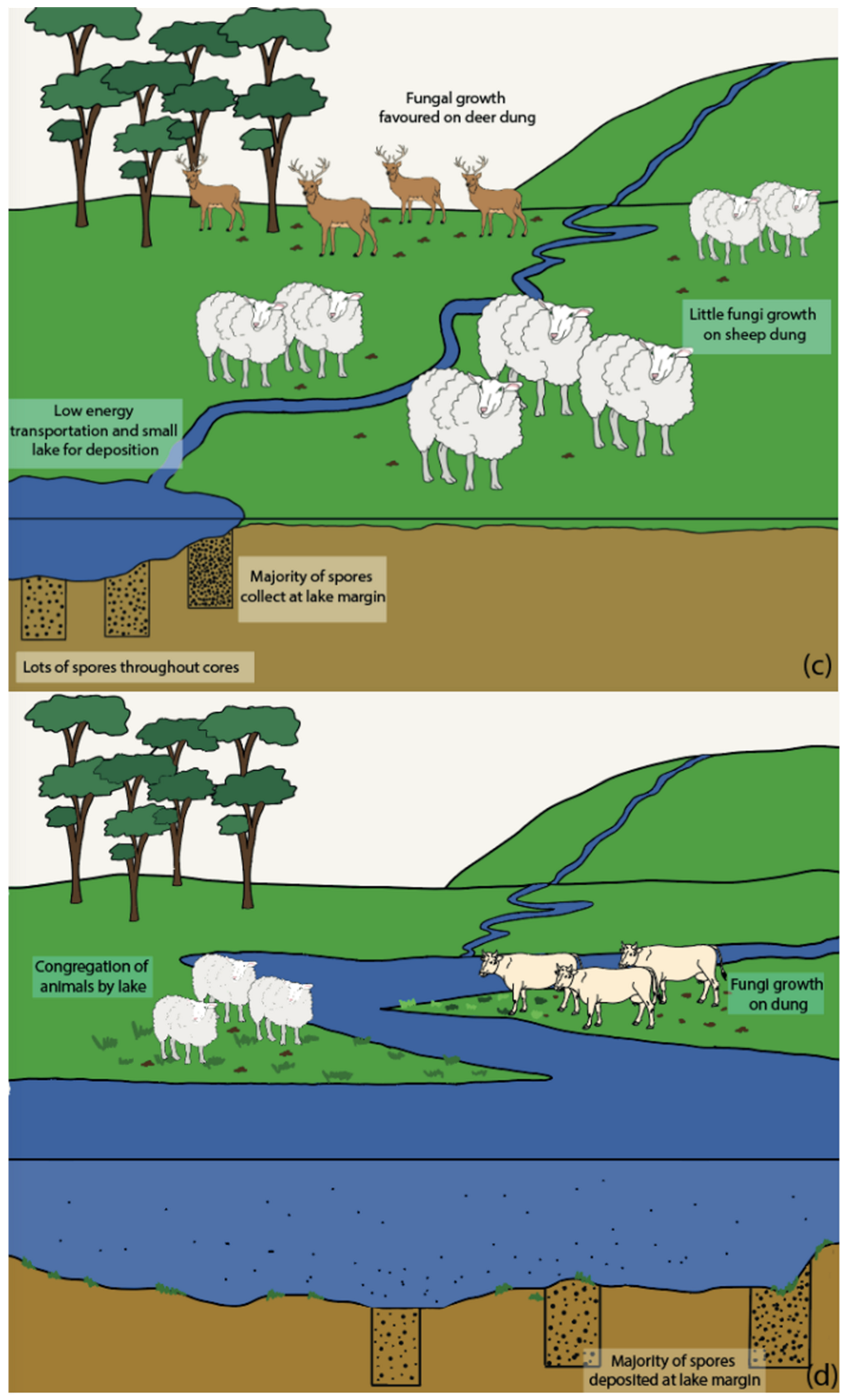
5.3. Influence of Lake Depositional Environments
5.4. Transportation of Spores
5.5. Climatic Influences
6. Outlook
6.1. Improve Understanding of SCF—Herbivore Relationships through the Study of Successions of Coprophilous Fungi
6.2. Improve Understanding of SCF—Climate Relationship
6.3. Improve ID of SCF through Morphological and DNA-Based Studies
6.4. Present SCF Data Independent of Other Proxies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdtman, G. Palynology. In Advances in Botanical Research; Preston, R.D., Ed.; Academic Press: Cambridge, MA, USA, 1963; Volume 1, pp. 149–208. [Google Scholar]
- Grant, M.J. Palynology. In The Encyclopedia of Archaeological Sciences; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 1–4. ISBN 978-1-119-18823-0. [Google Scholar]
- Riding, J. A Review of the Laboratory Preparation of Palynomorphs with a Description of an Effective Non-Acid Technique. Rev. Bras. Paleontol. 2004, 7, 13–44. [Google Scholar] [CrossRef]
- Van Geel, B. Non-Pollen Palynomorphs. In Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Bradley, R.S., Alverson, K., Eds.; Developments in Paleoenvironmental Research; Springer: Dordrecht, The Netherlands, 2001; pp. 99–119. ISBN 978-0-306-47668-6. [Google Scholar]
- Cook, E.J.; van Geel, B.; van der Kaars, S.; van Arkel, J. A Review of the Use of Non-Pollen Palynomorphs in Palaeoecology with Examples from Australia. Palynology 2011, 35, 155–178. [Google Scholar] [CrossRef]
- Van Geel, B. A Palaeoecological Study of Holocene Peat Bog Sections in Germany and The Netherlands, Based on the Analysis of Pollen, Spores and Macro- and Microscopic Remains of Fungi, Algae, Cormophytes and Animals. Rev. Palaeobot. Palynol. 1978, 25, 1–120. [Google Scholar] [CrossRef]
- Gosling, W.D.; McMichael, C.N.H.; Groenewoud, Z.; Roding, E.; Miller, C.S.; Julier, A.C.M. Preliminary Evidence for Green, Brown and Black Worlds in Tropical Western Africa during the Middle and Late Pleistocene. In Quaternary Vegetation Dynamics—The African Pollen Database; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-316276-6. [Google Scholar]
- Gillson, L. The Role of Palaeoecology in Conserving African Ecosystems. In Quaternary Vegetation Dynamics—The African Pollen Database; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-316276-6. [Google Scholar]
- Etienne, D.; Wilhelm, B.; Sabatier, P.; Reyss, J.-L.; Arnaud, F. Influence of Sample Location and Livestock Numbers on Sporormiella Concentrations and Accumulation Rates in Surface Sediments of Lake Allos, French Alps. J. Paleolimnol. 2013, 49, 117–127. [Google Scholar] [CrossRef]
- Hicks, S.; Hyvärinen, H. Pollen Influx Values Measured in Different Sedimentary Environments and Their Palaeoecological Implications. Grana 1999, 38, 228–242. [Google Scholar] [CrossRef]
- Van Asperen, E.N.; Kirby, J.R.; Hunt, C.O. The Effect of Preparation Methods on Dung Fungal Spores: Implications for Recognition of Megafaunal Populations. Rev. Palaeobot. Palynol. 2016, 229, 1–8. [Google Scholar] [CrossRef]
- Étienne, D.; Jouffroy-Bapicot, I. Optimal Counting Limit for Fungal Spore Abundance Estimation Using Sporormiella as a Case Study. Veg. Hist. Archaeobot. 2014, 23, 743–749. [Google Scholar] [CrossRef]
- Van Asperen, E.N.; Perrotti, A.; Baker, A. Coprophilous Fungal Spores: NPPs for the Study of Past Megaherbivores. Geol. Soc. Lond. Spec. Publ. 2021, 511, 245–267. [Google Scholar] [CrossRef]
- Perrotti, A.G.; van Asperen, E. Dung Fungi as a Proxy for Megaherbivores: Opportunities and Limitations for Archaeological Applications. Veg. Hist. Archaeobot. 2019, 28, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Richardson, M.J. Diversity and Occurrence of Coprophilous Fungi. Mycol. Res. 2001, 105, 387–402. [Google Scholar] [CrossRef]
- Bell, A. Dung Fungi: An Illustrated Guide to Coprophilous Fungi in New Zealand, 1st ed.; Victoria University Press: Wellington, New Zealand, 1983; ISBN 0-86473-001-2. [Google Scholar]
- Gauthier, E.; Bichet, V.; Massa, C.; Petit, C.; Vannière, B.; Richard, H. Pollen and Non-Pollen Palynomorph Evidence of Medieval Farming Activities in Southwestern Greenland. Veg. Hist. Archaeobot. 2010, 19, 427–438. [Google Scholar] [CrossRef]
- Davis, O.K.; Shafer, D.S. Sporormiella Fungal Spores, a Palynological Means of Detecting Herbivore Density. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 237, 40–50. [Google Scholar] [CrossRef]
- Wood, J.; Wilmshurst, J. Accumulation Rates or Percentages? How to Quantify Sporormiella and Other Coprophilous Fungal Spores to Detect Late Quaternary Megafaunal Extinction Events. Quat. Sci. Rev. 2013, 77, 1–3. [Google Scholar] [CrossRef]
- Johnson, C.N.; Rule, S.; Haberle, S.G.; Turney, C.S.M.; Kershaw, A.P.; Brook, B.W. Using Dung Fungi to Interpret Decline and Extinction of Megaherbivores: Problems and Solutions. Quat. Sci. Rev. 2015, 110, 107–113. [Google Scholar] [CrossRef]
- Pino, M.; Cossio, N.; Pinto, B. Sporormiella Fungal Spores as a Proxy for Megaherbivore Abundance and Decline at Pilauco. In Pilauco: A Late Pleistocene Archaeo-Paleontological Site; The Latin American Studies Book Series; Springer: Cham, Switzerland, 2020; pp. 95–109. ISBN 978-3-030-23917-6. [Google Scholar]
- Raczka, M.F.; Bush, M.B.; Folcik, A.M.; McMichael, C.H. Sporormiella as a Tool for Detecting the Presence of Large Herbivores in the Neotropics. Biota Neotrop. 2016, 16, e20150090. [Google Scholar] [CrossRef] [Green Version]
- Rozas-Davila, A.; Valencia, B.G.; Bush, M.B. The Functional Extinction of Andean Megafauna. Ecology 2016, 97, 2533–2539. [Google Scholar] [CrossRef]
- Birks, H.H.; van Geel, B.; Fisher, D.C.; Grimm, E.C.; Kuijper, W.J.; van Arkel, J.; van Reenen, G.B.A. Evidence for the Diet and Habitat of Two Late Pleistocene Mastodons from the Midwest, USA. Quat. Res. 2019, 91, 792–812. [Google Scholar] [CrossRef]
- Hudson, H.J. The Ecology of Fungi on Plant Remains Above the Soil. New Phytol. 1968, 67, 837–874. [Google Scholar] [CrossRef]
- Alexopoulos, C.J. Introductory Mycology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1962; ISBN 978-0-471-52229-4. [Google Scholar]
- Miranda, V.; Sede, S.; Aranda-Rickert, A.; Rothen, C.; Scervino, J.M.; Barros, J.; Fracchia, S. Taxonomy, Life Cycle and Endophytism of Coprophilous Fungi from an Underground Desert Rodent. Fungal Ecol. 2020, 43, 100872. [Google Scholar] [CrossRef]
- Taylor, T.N.; Taylor, E.L.; Krings, M. 3—Fungi, Bacteria, and Lichens. In Paleobotany, 2nd ed.; Taylor, T.N., Taylor, E.L., Krings, M., Eds.; Academic Press: London, UK, 2009; pp. 71–119. ISBN 978-0-12-373972-8. [Google Scholar]
- Davis, O.K.; Kolva, D.A.; Mehringer, P.J.J. Pollen Analysis of Wildcat Lake, Whitman County, Washington: The Last 1000 Years. Northwest. Sci. USA 1977, 51, 13–30. [Google Scholar]
- Van Geel, B.; Aptroot, A. Fossil Ascomycetes in Quaternary Deposits. Nova Hedwig. 2006, 82, 313–329. [Google Scholar] [CrossRef]
- Van Geel, B.; Gelorini, V.; Lyaruu, A.; Aptroot, A.; Rucina, S.; Marchant, R.; Damsté, J.S.S.; Verschuren, D. Diversity and Ecology of Tropical African Fungal Spores from a 25,000-Year Palaeoenvironmental Record in Southeastern Kenya. Rev. Palaeobot. Palynol. 2011, 164, 174–190. [Google Scholar] [CrossRef]
- Halbwachs, H.; Bässler, C. No Bull: Dung-Dwelling Mushrooms Show Reproductive Trait Syndromes Different from Their Non-Coprophilous Allies. Mycol. Prog. 2020, 19, 817–824. [Google Scholar] [CrossRef]
- Harper, J.E.; Webster, J. An Experimental Analysis of the Coprophilous Fungus Succession. Trans. Br. Mycol. Soc. 1964, 47, 511–530. [Google Scholar] [CrossRef]
- Dix, N.J. Fungal Ecology; Springer: Dordrecht, The Netherlands, 1995; ISBN 978-94-010-4299-4. [Google Scholar]
- Van Asperen, E.N.; Kirby, J.R.; Shaw, H.E. Relating Dung Fungal Spore Influx Rates to Animal Density in a Temperate Environment: Implications for Palaeoecological Studies. Holocene 2020, 30, 218–232. [Google Scholar] [CrossRef]
- Baker, A.G.; Cornelissen, P.; Bhagwat, S.A.; Vera, F.M.W.; Willis, K.J. Quantification of Population Sizes of Large Herbivores and Their Long-Term Functional Role in Ecosystems Using Dung Fungal Spores. Methods Ecol. Evol. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Angel, S.K.; Lussenhop, J. Fungal Community Expression in Lagomorph Versus Ruminant Feces. Mycologia 1980, 72, 1015–1021. [Google Scholar] [CrossRef]
- Gauthier, E.; Jouffroy-Bapicot, I. Detecting Human Impacts: Non-Pollen Palynomorphs as Proxies for Human Impact on the Environment. Geol. Soc. Lond. Spec. Publ. 2021, 511, 233–244. [Google Scholar] [CrossRef]
- Krstic, T. Pilobolus Crystallinus (F.H. Wigg.) Tode; Image Number 1013949 at Mushroom Observer. 2019. Available online: https://en.wikipedia.org/wiki/Pilobolus_crystallinus#/media/File:Pilobolus_crystallinus_(F.H._Wigg.)_Tode_1013949.jpg (accessed on 2 March 2022).
- AJC1. Eyelash Cup, Cheilymenia Fimicola. 2015. Available online: https://commons.wikimedia.org/wiki/File:Eyelash_Cup,_Cheilymenia_fimicola_(23519053553).jpg (accessed on 2 March 2022).
- Pixnio. Fungus, Mushroom, Grass, Nature, Vegetable, Soil. Available online: https://pixnio.com/flora-plants/fungi-mushrooms/fungus-mushroom-grass-nature-vegetable-soil (accessed on 2 March 2022).
- Richardson, M. The Ecology of the Zygomycetes and Its Impact on Environmental Exposure. Clin. Microbiol. Infect. 2009, 15, 2–9. [Google Scholar] [CrossRef]
- Krug, J.C.; Benny, G.L.; Keller, H.W. Coprophilous Fungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Academic Press: Cambridge, MA, USA, 2004; pp. 467–499. [Google Scholar]
- Nwe, N.; Furuike, T.; Tamura, H. Production, Properties and Applications of Fungal Cell Wall Polysaccharides: Chitosan and Glucan. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2011; Volume 244, pp. 187–207. ISBN 978-3-642-24060-7. [Google Scholar]
- Sinha, A.K. Botany for Degree Students Fungi; S. Chand Publishing: New Delhi, India, 1962; ISBN 978-81-219-2826-7. [Google Scholar]
- Peraza Reyes, L.; Berteaux-Lecellier, V. Peroxisomes and Sexual Development in Fungi. Front. Physiol. 2013, 4, 244. [Google Scholar] [CrossRef] [Green Version]
- Van Geel, B.; Guthrie, R.D.; Altmann, J.G.; Broekens, P.; Bull, I.D.; Gill, F.L.; Jansen, B.; Nieman, A.M.; Gravendeel, B. Mycological Evidence of Coprophagy from the Feces of an Alaskan Late Glacial Mammoth. Quat. Sci. Rev. 2011, 30, 2289–2303. [Google Scholar] [CrossRef]
- Van Geel, B.; Fisher, D.C.; Rountrey, A.N.; van Arkel, J.; Duivenvoorden, J.F.; Nieman, A.M.; van Reenen, G.B.A.; Tikhonov, A.N.; Buigues, B.; Gravendeel, B. Palaeo-Environmental and Dietary Analysis of Intestinal Contents of a Mammoth Calf (Yamal Peninsula, Northwest Siberia). Quat. Sci. Rev. 2011, 30, 3935–3946. [Google Scholar] [CrossRef]
- Kiffer, E.D.; Morelet, M. The Deuteromycetes—Mitosporic Fungi: Classification and Generic Keys; CRC Press: Boca Raton, FL, USA, 2011; pp. 6–33. ISBN 978-1-4822-9419-4. [Google Scholar]
- Barnes, E.H. The Deuteromycetes: (The Fungi Imperfecti). In Atlas and Manual of Plant Pathology; Barnes, E.H., Ed.; Springer: Boston, MA, USA, 1979; pp. 222–224. ISBN 978-1-4684-3495-8. [Google Scholar]
- Faegri, K.; Iversen, J. Textbook of Pollen Analysis, 4th ed.; John Wiley & Sons: Chichester, UK, 1989; Volume 5, ISBN 0-471-92178-5. [Google Scholar]
- Raper, D.; Bush, M. A Test of Sporormiella Representation as a Predictor of Megaherbivore Presence and Abundance. Quat. Res. 2009, 71, 490–496. [Google Scholar] [CrossRef]
- Parker, N.E.; Williams, J.W. Influences of Climate, Cattle Density, and Lake Morphology on Sporormiella Abundances in Modern Lake Sediments in the US Great Plains. Holocene 2012, 22, 475–483. [Google Scholar] [CrossRef]
- Van Geel, B.; Buurman, J.; Brinkkemper, O.; Schelvis, J.; Aptroot, A.; van Reenen, G.; Hakbijl, T. Environmental Reconstruction of a Roman Period Settlement Site in Uitgeest (The Netherlands), with Special Reference to Coprophilous Fungi. J. Archaeol. Sci. 2003, 30, 873–883. [Google Scholar] [CrossRef]
- Goethals, L.; Verschuren, D. Tracing Ancient Animal Husbandry in Tropical Africa Using the Fossil Spore Assemblages of Coprophilous Fungi: A Validation Study in Western Uganda. Veg. Hist. Archaeobot. 2020, 29, 509–526. [Google Scholar] [CrossRef]
- Ghosh, R.; Paruya, D.K.; Acharya, K.; Ghorai, N.; Bera, S. How Reliable Are Non-Pollen Palynomorphs in Tracing Vegetation Changes and Grazing Activities? Study from the Darjeeling Himalaya, India. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 475, 23–40. [Google Scholar] [CrossRef]
- Van Geel, B.; Zazula, G.D.; Schweger, C.E. Spores of Coprophilous Fungi from under the Dawson Tephra (25,300 14C Years BP), Yukon Territory, Northwestern Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 252, 481–485. [Google Scholar] [CrossRef]
- Baker, A.G.; Bhagwat, S.A.; Willis, K.J. Do Dung Fungal Spores Make a Good Proxy for Past Distribution of Large Herbivores? Quat. Sci. Rev. 2013, 62, 21–31. [Google Scholar] [CrossRef]
- Capo, E.; Giguet-Covex, C.; Rouillard, A.; Nota, K.; Heintzman, P.; Vuillemin, A.; Ariztegui, D.; Arnaud, F.; Belle, S.; Bertilsson, S.; et al. Lake Sedimentary DNA Research on Past Terrestrial and Aquatic Biodiversity: Overview and Recommendations. Quaternary 2021, 4, 6. [Google Scholar] [CrossRef]
- Kistler, L. Paleoethnobotany and Ancient DNA Analysis. In The Encyclopedia of Archaeological Sciences; American Cancer Society: Atlanta, GA, USA, 2018; pp. 1–5. ISBN 978-1-119-18823-0. [Google Scholar]
- Edwards, M.E. The Maturing Relationship between Quaternary Paleoecology and Ancient Sedimentary DNA. Quat. Res. 2020, 96, 39–47. [Google Scholar] [CrossRef]
- Epp, L.S.; Zimmermann, H.H.; Stoof-Leichsenring, K.R. Sampling and Extraction of Ancient DNA from Sediments. In Ancient DNA: Methods and Protocols; Shapiro, B., Barlow, A., Heintzman, P.D., Hofreiter, M., Paijmans, J.L.A., Soares, A.E.R., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 31–44. ISBN 978-1-4939-9176-1. [Google Scholar]
- Thomas, S.P.; Shanmuganathan, B.; Krishnan, S.; Goswami, K.; Dev, M.; Jaiswal, M.K.; Kumaresan, A.; Sadasivam, S.K. Metabarcoding of PalEnDNA as An Efficient Tool to Recover Ancient Bacterial Diversity. Geomicrobiol. J. 2018, 35, 798–803. [Google Scholar] [CrossRef]
- Pedersen, M.W.; Ruter, A.; Schweger, C.; Friebe, H.; Staff, R.A.; Kjeldsen, K.K.; Mendoza, M.L.Z.; Beaudoin, A.B.; Zutter, C.; Larsen, N.K.; et al. Postglacial Viability and Colonization in North America’s Ice-Free Corridor. Nature 2016, 537, 45–49. [Google Scholar] [CrossRef]
- Stavrou, A.A.; Mixão, V.; Boekhout, T.; Gabaldón, T. Misidentification of Genome Assemblies in Public Databases: The Case of Naumovozyma Dairenensis and Proposal of a Protocol to Correct Misidentifications. Yeast 2018, 35, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Cugny, C.; Mazier, F.; Galop, D. Modern and Fossil Non-Pollen Palynomorphs from the Basque Mountains (Western Pyrenees, France): The Use of Coprophilous Fungi to Reconstruct Pastoral Activity. Veg. Hist. Archaeobot. 2010, 19, 391–408. [Google Scholar] [CrossRef] [Green Version]
- Stockmarr, J. Tablets with Spores Used in Absolute Pollen Analysis. Pollen Spores 1971, 13, 615–621. [Google Scholar]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for Scientific Data Management and Stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [Green Version]
- Davis, O.K. Spores of the Dung Fungus Sporormiella: Increased Abundance in Historic Sediments and before Pleistocene Megafaunal Extinction. Quat. Res. 1987, 28, 290–294. [Google Scholar] [CrossRef]
- Van Asperen, E.N. Fungal Diversity on Dung of Tropical Animals in Temperate Environments: Implications for Reconstructing Past Megafaunal Populations. Fungal Ecol. 2017, 28, 25–32. [Google Scholar] [CrossRef]
- Von Arx, J.A.; van der Aa, H.A. Spororminula Tenerifae Gen. et Sp.Nov. Trans. Br. Mycol. Soc. 1987, 89, 117–120. [Google Scholar] [CrossRef]
- Bond, W.J. Open Ecosystems: Ecology and Evolution beyond the Forest Edge; Oxford University Press: Oxford, UK; New York, NY, USA, 2019; ISBN 978-0-19-881245-6. [Google Scholar]
- Oneto, D.L.; Golan, J.; Mazzino, A.; Pringle, A.; Seminara, A. Timing of Fungal Spore Release Dictates Survival during Atmospheric Transport. Proc. Natl. Acad. Sci. USA 2020, 117, 5134–5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazier, F.; Galop, D.; Brun, C.; Buttler, A. Modern Pollen Assemblages from Grazed Vegetation in the Western Pyrenees, France: A Numerical Tool for More Precise Reconstruction of Past Cultural Landscapes. Holocene 2006, 16, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Orbay-Cerrato, M.E.; Oswald, W.W.; Doughty, E.D.; Foster, D.R.; Hall, B.R. Historic Grazing in Southern New England, USA, Recorded by Fungal Spores in Lake Sediments. Veg. Hist. Archaeobot. 2017, 26, 159–165. [Google Scholar] [CrossRef]
- Stamets, P. Mycelium Running: How Mushrooms Can Help Save the World; Ten Speed Press: Berkeley, CA, USA, 2005; ISBN 978-1-58008-579-3. [Google Scholar]
- Calhim, S.; Halme, P.; Petersen, J.H.; Læssøe, T.; Bässler, C.; Heilmann-Clausen, J. Fungal Spore Diversity Reflects Substrate-Specific Deposition Challenges. Sci. Rep. 2018, 8, 5356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Geel, B.; Engels, S.; Martin-Puertas, C.; Brauer, A. Ascospores of the Parasitic Fungus Kretzschmaria Deusta as Rainstorm Indicators during a Late Holocene Beech-Forest Phase around Lake Meerfelder Maar, Germany. J. Paleolimnol. 2013, 50, 33–40. [Google Scholar] [CrossRef]
- Kuthubutheen, A.J.; Webster, J. Water Availability and the Coprophilous Fungus Succession. Trans. Br. Mycol. Soc. 1986, 86, 63–76. [Google Scholar] [CrossRef]
- Birks, H.J.B. Contributions of Quaternary Botany to Modern Ecology and Biogeography. Plant Ecol. Divers. 2019, 12, 189–385. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Mooney, H.A.; Agard, J.; Capistrano, D.; DeFries, R.S.; Díaz, S.; Dietz, T.; Duraiappah, A.K.; Oteng-Yeboah, A.; Pereira, H.M.; et al. Science for Managing Ecosystem Services: Beyond the Millennium Ecosystem Assessment. Proc. Natl. Acad. Sci. USA 2009, 106, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Mottl, O.; Grytnes, J.-A.; Seddon, A.W.R.; Steinbauer, M.J.; Bhatta, K.P.; Felde, V.A.; Flantua, S.G.A.; Birks, H.J.B. Rate-of-Change Analysis in Palaeoecology Revisited: A New Approach. Rev. Palaeobot. Palynol. 2021, 293, 104483. [Google Scholar] [CrossRef]
- Keen, H.F.; Gosling, W.D.; Hanke, F.; Miller, C.S.; Montoya, E.; Valencia, B.G.; Williams, J.J. A Statistical Sub-Sampling Tool for Extracting Vegetation Community and Diversity Information from Pollen Assemblage Data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 408, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Ficetola, G.F.; Poulenard, J.; Sabatier, P.; Messager, E.; Gielly, L.; Leloup, A.; Etienne, D.; Bakke, J.; Malet, E.; Fanget, B.; et al. DNA from Lake Sediments Reveals Long-Term Ecosystem Changes after a Biological Invasion. Sci. Adv. 2018, 4, eaar4292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shumilovskikh, L.S.; Shumilovskikh, E.S.; Schlütz, F.; van Geel, B. NPP-ID: Non-Pollen Palynomorph Image Database as a Research and Educational Platform. Veg. Hist. Archaeobot. 2022, 31, 323–328. [Google Scholar] [CrossRef]
- Wei, H.; Duan, R.; Xu, Q.; Yang, S.; Fan, Q.; Hou, G.; Du, Y.; Qin, Z.; Gao, J. Fungal Spore Indicators of Vegetation and Highland Pastoralism in Modern Topsoil and Dung, Eastern Tibetan Plateau. CATENA 2021, 202, 105231. [Google Scholar] [CrossRef]
- Montoya, E.; Rull, V.; van Geel, B. Non-Pollen Palynomorphs from Surface Sediments along an Altitudinal Transect of the Venezuelan Andes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Loughlin, N.J.D.; Gosling, W.D.; Montoya, E. Identifying Environmental Drivers of Fungal Non-Pollen Palynomorphs in the Montane Forest of the Eastern Andean Flank, Ecuador. Quat. Res. 2018, 89, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Walanus, A.; Nalepka, D. Information Content of Zero Pollen Counts in Holocene Profiles. Holocene 2013, 23, 732–738. [Google Scholar] [CrossRef]






| Quantification | Abbreviations | Units |
|---|---|---|
| Percentage of the sum of total pollen and total NPP | % TLP + TNPP | % [4,19,66] |
| Percentage of total pollen assemblage | % TPA or % TP | % [13,14] |
| Total spore concentration | - | Spores/cm3 [9,13,67] |
| Pollen influx or spore accumulation rate | PI | Spores/cm2/year [10,13,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.M.; van Geel, B.; Gosling, W.D. On the Use of Spores of Coprophilous Fungi Preserved in Sediments to Indicate Past Herbivore Presence. Quaternary 2022, 5, 30. https://doi.org/10.3390/quat5030030
Lee CM, van Geel B, Gosling WD. On the Use of Spores of Coprophilous Fungi Preserved in Sediments to Indicate Past Herbivore Presence. Quaternary. 2022; 5(3):30. https://doi.org/10.3390/quat5030030
Chicago/Turabian StyleLee, Claire M., Bas van Geel, and William D. Gosling. 2022. "On the Use of Spores of Coprophilous Fungi Preserved in Sediments to Indicate Past Herbivore Presence" Quaternary 5, no. 3: 30. https://doi.org/10.3390/quat5030030
APA StyleLee, C. M., van Geel, B., & Gosling, W. D. (2022). On the Use of Spores of Coprophilous Fungi Preserved in Sediments to Indicate Past Herbivore Presence. Quaternary, 5(3), 30. https://doi.org/10.3390/quat5030030








