Middle Holocene Environment on the Ozark Margin in Southeast Missouri: Deciphering a Testudine Testament
Abstract
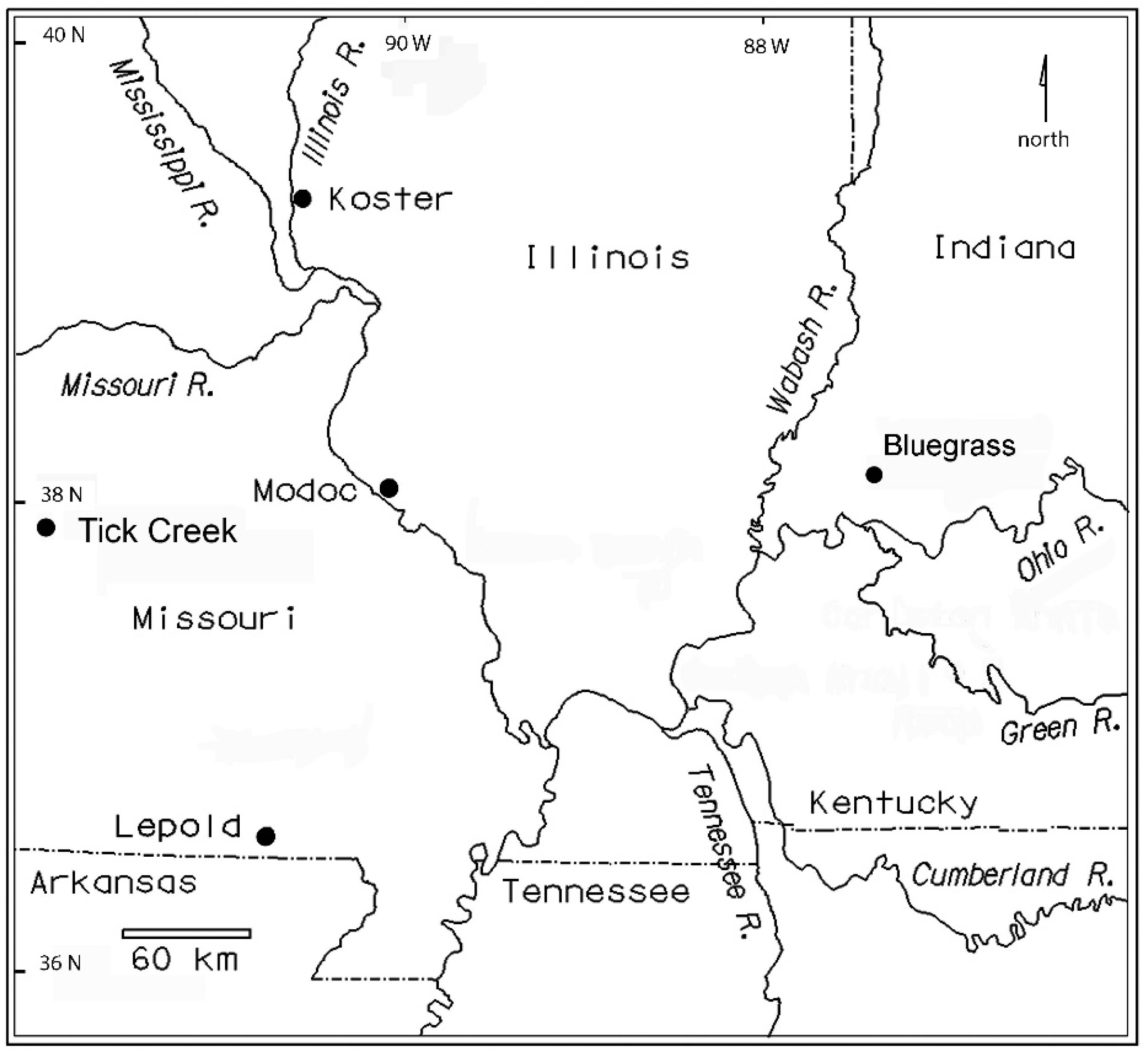
1. Introduction
2. Materials and Methods
2.1. Excavation Methods
2.2. Faunal Sample
2.3. Occupation History
2.4. Evidence of Local Environmental Conditions
3. Results
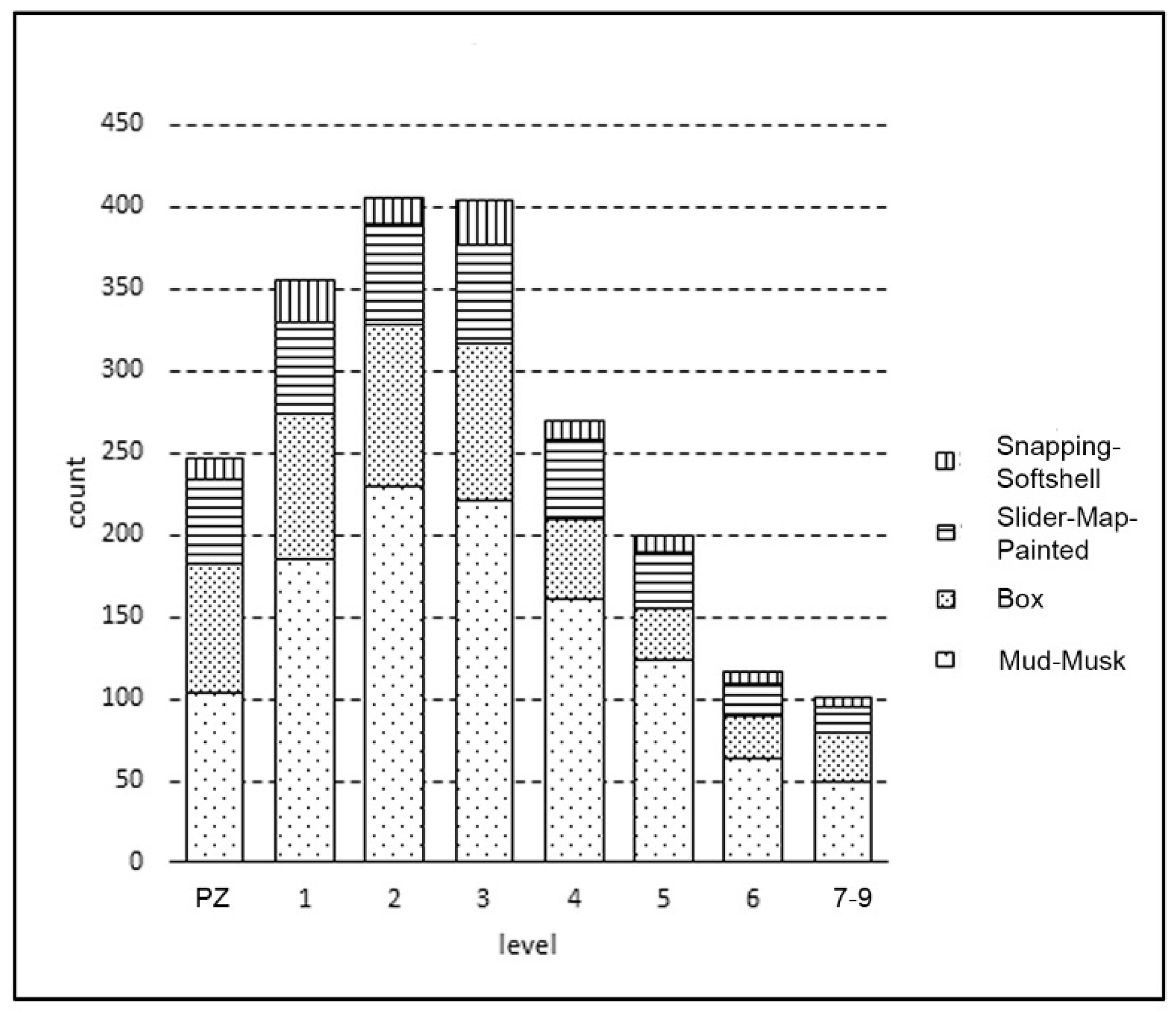
3.1. Turtle Bone Distribution
3.2. Taxa Represented in the Collection
3.3. Taxa Composition through Time
4. Discussion
4.1. Environment and Subsistence
4.2. Comparison to Other Archaeological Sites
4.3. Comparison to Taxon Composition of Living Communities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Transeau, E.N. The Prairie Peninsula. Ecology 1935, 16, 427–469. [Google Scholar] [CrossRef]
- Gleason, H.A. The Vegetation History of the Middle West. Ann. Assoc. Am. Geogr. 1922, 12, 39–85. [Google Scholar] [CrossRef]
- Borchert, J.R. Climate of the Central North American Grasslands. Ann. Am. Assoc. Geogr. 1950, 40, 1–39. [Google Scholar] [CrossRef]
- Changnon, S.A.; Kunkel, K.E.; Winstanley, D. Quantification of Climatic Conditions Important to the Tall Grass Prairie. Trans. Ill. Acad. Sci. 2003, 96, 41–54. [Google Scholar]
- Kunkel, K.; Changnon, S.A. Climate-Years in the True Prairie: Temporal Fluctuations of Ecologically Critical Climate Conditions. Clim. Chang. 2003, 61, 101–122. [Google Scholar] [CrossRef]
- King, J.E. Late Quaternary Vegetational History of Illinois. Ecol. Monogr. 1981, 51, 43–62. [Google Scholar] [CrossRef]
- Nelson, D.M.; Hu, F.S.; Grimm, E.C.; Curry, B.B.; Slate, J.E. The Influence of Aridity and Fire on Holocene Prairie Communities in the Eastern Prairie Peninsula. Ecology 2006, 87, 2523–2536. [Google Scholar] [CrossRef]
- Williams, J.W.; Shuman, B.; Bartlein, P.J.; Diffenbaugh, N.S.; Webb, T. III Rapid, Time-Transgressive, and Variable Responses to Early Holocene Midcontinental Drying in North America. Geology 2010, 38, 135–138. [Google Scholar] [CrossRef]
- Wright, H.E., Jr. Climate and Biota of Eastern North America. In Environment, Origins, and Population, Handbook of North American Indians; Ubelaker, D., Ed.; Smithsonian Institution Press: Washington, DC, USA, 2006; Volume 3, pp. 99–109. [Google Scholar]
- Wood, W.R.; McMillan, B.R. Prehistoric Man and His Environments: A Case Study in the Ozark Highlands; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- McMillan, R.B.; Klippel, W.E. Post-Glacial Environmental Change and Hunting-Gathering Societies of the Southern Prairie Peninsula. J. Archaeol. Sci. 1981, 8, 215–245. [Google Scholar] [CrossRef]
- Jefferies, R.W. The Status of Archaic Period Research in the Midwestern United States. Archaeol. East. N. Am. 1995, 23, 119–144. [Google Scholar]
- Milner, G.R. The Moundbuilders: Ancient Peoples of Eastern North America; Thames and Hudson: London, UK, 2004. [Google Scholar]
- Smith, B.D. The Archaeology of the Southeastern United States: From Dalton to de Soto, 10,500-500 B.P. Adv. World Archaeol. 1986, 5, 1–92. [Google Scholar]
- Stafford, C.R. Structural Changes in Archaic Landscape Use in the Dissected Uplands of Southwestern Indiana. Am. Antiq. 1994, 59, 219–237. [Google Scholar] [CrossRef]
- Butzer, K.W. Geomorphology of the Lower Illinois Valley as a Spatial-Temporal Context for the Koster Archaic Site; Reports of Investigations No. 34; Illinois State Museum: Springfield, IL, USA, 1977. [Google Scholar]
- Butzer, K.W. Changing Holocene Environments at the Koster Site: A Geo-Archaeological Perspective. Am. Antiq. 1978, 43, 408–413. [Google Scholar] [CrossRef][Green Version]
- Hajic, E.R. Koster Site Archaeology I: Stratigraphy and Landscape Evolution; Research Series Volume 8; Center for American Archaeology: Kampsville, IL, USA, 1990. [Google Scholar]
- Phillips, J.; Brown, J. (Eds.) Archaic Hunters and Gatherers in the American Midwest; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Munson, P.J. What Happened in the Archaic in the Midwestern United States? Rev. Anthropol. 1986, 13, 276–282. [Google Scholar] [CrossRef]
- Jefferies, R.W.; Thompson, V.D.; Milner, G.R. Archaic Hunter-Gatherer Landscape Use in West-Central Kentucky. J. Field Archaeol. 2005, 30, 2–23. [Google Scholar] [CrossRef]
- Milner, G.R.; Jefferies, R.W. The Read Archaic Shell Midden in Kentucky. Southeast. Archaeol. 1998, 17, 119–132. [Google Scholar]
- Morey, D.F.; Crothers, G.M.; Stein, J.K.; Fenton, J.P.; Herrmann, N.P. The Fluvial and Geomorphologic Context of Indian Knoll, an Archaic Shell Midden in West-Central Kentucky. Geoarchaeology 2002, 17, 521–553. [Google Scholar] [CrossRef]
- Denniston, R.F.; Gonzalez, L.; Semken, H.A., Jr.; Asmeron, Y.; Baker, R.G.; Recelli-Snyder, H.; Reagan, M.K.; Bettis, E.A., III. Integrating Stalagmite, Vertebrate, and Pollen Sequences to Investigate Holocene Vegetation and Climate Change in the Southern Midwestern United States. Quat. Res. 1999, 52, 381–387. [Google Scholar] [CrossRef]
- Denniston, R.F.; Gonzalez, L.A.; Asmeron, Y.; Reagan, M.K.; Recelli-Snyder, H. Speleothem Carbon Isotopic Records of Holocene Environments in the Ozark Highlands, USA. Quat. Int. 2000, 67, 21–27. [Google Scholar] [CrossRef]
- Brewer, D.J. Zooarchaeology: Method, Theory, Goals. Archaeol. Method Theory 1992, 4, 95–244. [Google Scholar]
- Cleland, C.E. The Prehistoric Animal Ecology and Ethnozoology of the Upper Great Lakes Region; Anthropological Papers No. 29; Museum of Anthropology, University of Michigan: Ann Arbor, Michigan, 1966. [Google Scholar]
- Lyman, R.L. Archaeofaunas and Subsistence Studies. Adv. Archaeol. Method Theory 1982, 5, 331–393. [Google Scholar]
- Lyman, R.L. Applied Zooarchaeology: An Archaeological Perspective from the Americas. World Archaeol. 1996, 28, 110–125. [Google Scholar] [CrossRef]
- Lyman, R.L. Paleozoology in the Service of Conservation Biology. Evol. Anthropol. 2006, 15, 11–19. [Google Scholar] [CrossRef]
- Bodie, J.R.; Semlitsch, R.D.; Renken, R.B. Diversity and Structure of Turtle Assemblages: Associations with Wetland Characters Across a Floodplain Landscape. Ecography 2000, 23, 444–456. [Google Scholar] [CrossRef]
- Cagle, F.R. Herpetological Fauna of Jackson and Union Counties, Illinois. Am. Midl. Nat. 1942, 10, 164–204. [Google Scholar] [CrossRef]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada, 2nd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2009. [Google Scholar]
- Johnson, T.R. The Amphibians and Reptiles of Missouri; Missouri Department of Conservation: Jefferson City, MO, USA, 1987. [Google Scholar]
- Adler, K. Turtles from Archeological Sites in the Great Lakes Region. Mich. Archaeol. 1968, 14, 147–163. [Google Scholar]
- Holman, J.A. The Status of Michigan Pleistocene Herpetofauna. Mich. Acad. 1988, 20, 125–132. [Google Scholar]
- Holman, J.A. North American Pleistocene Herpetofauna Stability and Its Impact on the Interpretation of Modern Herpetofaunas: An Overview. In Beamers, Bobwhites, and Blue-Points, Tributes to the Career of Paul W. Parmalee; James, R., Purdue, J.R., Walter, E., Klippel, W.E., Bonnie, W., Styles, B.W., Eds.; Reports of Investigations No. 23; Illinois State Museum: Springfield, IL, USA, 1991; pp. 227–235. [Google Scholar]
- Holman, J.A.; Richards, R.L. 1993 Herpetofauna of the Prairie Creek Site, Daviess County, Indiana. Proc. Indiana Acad. Sci. 1993, 102, 115–131. [Google Scholar]
- Parmalee, P.W. A Recent Cave Bone Deposit in Southwestern Illinois. Bull. Natl. Speleol. Soc. 1967, 29, 119–147. [Google Scholar]
- Parmalee, P.W.; Oesch, R.D.; Guildey, J. Pleistocene and Recent Vertebrate Fauna from Crankshaft Cave, Missouri; Reports of Investigations No. 14; Illinois State Museum: Springfield, IL, USA, 1969. [Google Scholar]
- Parmalee, P.W.; Ronald, D.; Oesch, R.D. Pleistocene and Recent Faunas from the Brynjulfson Caves, Missouri; Reports of Investigations No. 25; Illinois State Museum: Springfield, IL, USA, 1972. [Google Scholar]
- Fowler, M.L.; Parmalee, P.W. Ecological Interpretation of Data on Archaeological Sites: The Modoc Rock Shelter. Trans. Ill. State Acad. Sci. 1959, 52, 109–119. [Google Scholar]
- Dye, D.H.; Moore, K.H. Recovery Systems for Subsistence Data: Water Screening and Water Flotation. Tenn. Anthropol. 1978, 3, 59–69. [Google Scholar]
- Lynott, M.J.; Monk, S.M. The Lepold Site, 23RI59. Mo. Archaeol. Soc. Q. 1987, 4, 8–11. [Google Scholar]
- Jones, R.A.; Williams, J.W.; Jackson, S.T. Vegetation History Since the Last Glacial Maximum in the Ozark Highlands (USA): A New Record from Cupola Pond, Missouri. Quat. Sci. Rev. 2015, 170, 146–187. [Google Scholar] [CrossRef]
- Bettis, E.A. III Patterns in Holocene Colluvium and Alluvial Fans Across the Prairie-Forest Transition in the Midcontinent USA. Geoarchaeology 2003, 8, 779–797. [Google Scholar] [CrossRef]
- Jacobson, R.B.; Primm, A.T. Historical Land-Use Changes and Potential Effects on Stream Disturbance in the Ozark Plateaus, Missouri; Water Supply Paper No. 2484; US Department of the Interior, US Geological Survey: Reston, VA, USA, 1994. [Google Scholar]
- Kidder, T.R.; Adelsberger, K.A.; Arco, L.J.; Schilling, T.M. Basin-Scale Reconstruction of Geologi`cal Context of Human Settlement: An Example from the Lower Mississippi Valley, USA. Quat. Sci. Rev. 2008, 27, 1255–1270. [Google Scholar] [CrossRef]
- Van Arsdale, R.B.; Cupples, W.B.; Csontos, R.M. Pleistocene-Holocene Transition in the Central Mississippi River Valley. Geomorphology 2014, 214, 270–282. [Google Scholar] [CrossRef]
- Rittenour, T.M.; Blum, M.B.; Goble, R.J. Fluvial Evolution of the Lower Mississippi River Valley During the Last 100 K.Y. Glacial Cycle: Response to Glaciation and Sea-Level Change. Geol. Soc. Am. Bull. 2007, 119, 586–608. [Google Scholar] [CrossRef]
- Royall, P.D.; Delcourt, P.A.; Delcourt, H.R. Late-Quaternary Paleoecology and Paleoenvironments of the Central Mississippi Valley. Geol. Soc. Am. Bull. 1991, 103, 157–170. [Google Scholar] [CrossRef]
- Delcourt, H.R.; Delcourt, P.A.; Royall, P.D. Late Quaternary Vegetational History of the Western Lowlands. In Sloan, A Paleoindian Dalton Cemetery in Arkansas; Morse, D.F., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1997; pp. 103–122. [Google Scholar]
- Denniston, R.F.; DuPree, M.; Dorale, J.A.; Asmerom, Y.; Polyak, V.J.; Carpenter, S.J. Episodes of Late Holocene Aridity Recorded by Stalagmites from Devil’s Ice Box Cave, Central Missouri, USA. Quat. Res. 2007, 68, 45–52. [Google Scholar] [CrossRef]
- Grayson, D.K. Quantitative Zooarchaeology: Topics in the Analysis of Archaeological Faunas; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance; Harper and Row: New York, NY, USA, 1972. [Google Scholar]
- Whitaker, R.H. Communities and Ecosystems; Macmillan: New York, NY, USA, 1970. [Google Scholar]
- Risley, P.L. Observations on the Natural History of the Common Musk Turtle, Sternotherus odoratus (Latreille). Pap. Mich. Acad. Sci. Arts Lett. 1932, 17, 685–711. [Google Scholar]
- Skorepa, A.C.; Ozment, J. Habitat, Habits, and Variation of Kinosternon subrubrum in Southern Illinois. Trans. Ill. Acad. Sci. 1968, 61, 247–251. [Google Scholar]
- Cagle, F.R. The Life History of the Slider Turtle, Pseudemys scripta troostii (Holbrook). Ecol. Monogr. 1950, 20, 31–54. [Google Scholar] [CrossRef]
- Reagan, D.P. Habitat Selection in the Three-toed Box Turtle, Terrapene carolina triungus. Copeia 1974, 1974, 512–527. [Google Scholar] [CrossRef]
- Harris, K.A.; Clark, J.D.; Elmore, R.D.; Harper, C.A. Direct and Indirect Effects of Fire on Eastern Box Turtles. J. Wildl. Manag. 2020, 84, 1384–1395. [Google Scholar] [CrossRef]
- Guyette, R.P.; Muzika, R.M.; Dey, D.C. Dynamics of an Anthropogenic Fire Regime. Ecosystems 2002, 5, 472–486. [Google Scholar]
- Congdon, J.D.; Green, J.L.; Gibbons, J.W. Biomass of Freshwater Turtles: A Geographical Comparison. Am. Midl. Nat. 1986, 115, 165–173. [Google Scholar] [CrossRef]
- Dreslik, M.J.; Kuhns, A.R.; Phillips, C.A. Structure and Composition of a Southern Illinois Freshwater Turtle Assemblage. Northeast. Nat. 2005, 12, 173–186. [Google Scholar] [CrossRef]
- Glorioso, B.M.; Vaughn, A.J.; Waddle, J.H. The Aquatic Turtle Assemblage Inhabiting a Highly Altered Landscape in Southeast Missouri. J. Fish Wildl. Manag. 2010, 1, 161–168. [Google Scholar] [CrossRef]
- Nickerson, M.A.; Mitchell, J.C.; Glorioso, B.M. The Importance of Turtle Populations to Wetland Restoration in the Upper Mississippi Embayment of the Mississippi Alluvial Valley. Wetl. Ecol. Manag. 2019, 27, 683–692. [Google Scholar] [CrossRef]
- Clark, H.W.; Southall, J.R. Fresh-Water Turtles: A Source of Meat Supply; Document 889; Bureau of Fisheries, U.S. Department of Commerce: Washington, DC, USA, 1920. [Google Scholar]
- Cahn, A.R. The Turtles of Illinois; Illinois Biological Monographs, University of Illinois: Urbana, IL, USA, 1937; Volume 16. [Google Scholar]
- Reehl, M.; Thompson, J.; Tucker, J.K. A Three Year Survey of Aquatic Turtles in a Riverside Pond. Trans. Ill. State Acad. Sci. 2006, 99, 145–152. [Google Scholar]
- Parmalee, P.W. The Food Economy of Archaic and Woodland Peoples at the Tick Creek Cave Site, Missouri. Mo. Archaeol. 1965, 27, 1–34. [Google Scholar]
- Adler, K. The Influence of Prehistoric Man on the Distribution of the Box Turtle. Ann. Carnegie Mus. 1969, 41, 263–280. [Google Scholar]
- Congdon, J.D.; Dunham, A.E.; van Loben Sels, R.C. Demographics of Common Snapping Turtles (Chelydra serpentina): Implications and Management of Long-Lived Organisms. Am. Zool. 1994, 34, 397–408. [Google Scholar] [CrossRef]
- Hunter, M., Jr. Benchmarks for Managing Ecosystems: Are Human Activities Natural? Conserv. Biol. 1996, 10, 695–697. [Google Scholar] [CrossRef]
- Abrams, M.D. Fire and the Development of Oak Forests. Bioscience 1992, 42, 346–353. [Google Scholar] [CrossRef]
- Abrams, M.D.; Nowacki, G.J. Native Americans as Active and Passive Promoters of Mast and Fruit Trees in the Eastern USA. Holocene 2008, 18, 1123–1137. [Google Scholar] [CrossRef]
- Delcourt, P.A.; Delcourt, H.R.; Cridebaugh, P.A.; Chapman, J. Holocene Ethnobotanical and Paleoecological Record of Human Impact on Vegetation in the Little Tennessee Valley, Tennessee. Quat. Res. 1986, 25, 330–349. [Google Scholar] [CrossRef]
- Roberts, R.G. Tick Creek Cave an Archaic Site in the Gasconade River Valley of Missouri. Mo. Archaeol. 1965, 27, 1–52. [Google Scholar]
- Stafford, C.R.; Richards, R.L.; Anslinger, C.M. The Bluegrass Fauna and Changes in Middle Holocene Hunter-Gatherer Foraging in the Southern Midwest. Am. Antiq. 2000, 65, 317–336. [Google Scholar] [CrossRef]
- Dreslik, M.J.; Phillips, C.A. Turtle Communities in the Upper Midwest, USA. J. Freshw. Ecol. 2005, 20, 149–164. [Google Scholar] [CrossRef]
- Broadman, R.; Cortwright, S.; Resetar, A. Historical Changes of Reptiles and Amphibians of Northwest Indiana Fish and Wildlife Properties. Am. Midl. Nat 2002, 147, 135–144. [Google Scholar] [CrossRef]
- Stone, P.A.; Hauge, J.B.; Scott, A.F.; Guyer, C.; Donie, J.L. Temporal Changes in Two Turtle Assemblages. J. Herpetol. 1993, 27, 13–23. [Google Scholar] [CrossRef]
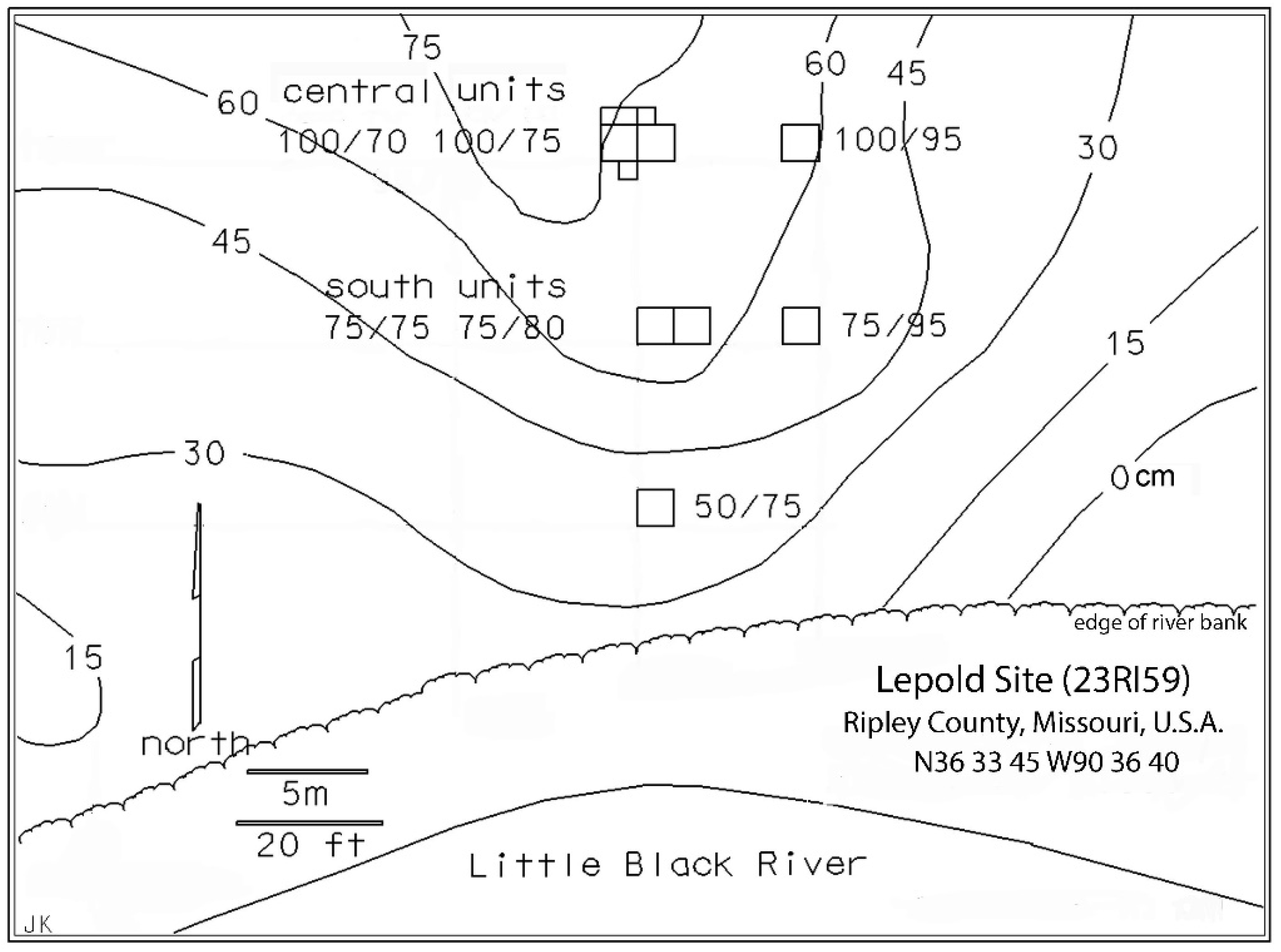



| Unit and Level | Depth (cm) | Lab No. | Material | RCY BP | 13C/12C | |
|---|---|---|---|---|---|---|
| 1981 | test | 20–40 | B-5601 | bone | 3900 ± 110 | −21.7 |
| 105/75 | 3 | 46–58 | B-104976 | nut shell | 6110 ± 60 | −25.2 |
| 100/70 | 5 | 70–82 | B-66800 | bone | 5630 ± 50 | −23.3 |
| 75/80 | 6 | 82–95 | B-66801 | bone | 6300 ± 80 | −23.5 |
| 75/80 | 7 | 95–107 | B-10975 | nut shell | 7260 ± 60 | −26.9 |
| 100/70 | 7 | 95–107 | B-10977 | nut shell | 7450 ± 60 | −27.4 |
| 75/75 | 8 | 107–119 | B-65936 | bone | 7235 ± 95 | −24.6 |
| Level | Total | Turtle | Percent Turtle |
|---|---|---|---|
| PZ | 5965 | 1219 | 20.4 |
| 1 | 5186 | 1524 | 29.4 |
| 2 | 5236 | 1611 | 30.8 |
| 3 | 4311 | 1418 | 32.9 |
| 4 | 3330 | 886 | 26.6 |
| 5 | 2475 | 697 | 28.2 |
| 6 | 1922 | 460 | 23.9 |
| 7 | 813 | 158 | 19.4 |
| 8 | 523 | 96 | 18.3 |
| 9 | 540 | 83 | 15.4 |
| Total | 30301 | 8152 | 26.9 |
| Common Name | Scientific Name | Characteristic Habitat |
|---|---|---|
| Snapping | Chelydra serpentina | Slow-moving shallow water, soft bottom with aquatic vegetation [33] (p. 115) |
| Eastern Box | Terrapene carolina | Open mesic woodland [33] (p. 411) |
| Common Musk | Sternotherus odoratus | Slow-moving shallow rivers and ponds with soft bottom [33] (p. 526) |
| Eastern Mud | Kinosternon subrubrum | Slow moving, shallow water, soft bottom with aquatic vegetation [33] (p. 501) |
| Smooth Softshell | Apalone nutica | Medium and large rivers [33] (pp. 614–615) |
| Spiny Softshell | Apalone spinifera | Rivers, side channels, and ponds [33] (p. 624) |
| Northern Map | Graptemys geographica | Large bodies of water, rivers and lakes, with basking sites [33] (p. 295) |
| False Map | Graptemys pseudographica | Large bodies of water, rivers and lakes, with basking sites and vegetation [33] (p. 327) |
| Pond Slider | Trachemys scripta | Ponds and slow rivers, 1 to 2 m deep, with aquatic vegetation [33] (p. 447) |
| Painted | Chrysemys picta | Slow-moving shallow water, soft bottom, with vegetation [33] (p. 188) |
| Level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | PZ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| T. carolina | 78 | 88 | 99 | 95 | 48 | 32 | 25 | 12 | 12 | 7 |
| T. scripta | 11 | 11 | 8 | 5 | 7 | 8 | 3 | 6 | 0 | 1 |
| G. geographica | 3 | 1 | 2 | 2 | 1 | 3 | 0 | 1 | 0 | 0 |
| C. picta | 28 | 30 | 30 | 38 | 26 | 18 | 12 | 1 | 1 | 1 |
| G. psedographica | 5 | 8 | 11 | 4 | 6 | 1 | 4 | 2 | 0 | 0 |
| Slider/Map/Painted | 5 | 6 | 11 | 12 | 9 | 4 | 2 | 0 | 0 | 2 |
| C. serpentina | 6 | 19 | 9 | 13 | 8 | 8 | 5 | 0 | 5 | 1 |
| K. subrubrum | 19 | 15 | 29 | 34 | 15 | 19 | 8 | 5 | 1 | 0 |
| S. odoratus | 7 | 15 | 22 | 13 | 14 | 9 | 2 | 2 | 1 | 0 |
| Kinosternidae | 78 | 155 | 178 | 174 | 132 | 95 | 54 | 18 | 14 | 8 |
| Apalone | 7 | 7 | 6 | 13 | 3 | 2 | 1 | 0 | 0 | 0 |
| Unknown | 972 | 1168 | 1206 | 1015 | 617 | 498 | 344 | 111 | 62 | 63 |
| T. carolina | Slider/Map/Painted | Chelydra/Trionychidae Kinosternidae | ||||||
|---|---|---|---|---|---|---|---|---|
| Level | ct | % | ct | % | ct | % | ct | % |
| PZ | 78 | 32 | 52 | 21 | 13 | 5 | 104 | 42 |
| 1 | 88 | 25 | 56 | 16 | 26 | 7 | 185 | 52 |
| 2 | 99 | 24 | 62 | 15 | 15 | 4 | 229 | 56 |
| 3 | 95 | 23 | 61 | 15 | 26 | 6 | 221 | 55 |
| 4 | 48 | 18 | 49 | 18 | 11 | 4 | 161 | 60 |
| 5 | 32 | 16 | 34 | 17 | 10 | 5 | 123 | 62 |
| 6 | 25 | 22 | 21 | 18 | 6 | 5 | 64 | 55 |
| 7–9 | 31 | 31 | 15 | 15 | 6 | 6 | 49 | 49 |
| observed | ||||||||
| Taxon | PZ | 1 | 2 | 3 | 4 | 5 | 6 | 7–9 |
| T. carolina | 78 | 88 | 99 | 95 | 48 | 32 | 25 | 31 |
| Slider/Map/Painted | 52 | 56 | 62 | 61 | 49 | 34 | 21 | 15 |
| Chelydra/Apalone | 13 | 26 | 15 | 26 | 11 | 10 | 6 | 6 |
| Kinosternidae | 104 | 185 | 229 | 221 | 161 | 123 | 64 | 49 |
| expected | ||||||||
| T. carolina | 58.5 | 84 | 96 | 95.4 | 63.7 | 47.1 | 27.5 | 23.9 |
| Slider/Map/Painted | 41 | 59.3 | 67.7 | 67.3 | 44.9 | 33.2 | 19.4 | 16.9 |
| Chelydra/Apalone | 13 | 19.1 | 21.8 | 21.7 | 14.5 | 10.7 | 6.3 | 5.4 |
| Kinosternidae | 133.9 | 192.5 | 219.6 | 218.5 | 145.8 | 107.9 | 62.9 | 54.8 |
| χ2 cell values | ||||||||
| T. carolina | 6.5 | 0.19 | 0.09 | 0 | 3 | 4.8 | 0.32 | 2.1 |
| Slider/Map/Painted | 2.9 | 0.18 | 0.48 | 0.59 | 0.37 | 0.02 | 0.13 | 0.21 |
| Chelydra/Apalone | 0 | 2.49 | 2.12 | 0.85 | 0.84 | 0.04 | 0.01 | 0.07 |
| Kinosternidae | 6.67 | 0.03 | 0.4 | 0.03 | 1.58 | 2.1 | 0.02 | 0.61 |
| Total | χ2 = 40.54; p 0.05 = 32.67; p. 0.01 = 38.93 | |||||||
| Taxa | PZ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T. carolina | 78 | 88 | 99 | 95 | 48 | 32 | 25 | 12 | 12 | 7 | 496 |
| Kinosternidae | 104 | 185 | 229 | 221 | 161 | 123 | 64 | 25 | 16 | 8 | 1136 |
| Kino/T. c. | 1.33 | 2.1 | 2.31 | 2.33 | 3.35 | 3.84 | 2.56 | 2.08 | 1.33 | 1.14 | 2.29 |
| Taxa | PZ | 1 | 2 | 3 | 4 | 5 | 6 | 7–9 |
|---|---|---|---|---|---|---|---|---|
| Max. poss. | 16 | 17 | 19 | 17 | 16 | 12 | 5 | 9 |
| Percent | 9.5 | 6.4 | 6.2 | 5.5 | 7.2 | 8.7 | 5.5 | 12.8 |
| Total count | 169 | 267 | 306 | 308 | 221 | 137 | 91 | 70 |
| Taxa | PZ | 1 | 2 | 3 | 4 | 5 | 6 | 7–9 |
|---|---|---|---|---|---|---|---|---|
| K. subrubrum | 19 | 15 | 29 | 34 | 15 | 19 | 8 | 6 |
| S. odoratus | 7 | 15 | 22 | 13 | 14 | 9 | 2 | 3 |
| Ratio K/S | 2.7 | 1 | 1.3 | 2.6 | 1.1 | 2.1 | 4 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakker, J.J.; Krakker, L.A. Middle Holocene Environment on the Ozark Margin in Southeast Missouri: Deciphering a Testudine Testament. Quaternary 2022, 5, 29. https://doi.org/10.3390/quat5030029
Krakker JJ, Krakker LA. Middle Holocene Environment on the Ozark Margin in Southeast Missouri: Deciphering a Testudine Testament. Quaternary. 2022; 5(3):29. https://doi.org/10.3390/quat5030029
Chicago/Turabian StyleKrakker, James J., and Linda A. Krakker. 2022. "Middle Holocene Environment on the Ozark Margin in Southeast Missouri: Deciphering a Testudine Testament" Quaternary 5, no. 3: 29. https://doi.org/10.3390/quat5030029
APA StyleKrakker, J. J., & Krakker, L. A. (2022). Middle Holocene Environment on the Ozark Margin in Southeast Missouri: Deciphering a Testudine Testament. Quaternary, 5(3), 29. https://doi.org/10.3390/quat5030029





