Acidification Assessment after Peat Bog Drainage in the Catalan Pyrenees (NE Iberia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
- Cambro-Ordovician sedimentary materials: Rhythmic alternation of quartzitic sandstones with feldspars and slaty lutitic layers, with thicknesses ranging from a few centimeters to few decimeters. They form a thick and monotonous series, with some intercalations of black shades or very dark gray with iron sulfides, which give reddish colours when altered.
- Sedimentary materials: Formed by carbonaceous black shales, very rich in organic remains and sulfides. They are characterized by being relatively soft, and have grayish or whitish colours due to their superficial alteration. They are interpreted as marine sediments, deposited in low oxygenated environments that favor the accumulation of organic matter and the precipitation of sulfides.
2.2. Soil Sampling, Morphology and Micromorphology
2.3. Laboratory Methods: Physical and Chemical Analysis
3. Results and Discussion
3.1. Morphology and General Soil Characteristics of the Profiles
3.2. Organic Matter
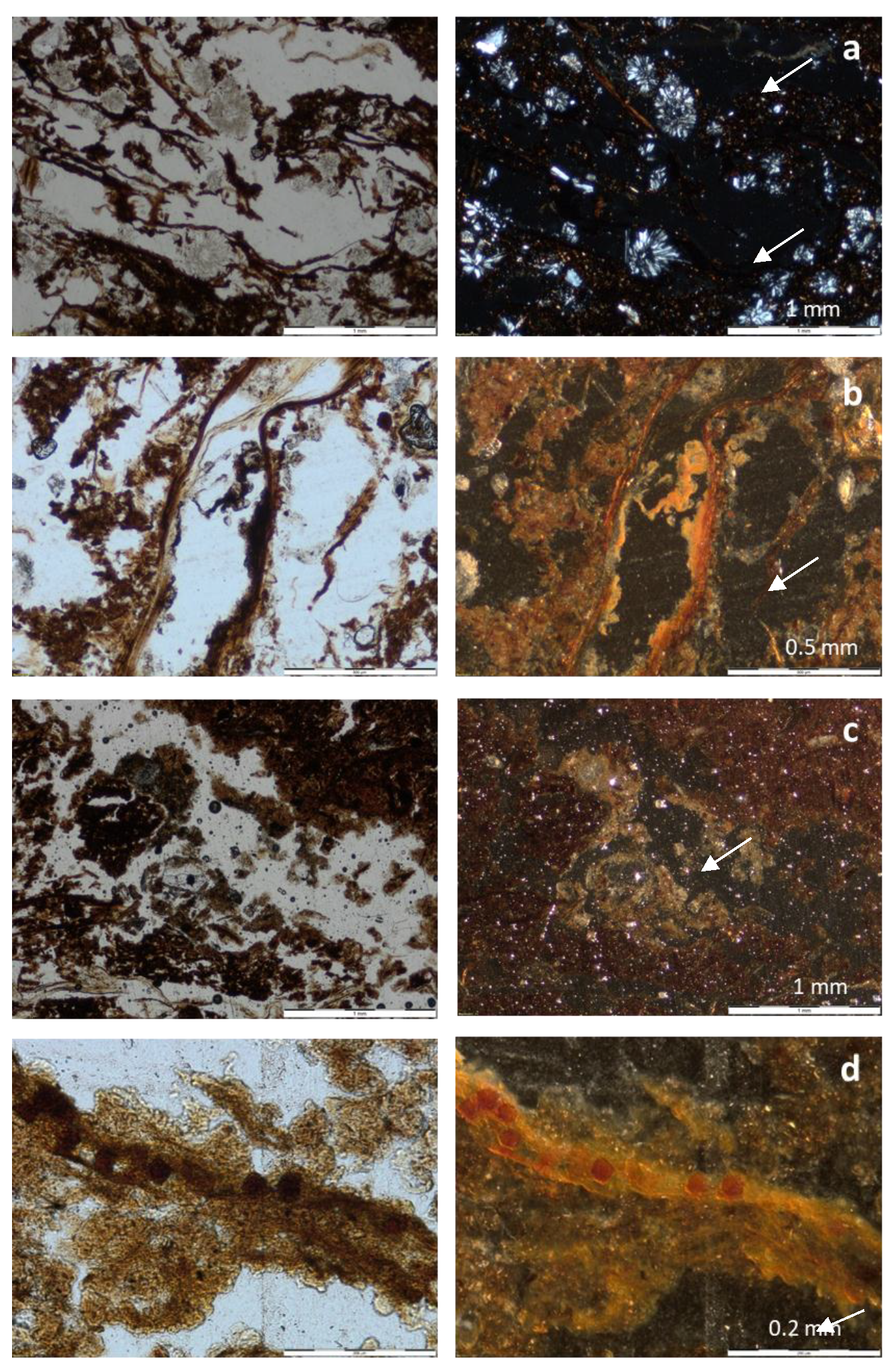
3.3. Micromorphology
3.4. Electrical Conductivities and Chloride and Sulfate Tests
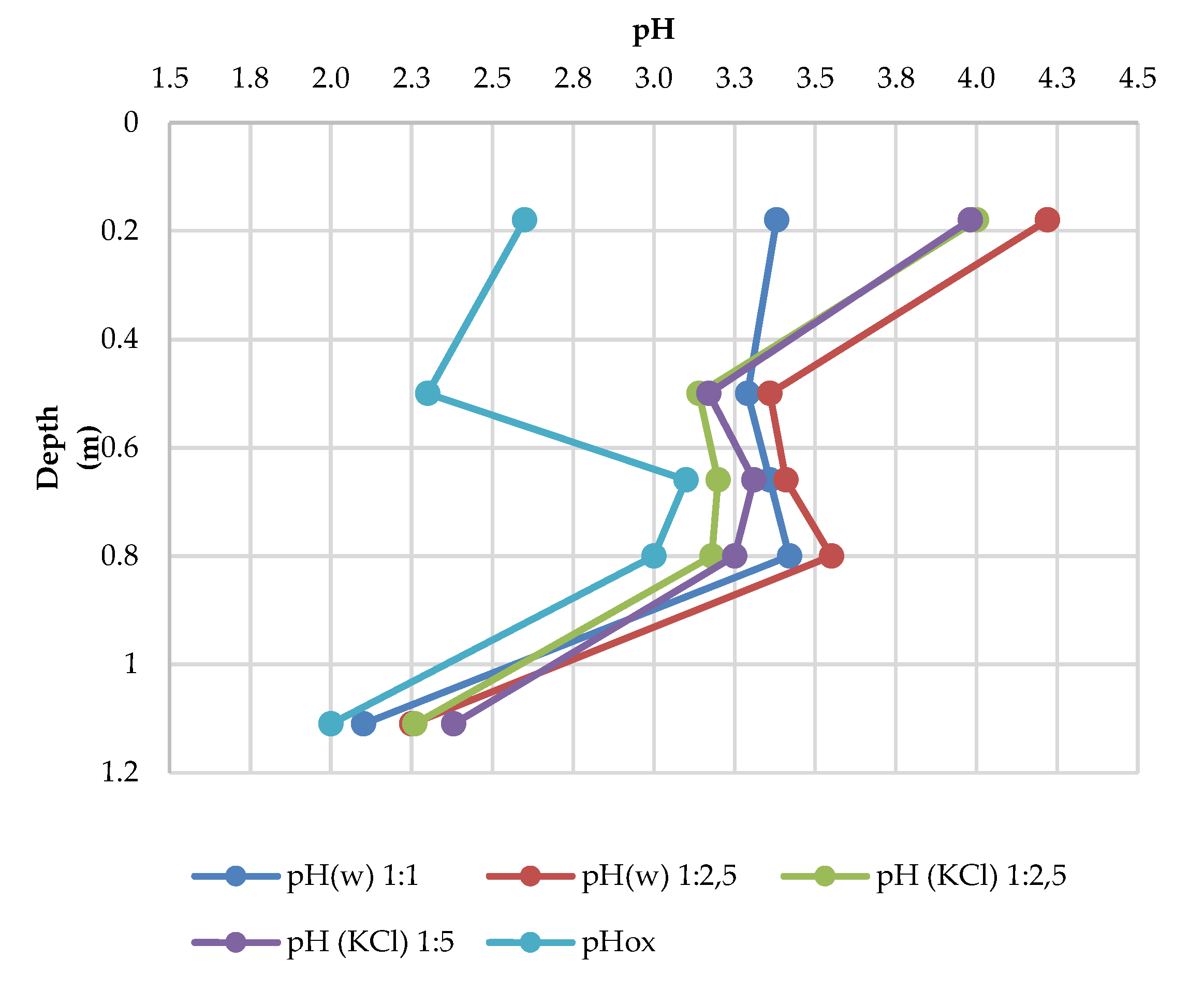
3.5. Soil Reaction
3.6. Peroxide Oxidation Combined Acidity and Sulfur (SPOCAS) Method
- The initial content of pyrite in the horizons. The successive accumulation of detrital materials from upslope combined with periods of peat formation could be the cause of varying pyrite contents along the profile, and therefore of variable potential acidity.
- Part of the pyrite content having already been oxidized due to the drainage works. Again, the degree of oxidation varies depending on local profile conditions and also on the position and characteristics of the horizons.
3.7. Implications for Acidification Risk Assessment in the Pyrenees
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pons, L.J. Outline of the genesis, characteristics, classification and improvement of acid sulphate soils. In Acid Sulphate Soils, Proceedings of the International Symposium, Wageningen, The Netherlands, 13–20 August 1972; Dost, H., Ed.; International Land Reclamation Institute Pub. 18: Wageningen, The Netherlands, 1973; Volume 1, pp. 3–27. [Google Scholar]
- Fanning, D.S.; Fanning, M.C.B. Soil Morphology, Genesis, and Classification; John Wiley and Sons: New York, NY, USA, 1989; p. 395. [Google Scholar]
- Dent, D.L.; Pons, L.J. A world perspective on acid sulfate soils. Geoderma 1995, 67, 263–276. [Google Scholar] [CrossRef]
- Isbell, R.F. The Australian Soil Classification System; CSIRO Publishing: Melbourne, VIC, Australia, 1996. [Google Scholar]
- Fanning, D.S.; Rabenhorst, M.C.; Burch, S.N.; Islam, K.R.; Tangren, S.A. Sulfides and sulfates. In Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; Soil Science Society of America Book Series #7: Madison, WI, USA, 2002; pp. 229–260. [Google Scholar]
- Powell, B.; Martens, M. A review of acid sulfate soil impacts, actions and policies that impact on water quality in Great Barrier Reef catchments, including a case study on remediation at East Trinity. Mar. Pollut. Bull. 2005, 51, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Andriesse, W.; Van Mensvoort, M.E.F. Acid sulfate soils: Distribution and extent. In Encyclopedia Soil Science; Lal, R., Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 1, pp. 14–19. [Google Scholar]
- Hall, K.C.; Baldwin, D.S.; Rees, G.N.; Richardson, A.J. Distribution of inland wetlands with sulfidic sediments in the Murray-Darling Basin, Australia. Sci. Total Environ. 2006, 370, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, R.W.; Fritsch, E.; Self, P.G. Interpretation of soil features produced by ancient and modern processes in degraded landscapes: V. Development of saline sulfidic features in non-tidal seepage areas. Geoderma 1996, 69, 1–29. [Google Scholar] [CrossRef]
- Bescansa, P.; Roquero, C. Characterization and classification of tidal marsh soils and plant communities in north-west Spain. Catena 1990, 17, 347–355. [Google Scholar] [CrossRef]
- Gómez-Miguel, V.D.; Badía-Villas, D. Soil Distribution and classification. In The Soils of Spain; Gallardo, J.F., Ed.; World Soils Book Series; Springer: Berlin Germany, 2007; Volume 2015, pp. 11–48. [Google Scholar]
- Rubio, J.L.; Sánchez, J.; Corteza, J. Proyecto LUCDEME: Mapa de suelos de la Comunidad Valenciana; Conselleria d’Agricultura i Medi Ambient: Valencia, Spain, 1996. [Google Scholar]
- Camarero, J.J.; Gutiérrez, E. Pace and pattern of recent treeline dynamics: Response of ecotones to climatic variability in the Spanish Pyrenees. Clim. Chang. 2004, 63, 181–200. [Google Scholar] [CrossRef]
- Garcés-Pastor, S.; Cañellas-Boltà, N.; Pèlachs, A.; Soriano, J.M.; Pérez-Obiol, R.; Pérez-Haase, A.; Calero, M.A.; Andreu, O.; Escolà, N.; Vegas-Vilarrúbia, T. Environmental history and vegetation dynamics in response to climate variations and human pressure during the Holocene in Bassa Nera, Central Pyrenees. Palaeogeogr. Palaeoclimatol. 2017, 479, 48–60. [Google Scholar] [CrossRef]
- Pèlachs Mañosa, A.; Pérez-Obiol, R.; Soriano López, J.M.; Bal, M.C. El paisatge vegetal de les Planes de Son i la mata de València: Una aproximació als darrers mil·lennis de geohistòria ambiental. In Els Sistemes Naturals de les Planes de Son i la Mata de València; Germain, J., Ed.; Treballs de la Institució Catalana d’Història Natural 16; Institució Catalana d’Història Natural: Barcelona, Spain, 2010; Volume 16, pp. 751–783. [Google Scholar]
- Institut Cartogràfic i Geològic de Catalunya (ICGC). Documents of the Centre d’Interpretació de Sòls dels Pirineus, Unpublished. Available online: http://www.icgc.cat/ca/L-ICGC/Agenda2/Centre-d-Interpretacio-dels-Sols-dels-Pirineus (accessed on 25 June 2019).
- Soil Survey Staff. Soil Taxonomy. A basic System of Soil Classification for Making and Interpreting Soil Surv. In Agriculture Handbook 436; US Department of Agriculture: Washington, DC, USA, 2014. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106, 192; FAO: Rome, Italy, 2015. [Google Scholar]
- Fanning, D.; Rabenhorst, M.; Fitzpatrick, R. Historical developments in the understanding of acid sulfate soils. Geoderma 2017, 308, 191–206. [Google Scholar] [CrossRef]
- Creeper, N.L.; Fitzpatrick, R.; Shand, P. A simplified incubation method using chip-trays as incubation vessels to identify sulphidic materials in acid sulphate soils. Soil Use Manag. 2012, 28, 401–408. [Google Scholar] [CrossRef]
- Institut Cartogràfic i Geològic de Catalunya (ICGC). Llegenda del Visualitzador Geoíndex-Sòls; ICGC: Barcelona, Spain, 2018; p. 43. Available online: https://www.icgc.cat/Administracio-i-empresa/Eines/Visualitzadors-Geoindex/Geoindex-Sols (accessed on 25 June 2019).
- Losantos, M. La geologia de les Planes de Son i la mata de València. In Els Sistemes Naturals de les Planes de Son i la Mata de València; Germain, J., Ed.; Treballs de la Institució Catalana d’Història Natural 16; Institució Catalana d’Història Natural: Barcelona, Spain, 2010; pp. 21–75. [Google Scholar]
- CBDSA (Comisión del Banco de Datos de Suelos y Aguas). SINEDARES, Sistema de Información Edafológica y Agronómica de España; Ministerio de Agricultura y Pesca: Madrid, Spain, 1981.
- Stone, Y.; Ahern, C.R.; Blunden, B. Acid Sulfate Soils Manual; Acid Sulfate Soils Management Advisory Committee: Wollongbar, NSW, Australia, 1998. [Google Scholar]
- Benyarku, C.A.; Stoops, G. Guidelines for Preparation of Rock and Soil Thin Sections and Polished Sections; Quaderns DMACS, 33; Universitat de Lleida: Lleida, Spain, 2005. [Google Scholar]
- Stoops, G. Guidelines for Analysis and Description of Soil and Regolith Thin Sections; Soil Science Society of America Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Ahern, C.R.; McElnea, A.E.; Sullivan, L.A. Acid Sulphate Soils: Laboratory Methods Guidelines; Department of Natural Resources, Mines and Energy: Indooroopilly, QLD, Australia, 2004.
- Jayalath, N. Laboratory Protocols For. Acid Sulfate Soils; CSIRO: Canberra, CBR, Australia, 2012. [Google Scholar]
- Porta, J.; López-Acevedo, M.; Rodríguez-Ochoa, R. Técnicas y Experimentos en Edafología; Col·legi Oficial d’Enginyers Agrònoms de Catalunya: Barcelona, Spain, 1986. [Google Scholar]
- Soil Survey Laboratory. Soil Survey Laboratory Methods Manual; United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2004.
- McElnea, A.E.; Ahern, C.R.; Menzies, N.W. The measurement of actual acidity in acid sulfate soils and the determination of sulfidic acidity in suspension after peroxide oxidation. Soil Res. 2002, 40, 1133–1157. [Google Scholar] [CrossRef]
- Zaiets, O.; Poch, R.M. Use of micromorphology for humus characterization and classification in some mediterranean calcareous soils. Appl. Soil Ecol. 2018, 123, 672–681. [Google Scholar] [CrossRef]
- Poch, R.M.; Artieda, O.; Lebedeva, M. Gypsic features. In Interpretation of Micromorphological Features of Soils and Regoliths; Stoops, G., Marcelino, V., Mees, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 259–287. [Google Scholar]
- Mees, F.; Stoops, G. Sulphidic and Sulphuric Materials. In Interpretation of Micromorphological Features of Soils and Regoliths; Stoops, G., Marcelino, V., Mees, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 347–376. [Google Scholar]
- Institu Cartogràfic i Geològic de Catalunya (ICGC). Les Pàtines Blanques a la Capçalera de la Noguera de Vallferrera. Composicions, Processos i Efectes Associats. Technical Report IGC-GAO-003/2012. Available online: http://parcsnaturals.gencat.cat/web/.content/home/alt_pirineu/coneix-nos/centre_de_documentacio/fons_documental/biblioteca_digital/hidrologia/les_patines_blanques_a_la_cap_alera_de_la_noguera_de_vallferrera/3les_patines_blanques_de_la_cap_alera_de_la_noguera_de_vallferrera.pdf (accessed on 17 September 2019).
- Jiménez Moya, M.; Madaula Izquierdo, E.; Molina García, O. Origen i Efectes Dels Drenatges Àcids a L’alta Muntanya. El Cas de la Capçalera Del Riu Freser. Bachelor’s Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2019. [Google Scholar]
- Bui, E.N. High-resolution mapping of acid sulfate soils in Northern Australia through predictive models. Environ. Chem. Lett. 2018, 16, 1449–1455. [Google Scholar] [CrossRef]

| Depth (cm) | Horizon | Munsell Colour (moist) | Bulk Density (Mg/m3) | EC(1:5) (dS/m at 25 °C) | Chloride Test | Sulfate Test | OM (%) W&B | OM (%) Calcination | pH(w) 1:1 | pH(w) 1:2.5 | pH(KCl) 1:2.5 | pH(ox) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Profile 1 | ||||||||||||
| 0–18 | Ap | 7.5YR 3/2 | 0.48 | 3.9 | Negative | Positive | 15.2 | 48.9 | 3.4 | 4.2 | 4.0 | 1.6 |
| 19–55 | H1 | 5YR 2/2 | 0.18 | 4.0 | Negative | Positive | 27.7 | 40.4 | 3.3 | 3.4 | 3.1 | 1.3 |
| 56–66 | H2 | 7.5YR 3/2 | 0.49 | 2.8 | Negative | Positive | 9.6 | 10.4 | 3.4 | 3.4 | 3.2 | 1.5 |
| 67–80 | 2C | 2.5YR 4/2 | 0.75 | 1.7 | Negative | Positive | 1.9 | - | 3.4 | 3.6 | 3.2 | 1.9 |
| 81–111 | H3 | 5YR 3/3 | 0.23 | 8.2 | Negative | Positive | 22.1 | 27.6 | 2.1 | 2.3 | 2.3 | 1.0 |
| Profile 2 | ||||||||||||
| 0–12 | Oi | 7.5YR 2/1 | 0.43 | 0.7 | Negative | Negative | 10.7 | 27.1 | 6.3 | 6.5 | 5.7 | - |
| 12–20 | H1 | 5YR 2/2 | 0.37 | 0.4 | Negative | Negative | 29.1 | 32.2 | 5.1 | 5.2 | 4.3 | 1.8 |
| 21–35 | H2 | 7.5YR 4/2 | 0.49 | 0.3 | Negative | Negative | 18.3 | 20.0 | 5.0 | 5.1 | 4.2 | 1.9 |
| 36–45 | 2Cg1 | 10YR 5/2 | 1.10 | 0.1 | Negative | Negative | 2.3 | - | 5.7 | 5.7 | 4.3 | 2.6 |
| 46–65 | 2Cg2 | 10YR 5/2 | 1.34 | 0.04 | Negative | Negative | 1.2 | - | 6.7 | 6.6 | 5.3 | 3.8 |
| Depth (cm) | Horizon | Munsell Colour (moist) | Textural Class (USDA) | EC (1:5) (dS/m at 25 °C) | OM (%) W&B | CEC (cmol(+)/kg) | pH(w) 1:2.5 | pH(w) 1:5 |
|---|---|---|---|---|---|---|---|---|
| 0–20 | A | 10YR 4/3 | Sandy loam | 1.7 | 4.0 | 11.5 | 3.7 | - |
| 21–38 | H1 | 10YR 2/1 | - | 4.7 | 30.2 | 54.8 | - | 2.8 |
| 39–50 | H2 | 2.5YR 3/2 | - | 9.2 | 38.4 | 49.2 | - | 2.3 |
| 51–70 | H3 | 10YR 2/1 | - | 5.9 | 33.9 | 61.4 | - | 3.4 |
| 71–100 | 2Bg | 5/5GY | Sandy loam | 1.4 | 2.2 | 5.6 | 3.7 | - |
| Feature | Profile 1, Horizon H2 | Profile 2, Horizon H2 |
|---|---|---|
| Porosity and microstructure | 50%, compound packing pores. Fine crumb structure. | 40%, compound packing pores and fissures. Juxtaposed fine crumb structure and fine subangular blocky. |
| Organs and tissues | Large (max 1 cm) reed remains, layered, moderately decomposed, tissue (parenchima) fragments, phlobaphenized, coarse sand size, random and layered. About 20% of the volume. | Medium (2 mm) plant remains, layered, mainly roots and shoots, phlobaphenized. Some fungal sclerotia. About 15% of the volume. |
| Amorphous organic material | Reddish amorphous material, speckled. Undifferentiated b-fabric. | Brownish amorphous material, speckled. Undifferentiated b-fabric. |
| Faunal excrements | Abundant small excrements, as infillings of channels and also associated with plant remains. | Frequent small excrements, as infillings of channels and associated with plant remains. |
| Mineral components | Medium sand of schists and quartzites | Medium sand of schists and quartzites |
| Other pedofeatures | Very abundant gypsum rosettes (fan-like) made of prismatic gypsum crystals, medium sand size. Nodules, coatings and impregnative hypocoatings of jarosite around plant remains. | Very few gypsum rosettes, in plant rests. Nodules, coatings and impregnative hypocoatings of jarosite around plant rests. Hypocoatings and nodules of iron oxides, associated with plant rests. |
| Profile and Horizon | pH(KCl) | pH(ox) | TAA | TPA | S(KCl) | S(p) | Ca(KCl) | Ca(p) | Mg(KCl) | Mg(p) | TSA | S(POS) | Ca(a) | Mg(a) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH Units | Mol H+/t | %S | %Ca | %Mg | Mol H+/t | %S | %Ca | %Mg | ||||||
| Profile 1 | ||||||||||||||
| A | 4.3 | 2.6 | 247.6 | 1336.0 | 0.57 | 1.2 | 0.99 | 1.29 | 0.04 | 0.06 | 1088.4 | 0.58 | 0.30 | 0.02 |
| H1 | 3.4 | 2.3 | 330.9 | 1490.0 | 0.70 | 1.5 | 0.69 | 0.83 | 0.01 | 0.03 | 1159.1 | 0.79 | 0.14 | 0.02 |
| H2 | 3.8 | 3.1 | 121.9 | 230.2 | 0.38 | 0.4 | 0.34 | 0.34 | 0.01 | 0.01 | 108.3 | 0.06 | 0.00 | 0.00 |
| 2C | 3.8 | 3.0 | 67.3 | 85.0 | 0.19 | 0.2 | 0.18 | 0.20 | 0.00 | 0.01 | 17.6 | 0.06 | 0.02 | 0.00 |
| H3 | 2.8 | 2.0 | 903.9 | 1580.0 | 1.78 | 2.5 | 0.70 | 0.76 | 0.04 | 0.05 | 676.2 | 0.73 | 0.06 | 0.01 |
| Profile 2 | ||||||||||||||
| Oi | 6.2 | 5.4 | 8.5 | 0.0 | 0.03 | 0.2 | 0.56 | 0.46 | 0.05 | 0.05 | < 0 | 0.16 | 0.00 | 0.00 |
| H1 | 4.4 | 3.5 | 144.2 | 572.5 | 0.05 | 0.4 | 0.32 | 1.11 | 0.02 | 0.04 | 428.3 | 0.31 | 0.79 | 0.02 |
| H2 | 4.5 | 3.6 | 63.8 | 272.5 | 0.03 | 0.2 | 0.28 | 0.34 | 0.02 | 0.03 | 208.8 | 0.17 | 0.06 | 0.01 |
| 2Cg1 | 4.6 | 4.1 | 12.3 | 0.0 | 0.02 | 0.0 | 0.06 | 0.08 | 0.01 | 0.01 | < 0 | 0.01 | 0.02 | 0.00 |
| 2Cg2 | 5.4 | 5.8 | 4.8 | 0.0 | 0.02 | 0.0 | 0.07 | 0.08 | 0.01 | 0.01 | < 0 | 0.02 | 0.02 | 0.00 |
| Horizon | s-TAA | a-SPOS | Net Acidity | Net Acidity |
|---|---|---|---|---|
| S% | mol H+/t | S% | mol H+/t | |
| Profile 1 | ||||
| A | 0.40 | 362.99 | 0.98 | 610.6 |
| H1 | 0.53 | 489.60 | 1.32 | 820.5 |
| H2 | 0.20 | 37.42 | 0.26 | 159.3 |
| 2C | 0.11 | 34.93 | 0.16 | 102.2 |
| H3 | 1.45 | 452.81 | 2.18 | 1356.7 |
| Profile 2 | ||||
| H1 | 0.23 | 192.72 | 0.54 | 337.0 |
| H2 | 0.10 | 102.91 | 0.27 | 166.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalán, A.; Antúnez, M.; Poch, R.M. Acidification Assessment after Peat Bog Drainage in the Catalan Pyrenees (NE Iberia). Quaternary 2019, 2, 32. https://doi.org/10.3390/quat2030032
Catalán A, Antúnez M, Poch RM. Acidification Assessment after Peat Bog Drainage in the Catalan Pyrenees (NE Iberia). Quaternary. 2019; 2(3):32. https://doi.org/10.3390/quat2030032
Chicago/Turabian StyleCatalán, Alba, Montserrat Antúnez, and Rosa M. Poch. 2019. "Acidification Assessment after Peat Bog Drainage in the Catalan Pyrenees (NE Iberia)" Quaternary 2, no. 3: 32. https://doi.org/10.3390/quat2030032
APA StyleCatalán, A., Antúnez, M., & Poch, R. M. (2019). Acidification Assessment after Peat Bog Drainage in the Catalan Pyrenees (NE Iberia). Quaternary, 2(3), 32. https://doi.org/10.3390/quat2030032





