1. Prevalence, Incidence and Mortality
1.1. Overview
Prostate cancer (PCa) is the second most common cancer among men in the world after lung cancer [
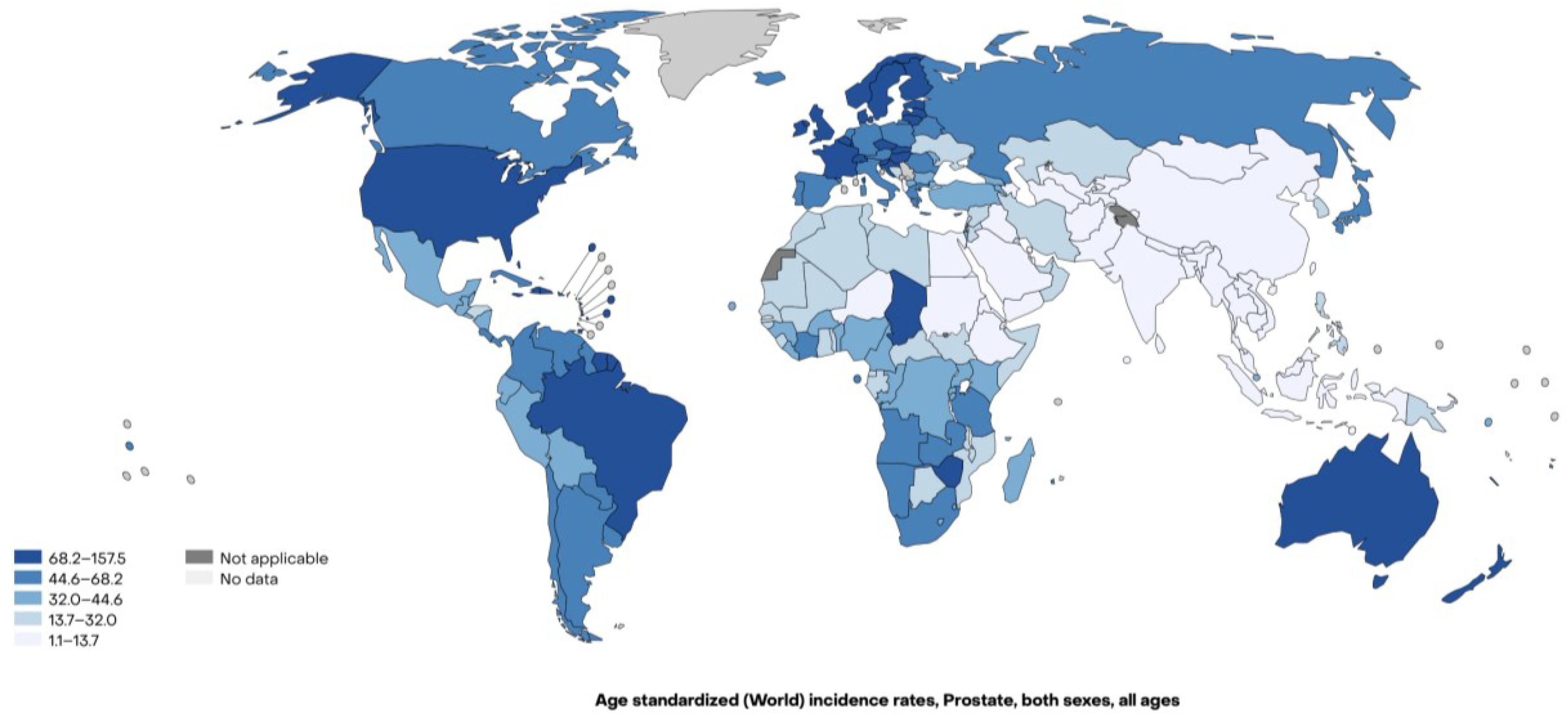
1]. In 2022, there were over 1.4 million new cases and the prevalence of men living with PCa is expected to be over 5 million by 2027. PCa is the most commonly diagnosed cancer in 118 of 185 of the countries of the world. However, the 2022 age-standardized incidence rates vary among continents (
Figure 1,
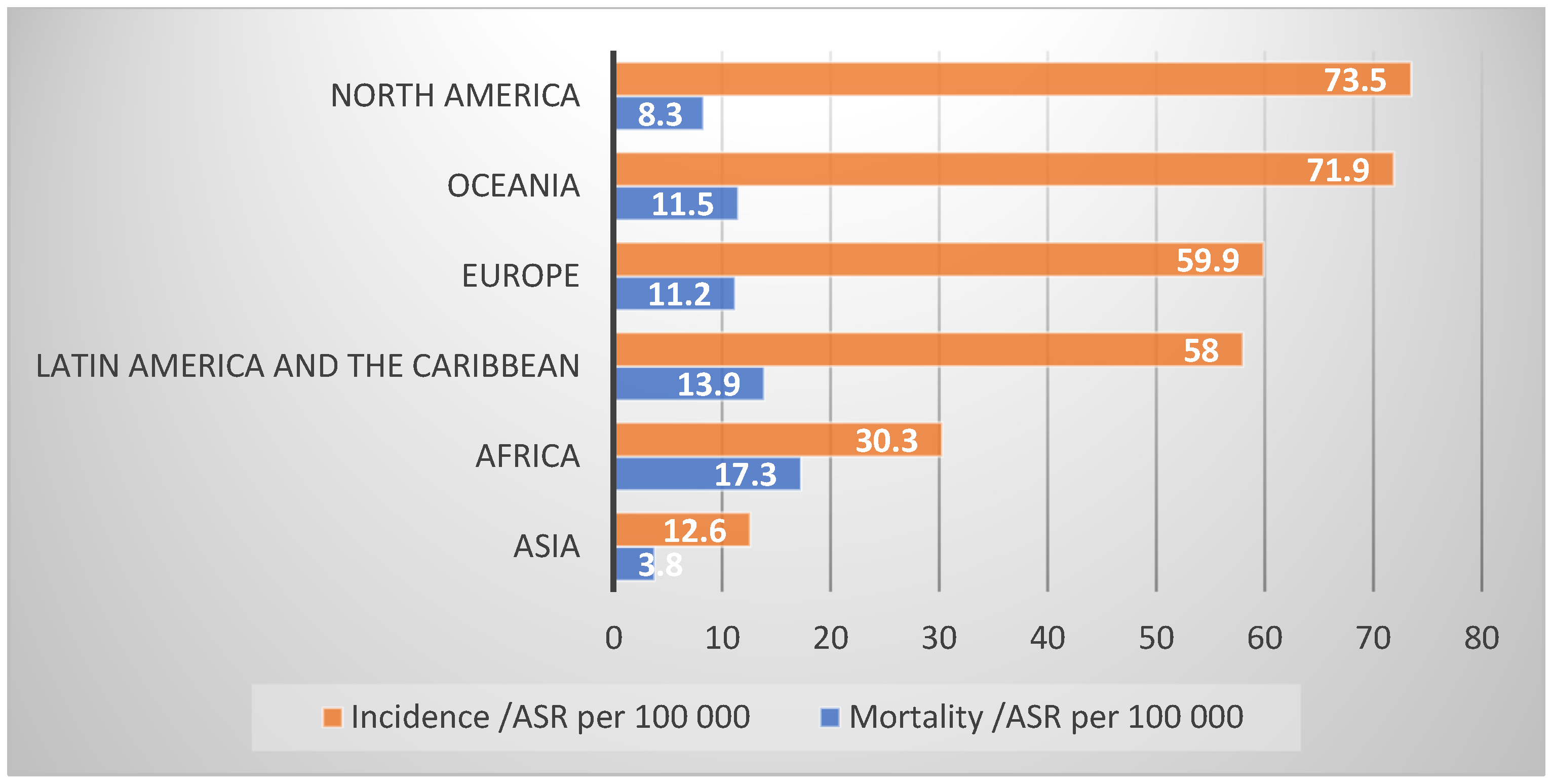
Table 1), being higher in Northern America (73.5 cases per 100,000 males) and Oceania (72.2 cases per 100,000 males), while lower in Asia (12.6 cases per 100,000 males) (
Figure 2) [
1,
2].
Worldwide, PCa is the fifth leading cause of death from cancer in men in 2022 with a wide variation across the globe (
Figure 3). It is the main cause of death from cancer in 52 of 185 of the countries and it is the leading cause of death from cancer in Africa (17.3 per 100,000 males) and Latin America and the Caribbean (13.9 per 100,000 males), while it is the seventh cause of mortality from cancer in Asia (3.7 per 100,000 males) (
Figure 2) [
1,
2].
1.2. Geographical Variation
There is substantial geographic variability in PCa incidence and mortality rates that can be attributed to variation in genetic susceptibility, life expectancy, PCa screening, access to medical care, available infrastructure in healthcare system and lifestyle factors [
3,
4]. Early detection of PCa is not standardized worldwide and there are differing recommendations between government agencies and specialty societies.
Socioeconomic status is also associated with PCa incidence rates and inversely associated with PCa mortality rates [
5].
Prostate cancer incidence in middle-aged men has also shown regional variations, with increasing diagnoses in men aged 45–64 years, particularly in high-income countries. Globally, the age-standardized incidence rate increased from 30.5 cases per 100,000 in 1990 to 37.9 cases per 100,000 in 2017. This increase is more pronounced in regions with a higher sociodemographic index, reflecting the influence of factors such as the expanded use of Prostate Specific Antigen (PSA) screening. However, in low-income regions, prostate cancer in middle-aged men is underreported due to limited access to healthcare services and diagnostic tools [
6].
Furthermore, PCa presents significant variability in its localized and metastatic forms across the globe. Localized PCa accounts for the majority of cases in regions with widespread screening practices, such as Northern America and Europe. In contrast, metastatic disease is more frequently diagnosed in regions such as Africa and parts of Asia, where access to screening and healthcare is limited. Developing Asian countries report a higher proportion of metastatic cases compared to Asian migrants in Western countries [
7]. This variation reflects disparities in healthcare infrastructure and early detection programs.
2. Northern America
Northern America has the highest incidence of PCa due to a high frequency of screening and early diagnosis and high quality of cancer registry data, and the PCa mortality rates are also one of the lowest in the world (8.3 per 100,000 males), following Asia rates (3.8 per 100,000 males) [
1]. In the US, the highest incidence is observed in the north and some regions of the southeast. Following a decrease in incidence rates in early 2010s related to changing screening recommendations, rates have increased in recent years. Moreover, incidence rates for advanced disease increased 4–6% per year in the last 5 years, leading to a slowdown in the decline in PCa mortality rates in the same period, following guideline recommendations against PSA screening [
8]. Moreover, in the US, the risk of being diagnosed with PCa is 2-fold higher in Black or African American men who are also more likely to die from PCa than non-Black men [
9].
3. Lattin America and the Caribbean
In Latin America and the Caribbean, PCa is the most common male cancer (58 per 100,000 males) and the most common cause of cancer death (13.9 per 100,000 males). One in fourteen men will develop PCa in their lifetime but the incidence rates vary between countries and the risk in some countries are among the highest in the world, such as those observed in Guadeloupe or Martinique, 19% (1 in 5 men) and 16.6% (1 in 6 men), respectively [
1]. There are disparities within healthcare systems, especially between public and private systems which affect PCa outcomes, with the population with private coverage having the same early diagnosis rates as developed countries [
10].
4. Europe
In Europe, the risk of being diagnosed with PCa before the age of 75 is 7.9% (1 in 13 men) and the highest PCa incidence rate in the world is found in Northern Europe, particularly Sweden (82.8 per 100,000 males) [
1]. Geographic variation, differences in the prevalence of risk factors, diagnostic practices, screening programs, and the effectiveness of national cancer control plans may all contribute to the observed disparities in the continence [
11].
5. Africa
Africa is the continent with the second lowest PCa incidence rates (30.3 per 100,000 males), but the highest PCa mortality rates (17.3 per 100,000 males) in the world. South Africa has the highest incidence rates (62.4 per 100,000 males), followed closely by Southern Africa (59.9 per 100,000 males) [
1]. Furthermore, PCa cases have increased by 60% from 2002 to 2018; and while Northern and Southern Africa have a high prevalence, the highest PCa mortality is found in Eastern, Western and Central Africa. Ethnicity, population origin, and limited access to PSA testing and effective treatment play a relevant role in the numbers of PCa and PCa deaths in Africa.
6. Oceania
In Oceania, PCa is the most common cancer in men (71.9 per 100,000 males), but the continent is second highest in PCa mortality (11.5 per 100,000 males) [
1]. A New Zealand study reported fluctuating PCa incidence over the last 20 years and a decline in PCa mortality rates [
12]. An Australian study observed that age-standardized incidence rates for localized PCa follow the trends in PSA screening rates and there was a decrease in PCa incidence rate after 2008 and a decrease in PCa mortality rate [
13]. As seen in other parts of the world, differences in treatment choices were identified between men diagnosed in private and public health services, which could not be explained by disease severity [
14].
7. Asia
Asia is the largest continent of the world and contains over 60% of the world’s population, which explains its impact on the number of cancer cases, accounting for 30.3% of new PCa cases and 18.3% of new PCa deaths from the disease worldwide despite it having the lowest PCa incidence (12.6 per 100,000 males) and PCa mortality in the world (3.8 per 100,000 males) [
1]. Asia comprises many different countries and has a high variation in PCa incidence and mortality rates, which reflects the lack of screening programs in many countries and the diversity in healthcare systems. PCa is the fifth most common cancer in Asian men and the seventh cause of mortality from cancer; however, it is the leading cancer in men in Japan, Lebanon, Kuwait and Israel [
7].
8. Risk Factors for Prostate Cancer
Little is known about PCa etiology, the understanding of which would be paramount for prevention measures. Age, race, family history and certain germline mutations are the few well-stablished risk factors for PCa, and environmental factors also contribute to PCa development [
4].
8.1. Non-Modifiable Risk Factors
Age
Prostate cancer incidence is known to increase with age and there is an association between advanced age and greater PCa aggressiveness [
15]. With the widespread use of PSA-based screening, the median age at diagnosis decreased and studies have shown a greater reduction in PCa mortality when PSA testing starts at earlier ages (e.g., before 55 instead of after) [
16]. Moreover, the burden differs between African Americans and patients with positive family history, who are affected at younger ages [
16,
17]. In autopsy studies, most men would have PCa by 100 years of age; however, localized PCa may remain asymptomatic before progressing to clinically significant disease [
18].
9. Family History as a Risk Factor for Prostate Cancer
The pooled relative risk of a prostate cancer diagnosis in first-degree relatives of men with prostate cancer is 2.5 (95% confidence interval 2.2–2.8) [
19]. The relative risk in men whose father or brother was diagnosed before age 60 years is 4.3 (95% confidence interval 2.9–6.3). In men with two affected relatives, it is 3.5 (95% confidence interval 2.6–4.8) [
19]. Having an affected brother increases the risk more than an affected father [
19]. The latter finding may be explained by X-linked and recessive inheritance, although shared environmental factors and detection bias may contribute.
More recent research has found that a family history of high-grade or metastatic disease increases the risk of high-grade and metastatic cancer more than a family history of low-grade, non-metastatic disease [
20]. Nonetheless, in a nationwide Swedish study, a family history of low-risk PCa in a brother increased the probability of high-risk disease by age 75 years to 8.0% (95% confidence interval 7.0% to 9.1%) from the average population risk of 5.2% [
20].
Not as many studies have investigated the association between family history and the risk of dying from PCa. In a nationwide register study, the hazard ratio for death from PCa was 1.6 for men with a father diagnosed with non-fatal PCa and 2.0 for men whose father died from PCa [
21]. The “true” risk increase may be greater than this, as frequent PSA testing and subsequent curative treatment of localized cancer among men with a family history most likely reduce their risk of dying from the disease.
Prostate cancer heritability is to some extent linked to breast cancer heritability. A systematic review and meta-analysis of 18 studies showed that having a sister or mother with breast cancer increased the risk of PCa 1.3-fold [
22]. Besides the association with breast cancer, no other cancer type has been shown to have a clinically relevant familiar association with PCa.
9.1. Clinical Consequences of Family History
Many clinical guidelines recommend regular PSA testing for men with a brother or father diagnosed with PCa initiated some years earlier than for men in general. In the Prostate, Lung, Colorectal and Ovarian (PLCO) screening trial, men with a family history of PCa had a significantly higher risk of dying from PCa if they were allocated to the non-screening arm compared with the screening arm (hazard ratio 1.9) [
23]. In contrast, two analyses from the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial did not show different outcomes for men with a family history of PCa [
24,
25]. It is possible that men with a strong family history of PCa already underwent screening and were diagnosed with PCa when invited to the ERSPC trial; if so, the trial underestimated the effect of screening in this subgroup.
A systematic review of nine North American studies, one European study and one Asian study, with a total of nearly 40,000 patients, concluded that a family history of PCa does not increase the risk of biochemical recurrence or death from PCa after surgery or radiotherapy for localized PCa [
26].
Another systematic review of six North American and European studies investigating the clinical consequences of family history in men who opted for active surveillance of localized PCa included 2400 patients [
27]. With a possible exception for African-American men, family history does therefore not seem to be an important factor when determining eligibility for active surveillance [
27]. These results do, however, not exclude that some rare germline mutations are associated with more rapid progression to incurable disease and studies are ongoing.
9.2. Modifiable
9.2.1. Diet
Dietary factors have been studied for possible associations with PCa risk. Studies have demonstrated an inverse association between a high intake of fruits, vegetables, nuts and low intake of meat and risk of PCa [
28]. A network meta-analysis that investigated the effect of ten antioxidants, including vitamins, folic-acid, selenium, beta-carotene and green tea catechins, demonstrated that the latter significantly reduced the risk of PCa, followed by vitamin D, vitamin B6 and folic acid [
29]. Also, fibers are considered to have anti-inflammatory properties through antioxidant activity; however, no association has been found between fiber intake and PCa risk [
30].
On the other hand, a proinflammatory diet, which includes Western dietary patterns, rich is processed food and sugar, has underlying mechanisms that potentially play a role in cancer development and a recent meta-analysis found that a proinflammatory diet increases the risk of PCa [
31]. Red and processed meat have harmful compounds that are produced during high-temperature or prolonged cooking, which are reported to be carcinogenic. While two previous meta-analyses did not find an association between red and processed meat and an increased incidence of PCa [
32,
33], Nouri-Majd et al. analyzed only prospective studies, comprising 25 studies and 1,900,910 patients; and found that high processed meat consumption is marginally associated with an increased risk of developing PCa (RR 1.06; 95% CI 1.01, 1.10) and advanced PCa (RR 1.17; 95% CI 1.09, 1.26). However, they observed a weak relationship between red meat and PCa risk (RR 1.05; 95% CI 0.98, 1.12) [
34].
9.2.2. Physical Activity
Physical activity is associated with a risk reduction for certain cancer types, although there is no strong evidence with regard to PCa. Benke et al. demonstrated a significant reduction in PCa risk with long-term physical activity; however, it was non-significant in a leave-one-out analysis. In addition, physical activity after PCa diagnosis was related to a 31% reduction in PCa mortality [
35]. Current physical activity guidelines recommend at least three sessions of exercise per week of aerobic and resistance exercises, but highlight the need for a better understanding regarding associations with PCa risk [
36].
9.2.3. Alcohol
Alcohol is considered a carcinogen associated with several cancers, including esophagus, larynx, breast, stomach and liver; and the risk of cancer is correlated with the amount of ethanol intake [
37]. With regard to PCa, the evidence is inconclusive. Although several studies found no association between alcohol consumption and PCa risk [
38], a recently published meta-analysis by D’Ecclesiis et al. focused on evaluating the effects of alcohol consumption on mortality from PCa and revealed no association overall (summary risk estimate -[SRE] 0.97; 95% CI 0.92, 1.03). However, when one of the studies that was responsible for the heterogeneity of the results was excluded from the pooled analysis, a direct association was found between alcohol intake and fatal PCa (SRE 1.33; 95% CI 1.12, 1.58) [
39]. Regardless of contrasting results, the evidence demonstrates a link between alcohol intake and the development of PCa [
40].
9.2.4. Smoking
Burning cigarettes have at least 70 carcinogens and smoking is a known risk factor of a variety of cancers, including genito-urinary cancers, such as kidney and bladder cancer. However, its association with PCa is still controversial, with a previous meta-analysis demonstrating an increased risk of PCa among former smokers (RR 1.09; 95% CI 1.02, 1.16), but not among current smokers (OR 1.04; 95% CI 0.87, 1.24) [
41]. Moreover, two recent meta-analyses had the same findings, suggesting an inverse association with PCa incidence [
42,
43] and a higher risk of PCa death (RR 1.42; 95% CI 1.20, 1.68) [
42], and both associated the results to the poor adherence to PCa screening among smokers.
9.2.5. Sexual Activity
Sexual behavior is a potential modifiable risk factor for PCa and studies that focused on the frequency of sexual activity reported that more frequent ejaculation (more than 20 times per month) could prevent PCa, which is related to a decrease in carcinogenic secretions in prostatic tissue [
44].
9.3. Medications and Vitamins
9.3.1. 5-Alpha Reductase Inhibitors (5ARIs)
5ARIs are medications that inhibit the conversion of testosterone to dihydrotestosterone, commonly used in benign prostatic hyperplasia and suggested as potential chemopreventive agents for PCa. A meta-analysis comprising 23 studies found a decreased risk of overall PCa in 5ARI users (RR 0.77; 95% CI 0.67, 0.88) but an increased risk of high-grade PCa (RR 1.19; 95% CI 1.01, 1.40) [
45]. Knijnik et al. confirmed the finding of a reduction of 26% in PCa diagnosis (RR 0.74; 95% CI 0.59, 0.97), but did not demonstrate an increase in high-grade PCa [
46]. None of the studies found an association with PCa mortality and none of the 5ARIs are approved as chemoprevention.
9.3.2. Statins
Statins are lipid-regulating agents that can decrease total cholesterol, low-density lipoprotein and triglycerides. Studies have associated high cholesterol with an increased incidence of high-risk PCa and since cholesterol is the precursor of androgen, it could affect androgen signaling pathways [
47]; however, the effect of statin usage on the risk of PCa is unclear. The most recent pooled analysis of 41 studies did not associate statin usage with PCa incidence (RR 0.87; 95% CI 0.82, 1.08). However, when higher doses and longer times of use were considered, statins were associated with a lower risk of PCa [
47].
9.3.3. Vitamin D
Vitamin D deficiency has been suggested to be associated with an increased risk of cancer, and supplementation of vitamin D has been associated with a decrease in PCa risk in experimental models [
48]. Furthermore, both deficiency and insufficiency have been associated with adverse pathology following radical prostatectomy [
49,
50].
9.3.4. Vitamin E
Vitamin E is a fat-soluble micronutrient with antioxidant effects and, to date, the data on its role in PCa are controversial. The most recent meta-analysis on this subject evaluated the effect of dietary and supplemental vitamin E intake on PCa and found non-significant results, except for a reduction in PCa risk in studies in Europe in a subgroup analysis [
51].
10. Conclusions
Prostate cancer ranks as the second most common cancer in men worldwide, with over 1.4 million new cases reported in 2022 and an anticipated prevalence exceeding 5 million by 2027. Incidence rates vary significantly across continents, with the highest rates observed in Northern America and Oceania, and the lowest in Asia. Geographical variability in PCa incidence can be attributed to a combination of genetic predisposition, screening practices, healthcare accessibility, and lifestyle factors. Socioeconomic status plays a pivotal role, with lower socioeconomic groups exhibiting higher mortality rates.
Established non-modifiable risk factors include familial history, age, and race, while modifiable factors comprise diet, physical activity, alcohol consumption, and smoking habits. Certain medications such as 5-alpha reductase inhibitors and statins, as well as dietary supplements such as vitamin D, demonstrate potential in reducing PCa risk. Ongoing research endeavors are imperative for a more comprehensive understanding of PCa risk factors and the development of effective preventive strategies on a global scale.
To address the growing global burden of prostate cancer, healthcare strategies should prioritize equitable access to early detection and treatment, particularly in low- and middle-income countries where mortality rates are disproportionately high. Public health campaigns must focus on raising awareness about modifiable risk factors, such as promoting healthy dietary patterns, increasing physical activity, and reducing smoking and alcohol consumption. Additionally, targeted interventions, including the integration of cost-effective screening programs like PSA testing in high-risk populations, can significantly improve early diagnosis. Future research should explore the genetic and environmental determinants of prostate cancer, with an emphasis on understanding disparities in incidence and outcomes. Longitudinal studies assessing the efficacy of preventive pharmacological agents and dietary supplements in diverse populations are crucial to inform evidence-based guidelines and tailored prevention strategies.
Author Contributions
Conceptualization, all authors; methodology, all authors; investigation, all authors; data curation, B.V.L.A.M., K.R.P., K.M., S.V.C.; writing—original draft preparation, B.V.L.A.M.; writing—review and editing, all authors.; visualization, B.V.L.A.M., S.V.C.; supervision, O.B., S.V.C.; funding acquisition, B.V.L.A.M., S.V.C. All authors have read and agreed to the published version of the manuscript.
Funding
The author’s work was supported in part by funding from the National Institutes of Health/National Cancer Institute (P30-CA008748) and from the CAPES Foundation (CAPES-PRINT—88887.835383/2023-00).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data is available for this manuscript.
Conflicts of Interest
SVC has received an honorarium and travel reimbursement from Ipsen, and has served on an advisory board for Prostatype Genomics, unrelated to the present manuscript. All other authors declare no conflict of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.fr/tomorrow (accessed on 13 February 2024).
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020, 8, 49–54. [Google Scholar] [CrossRef]
- Zhai, Z.; Zheng, Y.; Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Hao, Q.; Song, D.; Wu, Y.; et al. Incidence and disease burden of prostate cancer from 1990 to 2017: Results from the Global Burden of Disease Study 2017. Cancer 2020, 126, 1969–1978. [Google Scholar] [CrossRef]
- Zhu, Y.; Mo, M.; Wei, Y.; Wu, J.; Pan, J.; Freedland, S.J.; Zheng, Y.; Ye, D. Epidemiology and genomics of prostate cancer in Asian men. Nat. Rev. Urol. 2021, 18, 282–301. [Google Scholar] [CrossRef]
- Schafer, E.J.; Jemal, A.; Wiese, D.; Sung, H.; Kratzer, T.B.; Islami, F.; Dahut, W.L.; Knudsen, K.E. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur. Urol. 2023, 84, 117–126. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Reis, R.B.D.; Alías-Melgar, A.; Martínez-Cornelio, A.; Neciosup, S.P.; Sade, J.P.; Santos, M.; Villoldo, G.M. Prostate Cancer in Latin America: Challenges and Recommendations. Cancer Control 2020, 27, 1073274820915720. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Matti, B.; Chapman, D.; Zargar-Shoshtari, K. Ethnic and regional differences in the temporal trends of prostate cancer incidence and mortality in New Zealand. ANZ J. Surg. 2021, 91, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; O’Connell, D.L.; Yu, X.Q.; Kahn, C.; Caruana, M.; Pesola, F.; Sasieni, P.; Grogan, P.B.; Aranda, S.; Cabasag, C.J.; et al. Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: A statistical modelling study. Lancet Public Health 2022, 7, e537–e548. [Google Scholar] [CrossRef] [PubMed]
- Te Marvelde, L.; Milne, R.L.; Hornby, C.J.; Chapman, A.B.; Giles, G.G.; Haines, I.E. Differences in treatment choices for localised prostate cancer diagnosed in private and public health services. Med. J. Aust. 2020, 213, 411–417. [Google Scholar] [CrossRef]
- Godtman, R.A.; Kollberg, K.S.; Pihl, C.G.; Månsson, M.; Hugosson, J. The Association Between Age, Prostate Cancer Risk, and Higher Gleason Score in a Long-term Screening Program: Results from the Göteborg-1 Prostate Cancer Screening Trial. Eur. Urol. 2022, 82, 311–317. [Google Scholar] [CrossRef]
- Carlsson, S.V.; Arnsrud Godtman, R.; Pihl, C.G.; Vickers, A.; Lilja, H.; Hugosson, J.; Månsson, M. Young Age on Starting Prostate-specific Antigen Testing Is Associated with a Greater Reduction in Prostate Cancer Mortality: 24-Year Follow-up of the Göteborg Randomized Population-based Prostate Cancer Screening Trial. Eur. Urol. 2023, 83, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.; Satturwar, S.; Van der Kwast, T. Young-age prostate cancer. J. Clin. Pathol. 2015, 68, 511–515. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Rohrmann, S. Risk factors for the onset of prostatic cancer: Age, location, and behavioral correlates. Clin. Epidemiol. 2012, 4, 1–11. [Google Scholar] [CrossRef]
- Johns, L.E.; Houlston, R.S. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int. 2003, 91, 789–794. [Google Scholar] [CrossRef]
- Bratt, O.; Drevin, L.; Akre, O.; Garmo, H.; Stattin, P. Family History and Probability of Prostate Cancer, Differentiated by Risk Category: A Nationwide Population-Based Study. J. Natl. Cancer Inst. 2016, 108, djw110. [Google Scholar] [CrossRef]
- Hemminki, K.; Sundquist, J.; Brandt, A. Familial mortality and familial incidence in cancer. J. Clin. Oncol. 2011, 29, 712–718. [Google Scholar] [CrossRef]
- Ren, Z.J.; Cao, D.H.; Zhang, Q.; Ren, P.W.; Liu, L.R.; Wei, Q.; Wei, W.R.; Dong, Q. First-degree family history of breast cancer is associated with prostate cancer risk: A systematic review and meta-analysis. BMC Cancer 2019, 19, 871. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. Prostate Cancer Incidence and Mortality in Relationship to Family History of Prostate Cancer; Findings From The PLCO Trial. Clin. Genitourin. Cancer 2019, 17, e837–e844. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, M.; Muller, A.; Carlsson, S.; Eberli, D.; Huber, A.; Grobholz, R.; Manka, L.; Mortezavi, A.; Sulser, T.; Recker, F.; et al. A positive family history as a risk factor for prostate cancer in a population-based study with organised prostate-specific antigen screening: Results of the Swiss European Randomised Study of Screening for Prostate Cancer (ERSPC, Aarau). BJU Int. 2016, 117, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Saarimaki, L.; Tammela, T.L.; Maattanen, L.; Taari, K.; Kujala, P.M.; Raitanen, J.; Auvinen, A. Family history in the Finnish Prostate Cancer Screening Trial. Int. J. Cancer 2015, 136, 2172–2177. [Google Scholar] [CrossRef]
- Urabe, F.; Kimura, S.; Yamamoto, S.; Tashiro, K.; Kimura, T.; Egawa, S. Impact of family history on oncological outcomes in primary therapy for localized prostate cancer patients: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021, 24, 638–646. [Google Scholar] [CrossRef]
- Telang, J.M.; Lane, B.R.; Cher, M.L.; Miller, D.C.; Dupree, J.M. Prostate cancer family history and eligibility for active surveillance: A systematic review of the literature. BJU Int. 2017, 120, 464–467. [Google Scholar] [CrossRef]
- Diallo, A.; Deschasaux, M.; Latino-Martel, P.; Hercberg, S.; Galan, P.; Fassier, P.; Allès, B.; Guéraud, F.; Pierre, F.H.; Touvier, M. Red and processed meat intake and cancer risk: Results from the prospective NutriNet-Santé cohort study. Int. J. Cancer 2018, 142, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Peng, Y.; Qiao, Y.; Huang, Y.; Song, F.; Zhang, M. Consumption of flavonoids and risk of hormone-related cancers: A systematic review and meta-analysis of observational studies. Nutr. J. 2022, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Ziglioli, F.; Patera, A.; Isgrò, G.; Campobasso, D.; Guarino, G.; Maestroni, U. Impact of modifiable lifestyle risk factors for prostate cancer prevention: A review of the literature. Front. Oncol. 2023, 13, 1203791. [Google Scholar] [CrossRef]
- Dai, Y.N.; Yi-Wen Yu, E.; Zeegers, M.P.; Wesselius, A. The Association between Dietary Inflammatory Potential and Urologic Cancers: A Meta-analysis. Adv. Nutr. 2024, 15, 100124. [Google Scholar] [CrossRef]
- Alexander, D.D.; Mink, P.J.; Cushing, C.A.; Sceurman, B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr. J. 2010, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Bylsma, L.C.; Alexander, D.D. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr. J. 2015, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Majd, S.; Salari-Moghaddam, A.; Aminianfar, A.; Larijani, B.; Esmaillzadeh, A. Association Between Red and Processed Meat Consumption and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 801722. [Google Scholar] [CrossRef]
- Benke, I.N.; Leitzmann, M.F.; Behrens, G.; Schmid, D. Physical activity in relation to risk of prostate cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1154–1179. [Google Scholar] [CrossRef] [PubMed]
- Hamblen, A.J.; Bray, J.W.; Hingorani, M.; Saxton, J.M. Physical activity and dietary considerations for prostate cancer patients: Future research directions. Proc. Nutr. Soc. 2023, 82, 298–304. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Cancer Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic Drinks and the Risk of Cancer. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Alcoholic-Drinks.pdf (accessed on 31 January 2025).
- Rohrmann, S.; Linseisen, J.; Key, T.J.; Jensen, M.K.; Overvad, K.; Johnsen, N.F.; Tjønneland, A.; Kaaks, R.; Bergmann, M.M.; Weikert, C.; et al. Alcohol consumption and the risk for prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- D’Ecclesiis, O.; Pastore, E.; Gandini, S.; Caini, S.; Marvaso, G.; Jereczek-Fossa, B.A.; Corrao, G.; Raimondi, S.; Bellerba, F.; Ciceri, S.; et al. Association between Alcohol Intake and Prostate Cancer Mortality and Survival. Nutrients 2023, 15, 925. [Google Scholar] [CrossRef]
- Macke, A.J.; Petrosyan, A. Alcohol and Prostate Cancer: Time to Draw Conclusions. Biomolecules 2022, 12, 375. [Google Scholar] [CrossRef]
- Huncharek, M.; Haddock, K.S.; Reid, R.; Kupelnick, B. Smoking as a risk factor for prostate cancer: A meta-analysis of 24 prospective cohort studies. Am. J. Public Health 2010, 100, 693–701. [Google Scholar] [CrossRef]
- Al-Fayez, S.; El-Metwally, A. Cigarette smoking and prostate cancer: A systematic review and meta-analysis of prospective cohort studies. Tob. Induc. Dis. 2023, 21, 19. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Zhang, S.; Chen, X.; Sheng, Y.; Pang, J. Association of cigarette smoking habits with the risk of prostate cancer: A systematic review and meta-analysis. BMC Public Health 2023, 23, 1150. [Google Scholar] [CrossRef] [PubMed]
- Rider, J.R.; Wilson, K.M.; Sinnott, J.A.; Kelly, R.S.; Mucci, L.A.; Giovannucci, E.L. Ejaculation Frequency and Risk of Prostate Cancer: Updated Results with an Additional Decade of Follow-up. Eur. Urol. 2016, 70, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.H.; Yang, Z.Q.; Shao, Y.X.; Yang, W.X.; Li, X. Association of 5-alpha-reductase inhibitor and prostate cancer incidence and mortality: A meta-analysis. Transl. Androl. Urol. 2020, 9, 2519–2532. [Google Scholar] [CrossRef]
- Knijnik, P.G.; Brum, P.W.; Cachoeira, E.T.; Paludo, A.O.; Gorgen, A.R.H.; Burttet, L.M.; Neyeloff, J.L.; Neto, B.S. The impact of 5-alpha-reductase inhibitors on mortality in a prostate cancer chemoprevention setting: A meta-analysis. World J. Urol. 2021, 39, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; An, Y.; Liu, C.Q.; Xu, J.Z.; Zhong, X.Y.; Zeng, N.; Sun, J.X.; Xia, Q.D.; Wang, S.G. Association of Statin Use with the Risk of Incident Prostate Cancer: A Meta-Analysis and Systematic Review. J. Oncol. 2022, 2022, 7827821. [Google Scholar] [CrossRef]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef]
- Nyame, Y.A.; Murphy, A.B.; Bowen, D.K.; Jordan, G.; Batai, K.; Dixon, M.; Hollowell, C.M.; Kielb, S.; Meeks, J.J.; Gann, P.H.; et al. Associations Between Serum Vitamin D and Adverse Pathology in Men Undergoing Radical Prostatectomy. J. Clin. Oncol. 2016, 34, 1345–1349. [Google Scholar] [CrossRef]
- Kristal, A.R.; Till, C.; Song, X.; Tangen, C.M.; Goodman, P.J.; Neuhauser, M.L.; Schenk, J.M.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; et al. Plasma vitamin D and prostate cancer risk: Results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1494–1504. [Google Scholar] [CrossRef]
- Loh, W.Q.; Youn, J.; Seow, W.J. Vitamin E Intake and Risk of Prostate Cancer: A Meta-Analysis. Nutrients 2022, 15, 14. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).