1. Introduction
Radical prostatectomy (RP) has been the “gold standard” surgical treatment of localized prostate cancer, dating back to the first perineal prostatectomy described by Dr. Hugh Young in 1905 [
1]. For much of the 20th century, RP was considered a highly morbid operation marred by significant blood loss, poor visualization, as well as high rates of impotence and urinary incontinence [
2,
3]. In 1979, landmark anatomical studies by Dr. Walsh and colleagues provided a more detailed understanding of the prostate and its surrounding structures. They introduced surgical refinements, such as ligation of the dorsal vein complex (DVC) or nerve-sparing techniques, that drastically improved intraoperative blood loss and postoperative outcomes of retropubic prostatectomy. Towards the end of the 20th century, the advent of laparoscopy brought forth the next significant advance in RP, after which robotic instrumentation with the daVinci Surgical System enabled surgeons to overcome the challenging technical learning curve to laparoscopic RP. In 2000, the first robotic-assisted radical prostatectomy (RARP) was performed, and now RARP makes up 90% of all prostatectomies performed globally today [
4,
5,
6]. Compared to all other approaches, RARP offers significant advantages, including reduced blood loss, pain, and shorter hospital stays [
7].
Since its conception, several techniques for performing RARP have been proposed. The anterior, transperitoneal approach has become the standard of care approach for RARP. However, novel techniques such as the pelvic fascia-sparing technique and the Hood technique, aimed at minimizing damage to the periprostatic structures, haved gained popularity [
5,
8]. Single-port robotic systems have also been adopted, expanding potential approaches (i.e, transperitoneal, perineal, transvesical) and reducing incisional morbidity while retaining the established benefits of RARP [
9]. Since pelvic anatomy remains complex and widely variable, the preferred technique to maximize oncologic and functional outcomes when performing RARP has yet to be determined.
This review was adapted from a comprehensive committee chapter published in the 3rd World Urologic Oncology Federation (WUOF)/Societe International d’Urologie (SIU) International Consultation on Urologic Diseases on Localized Prostate Cancer in 2024. Authored by a panel of international experts in robotic prostatectomy, the original chapter offers an extensive overview of surgical consideration in this field. Here, we present a concise summary focused on both traditional and emerging techniques in RARP, along with a synthesis of their respective clinical outcomes.
2. Traditional Robotic Prostatectomy Techniques
2.1. Transperitoneal Multiport Robotic Prostatectomy
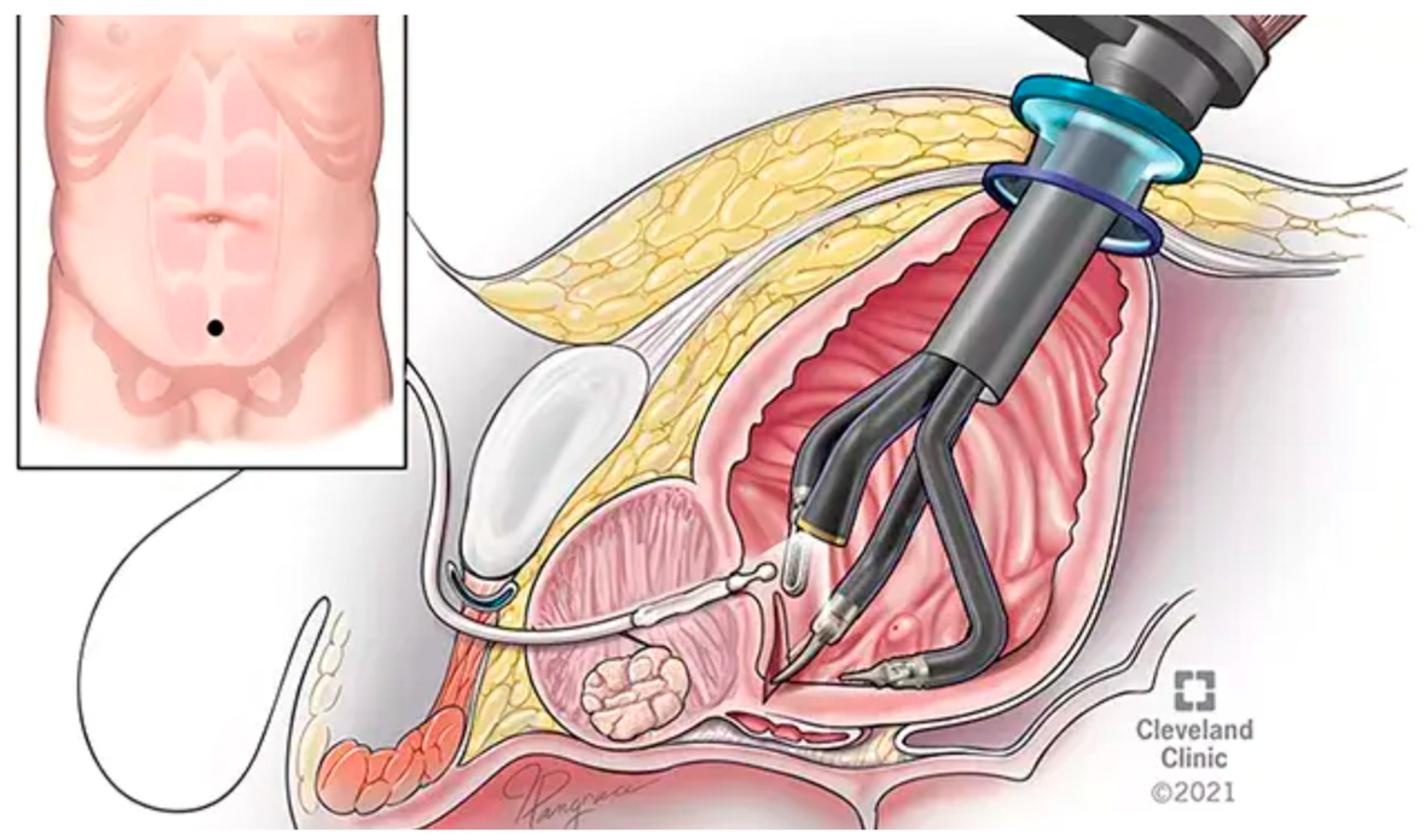
The standard approach to RARP (S-RARP) is the anterior transperitoneal approach, which involves releasing the bladder from its abdominal wall attachments by incising the anterior parietal peritoneum, thus entering the retropubic space. The bladder neck is divided to posteriorly access the vasa deferentia and seminal vesicles. This anterior approach necessitates the transection of the anterior detrusor apron, puboprostatic ligaments, and the DVC (
Figure 1) [
5]. The Montsouris technique is another commonly used modification of standard RARP, which involves a posterior/anterior approach. The dissection begins posteriorly via a low peritoneal retrovesical incision to isolate the vasa deferentia and the seminal vesicles before proceeding to the standard anterior approach [
10].
2.2. Extraperitoneal Multiport RARP
An extraperitoneal approach to RP was first performed laparoscopically in 1997 and quickly adapted to a robotic platform in the early 2000s [
11]. Today, extraperitoneal RARP is less commonly utilized. Similarly to an open, retropubic RP, the retropubic space is entered through an infraumbilical skin incision. A balloon dilator is introduced through the incision to create a preperitoneal space anterior to the bladder (
Figure 2). Once this working space is established, the remaining robotic trocars are placed without entering the peritoneal cavity. The subsequent surgical steps can proceed similarly to a retropubic RP or standard transperitoneal RARP. A key difference in this approach is the identification and dissection of the vasa deferentia and seminal vesicles, which are approached after transecting the posterior bladder neck [
12].
2.3. Outcomes of Traditional Robotic Prostatectomy
Compared to open or laparoscopic RP, RARP has demonstrated a clear advantage regarding perioperative outcomes such as blood loss, pain, and hospital length of stay [
7,
13]. Data on functional outcomes after RARP have been somewhat inconsistent due to variability in surgeon experience, outcome assessment tools, and patient populations. A large cohort study by Wu et al. that studied 1407 patients who underwent open retropubic RP (RRP), laparoscopic RP (LRP), and RARP showed that men who underwent RARP had several notable benefits. RARP patients had lower odds of erectile dysfunction 3 years after surgery, with an adjusted odds ratio of 0.74 compared to RRP and 0.60 compared to LRP. They also had lower odds of urinary incontinence, with an adjusted odds ratio of 0.93 compared to RRP and 0.60 compared to LRP [
14]. These outcomes are consistent with other studies, such as those by Ficarra et al. and Kowalczyk et al., which reported incontinence rates ranging from 4% to 31% and erectile dysfunction rates between 10% and 46% 1 year after RARP [
15,
16].
Another long-term study compared outcomes of open RRP and RARP in 4003 men with localized prostate cancer over an 8-year follow-up. At 8 years post-surgery, urinary incontinence rates were similar between groups, however erectile dysfunction was significantly lower in the RARP group, with 66% of patients reporting dysfunction compared to 70% in the RRP group (aRR 0.93, 95% CI 0.87–0.99). Notably, prostate cancer-specific mortality was also found to be lower in the RARP group. In high-risk patients, RARP was associated with better oncological outcomes, including lower rates of positive surgical margins (21% vs. 34%), biochemical recurrence (51% vs. 69%), and prostate cancer-specific mortality (11/77 vs. 14/220). These findings suggest comparable long-term functional outcomes among groups yet improved oncologic control in RARP, particularly for high-risk patients [
17].
Studies comparing extraperitoneal to standard transperitoneal RARP are mostly limited to single-surgeon or single-center experiences. Chung et al. found the extraperitoneal approach was associated with significantly shorter console time, lower postoperative pain scores, and decreased incidence of ileus and hernia compared to transperitoneal RARP. Functional and oncologic outcomes were similar between groups [
18]. Given the lack of high-level evidence showing the superiority of one traditional robotic approach over the other, either technique is acceptable, and surgeons should continue with the approach they are most familiar with.
3. Reconstruction Techniques in Traditional Prostatectomy
Despite improvements in perioperative outcomes associated with robotic surgery, there remains significant morbidity related to RARP, with urinary incontinence and impotence rates cited to be as high as 20% and 70%, respectively [
17,
19]. Greater insight into the function of specific pelvic structures has identified key reconstructive steps to restore functional relationships following RP. Techniques like bladder neck preservation, urethral length preservation, and various reconstructive maneuvers have been adapted to improve early urinary continence outcomes and sexual function postoperatively.
3.1. Bladder Neck Preservation
Bladder neck preservation (BNP) via meticulous dissection at the prostato–vesical junction during RARP has been associated with an earlier return of urinary continence [
20]. Freire et al. retrospectively compared 348 men undergoing BNP with 271 undergoing standard RARP and noted significantly improved urinary function with BNP at 4 and 24 months. Urinary continence was also considerably enhanced at 4 months (65.6 vs. 26.5%;
p < 0.001). Notably, there was no compromise in surgical margins when performing BNP, as suggested by older RRP and LRP series. Moreover, this series’ update demonstrated earlier continence recovery throughout the 2-year follow-up interval and no differences in prostate-specific antigen (PSA) recurrence-free survival up to 5 years following RARP. Other groups have reported similar results as well [
21,
22].
3.2. Urethral Preservation and Reconstruction
Various surgical techniques have been studied to improve postoperative continence outcomes by reconstructing the anterior and posterior periurethral structures during RP. As described by Patel et al., a simple technique involves a periurethral suspension stitch (PSS), performed by reversing the needle after ligation of the DVC and passing it through the perichondrium of the pubic symphysis. This maneuver can help control venous bleeding and mimic the function of puboprostatic ligaments, providing anterior urethral support. In their nonrandomized trial, Patel and colleagues showed earlier return of continence and higher continence rates at 3 months in men who underwent PSS, whereas similar urinary continence was observed at 1, 6, and 12 months. Therefore, although PSS may help immediate urinary continence recovery, it does not appear to affect long-term urinary continence [
20,
23].
A technique targeting posterior periurethral tissues was proposed by Rocco et al. during RRP and later adapted to RARP. This technique reconstructs the posterior musculofascial plate by suturing the median raphe of the urethra to the remnants of Denonvilliers’ fascia posterior to the bladder before urethrovesical anastomosis. Theoretically, this stitch restores the anatomical length of the rhabdosphincter and provides posterior support by fixing the structure into its natural position [
20]. Continence outcomes following posterior reconstruction (PR) have been mixed in the era of RARP. Coelho et al. and Nguyen et al. showed early return of continence in patients undergoing PR during RARP at 4 and 6 weeks, respectively [
24,
25]. However, Nguyen et al. found no difference in continence rates at 3 and 6 months. Evidence from several randomized controlled trials failed to show the effectiveness of PR during RARP in improving postoperative continence rates [
7,
20].
3.3. Nerve Sparing
Several techniques to preserve the neurovascular bundle (NVB) have been described. The Comparative Effectiveness Analysis of Surgery and Radiation (CEASAR) study highlighted significant improvements in functional outcomes for patients undergoing bilateral nerve-sparing (NS) prostatectomies compared to unilateral NS. This was confirmed through multivariate analysis, which showed that bilateral NS was associated with better overall urinary function scores and continence at 12 months postoperatively (
p ≤ 0.035) [
26,
27]. Additionally, studies have shown that both erectile function and urinary control are improved if the prostate pedicles and seminal vesicles are dissected athermally (using clips or sharp division), since both are closely intertwined with the NVB [
27].
4. Novel Robotic Prostatectomy Techniques
Innovation in the robotic era has focused on achieving oncologically safe prostate removal with minimal disruption to adjacent anatomical structures, thereby maximizing functional outcomes.
4.1. Pelvic Fascia-Sparing Prostatectomy
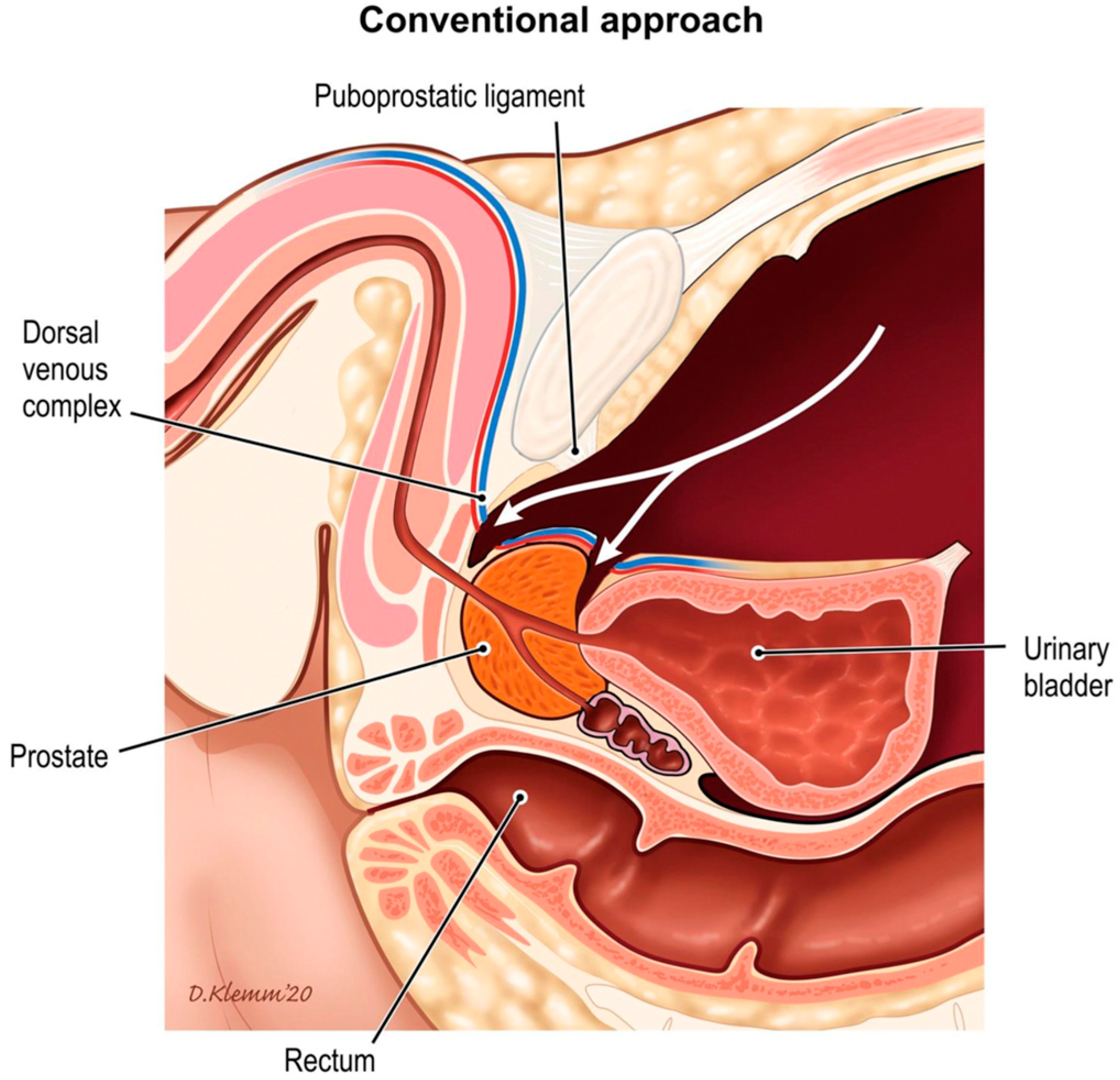
A novel robotic approach to prostatectomy was introduced in 2010 by Aldo Bocciardi and colleagues, which allowed natural preservation of the pelvic fascial anatomy without a need for reconstruction. This technique was named the pelvic fascia-paring, or Retzius-sparing, robotic-assisted radical prostatectomy (PFS-RARP) [
28]. This technique approaches the prostate in an antegrade fashion from the rectovesical pouch, first dissecting the seminal vesicles and subsequently progressing caudally behind the gland (
Figure 3). PFS-RARP aims to preserve all the anterior structures that may play a role in the mechanisms of continence and erection, particularly the puboprostatic ligaments, endopelvic fascia, detrusor apron, and anterolateral extension of the NVB.
The puboprostatic and pubovesical ligaments are thought to stabilize and support the bladder and urethra via suspension from the pubic bone. Leaving them untouched during RARP helps maintain the normal position of the bladder neck relative to the pubic symphysis. This anatomical support is crucial for maintaining continence as it reduces the pressure on the urethra during activities that increase intra-abdominal pressure, such as coughing or lifting, thereby minimizing the risk of stress urinary incontinence [
3,
5].
An increasing body of literature comparing S-RARP with PFS-RARP regarding surgical, oncological, and functional outcomes has become available. Robust recent pooled data from more than 1100 patients did not show significant differences between PFS- RARP and S-RARP regarding blood loss, operative time, complications, and hospital length of stay [
29]. Regarding urinary function, the current literature reports rates of immediate continence recovery up to 70% and 1-year continence recovery up to 95% [
30,
31,
32,
33,
34] following PFS-RARP. Data on erectile function are scarcer; only four studies including erectile function after radical prostatectomy are among the outcomes of interest [
34,
35,
36]. Despite heterogeneity in scoring systems (e.g., Sexual Health Inventory for Men (SHIM), International Index of Erectile Function (IIEF-5), Expanded Prostate cancer Index Composite (EPIC) score), all concluded the absence of substantial difference in potency recovery after PFS-RARP versus standard anterior RARP. PFS-RARP has been criticized with regards to its oncologic safety. Studies have shown a trend towards higher positive surgical margins (PSMs), although PFS-RARP did not reach the independent predictor status for increased PSMs [
37,
38,
39]. More robust conclusions will only be achieved once well-designed randomized clinical trials regarding PFS-RARP versus S-RARP are performed, such as the PARTIAL trial [
40].
4.2. Hood Technique RARP
Despite better PFS-RARP immediate urinary continence outcomes after catheter removal, many surgeons continue to prefer robotic prostatectomy using the standard anterior technique. Learning from the anatomical lessons of PFS-RARP, several surgeons have developed new surgical strategies to spare the puboprostatic ligaments, endopelvic fascia, and other anterior structures using an anterior standard approach [
41,
42,
43]. The “Hood Technique”, or anterior pelvic fascia-sparing (APFS) robotic prostatectomy, was first described by Tewari et al. For this approach, the bladder is dropped and the retropubic space is entered in standard fashion; however, the detrusor apron, arcus tendinous, puboprostatic ligaments, anterior vessels, and some detrusor muscle fibers are preserved (
Figure 4) [
42]. These structures create the appearance of a “hood”, which surrounds and safeguards the membranous urethra, external sphincter, and supportive structures.
Tewari et al. described the outcomes of this technique among 330 consecutive patients. Patients were followed for 2 years with continence defined as entirely pad-free. In the 300 patients who underwent the APFS technique, continence was 21%, 36%, 83%, 88%, 91%, and 94% at 1, 2, 4, 6, 12, and 24 weeks following catheter removal, respectively. The 30 patients who underwent RARP without APFS had respective continence rates of 12%, 22%, 76%, 85%, 86%, and 88%, highlighting the earlier return of urinary continence with this technique [
44]. Additional research and analysis are needed to better characterize the early return of urinary function and ensure adequate cancer control with the Hood/APFS technique. Additionally, there has been no direct comparison of APFS with PFS- RARP as of this publication.
4.3. Single-Port RARP
RARP is traditionally performed using a multiport (MP) system; the recent introduction of the novel purpose-built Single-Port (SP) robotic platform (Intuitive Surgical; Sunnyvale, CA, USA) has opened a new frontier in the minimally invasive surgical landscape of prostate cancer. The SP platform offers several unique features, including the narrow profile of a single robotic arm with four instrument drives that can simultaneously accommodate one double-jointed endoscopic camera and three robotic instruments with seven degrees of freedom, which allows for 360-degree anatomic access from a single pivot point. The movements of the endoscopic camera and the robotic instruments resemble human elbow and wrist movements, which aid intracorporeal triangulation and reduce the risk of instrument clashing [
45,
46,
47].
The SP platform’s maneuverability and ergonomics have proven helpful, especially when performing surgeries in shallow and more confined surgical working spaces. Hence, in addition to its utility for conventional RARP techniques, such as transperitoneal, extraperitoneal, and Retzius-sparing approaches, the SP platform has provided the unique opportunity for surgeons to develop more regionalized surgical approaches, with examples including transvesical and transperineal SP-RARP [
46,
47].
4.3.1. Transvesical Single-Port Prostatectomy
The transvesical single-port robotic-assisted radical prostatectomy (TV SP-RARP) was recently introduced by Kaouk et al. in 2020 [
48]. With TV SP-RARP, percutaneous bladder access is obtained via a suprapubic incision and insufflation is regionalized to involve only the bladder (
Figure 5). All surgical steps can be completed under direct vision from within the bladder without disrupting the supporting structures of the bladder and the extraperitoneal space [
48].
Several benefits of the transvesical approach have been recently demonstrated, including improved patient comfort, increasing rates of opioid-sparing, same-day discharges, earlier Foley catheter removal, and earlier return of urinary continence [
49]. The significantly improved continence outcomes, especially compared to multiport RARP, may be due to several anatomical factors. Of note, TV SP-RARP spares the retropubic space, allowing for maximal preservation of urethral length in addition to the anterior bladder attachments that hold the bladder and membranous urethra in their anatomical positions within the pelvis [
44,
49]. Furthermore, with a direct percutaneous entry into the bladder, the procedure has been an alternative for patients with previous abdominal surgeries or a frozen pelvis. The supine patient positioning has also allowed TV SP-RARP to be completed with patients awake under regional epidural anesthesia [
50].
Figure 5.
Sagittal view of transvesical single-port robotic-assisted radical prostatectomy (RARP) [
51].
Figure 5.
Sagittal view of transvesical single-port robotic-assisted radical prostatectomy (RARP) [
51].
4.3.2. Transperineal Single-Port Prostatectomy
The transperineal SP-RARP was first described by Lenfant et al. The patient is positioned in dorsal lithotomy with 10° Trendelenburg, and a semilunar incision is made between ischial tuberosities, followed by the dissection of the subcutaneous tissue and rectourethralis muscle. After the docking of the SP robot, dissection is carried posteriorly. Contrary to other radical prostatectomy approaches, the prostatic apex and urethra are dissected first, while bladder neck dissection is completed last. Although transperineal SP-RARP provides some advantages as an alternative surgical access for patients with previous abdominal surgeries or previous bladder injuries, the relatively steep learning curve of the surgery remains the most significant deterrent towards the broader adoption of the technique [
52].
5. Future Directions
The application of artificial intelligence (AI) in surgery together with the exponential increase in novel robotic systems (i.e., Hugo, Revo-I, Senhance, Versius) that aim to challenge the da Vinci monopoly signify a promising leap towards precision medicine in urology. Several AI-based tools are already being utilized to refine surgical techniques and decision making. For instance, AI-generated algorithms have been developed utilizing MRI to predict the pathological stage of prostate cancer with high sensitivity and specificity [
53]. Moreover, through image recognition and augmented reality (AR), AI is being developed to enhance the surgeon’s ability to visualize and preserve important anatomical structures in real time during RARP. For instance, Tanzi et al. were able to overlay 3D AR anatomical images of the urinary catether onto the surgeon’s console at the time of RARP, so that they could accurately excise additional tissue margins following removal of the prostate [
54]. AI-driven tools in RARP may enhance precision and reduce complications by helping surgeons identify critical anatomy. However, integrating AI into RARP raises ethical, legal, and regulatory considerations. Ensuring the privacy and security of patient data, maintaining transparency in AI’s decision-making processes, and developing clear guidelines for the clinical use of AI are paramount.
The journey from the origins of prostate surgery to contemporary robotic-assisted techniques underscores a profound shift toward precise, minimally invasive care. Advances in anatomical understanding, coupled with technological innovations, herald an exciting era in the treatment of prostate cancer, emphasizing the importance of balancing oncologic control with quality-of-life considerations.
Author Contributions
Conceptualization, K.J.K.; investigation, K.J.K. and B.M.-G.; resources, B.M.-G. and K.J.K.; writing—original draft preparation, B.M.-G.; writing—review and editing, B.M.-G. and K.J.K.; supervision, K.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to acknowledge all the remaining authors that contributed to the full-length version of this chapter in the 3rd WUOF/SIU International Consultation on Localized Prostate Cancer: Sylvia K. Alip; Antonio Galfano; Jim C. Hu; Jihad Kaouk; Koon Ho Rha; Nicolas A. Soputro; Oakley Strasser; Stefano Tappero.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RP | Radical prostatectomy |
| RRP | Retropubic radical prostatectomy |
| RARP | Robotic-assisted radical prostatectomy |
| DVC | Dorsal venous complex |
| RRP | Open retropubic radical prostatectomy |
| LRP | Laparoscopic radical prostatectomy |
| BNP | Bladder neck preservation |
| PSS | Periurethral suspension stitch |
| PR | Posterior reconstruction |
| NVB | Neurovascular bundle |
| NS | Nerve-sparing |
| PFS | Pelvic fascia-sparing |
| PSMs | Positive surgical margins |
| APFS | Anterior pelvic fascia-sparing |
| MP | Multiport |
| SP | Single-port |
| TV | Transvesical |
| AI | Artificial intelligence |
References
- Ercole, C.E.; Stephenson, A.J. Open Versus Robotic Prostatectomy. In Prostate Cancer; Academic Press: Cambridge, MA, USA, 2016; pp. 307–313. [Google Scholar]
- Holzbeierlein, J.M.; Langenstroer, P.; Porter Ii, H.J.; Thrasher, J.B. Case selection and outcome of radical perineal prostatectomy in localized prostate cancer. Int. Braz. J. Urol. 2003, 29, 291–299. [Google Scholar] [CrossRef]
- Reeves, F.; Everaerts, W.; Murphy, D.G.; Costello, A. Chapter 29—The Surgical Anatomy of the Prostate. In Prostate Cancer, 2nd ed.; Mydlo, J.H., Godec, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 253–263. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Xiong, Q.; Cheng, S. The progress of dorsal vascular complex control strategy in radical prostatectomy. J. Int. Med. Res. 2023, 51, 03000605231152091. [Google Scholar] [CrossRef]
- Davis, M.; Egan, J.; Marhamati, S.; Galfano, A.; Kowalczyk, K.J. Retzius-Sparing Robot-Assisted Robotic Prostatectomy: Past, Present, and Future. Urol. Clin. 2021, 48, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Cross, B.W. Hinman’s Atlas of Urologic Surgery, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2019; Available online: https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323655651000729 (accessed on 18 January 2025).
- Sood, A.; Jeong, W.; Peabody, J.O.; Hemal, A.K.; Menon, M. Robot-Assisted Radical Prostatectomy. Urol. Clin. N. Am. 2014, 41, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Rossanese, M.; Gilante, M.; Foti, M.; Macchione, L.; Mucciardi, G.; Martini, M.; Giannarini, G. Retzius-sparing vs. standard robot-assisted radical prostatectomy for clinically localised prostate cancer: A comparative study. Prostate Cancer Prostatic Dis. 2023, 26, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Kaouk, J.H.; Haber, G.P.; Autorino, R.; Crouzet, S.; Ouzzane, A.; Flamand, V.; Villers, A. A Novel Robotic System for Single-port Urologic Surgery: First Clinical Investigation. Eur. Urol. 2014, 66, 1033–1043. [Google Scholar] [CrossRef]
- Mann, M.; Lallas, C.D.; Trabulsi, E.J. Posterior Approach to Robotic-Assisted Laparoscopic Radical Prostatectomy. In Prostate Cancer; Academic Press: Cambridge, MA, USA, 2016; pp. 337–342. [Google Scholar]
- Gettman, M.T.; Hoznek, A.; Salomon, L.; Katz, R.; Borkowski, T.; Antiphon, P.; Lobontiu, A.; Abbou, C.-C. Laparoscopic radical prostatectomy: Description of the extraperitoneal approach using the da Vinci robotic system. J. Urol. 2003, 170, 416–419. [Google Scholar] [CrossRef]
- Pavlovich, C.P. The technique of robotic nerve sparing prostatectomy: Extraperitoneal approach. In Prostate Cancer; Academic Press: Cambridge, MA, USA, 2016; pp. 343–352. [Google Scholar]
- Kyriazis, I.; Spinos, T.; Tsaturyan, A.; Kallidonis, P.; Stolzenburg, J.U.; Liatsikos, E. Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes. Cancers 2022, 14, 1601. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chang, C.L.; Chen, C.I.; Huang, C.C. Comparison of Acute and Chronic Surgical Complications Following Robot-Assisted, Laparoscopic, and Traditional Open Radical Prostatectomy Among Men in Taiwan. JAMA Netw. Open 2021, 4, e2120156. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Ahlering, T.E.; Costello, A.; Eastham, J.A.; Graefen, M.; Guazzoni, G.; Menon, M.; Mottrie, A.; Patel, V.R.; et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 418–430. [Google Scholar] [CrossRef]
- Kowalczyk, K.J.; Huang, A.C.; Hevelone, N.D.; Lipsitz, S.R.; Yu, H.-Y.; Lynch, J.H.; Hu, J.C. Effect of minimizing tension during robotic-assisted laparoscopic radical prostatectomy on urinary function recovery. World J. Urol. 2013, 31, 515–521. [Google Scholar] [CrossRef]
- Lantz, A.; Bock, D.; Akre, O.; Angenete, E.; Bjartell, A.; Carlsson, S.; Modig, K.K.; Nyberg, M.; Kollberg, K.S.; Steineck, G.; et al. Functional and oncological outcomes after open versus robot-assisted laparoscopic radical prostatectomy for localised prostate cancer: 8-year follow-up. Eur. Urol. 2021, 80, 650–660. [Google Scholar] [CrossRef]
- Chung, J.S.; Kim, W.T.; Ham, W.S.; Yu, H.S.; Chae, Y.; Chung, S.H.; Choi, Y.D. Comparison of oncological results, functional outcomes, and complications for transperitoneal versus extraperitoneal robot-assisted radical prostatectomy: A single surgeon’s experience. J. Endourol. 2011, 25, 787–792. [Google Scholar] [CrossRef]
- Haglind, E.; Carlsson, S.; Stranne, J.; Wallerstedt, A.; Wilderäng, U.; Thorsteinsdottir, T.; Lagerkvist, M.; Damber, J.-E.; Bjartell, A.; Hugosson, J.; et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: A prospective, controlled, nonrandomised trial. Eur. Urol. 2015, 68, 216–225. [Google Scholar] [CrossRef]
- Vora, A.A.; Dajani, D.; Lynch, J.H.; Kowalczyk, K.J. Anatomic and technical considerations for optimizing recovery of urinary function during robotic-assisted radical prostatectomy. Curr. Opin. Urol. 2013, 23, 78. [Google Scholar] [CrossRef]
- Freire, M.P.; Weinberg, A.C.; Lei, Y.; Soukup, J.R.; Lipsitz, S.R.; Prasad, S.M.; Korkes, F.; Lin, T.; Hu, J.C. Anatomic bladder neck preservation during robotic-assisted laparoscopic radical prostatectomy: Description of technique and outcomes. Eur. Urol. 2009, 56, 972–980. [Google Scholar] [CrossRef]
- Friedlander, D.F.; Alemozaffar, M.; Hevelone, N.D.; Lipsitz, S.R.; Hu, J.C. Stepwise description and outcomes of bladder neck sparing during robot-assisted laparoscopic radical prostatectomy. J. Urol. 2012, 188, 1754–1760. [Google Scholar] [CrossRef]
- Patel, V.R.; Coelho, R.F.; Palmer, K.J.; Rocco, B. Periurethral Suspension Stitch During Robot-Assisted Laparoscopic Radical Prostatectomy: Description of the Technique and Continence Outcomes. Eur. Urol. 2009, 56, 472–478. [Google Scholar] [CrossRef]
- Coelho, R.F.; Chauhan, S.; Orvieto, M.A.; Sivaraman, A.; Palmer, K.J.; Coughlin, G.; Patel, V.R. Influence of modified posterior reconstruction of the rhabdosphincter on early recovery of continence and anastomotic leakage rates after robot-assisted radical prostatectomy. Eur. Urol. 2011, 59, 72–80. [Google Scholar] [CrossRef]
- Early Continence Outcomes of Posterior Musculofascial Plate Reconstruction During Robotic and Laparoscopic Prostatectomy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18190625/ (accessed on 10 January 2025).
- Kowalczyk, K.J.; Huang, A.C.; Hevelone, N.D.; Lipsitz, S.R.; Yu, H.-Y.; Ulmer, W.D.; Kaplan, J.R.; Patel, S.; Nguyen, P.L.; Hu, J.C. Stepwise approach for nerve sparing without countertraction during robot-assisted radical prostatectomy: Technique and outcomes. Eur. Urol. 2011, 60, 536–547. [Google Scholar] [CrossRef]
- Harbin, A.C.; Eun, D.D. Prostate Cancer: Science and Clinical Practice, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; Available online: https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780128000779000360 (accessed on 18 January 2025).
- Galfano, A.; Ascione, A.; Grimaldi, S.; Petralia, G.; Strada, E.; Bocciardi, A.M. A new anatomic approach for robot-assisted laparoscopic prostatectomy: A feasibility study for completely intrafascial surgery. Eur. Urol. 2010, 58, 457–461. [Google Scholar] [CrossRef]
- Barakat, B.; Othman, H.; Gauger, U.; Wolff, I.; Hadaschik, B.; Rehme, C. Retzius Sparing Radical Prostatectomy Versus Robot-assisted Radical Prostatectomy: Which Technique Is More Beneficial for Prostate Cancer Patients (MASTER Study)? A Systematic Review and Meta-analysis. Eur. Urol. Focus 2022, 8, 1060–1071. [Google Scholar] [CrossRef]
- Dalela, D.; Jeong, W.; Prasad, M.A.; Sood, A.; Abdollah, F.; Diaz, M.; Karabon, P.; Sammon, J.; Jamil, M.; Baize, B.; et al. A Pragmatic Randomized Controlled Trial Examining the Impact of the Retzius-sparing Approach on Early Urinary Continence Recovery After Robot-assisted Radical Prostatectomy. Eur. Urol. 2017, 72, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Dalela, D.; Jamil, M.; Diaz, M.; Tallman, C.; Abdollah, F.; Sood, A.; Lehtola, L.; Miller, D.; Jeong, W. Functional Recovery, Oncologic Outcomes and Postoperative Complications after Robot-Assisted Radical Prostatectomy: An Evidence-Based Analysis Comparing the Retzius Sparing and Standard Approaches. J. Urol. 2018, 199, 1210–1217. [Google Scholar] [CrossRef]
- Chang, L.W.; Hung, S.C.; Hu, J.C.; Chiu, K.Y. Retzius-sparing Robotic-assisted Radical Prostatectomy Associated with Less Bladder Neck Descent and Better Early Continence Outcome. Anticancer Res. 2018, 38, 345–351. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Y.; Chen, M.; Xu, L.; Guo, S.; Marra, G.; Rosenberg, J.E.; Ma, H.; Li, X.; Guo, H. Retzius-sparing robot-assisted radical prostatectomy improves early recovery of urinary continence: A randomized, controlled, single-blind trial with a 1-year follow-up. BJU Int. 2020, 126, 633–640. [Google Scholar] [CrossRef]
- Egan, J.; Marhamati, S.; Carvalho, F.L.F.; Davis, M.; O’neill, J.; Lee, H.; Lynch, J.H.; Hankins, R.A.; Hu, J.C.; Kowalczyk, K.J. Retzius-sparing Robot-assisted Radical Prostatectomy Leads to Durable Improvement in Urinary Function and Quality of Life Versus Standard Robot-assisted Radical Prostatectomy Without Compromise on Oncologic Efficacy: Single-surgeon Series and Step-by-step Guide. Eur. Urol. 2021, 79, 839–857. [Google Scholar] [CrossRef]
- Lim, S.K.; Kim, K.H.; Shin, T.Y.; Han, W.K.; Chung, B.H.; Hong, S.J.; Choi, Y.D.; Rha, K.H. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: Combining the best of retropubic and perineal approaches. BJU Int. 2014, 114, 236–244. [Google Scholar] [CrossRef]
- Umari, P.; Eden, C.; Cahill, D.; Rizzo, M.; Eden, D.; Sooriakumaran, P. Retzius-Sparing versus Standard Robot-Assisted Radical Prostatectomy: A Comparative Prospective Study of Nearly 500 Patients. J. Urol. 2021, 205, 780–790. [Google Scholar] [CrossRef]
- Sayyid, R.K.; Simpson, W.G.; Lu, C.; Terris, M.K.; Klaassen, Z.; Madi, R. Retzius-Sparing Robotic-Assisted Laparoscopic Radical Prostatectomy: A Safe Surgical Technique with Superior Continence Outcomes. J. Endourol. 2017, 31, 1244–1250. [Google Scholar] [CrossRef]
- Ota, Y.; Hamamoto, S.; Matsuyama, N.; Hamakawa, T.; Iwatsuki, S.; Etani, T.; Taguchi, K.; Naiki, T.; Ando, R.; Nakane, A.; et al. Pelvic Anatomical Features After Retzius-Sparing Robot-Assisted Radical Prostatectomy Intended for Early Recovery of Urinary Symptoms. J. Endourol. 2021, 35, 296–304. [Google Scholar] [CrossRef]
- Kowalczyk, K.J.; Madi, R.H.; Eden, C.G.; Sooriakumaran, P.; Fransis, K.; Raskin, Y.; Joniau, S.; Johnson, S.; Jacobsohn, K.; Galfano, A.; et al. Comparative Outcomes of Salvage Retzius-Sparing versus Standard Robotic Prostatectomy: An International, Multi-Surgeon Series. J. Urol. 2021, 206, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Stangl-Kremser, J.; Kowalczyk, K.; Schaeffer, E.M.; Allaf, M.; Scherr, D.; Yang, X.; Matoso, A.; Azumi, N.; Robinson, B.; Vickers, A.; et al. Study protocol for a prospective, multi-centered randomized controlled trial comparing pelvic fascia-sparing radical prostatectomy with conventional robotic-assisted prostatectomy: The PARTIAL trial. Contemp. Clin. Trials 2023, 128, 107168. [Google Scholar] [CrossRef]
- Kaouk, J.; Valero, R.; Sawczyn, G.; Garisto, J. Extraperitoneal single-port robot-assisted radical prostatectomy: Initial experience and description of technique. BJU Int. 2020, 125, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Wagaskar, V.G.; Mittal, A.; Sobotka, S.; Ratnani, P.; Lantz, A.; Falagario, U.G.; Martini, A.; Dovey, Z.; Treacy, P.-J.; Pathak, P.; et al. Hood Technique for Robotic Radical Prostatectomy—Preserving Periurethral Anatomical Structures in the Space of Retzius and Sparing the Pouch of Douglas, Enabling Early Return of Continence Without Compromising Surgical Margin Rates. Eur. Urol. 2021, 80, 213–221. [Google Scholar] [CrossRef]
- Cochetti, G.; Boni, A.; Barillaro, F.; Pohja, S.; Cirocchi, R.; Mearini, E. Full Neurovascular Sparing Extraperitoneal Robotic Radical Prostatectomy: Our Experience with PERUSIA Technique. J. Endourol. 2017, 31, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Jhaveri, J.; Rao, S.; Yadav, R.; Bartsch, G.; Te, A.; Ioffe, E.; Pineda, M.; Mudaliar, S.; Nguyen, L.; et al. Total reconstruction of the vesico-urethral junction. BJU Int. 2008, 101, 871–877. [Google Scholar] [CrossRef]
- Kaouk, J.; Bertolo, R.; Eltemamy, M.; Garisto, J. Single-Port Robot-Assisted Radical Prostatectomy: First Clinical Experience Using The SP Surgical System. Urology 2019, 124, 309. [Google Scholar] [CrossRef]
- Kaouk, J.; Aminsharifi, A.; Sawczyn, G.; Kim, S.; Wilson, C.A.; Garisto, J.; Fareed, K. Single-Port Robotic Urological Surgery Using Purpose-Built Single-Port Surgical System: Single-Institutional Experience with the First 100 Cases. Urology 2020, 140, 77–84. [Google Scholar] [CrossRef]
- Kaouk, J.; Garisto, J.; Bertolo, R. Robotic Urologic Surgical Interventions Performed with the Single Port Dedicated Platform: First Clinical Investigation. Eur. Urol. 2019, 75, 684–691. [Google Scholar] [CrossRef]
- Kaouk, J.; Beksac, A.T.; Zeinab, M.A.; Duncan, A.; Schwen, Z.R.; Eltemamy, M. Single Port Transvesical Robotic Radical Prostatectomy: Initial Clinical Experience and Description of Technique. Urology 2021, 155, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Carpinteyro, R.; Ferguson, E.L.; Chavali, J.S.; Geskin, A.; Kaouk, J. First 100 cases of transvesical single-port robotic radical prostatectomy. Asian J. Urol. 2023, 10, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Kaouk, J.; Ferguson, E.; Ramos-Carpinteyro, R.; Chavali, J.; Geskin, A.; Cummings, K.C.; Perilla, M. Transvesical Percutaneous Access Allows for Epidural Anesthesia Without Mechanical Ventilation in Single-Port Robotic Radical and Simple Prostatectomy. Urology 2023, 175, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Soputro, N.A.; Kaouk, J. Single-port robot-assisted radical prostatectomy. World J. Urol. 2024, 42, 245. [Google Scholar] [CrossRef]
- Lenfant, L.; Garisto, J.; Sawczyn, G.; Wilson, C.A.; Aminsharifi, A.; Kim, S.; Schwen, Z.; Bertolo, R.; Kaouk, J. Robot-assisted Radical Prostatectomy Using Single-port Perineal Approach: Technique and Single-surgeon Matched-paired Comparative Outcomes. Eur. Urol. 2021, 79, 384–392. [Google Scholar] [CrossRef]
- Moglia, A.; Georgiou, K.; Georgiou, E.; Satava, R.M.; Cuschieri, A. A systematic review on artificial intelligence in robot-assisted surgery. Int. J. Surg. 2021, 95, 106151. [Google Scholar] [CrossRef]
- Tanzi, L.; Piazzolla, P.; Porpiglia, F.; Vezzetti, E. Real-time deep learning semantic segmentation during intra-operative surgery for 3D augmented reality assistance. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 1435–1445. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).