Abstract
Background/Objectives: Prostate cancer is the most common cancer among men globally and a leading cause of cancer-related death. Germline genetic evaluation is increasingly recognized as essential for men with high-risk features such as a strong family history or advanced disease. Methods: Comprehensive genetic risk assessment should integrate three components: family history (FH), rare pathogenic mutations (RPMs), and polygenic risk scores (PRS). RPMs in DNA repair genes (e.g., BRCA2, CHEK2, ATM) can inform screening, prognosis, and treatment strategies, particularly for metastatic or aggressive disease. PRS, derived from common genetic variants, provides a personalized and independent measure of prostate cancer risk and may guide decisions on screening intensity and timing. Results: Although PRS cannot yet differentiate between indolent and aggressive cancer, it has the potential to stratify men into low and high-risk categories more effectively than FH or RPMs alone. Knowledge of specific RPMs can influence treatment decisions in clinically advanced prostate cancer. Challenges in clinical implementation include limited provider awareness, underutilization of genetic counseling, and lack of diversity in genomic datasets, which can lead to misdiagnoses. Emerging technologies and digital tools are being developed to streamline genetic testing and counseling. Population-level strategies and tailored screening protocols based on genetic risk are under active investigation. Conclusions: While early evidence suggests high satisfaction with genetic testing among patients, further studies in diverse populations are needed. Integration of germline genetic information into prostate cancer management offers promising avenues for personalized screening, surveillance, and treatment, ultimately aiming to reduce morbidity and mortality.
1. Introduction
Prostate cancer is the most common malignancy affecting men worldwide, and the second most common cause of cancer-related death [1]. For men with a strong family history of cancer (e.g., breast, ovarian, pancreatic, etc.), a germline genetic evaluation is recommended [2,3]. Genetic testing should also be offered for men diagnosed with high-risk or metastatic prostate cancer, those with first-degree relatives who died of prostate cancer before 75 years of age, and/or those with multiple primary cancers. The knowledge of germline testing results has multiple proven utilities, including (1) enabling the personalization of screening programs, (2) providing prognostic information for newly diagnosed men with lower-risk tumors, (3) informing treatment strategies for men with advanced cancer, and (4) providing information that family members can use to help improve cancer screening programs. While genetic testing is currently recommended by some authoritative guidelines, the exact make-up and gene constituents included in the testing panels remain somewhat ill defined. This review provides insight into the purpose of germline genetic testing, the considerations and strategies for implementation, and future directions in this field.
1.1. Genetic Assessment: Three Required Components
Many experts believe that a complete genetic risk assessment should include the following three components: (1) a family history (FH); (2) rare pathogenic mutations (RPMs); and (3) a single nucleotide polymorphism (SNP)-based polygenic risk score (PRS). These components offer the ability to better estimate the susceptibility and predict the responses and outcomes at almost every step during the prostate cancer journey, from screening to advanced disease.
1.2. Rare Pathogenic Mutations and Prostate Cancer Susceptibility
Germline testing for prostate cancer became feasible with two concurrent and significant events: (1) the technologies and cost for multigene testing capabilities improved and (2) precision medicine discoveries opened the door for advanced therapeutic options for patients with metastatic prostate cancer [4,5,6,7]. Identifying RPMs from hereditary cancer gene testing can significantly impact prostate cancer screening, the risk management for additional malignancies, the treatment of metastatic disease, and hereditary cancer assessments for men and their families [8,9,10].
The National Comprehensive Cancer Network (NCCN) guidelines currently recommend germline testing for high-risk individuals, including those with a strong FH of prostate cancer or other cancers, those of Ashkenazi Jewish ancestry, and those with a known FH of high-risk RPMs [11]. The guidelines suggest multigene panel testing for at least 10 different genes, including BRCA1, BRCA2, ATM, PALB2, CHEK2, HOXB13, MLH1, MSH2, MSH6, and PMS2. Mutations within these genes have been associated with diagnoses at earlier ages. However, it is important to understand that mutations within these genes do not all provide the same prognostic value. Some RPMs (e.g., those within HOXB13, ATM, BRCA2, and CHEK2) increase the disease risk, while others increase the risk of high-grade or aggressive disease (ATM, BRCA2, PALB2, CHEK2, and PMS2). However, most germline mutations are not associated with an increased risk of aggressive disease. That is to say, while RPMs may be associated with an increased risk of a prostate cancer diagnosis, not all RPMs are associated with worse prostate cancer outcomes, including metastasis and death.
1.3. Single Nucleotide Polymorphisms, Polygenic Risk Scores, and Prostate Cancer Susceptibility
Over 400 SNPs have been identified and are associated with an increased risk of prostate cancer. While the mechanism of the association with cancer susceptibility remains largely unknown, few SNPs are associated with aggressive disease risk. The polygenic risk score (PRS) is a broad term used to describe multiple methodologies that estimate the cumulative effect of these risk-associated SNPs. Many PRS methods for prostate cancer have been reported since 2008 [12]; while no one methodology is considered superior, each has unique advantages and potential limitations. Nonetheless, all PRS values, regardless of the methodology used, have been shown to offer independent and significantly improved prostate cancer risk predictions compared to FH information.
1.4. Key Characteristics of PRS for Prostate Cancer Risk Assessment
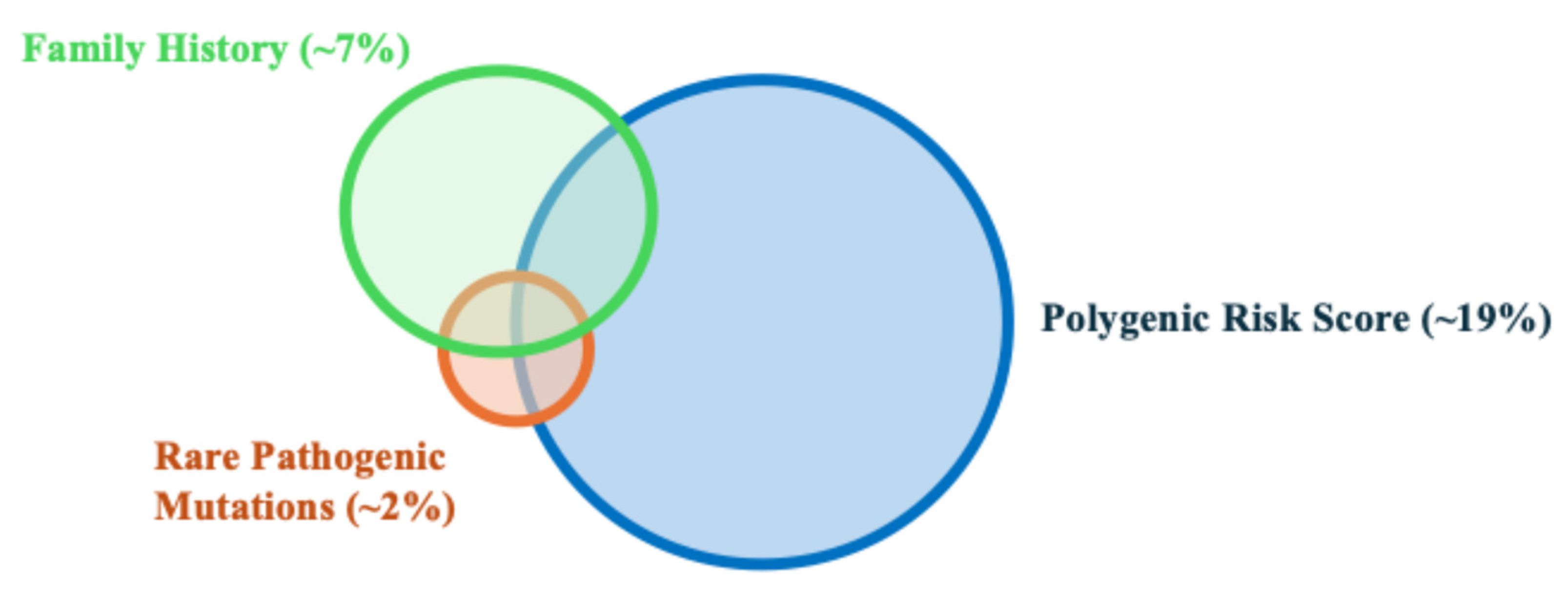
The PRS is a direct measurement of the genetic risk based on germline DNA, and is independent of environmental risk factors. Furthermore, due to a combination of hundreds to thousands of SNPs, the PRS is a personalized measurement of an individual’s genetic risk. Each person, even within the same family, may have a unique PRS value. This is different from FH, where all first-degree relatives are assigned the same genetic risk, even though they only share 50% of genetic material between them. Notably, the PRS provides additional information beyond FH and the currently known RPMs for stratifying prostate cancer risk. While FH and RPMs can identify ~7% and 2% of men at high risk for prostate cancer, respectively, the PRS can identify ~19% of men at a risk similar to the relative risk of FH and RPMs (Figure 1 and Figure 2) [13,14]. In addition, unlike FH and RPMs, where unaffected individuals are less informative and generally considered as having an average risk, PRS values can identify ~20% of men whose risk is half that of the general population. The ability to identify low-risk individuals who may benefit from delayed or less intense screening programs is unique to PRS values. Finally, PRS values have been inversely associated with the age of diagnosis, and therefore, men with higher PRS values may potentially benefit from more intensive and earlier screening [15].
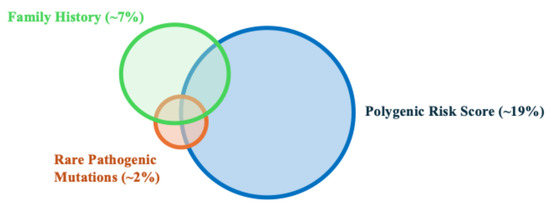
Figure 1.
Schematic Venn diagram showing the proportion of high-risk men in the general population that can be identified by family history, rare pathogenic mutations, and polygenic risk score.
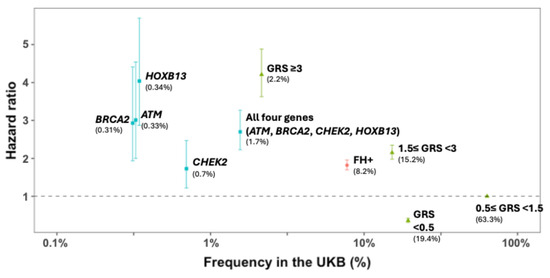
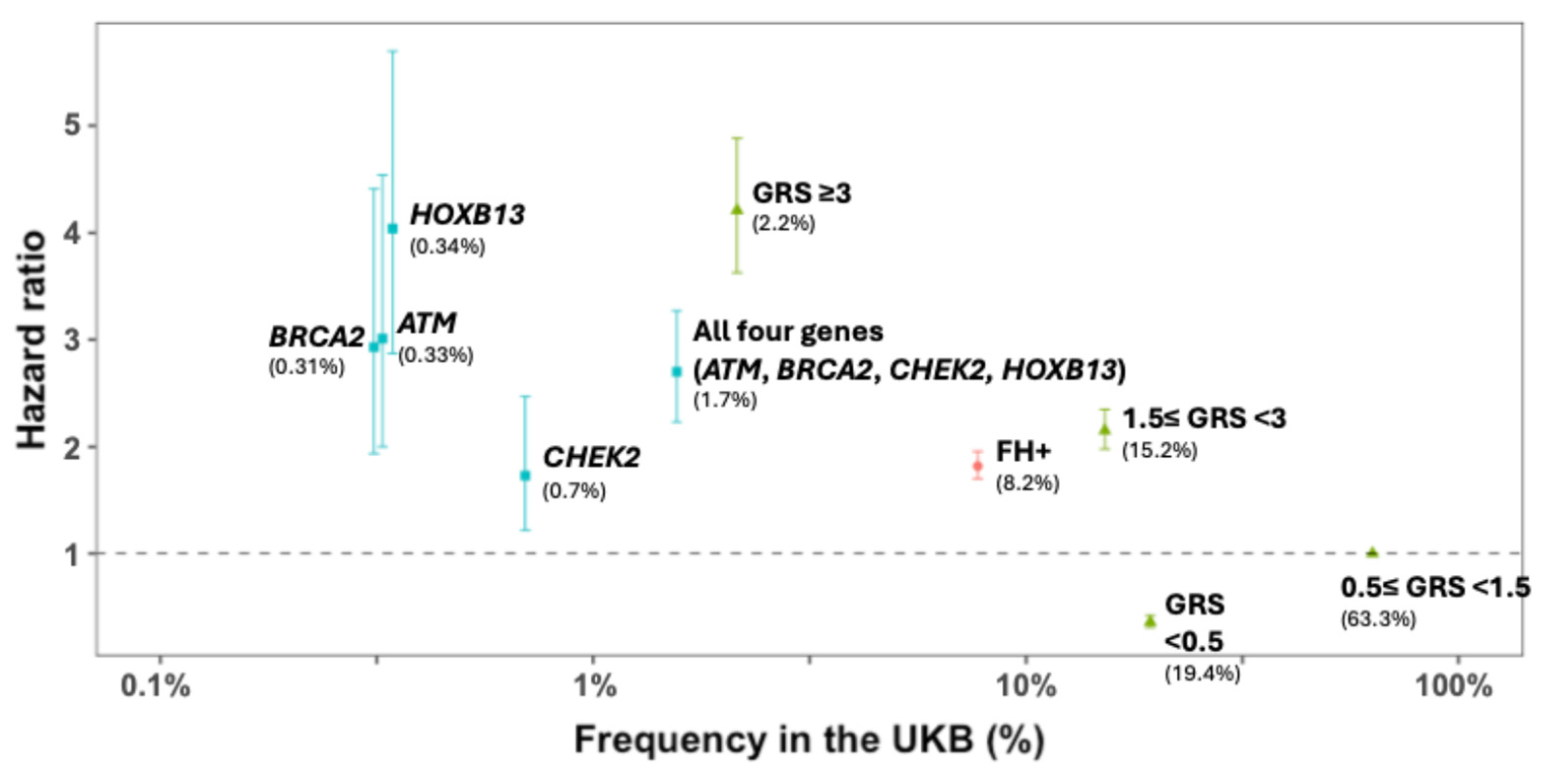
Figure 2.
Observed prostate cancer risk (hazard ratio) and 95% CI of three inherited risk measures for prostate cancer in a prostate cancer incidence cohort from the UK Biobank (European descent, N = 224,613). Source: [13]. FH = family history; GRS = genetic risk score; UKB: UK Biobank.
1.5. Inability to Differentiate Risk Between Indolent and Aggressive Prostate Cancer
It is important to note that the PRS is associated with susceptibility to prostate cancer, including both indolent and aggressive disease (metastatic and/or lethal disease) [13,14,16,17,18,19,20]. However, to date, none of the published PRS methods can routinely differentiate the risk between indolent and aggressive prostate cancer [13,17,19].
1.6. Challenges and Opportunities for PRS Adoption in the Clinic
Supplementing the PRS with FH and RPMs for a better genetic risk assessment has several potential clinical utilities, including an opportunity to develop a more personalized prostate cancer screening strategy [19], better estimate the penetrance of prostate cancer for carriers of RPMs [21], and better estimate the detection rate of prostate cancer from a prostate biopsy [13]. Despite these promising clinical utilities and consistent evidence for the PRS in risk assessment, the adoption of the PRS in clinical practice remains extremely limited. The continued education of care providers is necessary to increase its adoption.
1.7. Genetic Variation Among Different Racial Groups
It has been widely recognized that studies of genetics and genomics are not optimal if the data used to generate clinical interventions do not include ethnically diverse populations. Teo et al. [22] demonstrated how multi-ethnic studies in African populations can yield novel insights about the underlying genetic architecture of human disease because of the unique nature of the African genome. Furthermore, genomic studies based on African populations are more generalizable to other world populations compared to the same studies conducted on non-African populations due to the features of African genomic architecture [23,24]. Importantly, Kohane et al. [25] reported that genetic misdiagnoses may occur if genomic tests are developed using only White populations, and this misdiagnosis extends to both majority and minority populations. Thus, a limited diversity in research data can impact the accuracy of genetic testing and clinical decision making for all populations.
1.8. Clinical Implementation of Genetic Assessment: Utility for Unaffected Individuals
PRS values show great potential for identifying men who may or may not benefit from prostate cancer screening. In fact, the PRS has been shown to identify 19.4% of men as being at a low risk for prostate cancer and 17.4% of men as being at a high risk for prostate cancer [13]. If low-risk men were screened less frequently or not at all, and high-risk men were screened more frequently or beginning at a younger age, the PRS could change the screening decisions in approximately one third of men.
1.9. Clinical Implementation of Genetic Assessment: Utility for Patients on Active Surveillance
While PRS values do not routinely discriminate between aggressive and non-aggressive tumor types, it has been demonstrated that PRS values are positively associated with the multifocality of prostate tumors [26]. This information could potentially be used to inform management, where patients with low PRS values may be more suitable for active surveillance or emerging focal therapies, whereas individuals with higher PRS values may be more suitable for more invasive treatments, given the increased potential for multifocal disease [27]. However, prospective studies validating this concept are necessary.
1.10. Clinical Implementation of Genetic Assessment: Responsiveness to Treatment for Metastatic Cancer
In comparison to prostate cancer risk predictions, few studies have specifically evaluated the influence of RPMs or PRSs on the treatment response among individuals with localized or metastatic castration-sensitive prostate cancer. It would be surprising if the PRS predicted a disease response in these cohorts, based upon the fact that the PRS does not reliably distinguish aggressive tumors from non-aggressive tumors. However, DNA damage repair (DDR) genes have been associated with a shorter response time to ADT and a lower overall survival [28,29], which could provide important prognostic information when counseling patients.
Additionally, mismatch repair (MMR) gene alterations are present in 3.1% of advanced prostate tumors [30], and a significant proportion of these alterations are germline [31]. MMR defects have been associated with aggressive features and a more advanced disease at diagnosis [32,33,34]. However, conflicting results have been reported on the clinical outcomes of patients with MMR defects [33], suggesting either a favorable response to androgen deprivation therapy or the early development of castration resistance [32,35,36]. Notably, immune checkpoint inhibitors are now approved for patients with MMR defects and/or high microsatellite instability (dMMR/MSI-H), and the NCCN guidelines recommend their use in advanced disease [11].
1.11. Targeted Prostate Cancer Screening in Individuals with Alterations in Rare Variants
Germline genetic assessments for RPMs in men with prostate cancer have become more common following the finding that 4.6% of men with localized prostate cancer harbor mutations within DDR genes [37], and 11.8% of men with metastatic prostate cancer have DDR mutations. The most significant relative risks conferred by heritable mutations between metastatic cases and clinically localized cases were noted for BRCA2 and CHEK2. Xu and colleagues performed a meta-analysis on the available data, and concluded that a panel of mutations that included BRCA2, ATM, NBN, CHEK2, PALB2 was associated with an increased risk of metastatic and lethal cancer [28]. In surveillance patients, Carter et al. found that BRCA1/2 and ATM mutations are associated with an increased risk of grade reclassification, with a BRCA2 mutation conferring a relative risk of 2.74 (95% CI, 1.26–5.96) in a multivariable analysis [38]. Together, this suggests that specific mutations within panels of RPMs are associated with a more aggressive disease. Given the association between DDR RPMs and the risk of metastatic and lethal prostate cancer, a genetic assessment of these genes would provide critical information for treatment strategies.
Germline genetic testing can also indicate the prostate cancer risk in the screening setting. An international consortium of 65 centers in 20 countries (Identification of Men with a genetic predisposition to ProstAte Cancer: Targeted screening in BRCA1/2 mutation carriers and controls [IMPACT]) found that men with BRCA2 mutations and a prostate-specific antigen (PSA) > 3 ng/mL had a 48% probability of having prostate cancer on biopsy, twice that of the general population [39]. Furthermore, BRCA2 carriers had a higher cancer incidence rate per 1000 person years (19.4 vs. 12.0; p = 0.03); a younger age of diagnosis (61 vs. 64 yrs.; p = 0.04); and a higher likelihood of being diagnosed with clinically significant disease (77% vs. 40%; p = 0.01) [39]. No differences in age/tumor characteristics were seen in the BRCA1 carrier/control groups [40].
As a result of this report, a European Association of Urology (EAU) guideline now recommends PSA screening in BRCA2 mutation carriers starting at age 40. The NCCN also updated their guidelines to advise prostate cancer screening to start at age 45 for male carriers of BRCA2 mutations and to consider the same for carriers of BRCA1 mutations. However, there are no guidelines in the UK or internationally for carriers of MMR presently.
1.12. Targeted Prostate Cancer Screening in Individuals with Alterations in Common Variants
In contrast to targeted screening among the smaller proportion of men with RPMs, targeted screening based on common genetic risk variants has the potential for a significant population-wide impact. Recognizing this potential, Helfand and Catalona [41] proposed a PSA screening algorithm based on a 100 SNP score. Furthermore, professor Eeles’s UK group has initiated a suite of studies (the PROFILE and BARCODE 1 studies) that are offering intensive screening (e.g., a diffusion weighted-multi-parametric magnetic resonance imaging [DW-MPMRI], prostate biopsies, etc.) to individuals aged 40–69 with prostates, in addition to collecting biological samples. They have found that using PRS risk stratification improves the detection of clinically significant prostate cancer requiring treatment [42].
International guidelines are starting to be developed for targeted prostate cancer screening based on constitutional genetic variation. At present, the frequency of PSA testing, the use of magnetic resonance imaging (MRI), and whether some individuals will need to proceed directly to a prostate biopsy in the genetically higher risk groups is unknown. The results of trials of screening in the next few years will report the role of more extensive genetic profiles (both rare and common genetic variation) and the optimal screening algorithm.
1.13. Future of Genetic Information in Clinical Practice
Because genomic variation influences the prostate cancer risk and the disease presentation, prognosis, and response to treatment (including side effects), understanding and translating this knowledge into a clinical setting has the potential to provide patients with significant benefits in terms of morbidity and mortality in the future. Patients with prostate cancer have only recently begun to benefit from genetically informed clinical strategies or “personalized medicine”, including risk predictions and metastatic treatment decisions.
1.14. Implementation Strategies for Genetic Counseling and Germline Testing
Underscoring this idea, many studies have reported suboptimal referral rates for genetic counseling in the context of hereditary cancer [43]. This is particularly the case for prostate cancer, in which germline genetic evaluations are greatly underutilized. A systematic review found that knowledge is the most frequently cited barrier to referral for genetic counseling and genetic testing [43]. For prostate cancer specifically, research has shown critical gaps in provider knowledge, including which patients meet the criteria for a germline genetic evaluation [44]. Methods to integrate the collection and application of this information seamlessly in healthcare systems will be essential to its widespread adoption in clinical practice. In this regard, several technology-based tools have been created, such as the Helix application, which is an interactive platform to help providers assess a patient’s candidacy for germline testing [45].
1.15. Incorporating Germline Genetics into Healthcare Systems
The evolution of DNA sequencing has scaled our ability to assess the hereditary cancer risk. The Human Genome Project provided the first draft in 2001, but the cost was considerably high. Next-generation sequencing technologies have ushered in the modern-day approach to “panel testing”, where a patient has a genetic test performed that assesses multiple genes at once. Importantly, removing the historical gene-by-gene approach to testing has helped significantly with the accessibility of genetic information and risk assessments. Panels have become increasingly large over time, and efforts by ClinGen and ClinVar to ensure gene–disease associations are valid; additionally, ensuring the transparency of variant interpretation by labs has been important [46,47].
Health systems have taken population approaches to increase the incorporation of genetic testing, using targeted screening questionnaires in addition to broader “healthy population screening” to improve access [48,49,50]. Digital tools (platforms/chatbots) are increasingly being used to assist in raising awareness and improving access to genetic information [51,52,53]. Wang et al. examined four common workflows: (1) traditional referral, (2) point-of-care scheduling, (3) point-of-care counseling/telegenetics, and (4) point-of-care testing. They demonstrated that these can be scalable solutions for improving the uptake of genetic testing [54].
1.16. Satisfaction with Genetic Testing
The wide adoption of next-generation sequencing technology allows for an abundance of information to be gathered about a person, whether through commercial or research-sequencing efforts. In the genomics era of medicine, testing and generating sequencing data is not limited to a few highly penetrant genes, but rather can encompass the whole genome in healthy or affected individuals. This explosion of data has led to new challenges in providing appropriate consent and the return of results, given the potential clinical utility, benefit, and risks to the individual that can be context-specific, but that can also have indirect impacts for family members [55].
The patient experience is a critical component of care delivery, and communicating concepts related to genetic risk can be challenging. Most US-based studies reporting patient satisfaction with genetic testing for prostate cancer have evaluated novel methods of delivering genetic education compared to or in lieu of traditional pre- and/or post-test genetic counseling, with the aim of improving both access and speed [56]. While the early findings are encouraging and demonstrate high levels of satisfaction, these studies are primarily focused on non-Hispanic White, native English-speaking men with prostate cancer who are receiving care in academic settings. Further study is needed in non-White and non-native English-speaking populations. Also, satisfaction was assessed at relatively early time points in the genetic counseling and testing process, and therefore, this assessment did not include key areas such as support for future medical decision making and communicating results to at-risk family members. As such, future studies assessing satisfaction with genetic counseling and testing must include more diverse patients receiving care in a variety of settings, consider optimal ways to deliver culturally and linguistically concordant genetic services, and assess the satisfaction prior to, during, and after genetic testing.
2. Conclusions
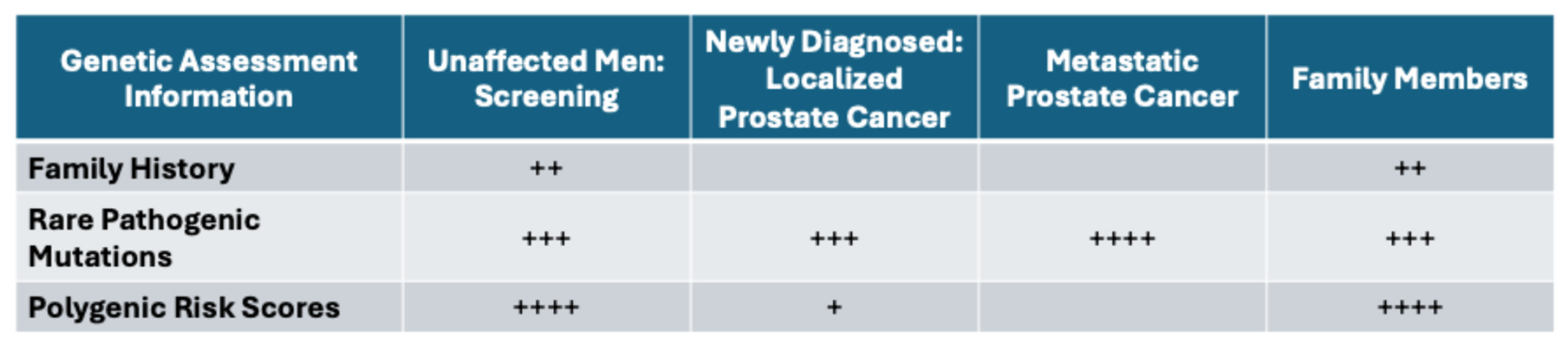
A complete genetic assessment for managing patients along the prostate cancer journey should include three components: family history information, the screening of RPMs, and the construction of a prostate-cancer-specific PRS. An evaluation of RPMs within DNA repair genes has become routinely included in authoritative guidelines for appropriate patients deemed “high-risk”. While our current evaluation of identifying patients at a “high risk” may be insufficient (e.g., FH information may not be broad enough to capture all patients who would benefit from genetic testing), the clinical impact of identifying an RPM upon genetic testing is several-fold (Figure 3). These results have the potential to influence the timing and frequency of screening, determine who has a propensity for developing aggressive tumors, and/or inform treatment decisions, including the choice for radical treatment, precision therapy (e.g., PARP inhibitors) and/or chemo- or immune therapies. Moreover, introducing a PRS evaluation could further advance and refine clinical decisions, with the potential to further optimize the outcomes for patients with prostate cancer.
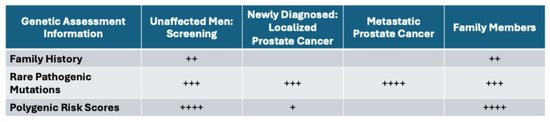
Figure 3.
Relative importance of genetic information informing various stages of prostate cancer, number of + signs indicate relative importance.
Author Contributions
Conceptualization: J.T.K. and B.T.H.; Methodology: J.T.K. and B.T.H.; Writing—Original Draft Preparation: J.T.K., A.A., E.C., R.A.E., L.M.F., P.J.H., S.L., C.P.P., T.R.R., Z.S., H.T., J.W., S.T.V., J.X. and B.T.H.; Writing—Review and Editing: J.T.K., A.A., E.C., R.A.E., L.M.F., P.J.H., S.L., C.P.P., T.R.R., Z.S., H.T., J.W., S.T.V., J.X. and B.T.H.; Visualization: J.T.K., J.X. and B.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Department of Defense (HT9425-23-1-1040 to S.L., S.V.), the Prostate Cancer Foundation (S.L.), the Sir Harold Cuthbertson Foundation (L.M.F.) and Gerald Harvey Bequest (L.M.F.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.S.; Bova, G.S.; Beaty, T.H.; Steinberg, G.D.; Childs, B.; Isaacs, W.B.; Walsh, P.C. Hereditary prostate cancer: Epidemiologic and clinical features. J. Urol. 1993, 150, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Bennett, R.L.; Buchanan, A.; Pearlman, R.; Wiesner, G.L. Guideline Development Group, American College of Medical Genetics and Genomics Professional Practice and Guidelines Committee and National Society of Genetic Counselors Practice Guidelines Committee. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: Referral indications for cancer predisposition assessment. Genet. Med. 2015, 17, 70–87. [Google Scholar] [PubMed]
- Giri, V.N.; Hegarty, S.E.; Hyatt, C.; O’Leary, E.; Garcia, J.; Knudsen, K.E.; Kelly, W.K.; Gomella, L.G. Germline genetic testing for inherited prostate cancer in practice: Implications for genetic testing, precision therapy, and cascade testing. Prostate 2018, 79, 333–339. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef]
- Agarwal, N.; A Azad, A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.C.; et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomised, placebo-controlled, phase 3 trial. Lancet 2023, 402, 291–303. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Clinical Pracice Guidelines in Oncology (NCCN Guidelines®): Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 3.2024). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1545 (accessed on 3 March 2024).
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prostate Cancer Early Detection Version 1. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf (accessed on 3 March 2024).
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prostate Cancer Version 1. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 3 March 2024).
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Zheng, S.L.; Sun, J.; Wiklund, F.; Smith, S.; Stattin, P.; Li, G.; Adami, H.-O.; Hsu, F.-C.; Zhu, Y.; Bälter, K.; et al. Cumulative association of five genetic variants with prostate cancer. N. Engl. J. Med. 2008, 358, 910–919. [Google Scholar] [CrossRef]
- Xu, J.; Resurreccion, W.K.; Shi, Z.; Wei, J.; Wang, C.-H.; Zheng, S.L.; Hulick, P.J.; Ross, A.E.; Pavlovich, C.P.; Helfand, B.T.; et al. Inherited risk assessment and its clinical utility for predicting prostate cancer from diagnostic prostate biopsies. Prostate Cancer Prostatic. Dis. 2022, 25, 422–430. [Google Scholar] [CrossRef]
- Shi, Z.; Platz, E.A.; Wei, J.; Na, R.; Fantus, R.J.; Wang, C.-H.; Eggener, S.E.; Hulick, P.J.; Duggan, D.; Zheng, S.L.; et al. Performance of Three Inherited Risk Measures for Predicting Prostate Cancer Incidence and Mortality: A Population-based Prospective Analysis. Eur. Urol. 2021, 79, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Na, R.; Labbate, C.; Yu, H.; Shi, Z.; Fantus, R.J.; Wang, C.-H.; Andriole, G.L.; Isaacs, W.B.; Zheng, S.L.; Helfand, B.T.; et al. Single-Nucleotide Polymorphism–Based Genetic Risk Score and Patient Age at Prostate Cancer Diagnosis. JAMA Netw. Open 2019, 2, e1918145. [Google Scholar] [CrossRef] [PubMed]
- Conti, D.V.; Darst, B.F.; Moss, L.C.; Saunders, E.J.; Sheng, X.; Chou, A.; Schumacher, F.R.; Al Olama, A.A.; Benlloch, S.; Dadaev, T.; et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat. Genet. 2021, 53, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Na, R.; Packiam, V.T.; Conran, C.A.; Jiang, D.; Tao, S.; Yu, H.; Lin, X.; Meng, W.; Zheng, S.L.; et al. Reclassification of prostate cancer risk using sequentially identified SNPs: Results from the REDUCE trial. Prostate 2017, 77, 1179–1186. [Google Scholar] [CrossRef]
- Plym, A.; Zhang, Y.; Stopsack, K.H.; Jee, Y.H.; Wiklund, F.; Kibel, A.S.; Kraft, P.; Giovannucci, E.; Penney, K.L.; Mucci, L.A. Family History of Prostate and Breast Cancer Integrated with a Polygenic Risk Score Identifies Men at Highest Risk of Dying from Prostate Cancer before Age 75 Years. Clin. Cancer Res. 2022, 28, 4926–4933. [Google Scholar] [CrossRef]
- Huynh-Le, M.-P.; Fan, C.C.; Karunamuni, R.; Thompson, W.K.; Martinez, M.E.; Eeles, R.A.; Kote-Jarai, Z.; Muir, K.; Schleutker, J.; Pashayan, N.; et al. Polygenic hazard score is associated with prostate cancer in multi-ethnic populations. Nat. Commun. 2021, 12, 1236. [Google Scholar] [CrossRef]
- Pagadala, M.S.; Lynch, J.; Karunamuni, R.; Alba, P.R.; Lee, K.M.; Agiri, F.Y.; Anglin, T.; Carter, H.; Gaziano, J.M.; Jasuja, G.K.; et al. Polygenic risk of any, metastatic, and fatal prostate cancer in the Million Veteran Program. J. Natl. Cancer Inst. 2023, 115, 190–199. [Google Scholar] [CrossRef]
- Darst, B.F.; Sheng, X.; Eeles, R.A.; Kote-Jarai, Z.; Conti, D.V.; Haiman, C.A. Combined Effect of a Polygenic Risk Score and Rare Genetic Variants on Prostate Cancer Risk. Eur. Urol. 2021, 80, 134–138. [Google Scholar] [CrossRef]
- Teo, Y.Y.; Small, K.S.; Kwiatkowski, D.P. Methodological challenges of genome-wide association analysis in Africa. Nat. Rev. Genet. 2010, 11, 149–160. [Google Scholar] [CrossRef]
- Lachance, J.; Berens, A.J.; Hansen, M.E.B.; Teng, A.K.; Tishkoff, S.A.; Rebbeck, T.R. Genetic Hitchhiking and Population Bottlenecks Contribute to Prostate Cancer Disparities in Men of African Descent. Cancer Res. 2018, 78, 2432–2443. [Google Scholar] [CrossRef]
- Kim, M.S.; Patel, K.P.; Teng, A.K.; Berens, A.J.; Lachance, J. Genetic disease risks can be misestimated across global populations. Genome. Biol. 2018, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Manrai, A.K.; Funke, B.H.; Rehm, H.L.; Olesen, M.S.; Maron, B.A.; Szolovits, P.; Margulies, D.M.; Loscalzo, J.; Kohane, I.S. Genetic Misdiagnoses and the Potential for Health Disparities. N. Engl. J. Med. 2016, 375, 655–665. [Google Scholar] [CrossRef]
- Xu, J.; Isaacs, W.B.; Mamawala, M.; Shi, Z.; Landis, P.; Petkewicz, J.; Wei, J.; Wang, C.; Resurreccion, W.K.; Na, R.; et al. Association of prostate cancer polygenic risk score with number and laterality of tumor cores in active surveillance patients. Prostate 2021, 81, 703–709. [Google Scholar] [CrossRef]
- Kearns, J.T.; Helfand, B.T.; Xu, J. Moving Prostate Cancer Polygenic Risk Scores from Research Towards Clinical Practice. Eur. Urol. Focus 2022, 8, 913–915. [Google Scholar] [CrossRef]
- Shi, Z.; Lu, L.; Resurreccion, W.K.; Yang, W.; Wei, J.; Wang, Q.; Engelmann, V.; Zheng, S.L.; Cooney, K.A.; Isaacs, W.B.; et al. Association of germline rare pathogenic mutations in guideline-recommended genes with prostate cancer progression: A meta-analysis. Prostate 2022, 82, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Saunders, E.J.; Dadaev, T.; Brook, M.N.; Wakerell, S.; Govindasami, K.; Rageevakumar, R.; Hussain, N.; Osborne, A.; Keating, D.; Lophatananon, A.; et al. Identification of Genes with Rare Loss of Function Variants Associated with Aggressive Prostate Cancer and Survival. Eur. Urol. Oncol. 2024, 7, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Kuzbari, Z.; Bandlamudi, C.; Loveday, C.; Garrett, A.; Mehine, M.; George, A.; Hanson, H.; Snape, K.; Kulkarni, A.; Allen, S.; et al. Germline-focused analysis of tumour-detected variants in 49,264 cancer patients: ESMO Precision Medicine Working Group recommendations. Ann. Oncol. 2023, 34, 215–227. [Google Scholar] [CrossRef]
- Ritch, E.; Fu, S.Y.; Herberts, C.; Wang, G.; Warner, E.W.; Schönlau, E.; Taavitsainen, S.; Murtha, A.J.; Vandekerkhove, G.; Beja, K.; et al. Identification of Hypermutation and Defective Mismatch Repair in ctDNA from Metastatic Prostate Cancer. Clin. Cancer Res. 2020, 26, 1114–1125. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Shaukat, F.; Velho, P.I.; Kaur, H.; Shenderov, E.; Pardoll, D.M.; Lotan, T.L. Clinical Features and Therapeutic Outcomes in Men with Advanced Prostate Cancer and DNA Mismatch Repair Gene Mutations. Eur. Urol. 2019, 75, 378–382. [Google Scholar] [CrossRef]
- Bancroft, E.K.; Page, E.C.; Brook, M.N.; Thomas, S.; Taylor, N.; Pope, J.; McHugh, J.; Jones, A.-B.; Karlsson, Q.; Merson, S.; et al. A prospective prostate cancer screening programme for men with pathogenic variants in mismatch repair genes (IMPACT): Initial results from an international prospective study. Lancet. Oncol. 2021, 22, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- van der Doelen, M.J.; Velho, P.I.; Slootbeek, P.H.; Naga, S.P.; Bormann, M.; van Helvert, S.; Kroeze, L.I.; van Oort, I.M.; Gerritsen, W.R.; Antonarakis, E.S.; et al. Impact of DNA damage repair defects on response to radium-223 and overall survival in metastatic castration-resistant prostate cancer. Eur. J. Cancer 2020, 136, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.N.; Rescigno, P.; Liu, D.; Yuan, W.; Carreira, S.; Lambros, M.B.; Seed, G.; Mateo, J.; Riisnaes, R.; Mullane, S.; et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J. Clin. Investig. 2018, 128, 5185. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Carter, H.B.; Helfand, B.; Mamawala, M.; Wu, Y.; Landis, P.; Yu, H.; Wiley, K.; Na, R.; Shi, Z.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur. Urol. 2019, 75, 743–749. [Google Scholar] [CrossRef]
- Bancroft, E.K.; Page, E.C.; Castro, E.; Lilja, H.; Vickers, A.; Sjoberg, D.; Assel, M.; Foster, C.S.; Mitchell, G.; Drew, K.; et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: Results from the initial screening round of the IMPACT study. Eur. Urol. 2014, 66, 489–499. [Google Scholar] [CrossRef]
- Page, E.C.; Bancroft, E.K.; Brook, M.N.; Assel, M.; Hassan Al Battat, M.; Thomas, S.; Taylor, N.; Chamberlain, A.; Pope, J.; Ni Raghallaigh, H.; et al. Interim Results from the IMPACT Study: Evidence for Prostate-specific Antigen Screening in BRCA2 Mutation Carriers. Eur. Urol. 2019, 76, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Helfand, B.T.; Catalona, W.J.; Xu, J. A genetic-based approach to personalized prostate cancer screening and treatment. Curr. Opin. Urol. 2015, 25, 53–58. [Google Scholar] [CrossRef]
- Eeles, R.A.; Bancroft, E.K.; McHugh, J.K.; Saunders, E.; Brook, M.; McGrowder, E.; Wakerell, S.; James, D.; Page, E.; Osborne, A.; et al. Effect of polygenic risk score for clinically significant prostate cancer in a screening program: The BARCODE 1 study results. JCO 2024, 42 (Suppl. S16), 10500. [Google Scholar] [CrossRef]
- Morrow, A.; Chan, P.; Tucker, K.M.; Taylor, N. The design, implementation, and effectiveness of intervention strategies aimed at improving genetic referral practices: A systematic review of the literature. Genet. Med. 2021, 23, 2239–2249. [Google Scholar] [CrossRef]
- Loeb, S.; Byrne, N.; Walter, D.; Makarov, D.V.; Wise, D.R.; Becker, D.; Giri, V.N. Knowledge and practice regarding prostate cancer germline testing among urologists: Gaps to address for optimal implementation✰,✰✰. Cancer Treat. Res. Commun. 2020, 25, 100212. [Google Scholar] [CrossRef] [PubMed]
- Giri, V.N.; Walker, A.; Gross, L.; Trabulsi, E.J.; Lallas, C.D.; Kelly, W.K.; Gomella, L.G.; Fischer, C.; Loeb, S. Helix: A Digital Tool to Address Provider Needs for Prostate Cancer Genetic Testing in Clinical Practice. Clin. Genitourin. Cancer 2022, 20, e104–e113. [Google Scholar] [CrossRef]
- Welcome to ClinGen. Available online: https://clinicalgenome.org/ (accessed on 22 March 2024).
- ** ClinVar **. Available online: http://www.clinvar.com/ (accessed on 22 March 2024).
- Lahiri, S.; Pirzadeh-Miller, S.; Moriarty, K.; Kubiliun, N. Implementation of a Population-Based Cancer Family History Screening Program for Lynch Syndrome. Cancer Control 2023, 30, 10732748231175011. [Google Scholar] [CrossRef]
- David, S.P.; Dunnenberger, H.M.; Choi, S.; DePersia, A.; Ilbawi, N.; Ward, C.; Wake, D.T.; Khandekar, J.D.; Shannon, Y.; Hughes, K.; et al. Personalized medicine in a community health system: The NorthShore experience. Front. Genet. 2023, 14, 1308738. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.; Hulick, P.J.; Wells, C.J. The integration of personalized medicine into health systems: Progress and a path forward. Pers. Med. 2021, 18, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.A.; Wu, R.R.; Myers, R.A.; Buchanan, A.H.; Henrich, V.C.; Hauser, E.R.; Ginsburg, G.S. Clinical utility of a Web-enabled risk-assessment and clinical decision support program. Genet. Med. 2016, 18, 1020–1028. [Google Scholar] [CrossRef]
- Webster, E.M.; Ahsan, M.D.; Perez, L.; Levi, S.R.; Thomas, C.; Christos, P.; Hickner, A.; Hamilton, J.G.; Babagbemi, K.; Cantillo, E.; et al. Chatbot Artificial Intelligence for Genetic Cancer Risk Assessment and Counseling: A Systematic Review and Meta-Analysis. JCO Clin. Cancer Inform. 2023, 7, e2300123. [Google Scholar] [CrossRef]
- Voils, C.I.; Coffman, C.J.; Wu, R.R.; Grubber, J.M.; Fisher, D.A.; Strawbridge, E.M.; Sperber, N.; Wang, V.; Scheuner, M.T.; Provenzale, D.; et al. A Cluster Randomized Trial of a Family Health History Platform to Identify and Manage Patients at Increased Risk for Colorectal Cancer. J. Gen. Intern. Med. 2023, 38, 1375–1383. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Bowen, D.J.; Xuan, Z. Implementing digital systems to facilitate genetic testing for hereditary cancer syndromes: An observational study of 4 clinical workflows. Genet. Med. 2023, 25, 100802. [Google Scholar] [CrossRef]
- Henderson, G.E.; Wolf, S.M.; Kuczynski, K.J.; Joffe, S.; Sharp, R.R.; Parsons, D.W.; Knoppers, B.M.; Yu, J.-H.; Appelbaum, P.S. The challenge of informed consent and return of results in translational genomics: Empirical analysis and recommendations. J. Law Med. Ethics 2014, 42, 344–355. [Google Scholar] [CrossRef]
- Russo, J.; Giri, V.N. Germline testing and genetic counselling in prostate cancer. Nat. Rev. Urol. 2022, 19, 331–343. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).