SIU-ICUD: Localized Prostate Cancer: Pathological Factors That Influence Outcomes and Management
Abstract
1. Introduction
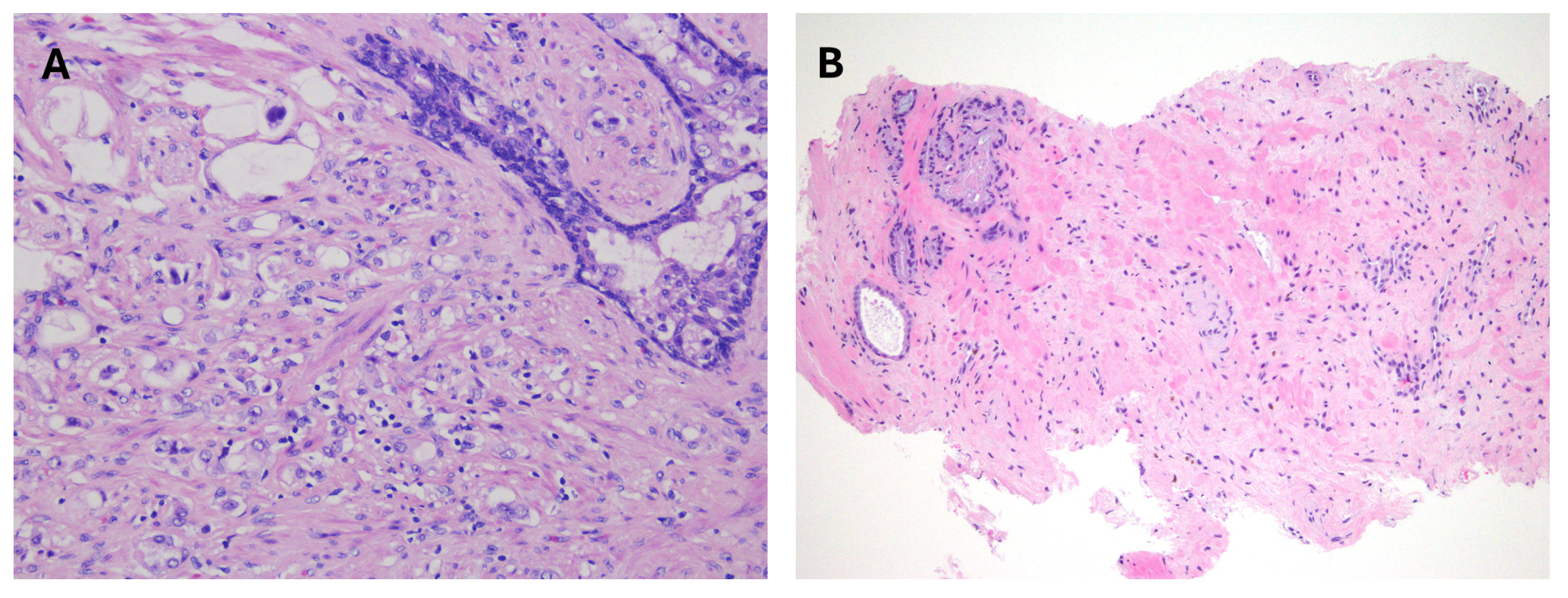
2. Acinar Adenocarcinoma and Subtypes
3. Modified Gleason Grading
3.1. Grade Group
3.2. Contemporary Gleason Patterns
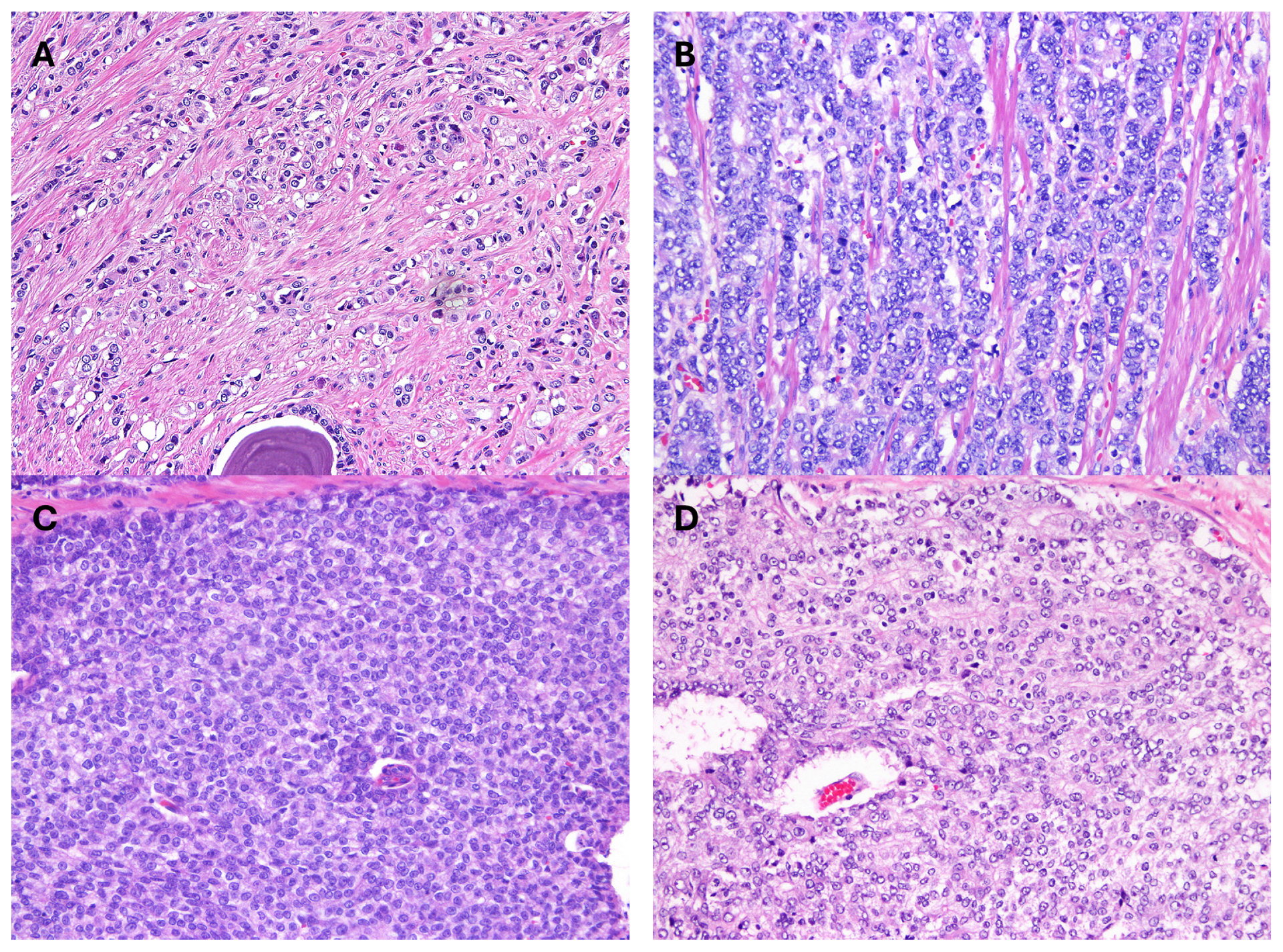
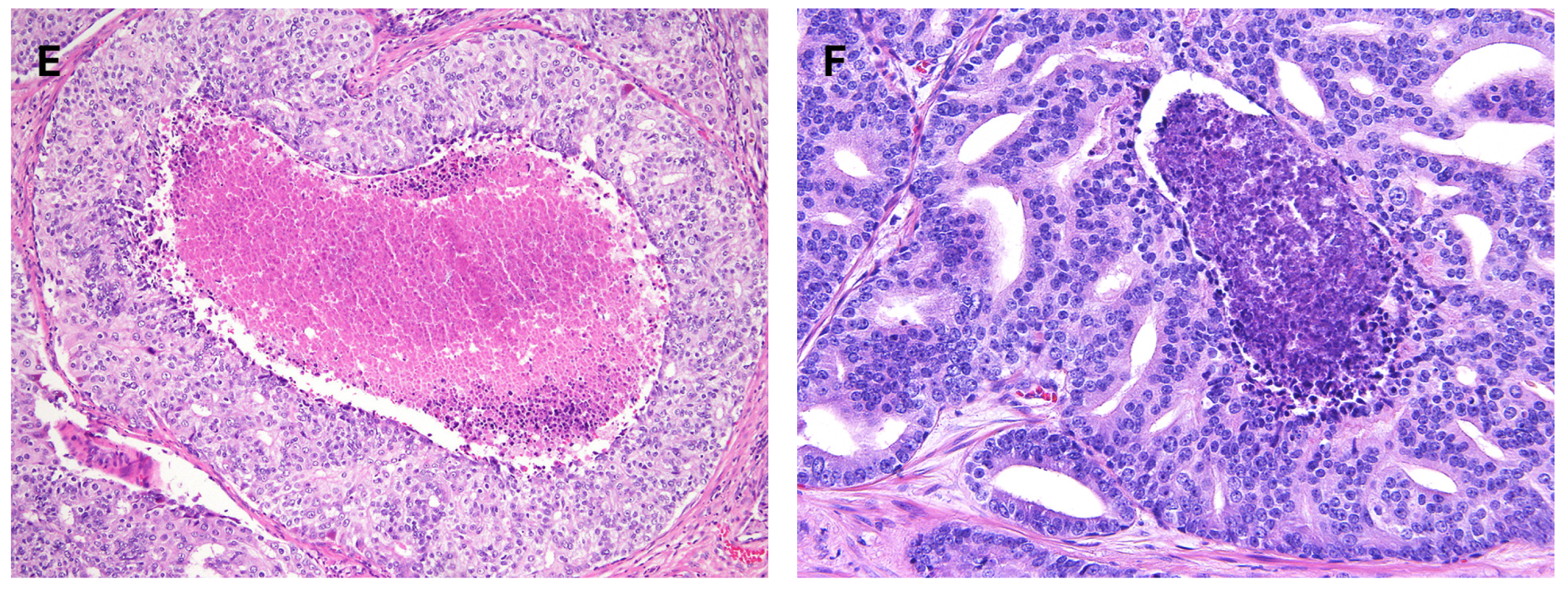
3.3. Modern Gleason Grading Rules
4. Reporting of Grades
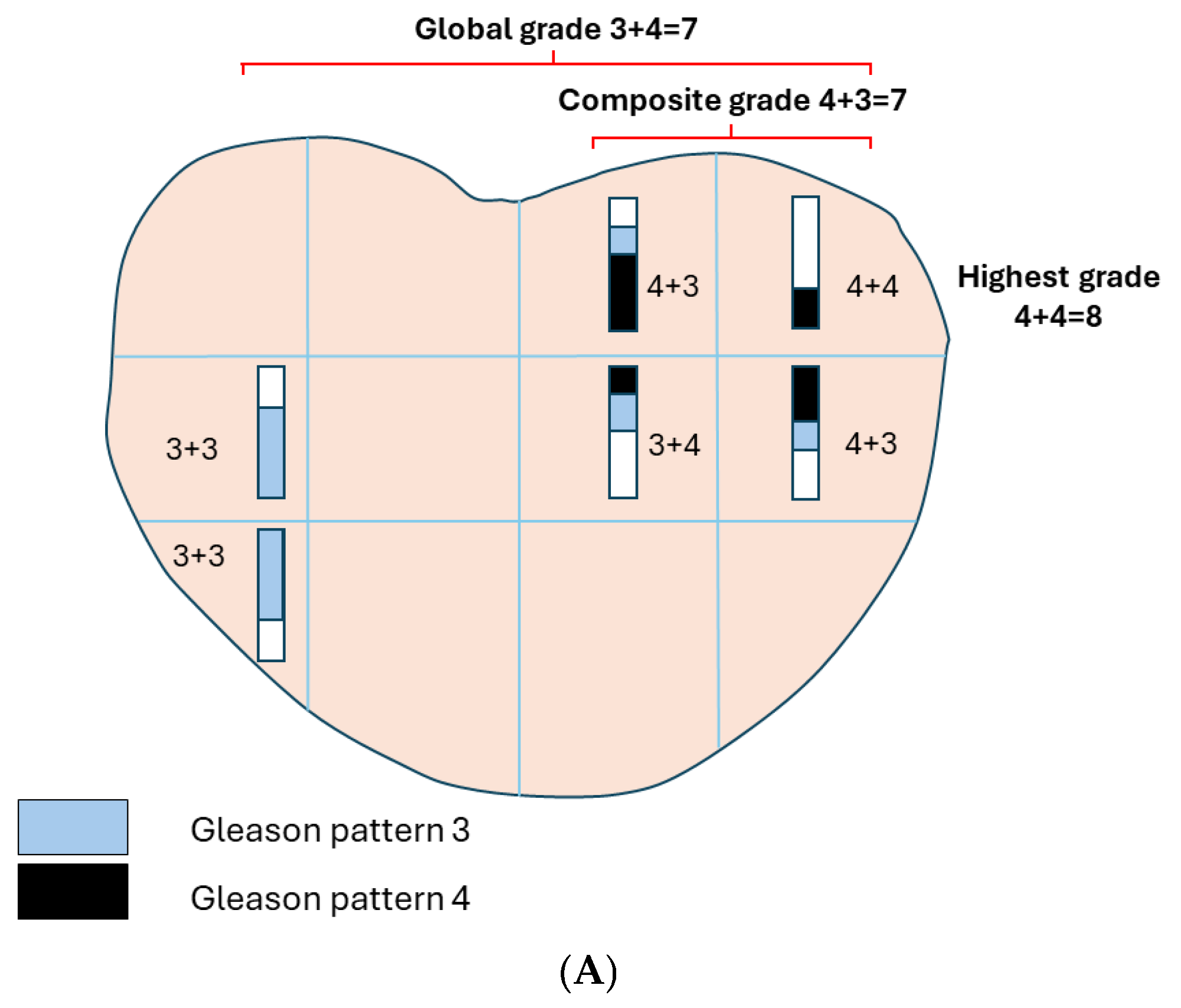
4.1. Reporting Grade in Biopsy
4.2. Reporting Grade in Radical Prostatectomy
5. Prognostic Impact of Gleason Patterns
5.1. Cribriform Pattern
5.2. Other Gleason Patterns
6. Quantitative Grading
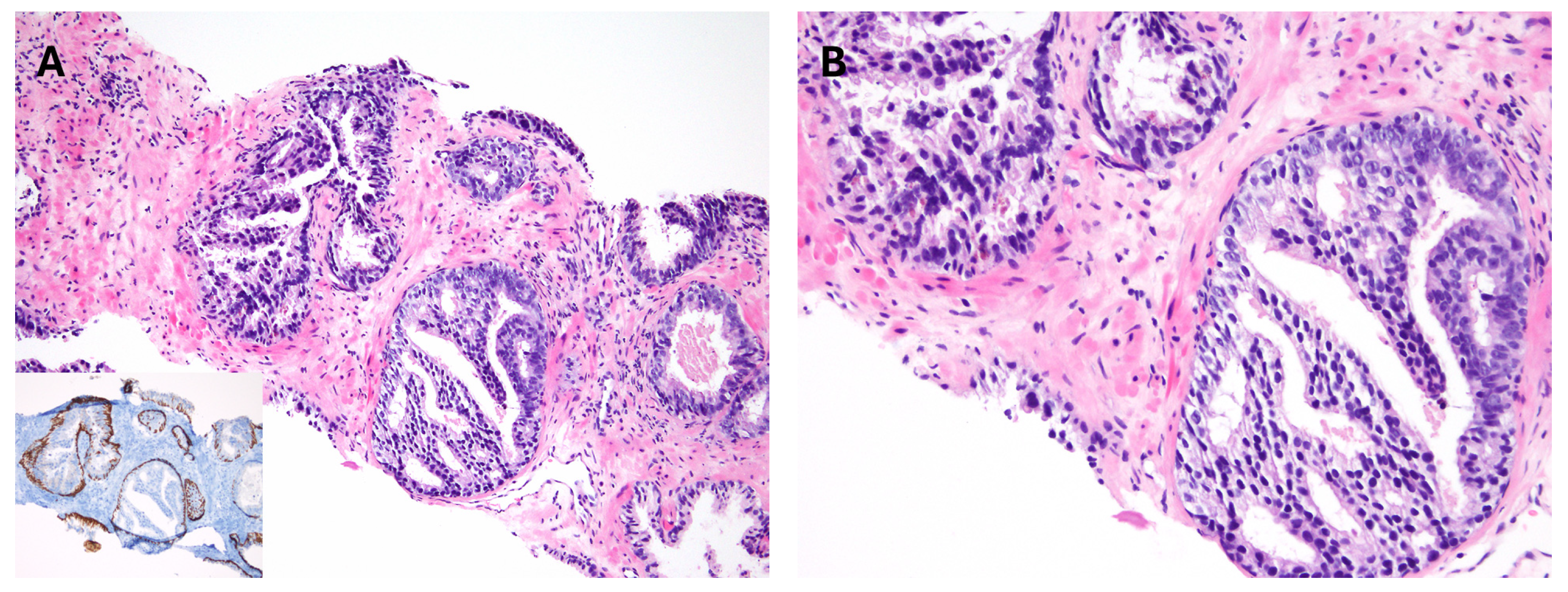
7. Intraductal Carcinoma
8. Atypical Intraductal Proliferation
9. Treatment-Related Effects
10. Tumor Volume or Extent
10.1. Tumor Volume in Biopsy
10.2. Tumor Volume in Radical Prostatectomy
11. Perineural Invasion
12. Lymphovascular Invasion
13. Margin Status in Radical Prostatectomy
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer. Available online: https://www.nccn.org (accessed on 31 January 2025).
- Epstein, J.I.; Walsh, P.C.; Carmichael, M.; Brendler, C.B. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Raymond, E.; O’Callaghan, M.E.; Vincent, A.D.; Beckmann, K.R.; Order, D.; Evans, S.; McNeil, J.; Millar, J.; Zalcberg, J.; et al. Optimum Tools for Predicting Clinical Outcomes in Prostate Cancer Patients Undergoing Radical Prostatectomy: A Systematic Review of Prognostic Accuracy and Validity. Clin. Genitourin. Cancer 2017, 15, e827–e834. [Google Scholar] [CrossRef]
- Willemse, P.M.; Davis, N.F.; Grivas, N.; Zattoni, F.; Lardas, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Dell’Oglio, P.; Donaldson, J.F.; et al. Systematic Review of Active Surveillance for Clinically Localised Prostate Cancer to Develop Recommendations Regarding Inclusion of Intermediate-risk Disease, Biopsy Characteristics at Inclusion and Monitoring, and Surveillance Repeat Biopsy Strategy. Eur. Urol. 2022, 81, 337–346. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Hilton, J.F.; Carroll, P.R. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer 2011, 117, 5039–5046. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J., Jr.; Dotan, Z.A.; DiBlasio, C.J.; Reuther, A.; Klein, E.A.; Kattan, M.W. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J. Clin. Oncol. 2005, 23, 7005–7012. [Google Scholar] [CrossRef]
- Lughezzani, G.; Briganti, A.; Karakiewicz, P.I.; Kattan, M.W.; Montorsi, F.; Shariat, S.F.; Vickers, A.J. Predictive and prognostic models in radical prostatectomy candidates: A critical analysis of the literature. Eur. Urol. 2010, 58, 687–700. [Google Scholar] [CrossRef]
- van Leenders, G.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N.; et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87–e99. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Fine, S.W.; Algaba, F.; Aron, M.; Baydar, D.E.; Beltran, A.L.; Brimo, F.; Cheville, J.C.; Colecchia, M.; et al. The 2019 Genitourinary Pathology Society (GUPS) White Paper on Contemporary Grading of Prostate Cancer. Arch. Pathol. Lab. Med. 2021, 145, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Amin, M.B.; Reuter, V.E.; Humphrey, P.A. Contemporary Gleason Grading of Prostatic Carcinoma: An Update with Discussion on Practical Issues to Implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2017, 41, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. In Urinary and Male Genital Tumours, 5th ed.; WHO Press: Geneva, Switzerland, 2022. [Google Scholar]
- Paner, G.P.; Gandhi, J.; Choy, B.; Amin, M.B. Essential Updates in Grading, Morphotyping, Reporting, and Staging of Prostate Carcinoma for General Surgical Pathologists. Arch. Pathol. Lab. Med. 2019, 143, 550–564. [Google Scholar] [CrossRef]
- Gleason, D.F. Histologic grading of prostate cancer: A perspective. Hum. Pathol. 1992, 23, 273–279. [Google Scholar] [CrossRef]
- Ross, H.M.; Kryvenko, O.N.; Cowan, J.E.; Simko, J.P.; Wheeler, T.M.; Epstein, J.I. Do adenocarcinomas of the prostate with Gleason score (GS) ≤6 have the potential to metastasize to lymph nodes? Am. J. Surg. Pathol. 2012, 36, 1346–1352. [Google Scholar] [CrossRef]
- Kweldam, C.F.; Wildhagen, M.F.; Bangma, C.H.; van Leenders, G.J. Disease-specific death and metastasis do not occur in patients with Gleason score ≤6 at radical prostatectomy. BJU Int. 2015, 116, 230–235. [Google Scholar] [CrossRef]
- Anderson, B.B.; Oberlin, D.T.; Razmaria, A.A.; Choy, B.; Zagaja, G.P.; Shalhav, A.L.; Meeks, J.J.; Yang, X.J.; Paner, G.P.; Eggener, S.E. Extraprostatic Extension Is Extremely Rare for Contemporary Gleason Score 6 Prostate Cancer. Eur. Urol. 2017, 72, 455–460. [Google Scholar] [CrossRef]
- Hassan, O.; Han, M.; Zhou, A.; Paulk, A.; Sun, Y.; Al-Harbi, A.; Alrajjal, A.; Baptista Dos Santos, F.; Epstein, J.I. Incidence of Extraprostatic Extension at Radical Prostatectomy with Pure Gleason Score 3 + 3 = 6 (Grade Group 1) Cancer: Implications for Whether Gleason Score 6 Prostate Cancer Should be Renamed “Not Cancer” and for Selection Criteria for Active Surveillance. J. Urol. 2018, 199, 1482–1487. [Google Scholar] [CrossRef]
- Paulk, A.; Giannico, G.; Epstein, J.I. PIN-like (Ductal) Adenocarcinoma of the Prostate. Am. J. Surg. Pathol. 2018, 42, 1693–1700. [Google Scholar] [CrossRef]
- Hudson, J.; Cao, D.; Vollmer, R.; Kibel, A.S.; Grewal, S.; Humphrey, P.A. Foamy gland adenocarcinoma of the prostate: Incidence, Gleason grade, and early clinical outcome. Hum. Pathol. 2012, 43, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Bronkema, C.; Arora, S.; Sood, A.; Dalela, D.; Keeley, J.; Borchert, A.; Baumgarten, L.; Rogers, C.G.; Peabody, J.O.; Menon, M.; et al. Rare Histological Variants of Prostate Adenocarcinoma: A National Cancer Database Analysis. J. Urol. 2020, 204, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ding, J.; Qi, J. Comparison of Typical Prostate Adenocarcinoma and Rare Histological Variant Prostate Cancer Showed Different Characteristics and Prognosis: A Surveillance, Epidemiology, and End Results Database Analysis. Eur. Urol. 2022, 82, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Bailar, J.C., 3rd; Mellinger, G.T.; Gleason, D.F. Survival rates of patients with prostatic cancer, tumor stage, and differentiation--preliminary report. Cancer Chemother. Rep. 1966, 50, 129–136. [Google Scholar]
- Gleason, D.F. Classification of prostatic carcinomas. Cancer Chemother. Rep. 1966, 50, 125–128. [Google Scholar]
- Epstein, J.I.; Allsbrook, W.C.; Amin, M.B., Jr.; Egevad, L.L.; Committee, I.G. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef]
- Smith, S.C.; Gandhi, J.S.; Moch, H.; Aron, M.; Compérat, E.; Paner, G.P.; McKenney, J.K.; Amin, M.B. Similarities and Differences in the 2019 ISUP and GUPS Recommendations on Prostate Cancer Grading: A Guide for Practicing Pathologists. Adv. Anat. Pathol. 2021, 28, 1–7. [Google Scholar] [CrossRef]
- Varma, M.; Shah, R.B.; Williamson, S.R.; Berney, D.M. 2019 Gleason grading recommendations from ISUP and GUPS: Broadly concordant but with significant differences. Virchows Arch. 2021, 478, 813–815. [Google Scholar] [CrossRef]
- Berney, D.M. Low Gleason score prostatic adenocarcinomas are no longer viable entities. Histopathology 2007, 50, 683–690. [Google Scholar] [CrossRef]
- Donin, N.M.; Laze, J.; Zhou, M.; Ren, Q.; Lepor, H. Gleason 6 prostate tumors diagnosed in the PSA era do not demonstrate the capacity for metastatic spread at the time of radical prostatectomy. Urology 2013, 82, 148–152. [Google Scholar] [CrossRef]
- Stark, J.R.; Perner, S.; Stampfer, M.J.; Sinnott, J.A.; Finn, S.; Eisenstein, A.S.; Ma, J.; Fiorentino, M.; Kurth, T.; Loda, M.; et al. Gleason score and lethal prostate cancer: Does 3 + 4 = 4 + 3? J. Clin. Oncol. 2009, 27, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- Danneman, D.; Drevin, L.; Robinson, D.; Stattin, P.; Egevad, L. Gleason inflation 1998-2011: A registry study of 97,168 men. BJU Int. 2015, 115, 248–255. [Google Scholar] [CrossRef]
- Pierorazio, P.M.; Walsh, P.C.; Partin, A.W.; Epstein, J.I. Prognostic Gleason grade grouping: Data based on the modified Gleason scoring system. BJU Int. 2013, 111, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Albertsen, P.C.; Moore, D.; Rotter, D.; Demissie, K.; Lu-Yao, G. Validation of a Contemporary Five-tiered Gleason Grade Grouping Using Population-based Data. Eur. Urol. 2017, 71, 760–763. [Google Scholar] [CrossRef]
- Leapman, M.S.; Cowan, J.E.; Simko, J.; Roberge, G.; Stohr, B.A.; Carroll, P.R.; Cooperberg, M.R. Application of a Prognostic Gleason Grade Grouping System to Assess Distant Prostate Cancer Outcomes. Eur. Urol. 2017, 71, 750–759. [Google Scholar] [CrossRef]
- Loeb, S.; Folkvaljon, Y.; Robinson, D.; Lissbrant, I.F.; Egevad, L.; Stattin, P. Evaluation of the 2015 Gleason Grade Groups in a Nationwide Population-based Cohort. Eur. Urol. 2016, 69, 1135–1141. [Google Scholar] [CrossRef]
- Delahunt, B.; Egevad, L.; Srigley, J.R.; Steigler, A.; Murray, J.D.; Atkinson, C.; Matthews, J.; Duchesne, G.; Spry, N.A.; Christie, D.; et al. Validation of International Society of Urological Pathology (ISUP) grading for prostatic adenocarcinoma in thin core biopsies using TROG 03.04 ‘RADAR’ trial clinical data. Pathology 2015, 47, 520–525. [Google Scholar] [CrossRef]
- Berney, D.M.; Beltran, L.; Fisher, G.; North, B.V.; Greenberg, D.; Møller, H.; Soosay, G.; Scardino, P.; Cuzick, J. Validation of a contemporary prostate cancer grading system using prostate cancer death as outcome. Br. J. Cancer 2016, 114, 1078–1083. [Google Scholar] [CrossRef]
- Harding-Jackson, N.; Kryvenko, O.N.; Whittington, E.E.; Whittington, E.E.; Eastwood, D.C.; Tjionas, G.A.; Jorda, M.; Iczkowski, K.A. Outcome of Gleason 3 + 5 = 8 Prostate Cancer Diagnosed on Needle Biopsy: Prognostic Comparison with Gleason 4 + 4 = 8. J. Urol. 2016, 196, 1076–1081. [Google Scholar] [CrossRef]
- Russo, G.I.; Soeterik, T.; Puche-Sanz, I.; Broggi, G.; Lo Giudice, A.; De Nunzio, C.; Lombardo, R.; Marra, G.; Gandaglia, G.; European Association of Urology Young Academic Urologists. Oncological outcomes of cribriform histology pattern in prostate cancer patients: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2023, 26, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; van Leenders, G.; Zattoni, F.; Kesch, C.; Rajwa, P.; Cornford, P.; van der Kwast, T.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; et al. Impact of Epithelial Histological Types, Subtypes, and Growth Patterns on Oncological Outcomes for Patients with Nonmetastatic Prostate Cancer Treated with Curative Intent: A Systematic Review. Eur. Urol. 2023, 84, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Kweldam, C.F.; Wildhagen, M.F.; Steyerberg, E.W.; Bangma, C.H.; van der Kwast, T.H.; van Leenders, G.J. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod. Pathol. 2015, 28, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Choy, B.; Pearce, S.M.; Anderson, B.B.; Shalhav, A.L.; Zagaja, G.; Eggener, S.E.; Paner, G.P. Prognostic Significance of Percentage and Architectural Types of Contemporary Gleason Pattern 4 Prostate Cancer in Radical Prostatectomy. Am. J. Surg. Pathol. 2016, 40, 1400–1406. [Google Scholar] [CrossRef]
- Leung, D.; Castellani, D.; Nicoletti, R.; Dilme, R.V.; Sierra, J.M.; Serni, S.; Franzese, C.; Chiacchio, G.; Galosi, A.B.; Mazzucchelli, R.; et al. The Oncological and Functional Prognostic Value of Unconventional Histology of Prostate Cancer in Localized Disease Treated with Robotic Radical Prostatectomy: An International Multicenter 5-Year Cohort Study. Eur. Urol. Oncol. 2024, 7, 581–588. [Google Scholar] [CrossRef]
- Iczkowski, K.A.; Paner, G.P.; Van der Kwast, T. The New Realization About Cribriform Prostate Cancer. Adv. Anat. Pathol. 2018, 25, 31–37. [Google Scholar] [CrossRef]
- van der Kwast, T.H.; van Leenders, G.J.; Berney, D.M.; Delahunt, B.; Evans, A.J.; Iczkowski, K.A.; McKenney, J.K.; Ro, J.Y.; Samaratunga, H.; Srigley, J.R.; et al. ISUP Consensus Definition of Cribriform Pattern Prostate Cancer. Am. J. Surg. Pathol. 2021, 45, 1118–1126. [Google Scholar] [CrossRef]
- Shah, R.B.; Cai, Q.; Aron, M.; Berney, D.M.; Cheville, J.C.; Deng, F.M.; Epstein, J.; Fine, S.W.; Genega, E.M.; Hirsch, M.S.; et al. Diagnosis of “cribriform” prostatic adenocarcinoma: An interobserver reproducibility study among urologic pathologists with recommendations. Am. J. Cancer Res. 2021, 11, 3990–4001. [Google Scholar]
- Egevad, L.; Delahunt, B.; Iczkowski, K.A.; van der Kwast, T.; van Leenders, G.J.L.H.; Leite, K.R.M.; Pan, C.C.; Samaratunga, H.; Tsuzuki, T.; Mulliqi, N.; et al. Interobserver reproducibility of cribriform cancer in prostate needle biopsies and validation of International Society of Urological Pathology criteria. Histopathology 2023, 82, 837–845. [Google Scholar] [CrossRef]
- De Vivar, A.D.; Sayeeduddin, M.; Rowley, D.; Cubilla, A.; Miles, B.; Kadmon, D.; Ayala, G. Histologic features of stromogenic carcinoma of the prostate (carcinomas with reactive stroma grade 3). Hum. Pathol. 2017, 63, 202–211. [Google Scholar] [CrossRef]
- McKenney, J.K.; Wei, W.; Hawley, S.; Auman, H.; Newcomb, L.F.; Boyer, H.D.; Fazli, L.; Simko, J.; Hurtado-Coll, A.; Troyer, D.A.; et al. Histologic Grading of Prostatic Adenocarcinoma Can Be Further Optimized: Analysis of the Relative Prognostic Strength of Individual Architectural Patterns in 1275 Patients From the Canary Retrospective Cohort. Am. J. Surg. Pathol. 2016, 40, 1439–1456. [Google Scholar] [CrossRef]
- Venkataraman, G.; Rycyna, K.; Rabanser, A.; Heinze, G.; Baesens, B.M.; Ananthanarayanan, V.; Paner, G.P.; Barkan, G.A.; Flanigan, R.C.; Wojcik, E.M. Morphometric signature differences in nuclei of Gleason pattern 4 areas in Gleason 7 prostate cancer with differing primary grades on needle biopsy. J. Urol. 2009, 181, 88–93; discussion 93–94. [Google Scholar] [CrossRef]
- van der Slot, M.A.; Hollemans, E.; den Bakker, M.A.; Hoedemaeker, R.; Kliffen, M.; Budel, L.M.; Goemaere, N.N.T.; van Leenders, G.J.L.H. Inter-observer variability of cribriform architecture and percent Gleason pattern 4 in prostate cancer: Relation to clinical outcome. Virchows Arch. 2021, 478, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Berney, D.M.; Beltran, L.; Sandu, H.; Soosay, G.; Møller, H.; Scardino, P.; Murphy, J.; Ahmad, A.; Cuzick, J.; Transatlantic Prostate Group. The percentage of high-grade prostatic adenocarcinoma in prostate biopsies significantly improves on Grade Groups in the prediction of prostate cancer death. Histopathology 2019, 75, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Kong, M.X.; Zhou, M.; Rosenkrantz, A.B.; Taneja, S.S.; Melamed, J.; Deng, F.M. Gleason score 3 + 4 = 7 prostate cancer with minimal quantity of gleason pattern 4 on needle biopsy is associated with low-risk tumor in radical prostatectomy specimen. Am. J. Surg. Pathol. 2014, 38, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Kench, J.; Al-Hussain, T.; Brimo, F.; Cimadamore, A.; Eggener, S.; Fine, S.W.; Kristiansen, G.; Leite, K.R.M.; Oxley, J.; et al. International Collaboration on Cancer Reporting. Prostate Core Needle Biopsy Histopathology Reporting Guide—Specimen Level Reporting. Available online: https://www.iccr-cancer.org/ (accessed on 31 January 2025).
- Paner, G.P.; Srigley, J.R.; Harik, L.R.; Amin, M.B.; Eggener, S.E.; Huang, J.; Montironi, R.; Pettus, J.R.; Giannico, G.A.; Sirintrapun, S.J.; et al. College of American Pathologists. Protocols for the Examination of Prostate Needle Biopsies from Patients with Carcinoma of the Prostate Gland: Specimen Level Reporting. Available online: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates (accessed on 31 January 2025).
- Kunz, G.M., Jr.; Epstein, J.I. Should each core with prostate cancer be assigned a separate gleason score? Hum. Pathol. 2003, 34, 911–914. [Google Scholar] [CrossRef]
- Kunju, L.P.; Daignault, S.; Wei, J.T.; Shah, R.B. Multiple prostate cancer cores with different Gleason grades submitted in the same specimen container without specific site designation: Should each core be assigned an individual Gleason score? Hum. Pathol. 2009, 40, 558–564. [Google Scholar] [CrossRef]
- Paner, G.P.; Kench, J.; Al-Hussain, T.; Brimo, F.; Cimadamore, A.; Eggener, S.; Fine, S.W.; Kristiansen, G.; Leite, K.R.M.; Oxley, J.; et al. International Collaboration on Cancer. Reporting Prostate Core Needle Biopsy Histopathology Reporting Guide—Case Level Reporting. Available online: https://www.iccr-cancer.org/ (accessed on 31 January 2025).
- Paner, G.P.; Srigley, J.R.; Harik, L.R.; Amin, M.B.; Eggener, S.E.; Huang, J.; Montironi, R.; Pettus, J.R.; Giannico, G.A.; Sirintrapun, S.J.; et al. College of American Pathologists. Protocols for the Examination of Prostate Needle Biopsies from Patients with Carcinoma of the Prostate Gland: Case Level Reporting. Available online: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates (accessed on 31 January 2025).
- Varma, M.; Narahari, K.; Mason, M.; Oxley, J.D.; Berney, D.M. Contemporary prostate biopsy reporting: Insights from a survey of clinicians’ use of pathology data. J. Clin. Pathol. 2018, 71, 874–878. [Google Scholar] [CrossRef]
- Tolonen, T.T.; Kujala, P.M.; Tammela, T.L.; Tuominen, V.J.; Isola, J.J.; Visakorpi, T. Overall and worst gleason scores are equally good predictors of prostate cancer progression. BMC Urol. 2011, 11, 21. [Google Scholar] [CrossRef]
- Arias-Stella, J.A., 3rd; Shah, A.B.; Montoya-Cerrillo, D.; Williamson, S.R.; Gupta, N.S. Prostate biopsy and radical prostatectomy Gleason score correlation in heterogenous tumors: Proposal for a composite Gleason score. Am. J. Surg. Pathol. 2015, 39, 1213–1218. [Google Scholar] [CrossRef]
- Deng, F.M.; Isaila, B.; Jones, D.; Ren, Q.; Kyung, P.; Hoskoppal, D.; Huang, H.; Mirsadraei, L.; Xia, Y.; Melamed, J. Optimal Method for Reporting Prostate Cancer Grade in MRI-targeted Biopsies. Am. J. Surg. Pathol. 2022, 46, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.; Pepe, L.; Pennisi, M.; Fraggetta, F. Which Prostate Biopsy in Men Enrolled in Active Surveillance? Experience in 110 Men Submitted to Scheduled Three-Years Transperineal Saturation Biopsy Combined with Fusion Targeted Cores. Clin. Genitourin. Cancer 2021, 19, 305–308. [Google Scholar] [CrossRef]
- Fiorentino, V.; Martini, M.; Dell’Aquila, M.; Musarra, T.; Orticelli, E.; Larocca, L.M.; Rossi, E.; Totaro, A.; Pinto, F.; Lenci, N.; et al. Histopathological Ratios to Predict Gleason Score Agreement between Biopsy and Radical Prostatectomy. Diagnostics 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.; Pepe, L.; Curduman, M.; Pennisi, M.; Fraggetta, F. Ductal prostate cancer staging: Role of PSMA PET/CT. Arch. Ital. Urol. Androl. 2024, 96, 12132. [Google Scholar] [CrossRef]
- Matsumoto, K.; Omura, M.; Takeda, T.; Kosaka, T.; Hashiguchi, A.; Takamatsu, K.; Yasumizu, Y.; Tanaka, N.; Morita, S.; Mizuno, R.; et al. Grading of Multifocal Prostate Cancer Cases in which the Largest Volume and the Highest Grade Do Not Coincide within One Lesion. J. Urol. 2021, 206, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Best, O.; Canagasingham, A.; Liu, Z.; Doan, P.; Haynes, A.M.; Delprado, W.; Maclean, F.; Yuen, C.; Stricker, P.; Thompson, J. Index grade group is superior to composite grade group for prediction of biochemical recurrence following radical prostatectomy. Pathology 2023, 55, 492–497. [Google Scholar] [CrossRef]
- Kweldam, C.F.; Kummerlin, I.P.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Incrocci, L.; van der Kwast, T.H.; Roobol, M.J.; van Leenders, J.V. Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3 + 4 = 7 prostate cancer. Mod. Pathol. 2017, 30, 1126–1132. [Google Scholar] [CrossRef]
- Kweldam, C.F.; Kummerlin, I.P.; Nieboer, D.; Verhoef, E.I.; Steyerberg, E.W.; van der Kwast, T.H.; Roobol, M.J.; van Leenders, G.J. Disease-specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod. Pathol. 2016, 29, 630–636. [Google Scholar] [CrossRef]
- Kweldam, C.F.; Kümmerlin, I.P.; Nieboer, D.; Verhoef, E.I.; Steyerberg, E.W.; Incrocci, L.; Bangma, C.H.; van der Kwast, T.H.; Roobol, M.J.; van Leenders, G.J. Prostate cancer outcomes of men with biopsy Gleason score 6 and 7 without cribriform or intraductal carcinoma. Eur. J. Cancer 2016, 66, 26–33. [Google Scholar] [CrossRef]
- Chan, E.; McKenney, J.K.; Hawley, S.; Corrigan, D.; Auman, H.; Newcomb, L.F.; Boyer, H.D.; Carroll, P.R.; Cooperberg, M.R.; Klein, E.; et al. Analysis of separate training and validation radical prostatectomy cohorts identifies 0.25 mm diameter as an optimal definition for “large” cribriform prostatic adenocarcinoma. Mod. Pathol. 2022, 35, 1092–1100. [Google Scholar] [CrossRef]
- Greenland, N.Y.; Cowan, J.E.; Zhang, L.; Carroll, P.R.; Chan, E.; Stohr, B.A.; and Simko, J.P. Expansile cribriform Gleason pattern 4 has histopathologic and molecular features of aggressiveness and greater risk of biochemical failure compared to glomerulation Gleason pattern 4. Prostate 2020, 80, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Hollemans, E.; Verhoef, E.I.; Bangma, C.H.; Rietbergen, J.; Helleman, J.; Roobol, M.J.; van Leenders, G. Large cribriform growth pattern identifies ISUP grade 2 prostate cancer at high risk for recurrence and metastasis. Mod. Pathol. 2019, 32, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hollemans, E.; Verhoef, E.I.; Bangma, C.H.; Rietbergen, J.; Osanto, S.; Pelger, R.C.M.; van Wezel, T.; van der Poel, H.; Bekers, E.; Helleman, J.; et al. Clinicopathological characteristics of glomeruloid architecture in prostate cancer. Mod. Pathol. 2020, 33, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Hollemans, E.; Verhoef, E.I.; Bangma, C.H.; Rietbergen, J.; Roobol, M.J.; Helleman, J.; van Leenders, G. Clinical outcome comparison of Grade Group 1 and Grade Group 2 prostate cancer with and without cribriform architecture at the time of radical prostatectomy. Histopathology 2020, 76, 755–762. [Google Scholar] [CrossRef]
- Aiyer, K.T.S.; Kroon, L.J.; van Leenders, G. Impact of comedonecrosis on prostate cancer outcome: A systematic review. Histopathology 2023, 83, 339–347. [Google Scholar] [CrossRef]
- Hansum, T.; Hollemans, E.; Verhoef, E.I.; Bangma, C.H.; Rietbergen, J.; Osanto, S.; Pelger, R.C.M.; van Wezel, T.; van der Poel, H.; Bekers, E.; et al. Comedonecrosis Gleason pattern 5 is associated with worse clinical outcome in operated prostate cancer patients. Mod. Pathol. 2021, 34, 2064–2070. [Google Scholar] [CrossRef]
- Zelic, R.; Giunchi, F.; Fridfeldt, J.; Carlsson, J.; Davidsson, S.; Lianas, L.; Mascia, C.; Zugna, D.; Molinaro, L.; Vincent, P.H.; et al. Prognostic Utility of the Gleason Grading System Revisions and Histopathological Factors Beyond Gleason Grade. Clin. Epidemiol. 2022, 14, 59–70. [Google Scholar] [CrossRef]
- Cole, A.I.; Morgan, T.M.; Spratt, D.E.; Palapattu, G.S.; He, C.; Tomlins, S.A.; Weizer, A.Z.; Feng, F.Y.; Wu, A.; Siddiqui, J.; et al. Prognostic Value of Percent Gleason Grade 4 at Prostate Biopsy in Predicting Prostatectomy Pathology and Recurrence. J. Urol. 2016, 196, 405–411. [Google Scholar] [CrossRef]
- Sauter, G.; Steurer, S.; Clauditz, T.S.; Krech, T.; Wittmer, C.; Lutz, F.; Lennartz, M.; Janssen, T.; Hakimi, N.; Simon, R.; et al. Clinical Utility of Quantitative Gleason Grading in Prostate Biopsies and Prostatectomy Specimens. Eur. Urol. 2016, 69, 592–598. [Google Scholar] [CrossRef]
- Sadimin, E.T.; Khani, F.; Diolombi, M.; Meliti, A.; Epstein, J.I. Interobserver Reproducibility of Percent Gleason Pattern 4 in Prostatic Adenocarcinoma on Prostate Biopsies. Am. J. Surg. Pathol. 2016, 40, 1686–1692. [Google Scholar] [CrossRef]
- Kir, G.; Seneldir, H.; Gumus, E. Outcomes of Gleason score 3 + 4 = 7 prostate cancer with minimal amounts (<6%) vs. >/=6% of Gleason pattern 4 tissue in needle biopsy specimens. Ann. Diagn. Pathol. 2016, 20, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Hirakawa, A.; Sato, H.; Hanazawa, R.; Naito, Y.; Tochigi, K.; Sano, T.; Ishida, S.; Funahashi, Y.; Fujita, T.; et al. Grade group 2 (10% >/= GP4) patients have very similar malignant potential with grade group 1 patients, given the risk of intraductal carcinoma of the prostate. Int. J. Clin. Oncol. 2021, 26, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.W.; Assel, M.; Sjoberg, D.D.; Vickers, A.J.; Al-Ahmadie, H.A.; Chen, Y.B.; Gopalan, A.; Sirintrapun, S.J.; Tickoo, S.K.; Eastham, J.A.; et al. Clinical Usefulness of Total Length of Gleason Pattern 4 on Biopsy in Men with Grade Group 2 Prostate Cancer. J. Urol. 2019, 201, 77–82. [Google Scholar] [CrossRef]
- Perera, M.; Assel, M.J.; Benfante, N.E.; Vickers, A.J.; Reuter, V.E.; Carlsson, S.; Laudone, V.; Touijer, K.A.; Eastham, J.A.; Scardino, P.T.; et al. Oncologic Outcomes of Total Length Gleason Pattern 4 on Biopsy in Men with Grade Group 2 Prostate Cancer. J. Urol. 2022, 208, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Iakymenko, O.A.; Lugo, I.; Briski, L.M.; Nemov, I.; Punnen, S.; Kwon, D.; Pollack, A.; Stoyanova, R.; Parekh, D.J.; Jorda, M.; et al. Percentage of Gleason pattern 4 and tumor volume predict adverse pathological stage and margin status at radical prostatectomy in grade Group 2 and grade Group 3 prostate cancers. Prostate 2021, 81, 866–873. [Google Scholar] [CrossRef]
- Deng, F.M.; Donin, N.M.; Pe Benito, R.; Melamed, J.; Le Nobin, J.; Zhou, M.; Ma, S.; Wang, J.; Lepor, H. Size-adjusted Quantitative Gleason Score as a Predictor of Biochemical Recurrence after Radical Prostatectomy. Eur. Urol. 2016, 70, 248–253. [Google Scholar] [CrossRef]
- Vickers, A.J.; Assel, M.; Cooperberg, M.R.; Fine, S.W.; Eggener, S. Amount of Gleason Pattern 3 Is Not Predictive of Risk in Grade Group 2-4 Prostate Cancer. Eur. Urol. 2024, 86, S0302–S2838. [Google Scholar] [CrossRef]
- Andolfi, C.; Vickers, A.J.; Cooperberg, M.R.; Carroll, P.R.; Cowan, J.E.; Paner, G.P.; Helfand, B.T.; Liauw, S.L.; Eggener, S.E. Blood Prostate-specific Antigen by Volume of Benign, Gleason Pattern 3 and 4 Prostate Tissue. Urology 2022, 170, 154–160. [Google Scholar] [CrossRef]
- Stamey, T.A.; Yemoto, C.M.; McNeal, J.E.; Sigal, B.M.; Johnstone, I.M. Prostate cancer is highly predictable: A prognostic equation based on all morphological variables in radical prostatectomy specimens. J. Urol. 2000, 163, 1155–1160. [Google Scholar] [CrossRef]
- Miura, N.; Mori, K.; Mostafaei, H.; Quhal, F.; Motlagh, R.S.; Pradere, B.; Laukhtina, E.; D’Andrea, D.; Saika, T.; Shariat, S.F. The Prognostic Impact of Intraductal Carcinoma of the Prostate: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 909–917. [Google Scholar] [CrossRef]
- Amin, B.; Edge, B.; Greene, L.; Byrd, R.; Brookland, K.; Washington, K.; Gershenwald, E.; Compton, C.; Hess, R.; Sullivan, C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer Nature: Cham, Switzerland, 2017. [Google Scholar]
- Kato, M.; Tsuzuki, T.; Kimura, K.; Hirakawa, A.; Kinoshita, F.; Sassa, N.; Ishida, R.; Fukatsu, A.; Kimura, T.; Funahashi, Y.; et al. The presence of intraductal carcinoma of the prostate in needle biopsy is a significant prognostic factor for prostate cancer patients with distant metastasis at initial presentation. Mod. Pathol. 2016, 29, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.J.; Wheeler, T.M.; Bonkhoff, H.; Rubin, M.A. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch. Pathol. Lab. Med. 2007, 131, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Epstein, J.I. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod. Pathol. 2006, 19, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.H.; Lawrence, M.G.; Ilic, D.; Clouston, D.; Bolton, D.M.; Frydenberg, M.; Murphy, D.G.; Pezaro, C.; Risbridger, G.P.; Taylor, R.A. Systematic Review Links the Prevalence of Intraductal Carcinoma of the Prostate to Prostate Cancer Risk Categories. Eur. Urol. 2017, 72, 492–495. [Google Scholar] [CrossRef]
- McNeal, J.E.; Yemoto, C.E. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am. J. Surg. Pathol. 1996, 20, 802–814. [Google Scholar] [CrossRef]
- Moch, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V.E. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- Lotan, T.L.; Gumuskaya, B.; Rahimi, H.; Hicks, J.L.; Iwata, T.; Robinson, B.D.; Epstein, J.I.; De Marzo, A.M. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod. Pathol. 2013, 26, 587–603. [Google Scholar] [CrossRef]
- Fine, S.W.; Al-Ahmadie, H.A.; Chen, Y.B.; Gopalan, A.; Tickoo, S.K.; Reuter, V.E. Comedonecrosis Revisited: Strong Association with Intraductal Carcinoma of the Prostate. Am. J. Surg. Pathol. 2018, 42, 1036–1041. [Google Scholar] [CrossRef]
- Miyai, K.; Divatia, M.K.; Shen, S.S.; Miles, B.J.; Ayala, A.G.; Ro, J.Y. Heterogeneous clinicopathological features of intraductal carcinoma of the prostate: A comparison between “precursor-like” and “regular type” lesions. Int. J. Clin. Exp. Pathol. 2014, 7, 2518–2526. [Google Scholar]
- Robinson, B.D.; Epstein, J.I. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: Emphasis on radical prostatectomy findings. J. Urol. 2010, 184, 1328–1333. [Google Scholar] [CrossRef]
- Cohen, R.J.; Shannon, B.A.; Weinstein, S.L. Intraductal carcinoma of the prostate gland with transmucosal spread to the seminal vesicle: A lesion distinct from high-grade prostatic intraepithelial neoplasia. Arch. Pathol. Lab. Med. 2007, 131, 1122–1125. [Google Scholar] [CrossRef]
- Grypari, I.M.; Logotheti, S.; Lazaris, A.C.; Kallidonis, P.; Fokaefs, E.; Melachrinou, M.; Zolota, V.; Tzelepi, V. Isolated Intraductal Carcinoma of the Prostate in Prostatectomy Specimens: Report of 2 Cases and Review of the Literature. Int. J. Surg. Pathol. 2020, 28, 918–924. [Google Scholar] [CrossRef]
- Khani, F.; Epstein, J.I. Prostate Biopsy Specimens with Gleason 3 + 3 = 6 and Intraductal Carcinoma: Radical Prostatectomy Findings and Clinical Outcomes. Am. J. Surg. Pathol. 2015, 39, 1383–1389. [Google Scholar] [CrossRef]
- Rijstenberg, L.L.; Hansum, T.; Hollemans, E.; Kweldam, C.F.; Kummerlin, I.P.; Bangma, C.H.; van der Kwast, T.H.; Roobol, M.J.; van Leenders, G. Intraductal carcinoma has a minimal impact on Grade Group assignment in prostate cancer biopsy and radical prostatectomy specimens. Histopathology 2020, 77, 742–748. [Google Scholar] [CrossRef]
- Cai, Q.; Shah, R.B. Cribriform Lesions of the Prostate Gland. Surg. Pathol. Clin. 2022, 15, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Kench, J.; Paner, G.P.; Srigley, J.R.; l-Hussain, T.; Brimo, F.; Cimadamore, A.; Eggener, S.; Fine, S.W.; Kristiansen, G.; Moreira Leite, K.; et al. International Collaboration on Cancer. Reporting Prostate Core Histopathology Reporting Guide Radical Prostatectomy Specimen. Available online: https://www.iccr-cancer.org/ (accessed on 31 January 2025).
- Shah, R.B.; Nguyen, J.K.; Przybycin, C.G.; Reynolds, J.P.; Cox, R.; Myles, J.; Klein, E.; McKenney, J.K. Atypical intraductal proliferation detected in prostate needle biopsy is a marker of unsampled intraductal carcinoma and other adverse pathological features: A prospective clinicopathological study of 62 cases with emphasis on pathological outcomes. Histopathology 2019, 75, 346–353. [Google Scholar] [CrossRef]
- Shah, R.B.; Magi-Galluzzi, C.; Han, B.; Zhou, M. Atypical cribriform lesions of the prostate: Relationship to prostatic carcinoma and implication for diagnosis in prostate biopsies. Am. J. Surg. Pathol. 2010, 34, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.B.; Zhou, M. Atypical cribriform lesions of the prostate: Clinical significance, differential diagnosis and current concept of intraductal carcinoma of the prostate. Adv. Anat. Pathol. 2012, 19, 270–278. [Google Scholar] [CrossRef]
- Shah, R.B.; Yoon, J.; Liu, G.; Tian, W. Atypical intraductal proliferation and intraductal carcinoma of the prostate on core needle biopsy: A comparative clinicopathological and molecular study with a proposal to expand the morphological spectrum of intraductal carcinoma. Histopathology 2017, 71, 693–702. [Google Scholar] [CrossRef]
- Morais, C.L.; Han, J.S.; Gordetsky, J.; Nagar, M.S.; Anderson, A.E.; Lee, S.; Hicks, J.L.; Zhou, M.; Magi-Galluzzi, C.; Shah, R.B.; et al. Utility of PTEN and ERG immunostaining for distinguishing high-grade PIN from intraductal carcinoma of the prostate on needle biopsy. Am. J. Surg. Pathol. 2015, 39, 169–178. [Google Scholar] [CrossRef]
- Miyai, K.; Divatia, M.K.; Shen, S.S.; Miles, B.J.; Ayala, A.G.; Ro, J.Y. Clinicopathological analysis of intraductal proliferative lesions of prostate: Intraductal carcinoma of prostate, high-grade prostatic intraepithelial neoplasia, and atypical cribriform lesion. Hum. Pathol. 2014, 45, 1572–1581. [Google Scholar] [CrossRef]
- Hickman, R.A.; Yu, H.; Li, J.; Kong, M.; Shah, R.B.; Zhou, M.; Melamed, J.; Deng, F.M. Atypical Intraductal Cribriform Proliferations of the Prostate Exhibit Similar Molecular and Clinicopathologic Characteristics as Intraductal Carcinoma of the Prostate. Am. J. Surg. Pathol. 2017, 41, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.; Cheng, L. Morphologic spectrum of treatment-related changes in prostate tissue and prostate cancer: An updated review. Hum. Pathol. 2022, 127, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J. Treatment effects in prostate cancer. Mod. Pathol. 2018, 31, S110–S121. [Google Scholar] [CrossRef]
- Srigley, J.R.; Delahunt, B.; Evans, A.J. Therapy-associated effects in the prostate gland. Histopathology 2012, 60, 153–165. [Google Scholar] [CrossRef]
- Ryan, P.; Finelli, A.; Lawrentschuk, N.; Fleshner, N.; Sweet, J.; Cheung, C.; van der Kwast, T.; Evans, A. Prostatic needle biopsies following primary high intensity focused ultrasound (HIFU) therapy for prostatic adenocarcinoma: Histopathological features in tumour and non-tumour tissue. J. Clin. Pathol. 2012, 65, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, V.; Martinez, C.H.; Williams, A.K.; Kwan, K.; Chin, J.L. Histological changes in the human prostate after radiotherapy and salvage high intensity focused ultrasound. Can. Urol. Assoc. J. 2010, 4, E100–E102. [Google Scholar] [CrossRef] [PubMed]
- Biermann, K.; Montironi, R.; Lopez-Beltran, A.; Zhang, S.; Cheng, L. Histopathological findings after treatment of prostate cancer using high-intensity focused ultrasound (HIFU). Prostate 2010, 70, 1196–1200. [Google Scholar] [CrossRef]
- Harnden, P.; Shelley, M.D.; Naylor, B.; Coles, B.; Mason, M.D. Does the extent of carcinoma in prostatic biopsies predict prostate-specific antigen recurrence? A systematic review. Eur. Urol. 2008, 54, 728–739. [Google Scholar] [CrossRef]
- Berney, D.M.; Finnegan, K.; Chu, K.; Fine, S.W.; Varma, M.; Cuzick, J.; Beltran, L.G. Transatlantic Prostate. Measuring cancer burden in prostatic needle core biopsies: Simplified assessments outperform complex measurements in assessing outcome: Evidence to assist pathologist efficiency and minimize datasets. Histopathology 2023, 82, 1021–1028. [Google Scholar] [CrossRef]
- Russo, G.I.; Cimino, S.; Castelli, T.; Favilla, V.; Urzi, D.; Veroux, M.; Madonia, M.; Morgia, G. Percentage of cancer involvement in positive cores can predict unfavorable disease in men with low-risk prostate cancer but eligible for the prostate cancer international: Active surveillance criteria. Urol. Oncol. 2014, 32, 291–296. [Google Scholar] [CrossRef]
- Morselli, S.; Sebastianelli, A.; Campi, R.; Liaci, A.; Gabellini, L.; Tasso, G.; Fantechi, R.; Venturini, S.; Spatafora, P.; Cito, G.; et al. Adverse pathology after radical prostatectomy: The prognostic role of cumulative cancer length >6-mm threshold in prostate cancer-positive biopsies. Prostate Int. 2019, 7, 143–149. [Google Scholar] [CrossRef]
- Vellekoop, A.; Loeb, S.; Folkvaljon, Y.; Stattin, P. Population based study of predictors of adverse pathology among candidates for active surveillance with Gleason 6 prostate cancer. J. Urol. 2014, 191, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Audenet, F.; Vertosick, E.A.; Fine, S.W.; Sjoberg, D.D.; Vickers, A.J.; Reuter, V.E.; Eastham, J.A.; Scardino, P.R.; Touijer, K.A. Biopsy Core Features are Poor Predictors of Adverse Pathology in Men with Grade Group 1 Prostate Cancer. J. Urol. 2018, 199, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Mamawala, M.; Patel, H.D.; Alam, R.; Epstein, J.I.; Ross, A.E.; Carter, H.B. Tumor Volume on Biopsy of Low Risk Prostate Cancer Managed with Active Surveillance. J. Urol. 2018, 199, 954–960. [Google Scholar] [CrossRef]
- Cooley, L.F.; Emeka, A.A.; Meyers, T.J.; Cooper, P.R.; Lin, D.W.; Finelli, A.; Eastham, J.A.; Logothetis, C.J.; Marks, L.S.; Vesprini, D.; et al. Factors Associated with Time to Conversion from Active Surveillance to Treatment for Prostate Cancer in a Multi-Institutional Cohort. J. Urol. 2021, 206, 1147–1156. [Google Scholar] [CrossRef]
- Arias-Stella, J.A., 3rd; Varma, K.R.; Montoya-Cerrillo, D.; Gupta, N.S.; Williamson, S.R. Does discontinuous involvement of a prostatic needle biopsy core by adenocarcinoma correlate with a large tumor focus at radical prostatectomy? Am. J. Surg. Pathol. 2015, 39, 281–286. [Google Scholar] [CrossRef]
- Schultz, L.; Maluf, C.E.; da Silva, R.C.; Falashi Rde, H.; da Costa, M.V.; Schultz, M.I. Discontinuous foci of cancer in a single core of prostatic biopsy: When it occurs and performance of quantification methods in a private-practice setting. Am. J. Surg. Pathol. 2013, 37, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Karram, S.; Trock, B.J.; Netto, G.J.; Epstein, J.I. Should intervening benign tissue be included in the measurement of discontinuous foci of cancer on prostate needle biopsy? Correlation with radical prostatectomy findings. Am. J. Surg. Pathol. 2011, 35, 1351–1355. [Google Scholar] [CrossRef]
- Yuk, H.D.; Byun, S.S.; Hong, S.K.; Lee, H. The tumor volume after radical prostatectomy and its clinical impact on the prognosis of patients with localized prostate cancer. Sci. Rep. 2022, 12, 6003. [Google Scholar] [CrossRef]
- Baba, H.; Sakamoto, S.; Zhao, X.; Yamada, Y.; Rii, J.; Fujimoto, A.; Kanesaka, M.; Takeuchi, N.; Sazuka, T.; Imamura, Y.; et al. Tumor Location and a Tumor Volume over 2.8 cc Predict the Prognosis for Japanese Localized Prostate Cancer. Cancers 2022, 14, 5823. [Google Scholar] [CrossRef]
- Alenezi, A.; Ismail, M.; Eden, C. Can Tumour Volume Percentage in Radical Prostatectomy Predict Cancer Biochemical Recurrence? Determining a Cut-off Point and Composite Risk Factors Approach. Res. Rep. Urol. 2021, 13, 445–455. [Google Scholar] [CrossRef]
- Ettel, M.; Kong, M.; Lee, P.; Zhou, M.; Melamed, J.; Deng, F.M. Modification of the pT2 substage classification in prostate adenocarcinoma. Hum. Pathol. 2016, 56, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.B.; Hamstra, D.A.; Jackson, W.C.; Zhou, J.; Foster, B.; Foster, C.; Song, Y.; Li, D.; Palapattu, G.S.; Kunju, L.; et al. Larger maximum tumor diameter at radical prostatectomy is associated with increased biochemical failure, metastasis, and death from prostate cancer after salvage radiation for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Billis, A.; Meirelles, L.R.; Freitas, L.L.; Polidoro, A.S.; Fernandes, H.A.; Padilha, M.M.; Magna, L.A.; Ferreira, U. Prostate total tumor extent versus index tumor extent--which is predictive of biochemical recurrence following radical prostatectomy? J. Urol. 2013, 189, 99–104. [Google Scholar] [CrossRef]
- van der Kwast, T.H.; Amin, M.B.; Billis, A.; Epstein, J.I.; Griffiths, D.; Humphrey, P.A.; Montironi, R.; Wheeler, T.M.; Srigley, J.R.; Egevad, L.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 2: T2 substaging and prostate cancer volume. Mod. Pathol. 2011, 24, 16–25. [Google Scholar] [CrossRef]
- Ito, Y.; Vertosick, E.A.; Sjoberg, D.D.; Vickers, A.J.; Al-Ahmadie, H.A.; Chen, Y.A.; Gopalan, A.; Sirintrapun, S.J.; Tickoo, S.K.; Eastham, J.A.; et al. In Organ-confined Prostate Cancer, Tumor Quantitation Not Found to Aid in Prediction of Biochemical Recurrence. Am. J. Surg. Pathol. 2019, 43, 1061–1065. [Google Scholar] [CrossRef]
- Billis, A.; Meirelles, L.; Freitas, L.L.; Magna, L.A.; Ferreira, U.; Reis, L.O. Tumor extent in radical prostatectomy specimens: Is it an independent prognostic factor for biochemical (PSA) progression following surgery? Int. Urol. Nephrol. 2011, 43, 417–422. [Google Scholar] [CrossRef]
- Wolters, T.; Roobol, M.J.; van Leeuwen, P.J.; van den Bergh, R.C.; Hoedemaeker, R.F.; van Leenders, G.J.; Schroder, F.H.; van der Kwast, T.H. Should pathologists routinely report prostate tumour volume? The prognostic value of tumour volume in prostate cancer. Eur. Urol. 2010, 57, 821–829. [Google Scholar] [CrossRef]
- Salomon, L.; Levrel, O.; Anastasiadis, A.G.; Irani, J.; De La Taille, A.; Saint, F.; Vordos, D.; Cicco, A.; Hoznek, A.; Chopin, D.; et al. Prognostic significance of tumor volume after radical prostatectomy: A multivariate analysis of pathological prognostic factors. Eur. Urol. 2003, 43, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Parameshwaran, V.; Beltran, L.; Fisher, G.; North, B.V.; Greenberg, D.; Soosay, G.; Moller, H.; Scardino, P.; Cuzick, J.; et al. Should reporting of peri-neural invasion and extra prostatic extension be mandatory in prostate cancer biopsies? correlation with outcome in biopsy cases treated conservatively. Oncotarget 2018, 9, 20555–20562. [Google Scholar] [CrossRef]
- DeLancey, J.O.; Wood, D.P., Jr.; He, C.; Montgomery, J.S.; Weizer, A.Z.; Miller, D.C.; Jacobs, B.L.; Montie, J.E.; Hollenbeck, B.K.; Skolarus, T.A. Evidence of perineural invasion on prostate biopsy specimen and survival after radical prostatectomy. Urology 2013, 81, 354–357. [Google Scholar] [CrossRef]
- Feng, F.Y.; Qian, Y.; Stenmark, M.H.; Halverson, S.; Blas, K.; Vance, S.; Sandler, H.M.; Hamstra, D.A. Perineural invasion predicts increased recurrence, metastasis, and death from prostate cancer following treatment with dose-escalated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e361–e367. [Google Scholar] [CrossRef]
- Strom, P.; Nordstrom, T.; Delahunt, B.; Samaratunga, H.; Gronberg, H.; Egevad, L.; Eklund, M. Prognostic value of perineural invasion in prostate needle biopsies: A population-based study of patients treated by radical prostatectomy. J. Clin. Pathol. 2020, 73, 630–635. [Google Scholar] [CrossRef]
- Ciftci, S.; Yilmaz, H.; Ciftci, E.; Simsek, E.; Ustuner, M.; Yavuz, U.; Muezzinoglu, B.; Dillioglugil, O. Perineural invasion in prostate biopsy specimens is associated with increased bone metastasis in prostate cancer. Prostate 2015, 75, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, X.; Lin, S.X.; Lu, M.; Deng, T.; Wang, Z.; Olumi, A.F.; Dahl, D.M.; Wang, D.; Blute, M.L.; et al. Impact of biopsy perineural invasion on the outcomes of patients who underwent radical prostatectomy: A systematic review and meta-analysis. Scand. J. Urol. 2019, 53, 287–294. [Google Scholar] [CrossRef]
- Trpkov, C.; Yilmaz, A.; Trpkov, K. Perineural invasion in prostate cancer patients who are potential candidates for active surveillance: Validation study. Urology 2014, 84, 149–152. [Google Scholar] [CrossRef]
- Moreira, D.M.; Fleshner, N.E.; Freedland, S.J. Baseline Perineural Invasion is Associated with Shorter Time to Progression in Men with Prostate Cancer Undergoing Active Surveillance: Results from the REDEEM Study. J. Urol. 2015, 194, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M., 2nd; Yecies, T.S.; Yabes, J.G.; Ristau, B.T.; Woldemichael, E.; Davies, B.J.; Jacobs, B.L.; Nelson, J.B. Biopsy Perineural Invasion in Prostate Cancer Patients Who Are Candidates for Active Surveillance by Strict and Expanded Criteria. Urology 2017, 102, 173–177. [Google Scholar] [CrossRef]
- de la Calle, C.M.; Mamawala, M.M.; Landis, P.; Landis, P.; Macura, K.J.; Trock, B.J.; Epstein, J.I.; Pavlovich, C.P. Clinical Significance of Perineural Invasion in Men with Grade Group 1 Prostate Cancer on Active Surveillance. J. Urol. 2023, 209, 180–186. [Google Scholar] [CrossRef]
- Baraban, E.; Erak, E.; Fatima, A.; Akbari, A.; Zhao, J.; Fletcher, S.A.; Bhanji, Y.; de la Calle, C.M.; Mamawala, M.; Landis, P.; et al. Identifying Men Who Can Remain on Active Surveillance Despite Biopsy Reclassification to Grade Group 2 Prostate Cancer. J. Urol. 2023, 210, 99–107. [Google Scholar] [CrossRef]
- Wu, S.; Xie, L.; Lin, S.X.; Wirth, G.J.; Lu, M.; Zhang, Y.; Blute, M.L.; Dahl, D.M.; Wu, C.L. Quantification of perineural invasion focus after radical prostatectomy could improve predictive power of recurrence. Hum. Pathol. 2020, 104, 96–104. [Google Scholar] [CrossRef]
- Stankovic, M.; Wolff, L.; Wieder, T.; Mendes, J.; Schumacher, B. Perineural invasion as predictor of biochemical recurrence in prostate cancer following open radical prostatectomy: A single-center experience. World J. Urol. 2022, 40, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Oh, J.J.; Lee, S.; Hong, S.K.; Lee, S.E.; Byun, S.S. Perineural Invasion and Lymphovascular Invasion are Associated with Increased Risk of Biochemical Recurrence in Patients Undergoing Radical Prostatectomy. Ann. Surg. Oncol. 2016, 23, 2699–2706. [Google Scholar] [CrossRef]
- Jamil, M.; Rakic, N.; Sood, A.; Keeley, J.; Modonutti, D.; Novara, G.; Jeong, W.; Menon, M.; Rogers, C.G.; Abdollah, F. Impact of Lymphovascular Invasion on Overall Survival in Patients with Prostate Cancer Following Radical Prostatectomy: Stage-per-Stage Analysis. Clin. Genitourin. Cancer 2021, 19, e319–e325. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, H.S.; Jang, W.S.; Kwon, J.K.; Yoon, C.Y.; Lee, J.Y.; Cho, K.S.; Ham, W.S.; Choi, Y.D. Impact of lymphovascular invasion on lymph node metastasis for patients undergoing radical prostatectomy with negative resection margin. BMC Cancer 2017, 17, 321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, L.; Wu, B.; Zha, Z.; Zhao, H.; Jun, Y.; Jiang, Y. The impact of lymphovascular invasion in patients with prostate cancer following radical prostatectomy and its association with their clinicopathological features: An updated PRISMA-compliant systematic review and meta-analysis. Medicine 2018, 97, e13537. [Google Scholar] [CrossRef]
- Kawase, M.; Ebara, S.; Tatenuma, T.; Sasaki, T.; Ikehata, Y.; Nakayama, A.; Toide, M.; Yoneda, T.; Sakaguchi, K.; Teishima, J.; et al. Prognostic Importance of Lymphovascular Invasion for Specific Subgroup of Patients with Prostate Cancer After Robot-Assisted Radical Prostatectomy (The MSUG94 Group). Ann. Surg. Oncol. 2024, 31, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, S.; Rogers, C.G.; Trinh, Q.D.; Sammon, J.; Sood, A.; Stricker, H.; Peabody, J.O.; Menon, M.; Diaz-Insua, M. Oncological outcomes after robot-assisted radical prostatectomy: Long-term follow-up in 4803 patients. BJU Int. 2014, 114, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, J.; Wu, S.; Guo, M.; Zhang, Y.; Liu, R. The impact of surgical margin status on prostate cancer-specific mortality after radical prostatectomy: A systematic review and meta-analysis. Clin. Transl. Oncol. 2020, 22, 2087–2096. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.; Zha, Z.; Zhao, H.; Yuan, J.; Jiang, Y.; Yang, W. Surgical margin status and its impact on prostate cancer prognosis after radical prostatectomy: A meta-analysis. World J. Urol. 2018, 36, 1803–1815. [Google Scholar] [CrossRef]
- Yossepowitch, O.; Briganti, A.; Eastham, J.A.; Epstein, J.; Graefen, M.; Montironi, R.; Touijer, K. Positive surgical margins after radical prostatectomy: A systematic review and contemporary update. Eur. Urol. 2014, 65, 303–313. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Lim, A.; Catterwell, R.; Selth, L.; O’Callaghan, M. Length of positive surgical margins after radical prostatectomy: Does size matter?—A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2023, 26, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Servoll, E.; Vlatkovic, L.; Saeter, T.; Nesland, J.M.; Axcrona, U.; Waaler, G.; Axcrona, K. The length of a positive surgical margin is of prognostic significance in patients with clinically localized prostate cancer treated with radical prostatectomy. Urol. Int. Urol. Int. 2014, 93, 289–295. [Google Scholar] [CrossRef]
- Kozal, S.; Peyronnet, B.; Cattarino, S.; Seisen, T.; Comperat, E.; Vaessen, C.; Mozer, P.; Renard-Penna, R.; Cussenot, O.; Roupret, M.; et al. Influence of pathological factors on oncological outcomes after robot-assisted radical prostatectomy for localized prostate cancer: Results of a prospective study. Urol. Oncol. 2015, 33, 330.e1–330.e7. [Google Scholar] [CrossRef] [PubMed]
- Kurose, H.; Ueda, K.; Ogasawara, N.; Chikui, K.; Nakiri, M.; Nishihara, K.; Matsuo, M.; Suekane, S.; Kusano, H.; Akiba, J.; et al. Impact of Gleason score of the tumor at the positive surgical margin as a prognostic factor. Mol. Clin. Oncol. 2022, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- John, A.; John, H.; Catterwell, R.; Selth, L.A.; Callaghan, M.O. Primary Gleason grade and Gleason grade group at positive surgical margins: A systematic review and meta-analysis. BJU Int. 2021, 127 (Suppl. S1), 13–22. [Google Scholar] [CrossRef]
- Lysenko, I.; Mori, K.; Mostafaei, H.; Enikeev, D.V.; Karakiewicz, P.I.; Briganti, A.; Quhal, F.; Janisch, F.; Shariat, S.F. Prognostic Value of Gleason Score at Positive Surgical Margin in Prostate Cancer: A Systematic Review and Meta-analysis. Clin. Genitourin. Cancer 2020, 18, e517–e522. [Google Scholar] [CrossRef]

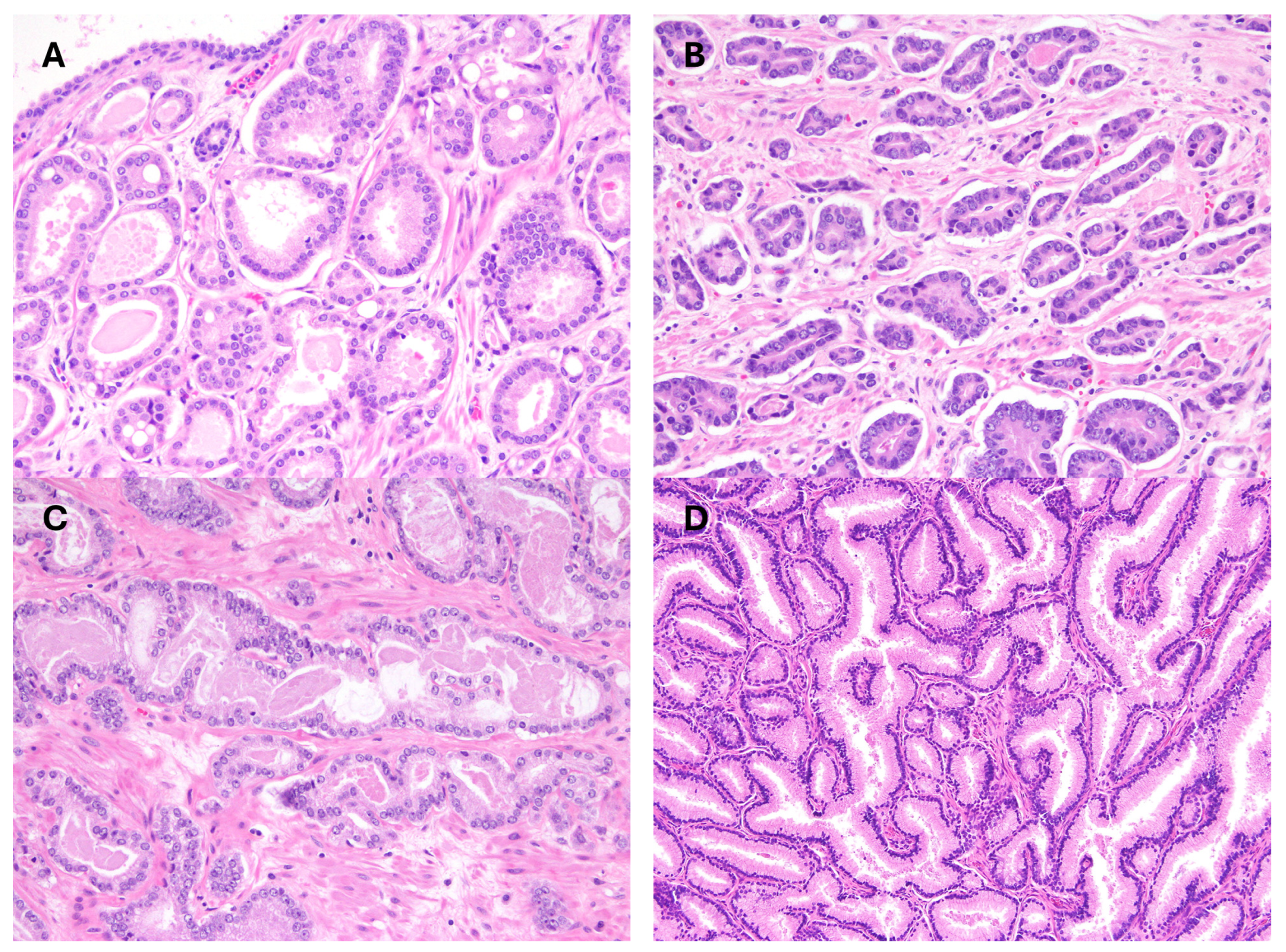





| Carcinoma Types | Increases Risk * | Likelihood of Extent at Diagnosis |
|---|---|---|
| ||
| No | Localized > non-localized |
| ||
| Yes | Non-localized > localized |
| Yes | Non-localized > localized |
| Yes | Non-localized > localized |
| No | Localized > non-localized (EPE in 46.1%) |
| ||
| No | Localized > non-localized |
| No | Localized > non-localized |
| No | Localized > non-localized |
| No | Localized > non-localized |
| No | Localized > non-localized (≥pT3 in 46%) |
| No | Localized > non-localized |
| Yes | Non-localized > localized |
| Yes | Non-localized > localized |
| Yes | Non-localized > localized |
| ||
| Yes | Non-localized > localized |
| Yes | Non-localized > localized |
| Yes | Non-localized > localized |
| Grade Group | Gleason Score | Definition |
|---|---|---|
| 1 | 6 | Only individual, discrete, well-formed glands |
| 2 | 3 + 4 = 7 | Predominantly well-formed glands with a lesser component of poorly formed/fused/cribriform/glomeruloid glands |
| 3 | 4 + 3 = 7 | Predominantly poorly formed/fused/cribriform/glomeruloid glands with a lesser component of well-formed glands 1 |
| 4 | 4 + 4 = 8, 3 + 5 = 8, 5 + 3 = 8 | Only poorly formed/fused cribriform/glomeruloid glands or Predominantly well-formed glands and a lesser component lacking glands 2 or Predominantly lacking glands with a lesser component of well-formed glands 2 |
| 5 | 4 + 5 = 9, 5 + 4 = 9, 5 + 5 = 10 | Lack of gland formation (or with necrosis) with or without poorly formed/fused/cribriform glands 1 |
| Gleason Pattern (or Grade) | Gleason Architectural Patterns |
|---|---|
| 3 | Well-formed glands, branched well-formed glands |
| 4 | Cribriform, glomeruloid, fused, poorly formed glands Hypernephromatoid cancer no longer used |
| 5 | Single cells, cords, solid sheets, small solid cylinders, and solid medium-tolarge-sized nests with rosette-like spaces Unequivocal comedonecrosis, even if focal |
| Authors | Cribriform Definition |
|---|---|
| van der Kwast et al. (ISUP) [49] | A confluent sheet of contiguous malignant epithelial cells with multiple glandular lumina easily visible at lower power (objective magnification 10×). There should be no intervening stroma or mucin separating individual or fused gland structures. |
| Shah et al. [50] | A dense sheet of tumor cells forming multiple lumens with transluminal bridging, imparting a “sieve-like” architecture, in which a majority of intraglandular cells are not in direct contact with stroma or mucin, and a clear luminal space along the periphery of the gland accounts for <50% of the glandular circumference. |
| Number of GP Present | Biopsy | RP | Example Scenarios |
|---|---|---|---|
| One | Double the GP as primary (first addend) and secondary (second addend) GPs | Similar | 100% GP 3 GS 3 + 3 = 6 |
| Two | Primary GP is most prevalent Secondary GP is less prevalent | Similar | 60% GP 3 40% GP 4 GS 3 + 4 = 7 |
| Exception: secondary GP not included in GS if of lower grade and minimal (≤5%) | Similar | 95% GP 4 5% GP 3 GS 4 + 4 = 8 | |
| Exception: secondary GP if of higher GP and minimal (≤5%) is not included in GS and is reported as minor GP (ISUP only) | 95% GP 3 5% GP 4 Biopsy: GS 3 + 4 = 7 RP: GS 3 + 4 = 7 or GS 3 + 3 = 6, with minor GP 4 (ISUP only) | ||
| Three (GPs 3, 4 and 5) | Primary GP is most prevalent Secondary GP is the second most prevalent | Similar | 65% GP 4 25% GP 5 10% GP 3 GS 4 + 5 = 9 |
| Exception: If tertiary GP (least prevalent) is higher than secondary GP, it is included in GS as secondary GP | Exception: If tertiary GP is higher than secondary GP and is >5%, it is included in GS as secondary GP | 60% GP 4 30% GP 3 10% GP 5 Biopsy and RP: GS 4 + 5 = 9 60% GP 4 37% GP 3 3% GP c5 Biopsy: GS 4 + 5 = 9 RP: GS 4 + 3 = 7, with minor tertiary GP 5 | |
| Exception: If tertiary GP is higher than secondary GP but is ≤5%, it is not included in GS and reported as minor tertiary GP |
| Grade | Definition |
|---|---|
| Highest or worst grade | Highest grade in any positive specimen in a biopsy set. |
| Global or overall grade | Grade derived by considering all positive specimens in a biopsy set. |
| Grade in the largest-volume cancer | Grade of the specimen with the largest tumor volume in a biopsy set. |
| Composite grade | Assign a grade to the entire biopsy set on the basis of positive cores from contiguous anatomic locations of the presumed dominant nodule. Tumor morphology in these separate cores is required to be similar to be included. |
| Grade | Definition |
|---|---|
| Highest grade | Highest grade among the multiple tumor nodules. |
| Grade of the largest tumor | Grade of the largest among the multiple tumor nodules. |
| Grade of the highest-stage (pT) tumor | Grade of tumor nodule with extraprostatic extension or seminal vesicle extension. |
| Global grade | Aggregate grade of all the tumor nodules. |
| Measure | Definition |
|---|---|
| Individual % GP 4 | mm of GP 4 tissue/total mm of cancer in a core or site |
| Overall % GP 4 | mm of GP 4 tissue (all cores)/total mm of cancer (all cores) |
| Maximum % GP 4 | Single core with the greatest involvement by GP 4 |
| Total length (mm) GP 4 | Sum of the total length in mm of GP 4 across all cores |
| Length (mm) of GP in greatest core | Length in mm of GP 4 in greatest core with highest GP 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paner, G.P.; Compérat, E.M.; Fine, S.W.; Kench, J.G.; Kristiansen, G.; Shah, R.B.; Smith, S.C.; Srigley, J.R.; van Leenders, G.J.L.H.; Varma, M.; et al. SIU-ICUD: Localized Prostate Cancer: Pathological Factors That Influence Outcomes and Management. Soc. Int. Urol. J. 2025, 6, 41. https://doi.org/10.3390/siuj6030041
Paner GP, Compérat EM, Fine SW, Kench JG, Kristiansen G, Shah RB, Smith SC, Srigley JR, van Leenders GJLH, Varma M, et al. SIU-ICUD: Localized Prostate Cancer: Pathological Factors That Influence Outcomes and Management. Société Internationale d’Urologie Journal. 2025; 6(3):41. https://doi.org/10.3390/siuj6030041
Chicago/Turabian StylePaner, Gladell P., Eva M. Compérat, Samson W. Fine, James G. Kench, Glen Kristiansen, Rajal B. Shah, Steven Christopher Smith, John R. Srigley, Geert J. L. H. van Leenders, Murali Varma, and et al. 2025. "SIU-ICUD: Localized Prostate Cancer: Pathological Factors That Influence Outcomes and Management" Société Internationale d’Urologie Journal 6, no. 3: 41. https://doi.org/10.3390/siuj6030041
APA StylePaner, G. P., Compérat, E. M., Fine, S. W., Kench, J. G., Kristiansen, G., Shah, R. B., Smith, S. C., Srigley, J. R., van Leenders, G. J. L. H., Varma, M., Zhou, M., & Amin, M. B. (2025). SIU-ICUD: Localized Prostate Cancer: Pathological Factors That Influence Outcomes and Management. Société Internationale d’Urologie Journal, 6(3), 41. https://doi.org/10.3390/siuj6030041







