Abstract
Objectives: It has been shown that concomitant autologous blood and dextranomer/hyaluronic acid (Deflux®) injection, hydrodistension autologous blood injection technique (HABIT), had a better mound preservation and treatment success compared to the hydrodistension injection technique (HIT) in vesicoureteral reflux (VUR) correction. In this study, we aimed to show microscopically whether the concomitant injection of autologous blood decreases the leakage of Deflux® particles. Methods: Children with VUR who underwent HIT or HABIT between March 2020 and January 2023 were enrolled. Following the completion of the procedure on each ureter, the bladder was irrigated for 3 to 5 min, and the retrieved sample of irrigation fluid was evaluated for dextranomer particle count as “immediate leakage”. A Foley catheter was placed, and a urine sample after 12 h was collected as “early leakage”. Results: A total of 86 children with a median age of 3.0 years (interquartile range = 4.6) were included. Overall, 66 patients underwent HABIT, and 20 children underwent HIT. Rupture was observed in five patients during the procedure, and re-injection was conducted successfully in these cases. Immediate, early, and total particle leakage in the first 12 h of the injection were significantly less in the HABIT group compared to the HIT group. In the regression analysis, only the injection technique (HIT/HABIT) and rupture were significantly associated with the total particle leakage in the first 12 h. Conclusions: Immediate injection of autologous blood into the mound following an endoscopic correction of VUR in children is associated with significantly less Deflux® particle leakage from the injection site regardless of the VUR grade. We hypothesize that a concomitant blood injection into the Deflux® mound will create a blood clot while the needle is kept in situ and help to stabilize the mound and decrease treatment failure by minimizing particle leakage from the injection site.
1. Introduction
Primary vesicoureteral reflux (VUR) is the most common urological anomaly in children. It is found in 30% to 40% of children investigated for febrile urinary tract infection (UTI) [1]. VUR nephropathy is the cause of end-stage renal failure in 3% to 25% of children [2]. The primary goal in managing VUR is to preserve renal function by preventing pyelonephritis, renal scarring, and hypertension [3]. Since 1984, endoscopic correction by bulking agent injection has been described as a minimally invasive approach and has gained popularity among pediatric urologists. However, this technique has yet to achieve the success rate of ureteral reimplantation [4]. Out of the various bulking agents used in endoscopic modality, dextranomer/hyaluronic acid copolymer (Deflux®) is the only bulking agent material approved by the Food and Drug Administration in the United States and has been generalized since 2001 [5].
Several techniques have been reported for the endoscopic treatment of VUR. The first technique was a subureteric Teflon injection, known as “The Sting” procedure [6]. It was further modified by the hydrodistension injection technique (HIT) in 2004 [7]. Then, we introduced a new technique by administrating concomitant blood and bulking agent injection using the hydrodistension autologous blood injection technique (HABIT) [8]. In this technique, following the Deflux® injection, the needle was not pulled back from the injection site. Instead, was advanced again proximally to the depth of the ureteral mucosa, and 0.5 to 1 mL autologous blood was injected slowly in the plane between the ureteral mucosa and the Deflux® implant.
In our previous research, we introduced the HABIT technique and compared its results with HIT; we observed that the success rate was significantly higher in the HABIT group after the first injection [8]. Later, we showed that an autologous blood injection led to better immediate and long-term mound preservation—a possible reason for the higher success rate of HABIT compared to HIT [9]. Here, we did not aim to report the response rate of VUR to the Deflux® injection nor to compare the success rate of HABIT and HIT. In fact, we aimed to show microscopically whether the concomitant injection of autologous blood decreases the leakage of Deflux® particles. This investigation uses a novel assessment method for the previously shown better clinical results and mound preservation of HABIT compared to HIT.
2. Materials and Methods
2.1. Participants and Study Design
A total of 86 children who had proven primary grade I to V VUR on voiding cystourethrography (VCUG) and who underwent HIT or HABIT between March 2020 and January 2023 were enrolled in this cross-sectional study. Of the total patients, 71 patients (82.6%) had bilateral VUR. And 37 children (43.0%) had a maximal VUR grade of Ⅰ–Ⅲ, while 49 (57.0%) had a maximal VUR grade of Ⅳ–Ⅴ. Children with grades I and Ⅱ reflux were included only if they had recurrent UTIs despite antibiotic therapy, renal scarring, or high-grade VUR in the contralateral ureter. All patients underwent urologic workups, including serum biochemical analysis, urine analysis, culture, renal ultrasonography assessment, VCUG, and 99 m technetium dimercaptosuccinic acid scan. Children with secondary VUR due to urethral valves, neuropathic bladder, bladder exstrophy complex, ureterocele, bladder diverticula, duplex system, and failed ureteral reimplantation were excluded from this study. We also excluded patients with any clinical evidence of voiding dysfunction. A simple explanation about the nature of the VUR, both procedures, potential risks and benefits, success rate, and complications were given to the parents, and written informed consent was obtained. When parents disagreed with the HABIT procedure or if a central line was needed for blood drawing in young infants, the HIT procedure was chosen. All patients were followed for at least one year following the procedure, based on recommendations that were provided by American Urological Association guidelines on the management of primary VUR in children [10]. The Ethics Committee of Tehran University of Medical Sciences approved the study protocol (number: 27518), and all the patients were treated in accordance with the Helsinki Declaration of the World Medical Association (1983).
2.2. Patient Selection and Surgical Technique
In patients under the age of one year, in case the patient was admitted for a febrile UTI with positive urine culture, endoscopic treatment was performed three weeks after the patient’s urine culture had become negative based on the protocol at our center. The reason for this cautious approach is that we have seen that in patients with an age of less than one year that a febrile UTI or pyelonephritis can leave permanent effects on the kidney. On the other hand, bladder mucosa edema due to an acute UTI may prevent a good volcano-shape formation following Deflux® injection.
The HIT technique was performed as previously described [9]. During routine cystoscopy, a 3 Fr cystoscopy injection needle was advanced through the working channel of a 6 Fr to 7.5 Fr neonatal cystourethroscope (Wolf GmbH, Knittlingen, Germany). After hydrodistension of the refluxing ureter and direct visualization of the injection site, the needle was inserted into the intraluminal wall of the ureter at the 6 o’clock position, and Deflux® was injected. In HABIT, following Deflux® injection, the needle was not pulled back from the injection site. Instead, it was advanced again proximally to the depth of the ureteral mucosa. In this group, (0.5–1 mL) autologous blood drawn intraoperatively was injected slowly in the plane between the ureteral mucosa and the Deflux® implant. Blood was provided intraoperatively and injected before clot formation about 3 to 4 min after being drawn. The needle was kept in place for up to 3 min to allow coagulation and minimize material leakage (Supplementary Video S1). The needle was also kept in position for the same amount of time in the HIT group to minimize fluid extravasation. Afterward, the bladder was irrigated with a normal saline flush for 3 to 5 min. Irrigated fluid volume was estimated based on the expected bladder capacity, which was measured following the International Children’s Continence Society recommendation [11].
The irrigation fluid sample was sent to the laboratory for Deflux® particle count. This count was regarded as “immediate leakage”. The same procedure was performed for patients undergoing a second contralateral injection. A Foley catheter was placed, and the urine sample taken 12 h after the procedure was also examined for particle count and regarded as “early leakage”. We also cleaned the wall of the Foley catheter bags after 12 h of urine collection and collected the particles adhered to their walls so that those particles would not be missed for early leakage. Keeping the Foley catheter for 12 h is routine in our setting. This is because of the possibility of urinary retention as a consequence of irritation and edema following the intervention. In addition, the patients were admitted for injection and were discharged the next day.
All of the injections were performed in a single-puncture fashion; in cases where Deflux® was not injected in the proper plane or the desired coaptation was not achieved, we avoided reinserting the needle into a different location. Instead, the needle was repositioned through the same former injection site. As soon as we observed that the injection site was not appropriate, we repositioned the needle so that an insignificant volume was injected in the wrong direction; this did not cause any excess Deflux® injection. Of note, the malpositioning was related to the moment when the needle entered to make the initial puncture for the Deflux® injection, and the injection of blood after the Deflux® was not related to this issue. If a rupture was observed during the operation, we did not finish the procedure, and the injection was repeated (next to and under the first try) until a mound of 8 to 10 mm was visualized.
2.3. Fluid Preparation and Particle Count
A pilot study was carried out to obtain the most appropriate method for particle count. It was primarily tried to count the leaked Deflux® particles with a Neubauer slide through the microscope. This method was unsuccessful due to particle attachment to the edge of the cover slide. In the next attempt, the irrigated fluid was examined for Deflux® particles by volume and weight estimation, but it was not a precise method due to accompanying cells and mucous membranes. Finally, the irrigated fluid was collected and centrifuged (Hettich, Tuttlingen, Germany) at 1000× g for 5 min. The volume was reduced to 10 mL by discarding the extra volume of supernatant after centrifugation using a pipette. Then, the remaining volume was resuspended in the tube and transferred into a 100 mm Petri dish. Deflux® particles were counted under an inverted microscope (Nikon, Tokyo, Japan) (Figure 1A). Pilot samples were not included in the final study.
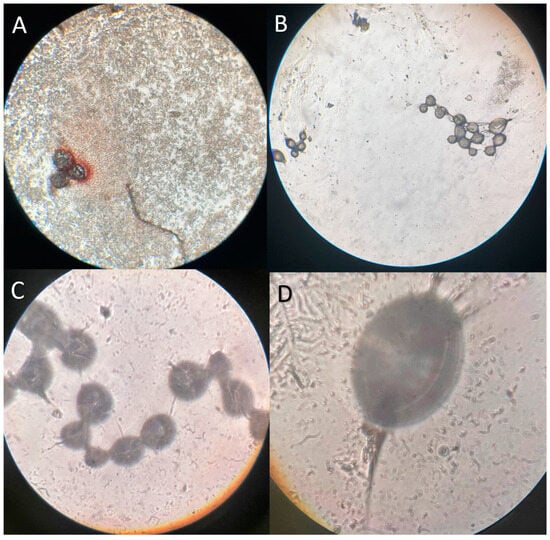
Figure 1.
Microscopic images of dextranomer/hyaluronic acid copolymer (Deflux®) particles; (A) Immediate leakage fluid sample after the hydrodistension autologous blood injection technique (HABIT) under an inverted microscope; (B–D) Diluted (1:100) Deflux® solution under an inverted microscope (10×, 40×, 100×).
2.4. Deflux® Solution Preparation and Particle Count
Firstly, we diluted Deflux® in distilled water at a ratio of 1:100 (0.1 mL Deflux® in 10 mL distilled water). It was on the shaker for one day to make the solution uniform. After that, the solution was centrifuged (Hettich, Tuttlingen, Germany) at 1500× g for 6 min. The volume was reduced to 10 mL by discarding the extra volume of supernatant after centrifugation using a pipette. Then, the remaining volume was suspended in the tube and transferred into a 100 mm Petri dish.
We poured 10 Landa with a sampler from the test tube onto the Neubauer slide. We tried to count the leaked Deflux® particles with a Neubauer slide through the microscope (Nikon, Tokyo, Japan) (Figure 1B–D). We repeated this stage four more times, so we had five total counts and took an average of them. For each count, we conducted it through the microscope. We use the following formula to obtain real Deflux® count:
Deflux® count under microscope × 10.1 mL (Deflux® + distilled water) × 104 = real Deflux® count
The average Deflux® particle count after the 5-time count was 17,912,350 particles in 0.1 mL Deflux®. So, there were approximately 179,123,500 particles in 1 mL of Deflux®. 0.1 mL Deflux® was equal to 0.118 g.
2.5. Particle Size
Samples of as-received Deflux® gel were applied as thin layers to glass substrates and were air dried prior to gold coating. These samples were then studied in a scanning electron microscope (AIS-2100, Seron Technology, Uiwang-si, South Korea). The size of dextranomer microspheres embedded in the carrier gel and the surface structure of microspheres are visible in these figures (Figure 2).
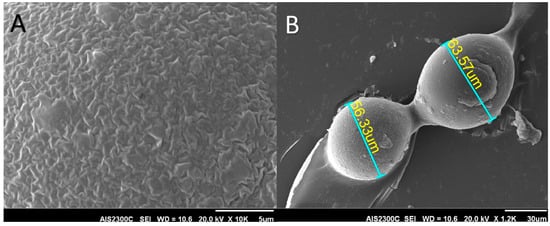
Figure 2.
Scanning electron microscope images showing the size and shape of dextranomer microsphere; (A) The surface and; (B) Size of the particles.
2.6. Statistical Analysis
Data were analyzed using IBM SPSS Statistics (version 26). Normality was assessed with Kolmogorov–Smirnov/Shapiro–Wilk test. Categorical variables were expressed as numbers and percentages. Continuous variables were expressed as the median and interquartile range (IQR) or mean ± standard deviation. Chi-Square/Fisher’s exact test and Mann–Whitney U tests were used to compare data between groups. Associations between variables and total particle leakage were assessed with quantile regression analysis (at a quantile value of 0.5). A p value of <0.05 was considered statistically significant.
3. Results
3.1. Baseline Information
A total of 86 children (61 girls and 25 boys) with a median age of 3.0 years (IQR = 4.6; range: 2 months to 14 years) were studied. Overall, 66 patients underwent HABIT, and 20 children underwent HIT. Table 1 shows the demographic and clinical information of the included patients. In total, 15 patients (17.4%) had unilateral VUR, while 71 (82.6%) children had bilateral VUR on VCUG. Rupture was observed in five patients during the procedure; four of them were in the HABIT group (three bilateral and one unilateral VUR), and one of them was in the HIT group (bilateral VUR). All ruptures in the HABIT group were after Deflux® injection and not after autologous blood injection. These five patients consisted of four females and a male (3 years old), and four had bilateral VUR. Children in the HIT group were younger than those in the HABIT group (median [IQR]: 1.6 [1.0] and 3.5 [4.4], respectively). No other procedure-related complications or migration was observed in either group. The procedures were performed on an outpatient basis, and all of the children recovered from the surgery. Among 86 children, 80 had a 6-month radionuclide cystogram, and six were lost to follow-up. In the 6-month radionuclide cystogram, 11 patients (13.8%) had persistent VUR; all patients had mild asymptomatic VUR. Comparing HABIT and HIT, only three cases (4.9%) had persistent VUR in the HABIT groups, while persistent VUR was observed in eight patients (42.1%) in the HIT groups (p-value < 0.001).

Table 1.
Information of all patients.
3.2. Particle Leakage
Table 2 compares the immediate, early, and total leakage of Deflux® particles between children who underwent HIT and HABIT procedures. The total particle count of immediate leakage had a median of 2,407,100 (IQR: 3,029,112) and 22,805 (IQR: 140,250) in the HIT and HABIT groups, respectively. In addition, the median particle count of early (12 h) leakage was 6,976,091 (IQR: 7,549,425) in the HIT group and 51,600 (IQR: 213,664) in the HABIT group. So, total counts of 9,445,441 (IQR: 9,324,535) in HIT and 108,882 (IQR: 374,348) in HABIT were calculated. As demonstrated, in all of them, particle leakage in the HABIT group was significantly less than that of the HIT group (p-value < 0.001).

Table 2.
The impact of autologous blood injection on dextranomer/hyaluronic acid copolymer particle leakage.
As mentioned, there were 179,123,500 particles in each 1 mL of Deflux®; therefore, the calculated range of particle leakage in the first 12 h (immediate + early) was 0–43% of the total volume of injected Deflux® for each patient. The cases with rupture had a total particle count ranging from 7,762,600 to 130,802,400 (median: 29,809,182), which was calculated to have a median of 16% (range: 3–43%) of the total volume of injected Deflux®.
3.3. Factors Related to Particle Leakage
To evaluate the association of each factor when adjusted with other variables, quantile regression at the quantile value of 0.5 was conducted. The analysis showed that among the covariates of age, gender, total volume injection of Deflux®, injection technique, uni- or bilaterality of VUR, maximal VUR grade, and rupture, only injection technique and rupture had significant associations with the total particle leakage in the first 12 h (immediate + early) (both p-values < 0.001; pseudo R squared = 0.388).
4. Discussion
Primary VUR is a common uropathy in the pediatric setting. Although there is no consensus on the optimal management plan, endoscopic management of VUR has become the treatment of choice in many cases, considering its lower cost, no need for long-term antibiotic therapy, shorter hospital stays, and reduced morbidity. In addition, there are no differences in UTI episodes after VUR treatment in patients undergoing subureteric bulking agent endoscopic injections compared with VUR reimplantation [12]. Various bulking agents have been suggested in the literature; however, many of them, such as Teflon, currently have limited application due to potential migration. Deflux® is now the most widely used biocompatible non-immunogenic tissue-augmenting substance with no potential for malignant transformation and a good long-term success rate in primary and secondary VUR [13,14,15].
Modifications have been applied in the endoscopic approach to increase the success rate by preserving ureteral coaptation intraoperatively. As mentioned, several techniques, including “The Sting” and HIT, have been previously introduced for the endoscopic management of vesicoureteral reflux. In addition, by elaborating HIT, the double-HIT technique was introduced as two injections performed, one proximally and one more distally within the ureter [16]. However, extrusion of the material immediately after implantation and caudal migration of the Deflux® implant remain a concern for urologists and account for a prominent cause of treatment failure [17,18]. Although migration can be attributed to bladder contraction or normal ureteral peristalsis, a necessary part of the migration is that the Deflux® particles first detach from the injection site. The previously introduced technique, HABIT, aims to minimize the chance of particle detachment by providing a safe, nonimmunogenic sealant formed from the patient’s own blood [9]. HABIT was the result of a modification in the HIT technique to achieve better outcomes. However, in our opinion, other techniques, such as the double-HIT technique, can be modified by adding an autologous blood injection following Deflux® injection to possibly achieve a better VUR resolution. Of note, autologous blood injection can help as long as it does not obstruct the lumen of the ureter. By decreasing particle detachment and stabilizing the mound in the future, we might be able to decrease the amount of bulking agent needed, which many developing countries will benefit from. Also, it is important to understand that even one less necessary procedure in a low-resource setting makes a difference. In addition, this issue would also be beneficial to all in terms of the differential cost of Deflux® and the risk of repeat anesthesia.
The first technique was subureteric Teflon injection, known as “The Sting” procedure [6]. It was further modified by the hydrodistension injection technique (HIT) in 2004 [7]. Then, we introduced a new technique by administrating concomitant blood and bulking agent injection using the hydrodistension autologous blood injection technique (HABIT) [8].
Myofibroblast functional studies suggest fibrin gel as a suitable matrix for promoting myofibroblast differentiation [19]. Autologous fibrin has been widely studied as a potential material to improve hemostasis and sealing and to promote tissue adhesion, such as mesh fixation in laparoscopic surgeries [20]. However, there is no dedicated study in the literature involving the role of autologous blood as a potentially rich source of fibrin in enhancing granulation formation or preventing particle leak following the Deflux® injection. The present study was carried out to investigate whether the injection of autologous blood following Deflux® injection has a protective effect on the leakage of Deflux® particles. In our patients, the injection of autologous blood reduced immediate and early leakage, and, as a result, reduced the total leakage of Deflux® particles in samples obtained during and following the procedure. Additionally, rupture and injection techniques were the only variables significantly contributing to the particle leakage.
HABIT was established as the preferred method in our center. However, in the case of parents’ preference or young infants, when concomitant blood injection required venous blood drawing, HIT was used to prevent further morbidity. That was the reason why children in the HIT group were younger than cases in the HABIT group in our series. Of note, age itself did not have a significant association with particle leakage when adjusted with other variables.
Volume retention of the mound is suggested to correlate with the higher rate of VUR resolution [21]. In a recent report by Zambaiti et al. on children with moderate to high VUR, mound height was the major predictive parameter for reflux resolution (sensitivity 100%, specificity 65.9%) [22]. We had previously reported that the HABIT procedure was associated with a lower rate of caudal migration and mound dissolution compared to the HIT procedure [9]. Using the HABIT procedure, the bulking agent was further stabilized, and the risks of volume loss and mound displacement were minimized. We believe that the smaller number of leaked particles, shown in the present study, was the reason for the aforementioned observations on ultrasonographic imaging and, more importantly, lower treatment failure rates in the HABIT procedure.
In our center, VCUG is not currently in the follow-up protocol of asymptomatic patients who received Deflux® injections due to the invasive nature of this modality. Arlen et al. also suggested that postoperative VCUG could be safely avoided in the majority of children undergoing endoscopic treatment of primary VUR [23]. Bladder ultrasonography is highly sensitive for detecting Deflux® implants and can anticipate the resolution of reflux with an accuracy of 88.95% in low-grade VUR and 36.67% in high-grade VUR [24]. Postoperative bladder ultrasonography could be modified as a screening tool in the follow up of patients with a primary low-grade VUR treated by Deflux® injection, while VCUG could be used for the follow up of high-grade VUR patients [24]. In our setting, we used a radionuclide cystogram to follow the resolution of vesicoureteral reflux in our patients.
We have previously reported the higher success rate of the HABIT technique by follow-up VCUG in another report [8]. Also, we showed an improved immediate and long-term mound preservation in concomitant autologous blood injection [9]. Herein, the present study was designed to evaluate whether this method results in less immediate and early Deflux® particle leak. Based on our data, we hypothesize that using concomitant blood in the endoscopic approach helps to stabilize the mound [9] and decrease treatment failure [8] by minimizing particle leakage from the injection site. Nevertheless, one should bear in mind that there is a learning curve with any new technique or modification, and to achieve comparable results, appropriate practice is warranted to ensure sufficient expertise in any institution [25].
Limitations and Suggestions
One of the limitations of this study is the age difference between the two groups, which was mainly due to parents’ preference for infants as many of them were scared regarding drawing venous blood, and we did not want to put any further pressure on them. However, the data analysis showed that the difference between particles in the two groups was not due to the age difference. In addition, this study was not a clinical study aiming at the long-term results of endoscopic treatment. Therefore, the lack of a detailed outcome for patients in the present study accounts for one of its major limitations, as the clinical outcomes cannot be directly associated with the particle leakage in each group. The clinical results of these two techniques with long-term follow up and a large sample size of 341 patients were provided in our previous study [8]. Although our center admits a large number of children with VUR for endoscopic Deflux® injection annually, this study was performed on a sample size of 86 patients, as the laboratory investigation in this experiment was expensive and, thus, not practical in more cases. Moreover, only children without congenital anomalies and any previous VUR correction were recruited in this study; therefore, this study does not provide information on the impact of underlying urological abnormalities on particle leakage. Future longitudinal studies are warranted using histological evaluation to study the correlation between particle leakage and obstruction. It is worth mentioning that in cases of bilateral injection, the immediate Deflux® count may be influenced by the fact that two injections were conducted. To prevent the effect of bilateral injection on total particle leakage, we included the uni- and bilaterality of VUR as one of the covariates for the regression analysis.
5. Conclusions
This report presents evidence that using autologous blood in the endoscopic correction of VUR in children is associated with significantly less immediate and early Deflux® particle extravasation from the injection site regardless of VUR grade. We hypothesize that concomitant blood helps to stabilize the mound and decrease treatment failure by minimizing particle leakage from the injection site.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/siuj5010007/s1, Video S1: Blood was provided intraoperatively and injected before clot formation about 3–4 min after being drawn. The video shows the blood injection, which the needle was kept in place for up to 3 min to allow coagulation and minimize material leakage. Of note, in this case, a ureteral stent was inserted because it was a single kidney case; inserting a ureteral stent is not routine for other patients in our setting.
Author Contributions
Conceptualization, A.-M.K.; formal analysis, H.K.; investigation, H.K., N.T. and S.E.; writing—original draft preparation, N.T. and S.E.; writing—review and editing, H.K.; supervision, A.-M.K., P.H. and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethics Committee of Tehran University of Medical Sciences approved the study protocol (number 27518), and all the patients were treated in accordance with the Helsinki Declaration of the World Association (1983).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Deflux®: dextranomer/hyaluronic acid copolymer; HABIT: hydrodistension autologous blood injection technique; HIT: hydrodistension injection technique; IQR: interquartile range; UTI: urinary tract injection; VCUG: voiding cystourethrography; VUR: vesicoureteral reflux.
References
- Koyle, M.A.; Shifrin, D. Issues in Febrile Urinary Tract Infection Management. Pediatr. Clin. N. Am. 2012, 59, 909–922. [Google Scholar] [CrossRef]
- Ardissino, G.; Avolio, L.; Dacco, V.; Testa, S.; Marra, G.; ViganÒ, S.; Loi, S.; Caione, P.; De Castro, R.; De Pascale, S. Long-term outcome of vesicoureteral reflux associated chronic renal failure in children. Data from the ItalKid Project. J. Urol. 2004, 172, 305–310. [Google Scholar] [CrossRef][Green Version]
- Lopez, P.-J.; Celis, S.; Reed, F.; Zubieta, R. Vesicoureteral reflux: Current management in children. Curr. Urol. Rep. 2014, 15, 447. [Google Scholar] [CrossRef]
- Elder, J.S.; Diaz, M.; Caldamone, A.A.; Cendron, M.; Greenfield, S.; Hurwitz, R.; Kirsch, A.; Koyle, M.A.; Pope, J.; Shapiro, E. Endoscopic therapy for vesicoureteral reflux: A meta-analysis. I. Reflux resolution and urinary tract infection. J. Urol. 2006, 175, 716–722. [Google Scholar] [CrossRef]
- Celik, O.; Ipekci, T.; Aydogdu, O.; Yucel, S. Current medical diagnosis and management of vesicoureteral reflux in children. Nephro-Urol. Mon. 2014, 6, e13534. [Google Scholar] [CrossRef]
- O’Donnell, B.; Puri, P. Treatment of vesicoureteric reflux by endoscopic injection of Teflon. Br. Med. J. (Clin. Res. Ed.) 1984, 289, 7–9. [Google Scholar] [CrossRef]
- Kirsch, A.J.; Perez-Brayfield, M.; Smith, E.A.; Scherz, H.C. The modified sting procedure to correct vesicoureteral reflux: Improved results with submucosal implantation within the intramural ureter. J. Urol. 2004, 171, 2413–2416. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A.-M.; Tourchi, A. Usefulness of concomitant autologous blood and dextranomer/hyaluronic acid copolymer injection to correct vesicoureteral reflux. J. Urol. 2012, 188, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A.-M.; Aryan, Z.; Tourchi, A.; Alizadeh, H. Long-term Ultrasound Appearance of Concomitant Autologous Blood and Dextranomer/Hyaluronic Acid Copolymer Implants: Is It Associated with Successful Correction of Vesicoureteral Reflux? Urology 2013, 81, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.A.; Skoog, S.J.; Arant, B.S.; Copp, H.L.; Elder, J.S.; Hudson, R.G.; Khoury, A.E.; Lorenzo, A.J.; Pohl, H.G.; Shapiro, E. Summary of the AUA guideline on management of primary vesicoureteral reflux in children. J. Urol. 2010, 184, 1134–1144. [Google Scholar] [CrossRef]
- Austin, P.F.; Bauer, S.B.; Bower, W.; Chase, J.; Franco, I.; Hoebeke, P.; Rittig, S.; Walle, J.V.; von Gontard, A.; Wright, A. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the Standardization Committee of the International Children’s Continence Society. J. Urol. 2014, 191, 1863–1865.e13. [Google Scholar] [CrossRef]
- Mina-Riascos, S.H.; Fernández, N.; García-Perdomo, H.A. Effectiveness and risks of endoscopic management compared to vesicoureteral reimplantation in patients with high-grade vesicoureteral reflux: Systematic review and network meta-analysis. Eur. J. Pediatr. 2021, 180, 1383–1391. [Google Scholar] [CrossRef]
- Polackwich, A.S.; Skoog, S.; Austin, J.C. Long-term followup after endoscopic treatment of vesicoureteral reflux with dextranomer/hyaluronic acid copolymer in patients with neurogenic bladder. J. Urol. 2012, 188, 1511–1515. [Google Scholar] [CrossRef]
- Kaye, J.D.; Srinivasan, A.K.; Delaney, C.; Cerwinka, W.H.; Elmore, J.M.; Scherz, H.C.; Kirsch, A.J. Clinical and radiographic results of endoscopic injection for vesicoureteral reflux: Defining measures of success. J. Pediatr. Urol. 2012, 8, 297–303. [Google Scholar] [CrossRef]
- Chertin, B.; Kocherov, S.; Chertin, L.; Natsheh, A.; Farkas, A.; Shenfeld, O.Z.; Halachmi, S. Endoscopic bulking materials for the treatment of vesicoureteral reflux: A review of our 20 years of experience and review of the literature. Adv. Urol. 2011, 2011, 309626. [Google Scholar] [CrossRef]
- Cerwinka, W.H.; Scherz, H.C.; Kirsch, A.J. Dynamic hydrodistention classification of the ureter and the double hit method to correct vesicoureteral reflux. Arch. Esp. Urol. 2008, 61, 882–887. [Google Scholar] [CrossRef]
- Diamond, D.A.; Caldamone, A.A.; Bauer, S.B.; Retik, A.B. Mechanisms of failure of endoscopic treatment of vesicoureteral reflux based on endoscopic anatomy. J. Urol. 2003, 170, 1556–1559. [Google Scholar] [CrossRef]
- Elmore, J.M.; Scherz, H.C.; Kirsch, A.J. Dextranomer/hyaluronic acid for vesicoureteral reflux: Success rates after initial treatment failure. J. Urol. 2006, 175, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Kilarski, W.W.; Jura, N.; Gerwins, P. An ex vivo model for functional studies of myofibroblasts. Lab. Investig. 2005, 85, 643–654. [Google Scholar] [CrossRef] [PubMed]
- De Hingh, I.; Nienhuijs, S.; Overdevest, E.; Scheele, K.; Everts, P. Mesh fixation with autologous platelet-rich fibrin sealant in inguinal hernia repair. Eur. Surg. Res. 2009, 43, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Jeon, T.; Yoo, S.-Y.; Kim, J.; Eo, H.; Song, K. The appearance of dextranomer–hyaluronic acid copolymer implants on ultrasound may predict resolution of vesicoureteral reflux after injection therapy. Clin. Radiol. 2014, 69, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Zambaiti, E.; Pensabene, M.; Montano, V.; Casuccio, A.; Sergio, M.; Cimador, M. Ultrasonographic mound height as predictor of vesicoureteral reflux resolution after endoscopic treatment in children. J. Pediatr. Surg. 2016, 51, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Arlen, A.M.; Scherz, H.C.; Filimon, E.; Leong, T.; Kirsch, A.J. Is routine voiding cystourethrogram necessary following double hit for primary vesicoureteral reflux? J. Pediatr. Urol. 2015, 11, e1–e40. [Google Scholar] [CrossRef] [PubMed]
- Aboutaleb, H.; Eldib, D.B.; Farahat, Y.; Abouelgreed, T.A.; Zanaty, F. Efficacy of Bladder Ultrasound in Prediction of Resolution of Vesicoureteral Reflux After Endoscopic Subureteral Hyaluronic Acid/Dextranomer (Deflux) Injection. Urology 2022, 165, 299–304. [Google Scholar] [CrossRef]
- Chertin, B.; Colhoun, E.; Velayudham, M.; Puri, P. Endoscopic treatment of vesicoureteral reflux: 11 to 17 years of followup. J. Urol. 2002, 167, 1443–1446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).