Perioperative Outcomes of Anatomic Endoscopic Enucleation of the Prostate, Robotic and Open Simple Prostatectomy from a Multi-Institutional Database
Abstract
:1. Introduction
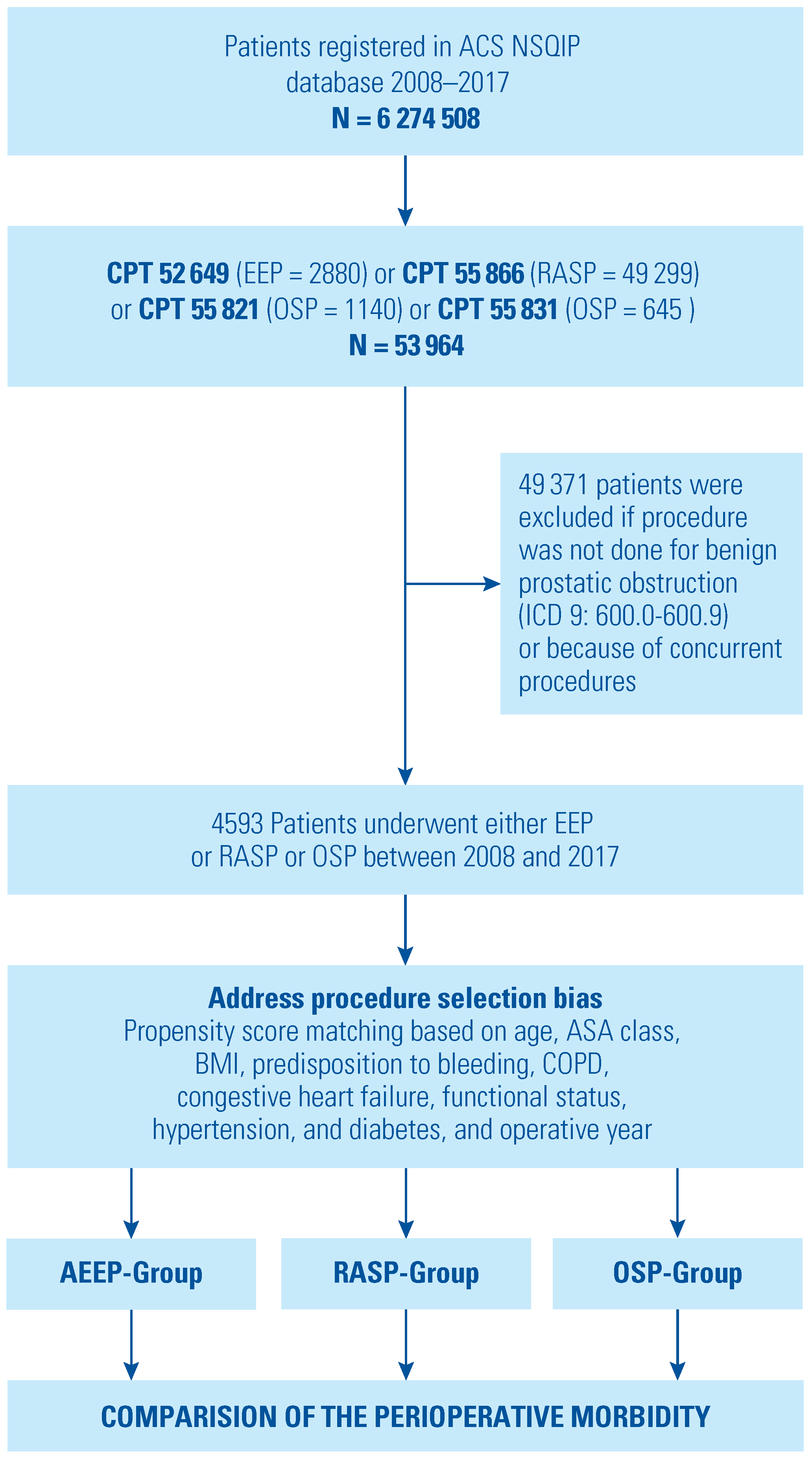
2. Materials and Methods
Statistics
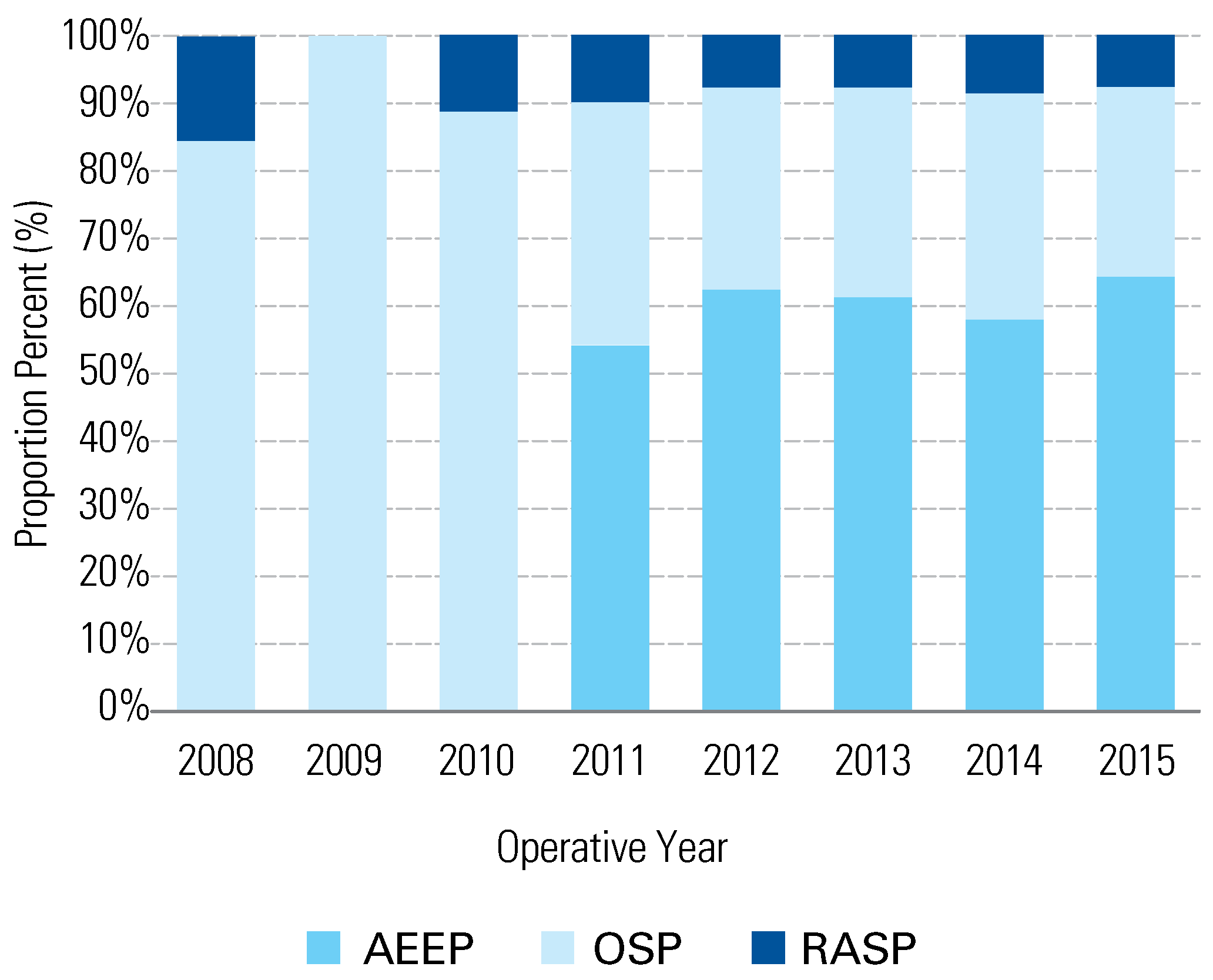
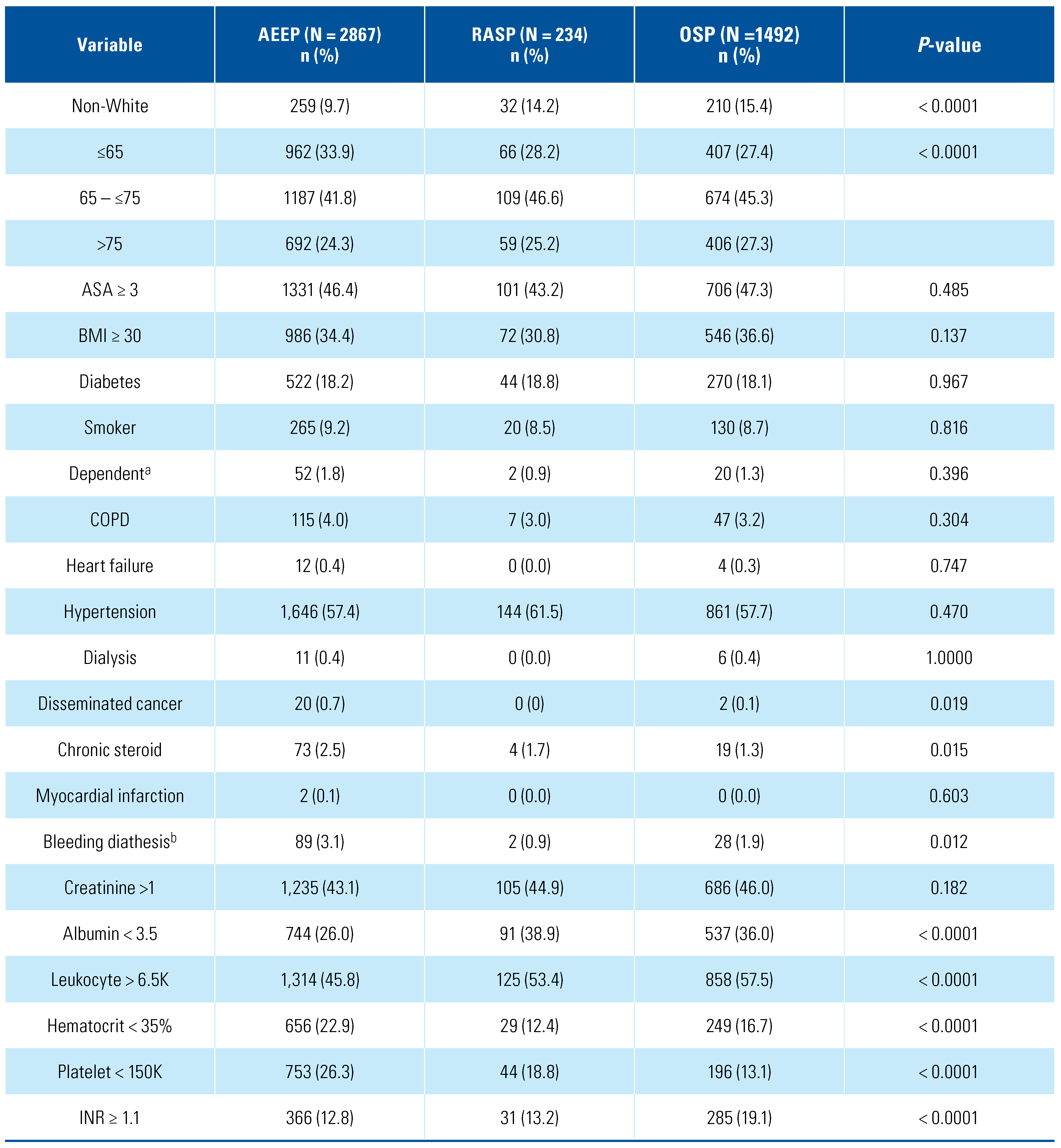
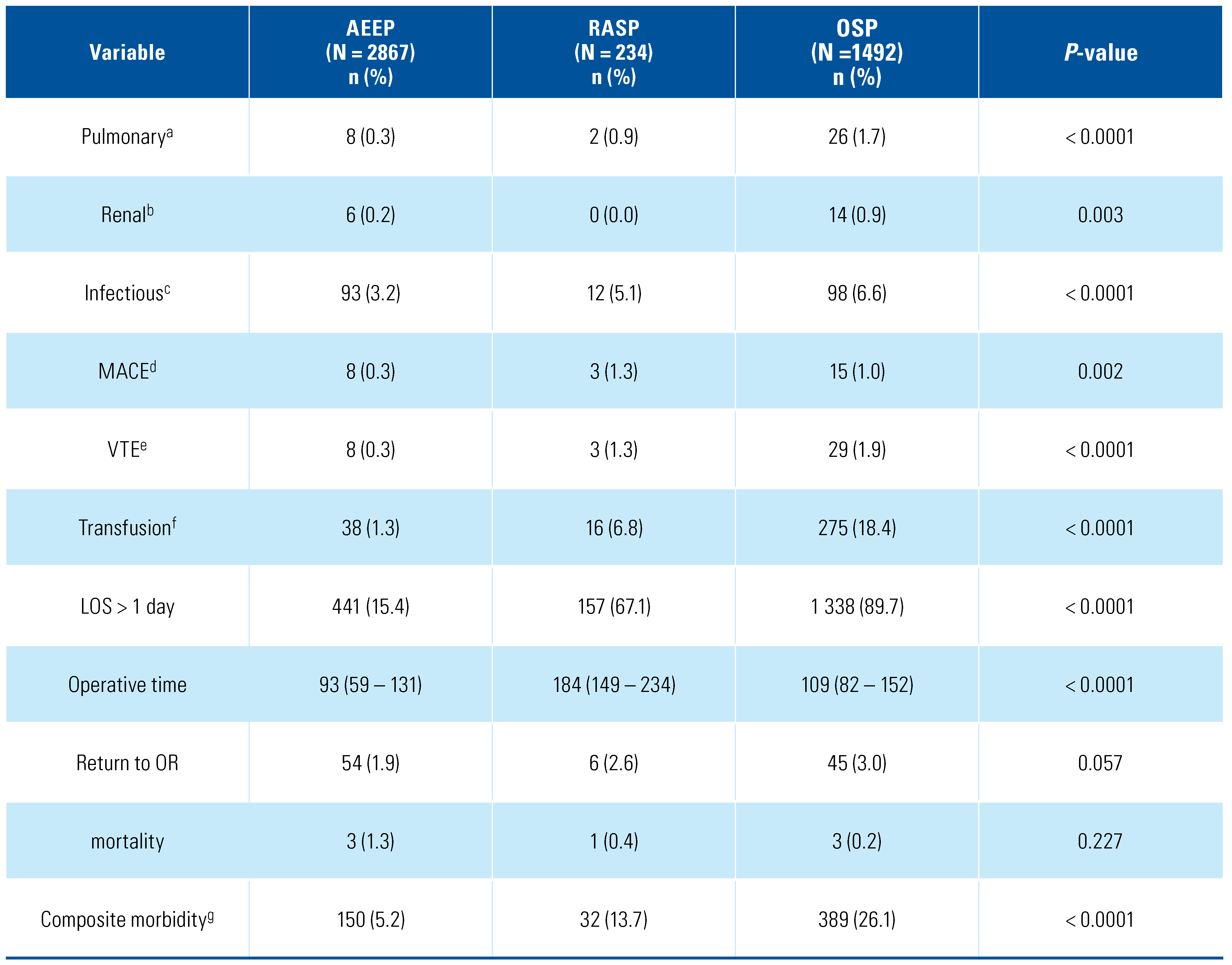
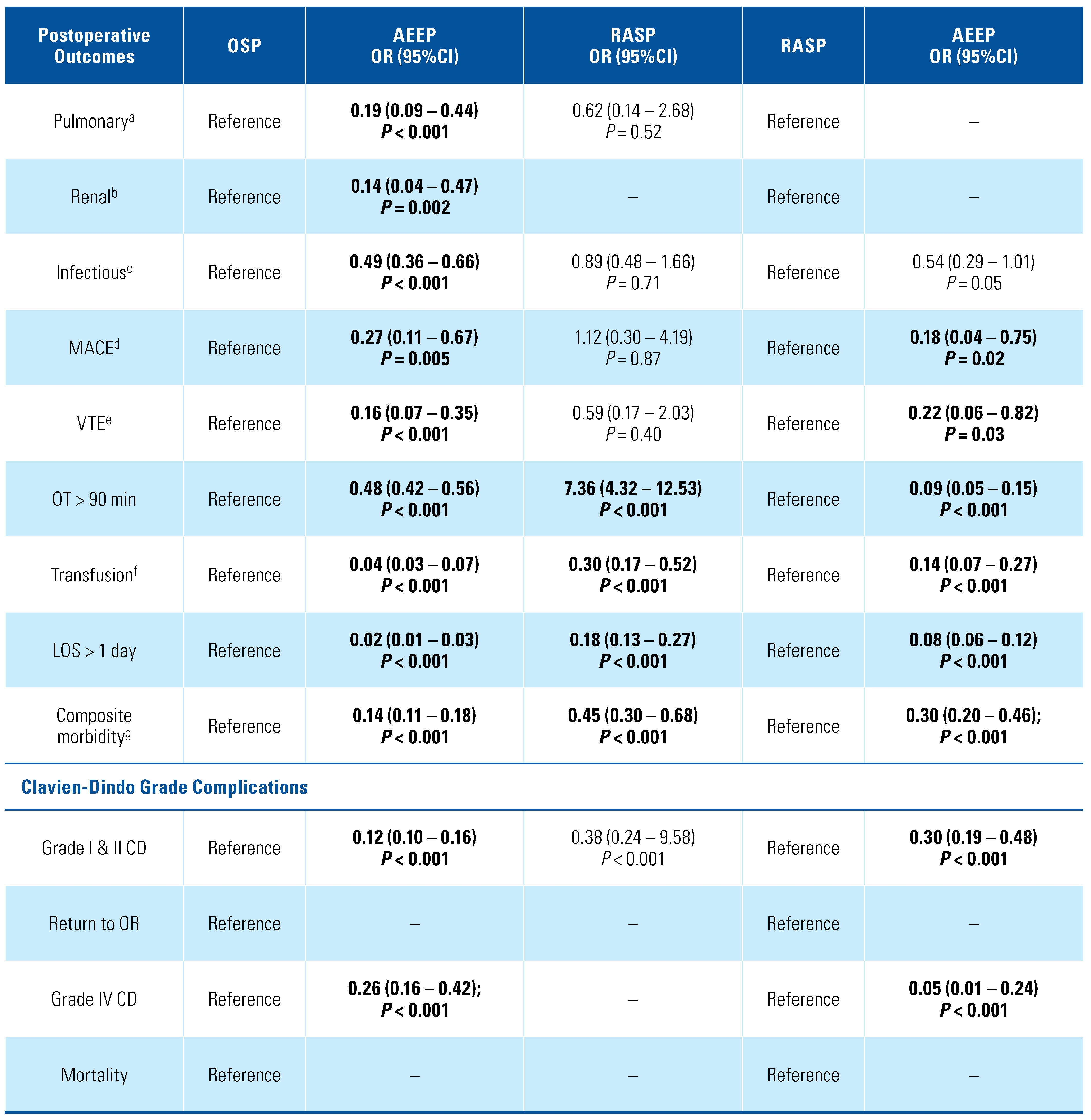
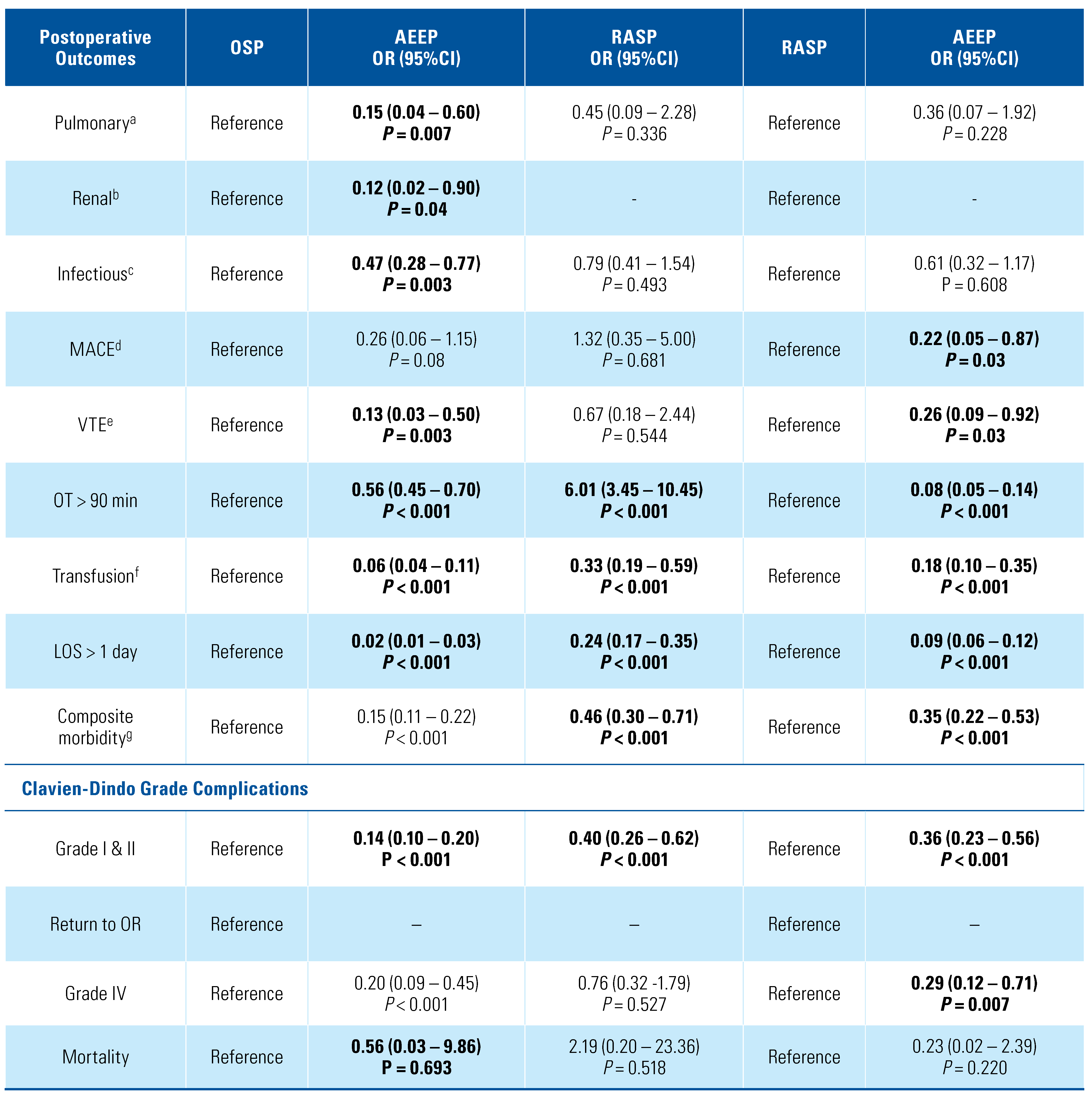
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Egan, K.B. The Epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. 2016, 43, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; Calhoun, E.; Jacobsen, S.J. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2008, 179 (Suppl. 5), S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Bachmann, A.; Descazeaud, A.; Drake, M.J.; Madersbacher, S.; Mamoulakis, C.; et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015, 67, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- AUA Announces Updates to Clinical Guidance for Surgical Management of LUTS Attributed to BPH. 2020.
- Stolzenburg, J.U.; Kallidonis, P.; Qazi, H.; Ho Thi, P.; Dietel, A.; Liatsikos, E.N.; et al. Extraperitoneal approach for robotic-assisted simple prostatectomy. Urology. 2014, 84, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Gahan, J.C.; Sorokin, I. Comparison of robot-assisted versus open simple prostatectomy for benign prostatic hyperplasia. Curr Urol Rep. 2018, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Mourmouris, P.; Keskin, S.M.; Skolarikos, A.; Argun, O.B.; Karagiannis, A.A.; Tufek, I.; et al. A prospective comparative analysis of robot-assisted vs open simple prostatectomy for benign prostatic hyperplasia. BJU Int. 2019, 123, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, X.; Xu, A.; Ren, R.; Zhou, X.; Wen, Y.; et al. Transurethral enucleation of the prostate versus transvesical open prostatectomy for large benign prostatic hyperplasia: a systematic review and meta- analysis of randomized controlled trials. World J Urol. 2016, 34, 1207–19. [Google Scholar] [CrossRef] [PubMed]
- Gilling, P.J.; Cass, C.B.; Cresswell, M.D.; Fraundorfer, M.R. Holmium laser resection of the prostate: preliminary results of a new method for the treatment of benign prostatic hyperplasia. Urology. 1996, 47, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Gilling, P.J.; Wilson, L.C.; King, C.J.; Westenberg, A.M.; Frampton, C.M.; Fraundorfer, M.R. Long-term results of a randomized trial comparing holmium laser enucleation of the prostate and transurethral resection of the prostate: results at 7 years. BJU Int. 2012, 109, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.W.; El Tayeb, M.M.; Borofsky, M.S.; Dauw, C.A.; Wagner, K.R.; Lowry, P.S.; et al. Comparison of perioperative outcomes between holmium laser enucleation of the prostate and robot-assisted simple prostatectomy. J Endourol. 2017, 31, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Umari, P.; Fossati, N.; Gandaglia, G.; Pokorny, M.; De Groote, R.; Geurts, N.; et al. Robotic assisted simple prostatectomy versus holmium laser enucleation of the prostate for lower urinary tract symptoms in patients with large volume prostate: a comparative analysis from a high volume center. J Urol. 2017, 197, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Baldini, A.; Fassi-Fehri, H.; Duarte, R.C.; Crouzet, S.; Ecochard, R.; Abid, N.; et al. Holmium laser enucleation of the prostate versus laparoscopic transcapsular prostatectomy: perioperative results and three- month follow-up. Curr Urol. 2017, 10, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, I.; Sundaram, V.; Singla, N.; Walker, J.; Margulis, V.; Roehrborn, C.; et al. Robot-assisted versus open simple prostatectomy for benign prostatic hyperplasia in large glands: a propensity score- matched comparison of perioperative and short-term outcomes. J Endourol. 2017, 31, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Kordan, Y.; Canda, A.E.; Köseoglu, E.; Balbay, D.; Laguna, M.P.; de la Rosette, J. Robotic-assisted simple prostatectomy: a systematic review. J Clin Med. 2020, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surgeons ACo. ACS NSQIP Hospitals 2020. Available online: https://www.facs.org/search/nsqip-participants?allresults= (accessed on 20 July 2020).
- Lerner, L.B.; Rajender, A. Laser prostate enucleation techniques. Can J Urol. 2015, 22 (Suppl 1), 53–59. [Google Scholar] [PubMed]
- Stuart, E.A. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010, 25, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Simpson, A.; Bhagnani, T.; Leeper, N.J.; Murphy, B.; Nordstrom, B.; et al. Incidence and cost of major adverse cardiovascular events and major adverse limb events in patients with chronic coronary artery disease or peripheral artery disease. Am J Cardiol. 2019, 123, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shahait, M.; Labban, M.; Dobbs, R.W.; Cheaib, J.G.; Lee, D.I.; Tamim, H.; et al. A 5-Item frailty index for predicting morbidity and mortality after radical prostatectomy: an analysis of the American College of Surgeons national surgical quality improvement program database. J Endourol. 2021. [CrossRef] [PubMed]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Weprin, S.; Zukovski, E.B.; Porpiglia, F.; Hampton, L.J.; Autorino, R. Rationale for robotic-assisted simple prostatectomy for benign prostatic obstruction. Eur Urol Focus. 2018, 4, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Lucca, I.; Shariat, S.F.; Hofbauer, S.L.; Klatte, T. Outcomes of minimally invasive simple prostatectomy for benign prostatic hyperplasia: a systematic review and meta-analysis. World J Urol. 2015, 33, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Autorino, R.; Zargar, H.; Mariano, M.B.; Sanchez-Salas, R.; Sotelo, R.J.; Chlosta, P.L.; et al. Perioperative outcomes of robotic and laparoscopic simple prostatectomy: a European–American multi-institutional analysis. Eur Urol. 2015, 68, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Elshal, A.M.; Elmansy, H.M.; Elhilali, M.M. Transurethral laser surgery for benign prostate hyperplasia in octogenarians: safety and outcomes. Urology. 2013, 81, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Sivarajan, G.; Borofsky, M.S.; Shah, O.; Lingeman, J.E.; Lepor, H. The role of minimally invasive surgical techniques in the management of large-gland benign prostatic hypertrophy. Rev Urol. 2015, 17, 140–149 PMID: 26543428. [Google Scholar] [PubMed]
- Rivera, M.; Krambeck, A.; Lingeman, J. Holmium laser enucleation of the prostate in patients requiring anticoagulation. Curr Urol Rep. 2017, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- El Tayeb, M.M.; Jacob, J.M.; Bhojani, N.; Bammerlin, E.; Lingeman, J.E. holmium laser enucleation of the prostate in patients requiring anticoagulation. J Endourol. 2016, 30, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, D.; Peng, L.; Ren, Z.; Gou, H.; Li, Y.; et al. comparison between minimally invasive simple prostatectomy and open simple prostatectomy for large prostates: a systematic review and meta- analysis of comparative trials. J Endourol. 2019, 33, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Juaneda, R.; Thanigasalam, R.; Rizk, J.; Perrot, E.; Theveniaud, P.E.; Baumert, H. Holmium laser enucleation versus laparoscopic simple prostatectomy for large adenomas. Actas Urol Esp. 2016, 40, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kampantais, S.; Dimopoulos, P.; Tasleem, A.; Acher, P.; Gordon, K.; Young, A. Assessing the learning curve of holmium laser enucleation of prostate (HoLEP). A systematic review. Urology. 2018, 120, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Robert, G.; Comat, V.; Rouprêt, M.; Gomez-Sancha, F.; Cornu, J.-N.; et al. Learning curves and perioperative outcomes after endoscopic enucleation of the prostate: a comparison between GreenLight 532-nm and holmium lasers. World J Urol. 2017, 35, 973–83. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, M.R.; Miller, N.L.; Handa, S.E.; Terry, C.; Munch, L.C.; Lingeman, J.E. Holmium laser enucleation of the prostate--outcomes independent of prostate size? J Urol. 2008, 180, 2431–2435. [Google Scholar] [CrossRef] [PubMed]
- Heidar, N.A.; Labban, M.; Misrai, V.; Mailhac, A.; Tamim, H.; El-Hajj, A. Laser enucleation of the prostate versus transurethral resection of the prostate: perioperative outcomes from the ACS NSQIP database. World J Urol. 2020. [CrossRef]
- Nestler, S.; Bach, T.; Herrmann, T.; Jutzi, S.; Roos, F.C.; Hampel, C.; et al. Surgical treatment of large volume prostates: a matched pair analysis comparing the open, endoscopic (ThuVEP) and robotic approach. World J Urol. 2019, 37, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.; Perez, D.S.; Weeks, D.C. Robot-assisted simple prostatectomy for severe benign prostatic hyperplasia. J Endourol. 2011, 25, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Suardi, N.; Naspro, R.; Mazzoccoli, B.; Zanni, G.; Gallina, A.; et al. Holmium laser enucleation versus open prostatectomy for benign prostatic hyperplasia: an inpatient cost analysis. Urology. 2006, 68, 302–306. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
 |
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2021 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Labban, M.; Heidar, N.A.; Misrai, V.; Najdi, J.; Tamim, H.; El-Hajj, A. Perioperative Outcomes of Anatomic Endoscopic Enucleation of the Prostate, Robotic and Open Simple Prostatectomy from a Multi-Institutional Database. Soc. Int. Urol. J. 2021, 2, 196-209. https://doi.org/10.48083/LKVV8843
Labban M, Heidar NA, Misrai V, Najdi J, Tamim H, El-Hajj A. Perioperative Outcomes of Anatomic Endoscopic Enucleation of the Prostate, Robotic and Open Simple Prostatectomy from a Multi-Institutional Database. Société Internationale d’Urologie Journal. 2021; 2(4):196-209. https://doi.org/10.48083/LKVV8843
Chicago/Turabian StyleLabban, Muhieddine, Nassib Abou Heidar, Vincent Misrai, Jad Najdi, Hani Tamim, and Albert El-Hajj. 2021. "Perioperative Outcomes of Anatomic Endoscopic Enucleation of the Prostate, Robotic and Open Simple Prostatectomy from a Multi-Institutional Database" Société Internationale d’Urologie Journal 2, no. 4: 196-209. https://doi.org/10.48083/LKVV8843
APA StyleLabban, M., Heidar, N. A., Misrai, V., Najdi, J., Tamim, H., & El-Hajj, A. (2021). Perioperative Outcomes of Anatomic Endoscopic Enucleation of the Prostate, Robotic and Open Simple Prostatectomy from a Multi-Institutional Database. Société Internationale d’Urologie Journal, 2(4), 196-209. https://doi.org/10.48083/LKVV8843




