Pressurized Metered-Dose Inhaler Versus Dry Powder Inhaler Adherence Among Individuals with Asthma and COPD
Abstract
Highlights
- Analysis of ICS + LABA inhaler purchases over 12 months showed that overall utilization was very low among individuals with asthma and COPD. The highest purchase rates were observed in those with asthma–COPD overlap.
- Dry powder inhalers (DPIs) were purchased significantly more frequently than pressurized metered-dose inhalers (pMDIs), with once-a-day DPI formulations demonstrating the highest purchase rates.
- When prescribing maintenance ICS + LABA inhaler therapy, healthcare professionals should carefully assess actual inhaler purchases at each visit, in addition to evaluating inhaler technique and patient preferences.
- Individuals with high ICS + LABA inhaler purchase rates may either be better-controlled or experiencing more severe symptoms. In such cases, the possibility of asthma–COPD overlap should be considered.
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CHS | Clalit Health Services |
| CCI | Charlson comorbidity index |
| CI | confidence interval |
| COPD | chronic obstructive pulmonary disease |
| DPI | dry powder inhaler |
| GINA | Global Initiative for Asthma |
| GOLD | Global Initiative for Chronic Obstructive Lung Diseases |
| HMO | health maintenance organization |
| ICS | inhaled corticosteroids |
| IRB | institutional review board |
| IQR | interquartile range |
| LABA | long-acting β2-agonist |
| LAMA | long-acting muscarinic antagonist |
| MPR | medication possession ratio |
| OR | odds ratio |
| PDC | proportion of days covered |
| pMDI | pressurized metered-dose inhaler |
| SD | standard deviation |
| SES | socioeconomic status |
References
- Ferkol, T.; Schraufnagel, D. The global burden of respiratory disease. Ann. Am. Thorac. Soc. 2014, 11, 404–406. [Google Scholar] [CrossRef]
- World Health Organization. Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 10 February 2025).
- GINA—Global Initiative for Astma. 2024 GINA Main Report. Available online: https://ginasthma.org/2024-report/ (accessed on 10 February 2025).
- Global Initiative for Chronic Obstructive Lung Disease. 2025 GOLD Report. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 10 February 2025).
- Sanchis, J.; Gich, I.; Pedersen, S. Systematic review of errors in inhaler use: Has patient technique improved over time? Chest 2016, 150, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Rosenbaum, I.; Oberman, B.; Katz, I.; Sharabi, N.; Shlomi, D. The impact of pharmacist-guided inhaler technique education on chronic obstructive pulmonary disease and asthma patients. J. Pharm. Health Serv. Res. 2023, 14, 198–204. [Google Scholar] [CrossRef]
- Usmani, O.S.; Lavorini, F.; Marshall, J.; Dunlop, W.C.N.; Heron, L.; Farrington, E.; Dekhuijzen, R. Critical inhaler errors in asthma and COPD: A systematic review of impact on health outcomes. Respir. Res. 2018, 19, 10. [Google Scholar] [CrossRef]
- Poplicean, E.; Crișan, A.F.; Tudorache, E.; Hogea, P.; Mladin, R.; Oancea, C. Unlocking Better Asthma Control: A Narrative Review of Adherence to Asthma Therapy and Innovative Monitoring Solutions. J. Clin. Med. 2024, 13, 6699. [Google Scholar] [CrossRef]
- Chrystyn, H.; Van Der Palen, J.; Sharma, R.; Barnes, N.; Delafont, B.; Mahajan, A.; Thomas, M. Device errors in asthma and COPD: Systematic literature review and meta-analysis. Npj Prim. Care Respir. Med. 2017, 27, 22. [Google Scholar] [CrossRef] [PubMed]
- Lindh, A.; Theander, K.; Arne, M.; Lisspers, K.; Lundh, L.; Sandelowsky, H.; Ställberg, B.; Westerdahl, E.; Zakrisson, A.B. Errors in inhaler use related to devices and to inhalation technique among patients with chronic obstructive pulmonary disease in primary health care. Nurs. Open 2019, 6, 1519. [Google Scholar] [CrossRef]
- Duarte-de-Araújo, A.; Teixeira, P.; Hespanhol, V.; Correia-de-Sousa, J. COPD: Analysing factors associated with a successful treatment. Pulmonology 2020, 26, 66–72. [Google Scholar] [CrossRef]
- Latorre, M.; Parri, G.; Paggiaro, P. Is adherence to treatment influenced by the ability to use inhaled devices in patients with COPD correctly? Pulmonology 2020, 26, 63–65. [Google Scholar] [CrossRef]
- Lavorini, F.; Magnan, A.; Christophe Dubus, J.; Voshaar, T.; Corbetta, L.; Broeders, M.; Dekhuijzen, R.; Sanchis, J.; Viejo, J.L.; Barnes, P.; et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir. Med. 2008, 102, 593–604. [Google Scholar] [CrossRef]
- Al-Showair, R.A.M.; Tarsin, W.Y.; Assi, K.H.; Pearson, S.B.; Chrystyn, H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir. Med. 2007, 101, 2395–2401. [Google Scholar] [CrossRef]
- Monteiro, C.; Maricoto, T.; Prazeres, F.; Augusto Simões, P.; Augusto Simões, J. Determining factors associated with inhaled therapy adherence on asthma and COPD: A systematic review and meta-analysis of the global literature. Respir. Med. 2022, 191, 106724. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, J.; Kim, H.; Woo, S.D. Comparative analysis of real-world data on the efficacy and safety of and adherence to ICS/LABA combinations in asthma management. Respir. Res. 2025, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Zaib, M.; Ullah, R.; Salam, S.; Khan, R.N.; Naz, S.; Ghaffar, T.; Amin, S. Comparison of the Adherence to Metered Dose Inhalers Versus Dry Powder Inhalers in Patients with Newly Diagnosed Chronic Obstructive Pulmonary Disease. J. Saidu Med. Coll. 2018, 8, 94–98. [Google Scholar]
- Roy, A.; Battle, K.; Lurslurchachai, L.; Halm, E.A.; Wisnivesky, J.P. Inhaler device, administration technique, and adherence to inhaled corticosteroids in patients with asthma. Prim. Care Respir. J. 2011, 20, 148–154. [Google Scholar] [CrossRef]
- Toh, M.R.; Ng, G.X.Z.; Goel, I.; Lam, S.W.; Wu, J.T.; Lee, C.F.; Ong, M.E.H.; Matchar, D.B.; Tan, N.C.; Loo, C.M.; et al. Asthma prescribing trends, inhaler adherence and outcomes: A Real-World Data analysis of a multi-ethnic Asian Asthma population. Npj Prim. Care Respir. Med. 2024, 34, 35. [Google Scholar] [CrossRef]
- Gemicioglu, B.; Gungordu, N.; Can, G.; Alp Yıldırım, F.I.; Uydeş Doğan, B.S. Evaluation of real-life data on the use of inhaler devices, including satisfaction and adherence to treatment, by community pharmacists in partnership with pulmonary disease specialists. J. Asthma 2023, 60, 1326–1335. [Google Scholar] [CrossRef]
- Woo, S.D.; Ye, Y.M.; Lee, Y.; Lee, S.H.; Shin, Y.S.; Park, J.H.; Choi, H.; Lee, H.Y.; Shin, H.J.; Park, H.S. Efficacy and safety of a pressurized metered-dose inhaler in older asthmatics: Comparison to a dry powder inhaler in a 12-week randomized trial. Allergy Asthma Immunol. Res. 2020, 12, 454–466. [Google Scholar] [CrossRef]
- Darbà, J.; Ramírez, G.; Sicras, A.; Francoli, P.; Torvinen, S.; Sánchez-de la Rosa, R. The importance of inhaler devices: The choice of inhaler device may lead to suboptimal adherence in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, H.; Vuong, V.; Kaye, L.; Anderson, W.C., 3rd; Szefler, S.; Stempel, D.A. Is Once Versus Twice Daily Dosing Better for Adherence in Asthma and Chronic Obstructive Pulmonary Disease? J. Allergy Clin. Immunol. Pract. 2023, 11, 2087–2093.e3. [Google Scholar] [CrossRef] [PubMed]
- Averell, C.M.; Stanford, R.H.; Laliberté, F.; Wu, J.W.; Germain, G.; Duh, M.S. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. J. Asthma 2021, 58, 102–111. [Google Scholar] [CrossRef]
- Stanford, R.H.; Averell, C.; Parker, E.D.; Blauer-Peterson, C.; Reinsch, T.K.; Buikema, A.R. Assessment of Adherence and Asthma Medication Ratio for a Once-Daily and Twice-Daily Inhaled Corticosteroid/Long-Acting β-Agonist for Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1488–1496.e7. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Lee, L.Y.; DiRocco, K.K.; Germain, G.; Klimek, J.; Laliberté, F.; Lejeune, D.; Noorduyn, S.G.; Paczkowski, R. Adherence and Persistence with Single-Inhaler Triple Therapy Among Patients with COPD Using Commercial and Medicare Advantage US Health Plan Claims Data. Adv. Ther. 2024, 42, 830–848. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Robertson, A.; Bullen, K.; Rand, C.; Horne, R.; Staudinger, H. Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: A randomized open-label study. BMC Pulm. Med. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Murillo, E.R.; Mora, J.R.M.; Bachiller, M.M.; Santiago, L.G.; Braqué, N.N.; Bravo, M.O. Factors associated with low adherence to inhaled therapy in patients with chronic respiratory diseases: A cross-sectional study. BMC Pulm. Med. 2025, 25, 94. [Google Scholar] [CrossRef]
- Bhattarai, B.; Walpola, R.; Mey, A.; Anoopkumar-Dukie, S.; Khan, S. Barriers and Strategies for Improving Medication Adherence Among People Living With COPD: A Systematic Review. Respir. Care 2020, 65, 1738–1750. [Google Scholar] [CrossRef]
- Kerwin, E.M.; Preece, A.; Brintziki, D.; Collison, K.A.; Sharma, R. ELLIPTA Dry Powder Versus Metered-Dose Inhalers in an Optimized Clinical Trial Setting. J. Allergy Clin. Immunol. Pract. 2019, 7, 1843–1849. [Google Scholar] [CrossRef]
- Morice, A.H.; Peterson, S.; Beckman, O.; Osmanliev, D. Therapeutic comparison of a new budesonide/formoterol pMDI with budesonide pMDI and budesonide/formoterol DPI in asthma. Int. J. Clin. Pract. 2007, 61, 1874. [Google Scholar] [CrossRef]
- Koehorst-Ter Huurne, K.; Movig, K.; Van Der Valk, P.; van der Palen, J.; Brusse-Keizer, M. The influence of type of inhalation device on adherence of COPD patients to inhaled medication. Expert. Opin. Drug. Deliv. 2016, 13, 469–475. [Google Scholar] [CrossRef]
- Turégano-Yedro, M.; Trillo-Calvo, E.; Navarro IRos, F.; Maya-Viejo, J.D.; González Villaescusa, C.; Echave Sustaeta, J.M.; Doña, E.; Alcázar Navarrete, B. Inhaler Adherence in COPD: A Crucial Step Towards the Correct Treatment. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 2887–2893. [Google Scholar] [CrossRef]
- Stanford, R.H.; Parker, E.D.; Reinsch, T.K.; Buikema, A.R.; Blauer-Peterson, C. Assessment of COPD-related outcomes in patients initiating a once daily or twice daily ICS/LABA. Respir. Med. 2019, 150, 1–7. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Beeh, K.-M.; Schultze, M.; Kossack, N.; Richter, L.M.; Claussen, J.; Compton, C.; Noorduyn, S.G.; Ismaila, A.S.; Requena, G. Evaluation of Adherence and Persistence to Triple Therapy in Patients with COPD: A German Claims Data Study. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Alshabanat, A.; Zafari, Z.; Albanyan, O.; Dairi, M.; FitzGerald, J.M. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PLoS ONE 2015, 10, e0136065. [Google Scholar] [CrossRef]
- Vanoverschelde, A.; Van Der Wel, P.; Putman, B.; Lahousse, L. Determinants of poor inhaler technique and poor therapy adherence in obstructive lung diseases: A cross-sectional study in community pharmacies. BMJ Open Respir. Res. 2021, 8, e000823. [Google Scholar] [CrossRef]
- Nili, M.; Lemasters, T.J.; Adelman, M.; Dwibedi, N.; Madhavan, S.S.; Sambamoorthi, U. Initial maintenance therapy adherence among older adults with asthma-COPD overlap. Am. J. Manag. Care 2021, 27, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, H.K.; Azapagic, A. Life cycle environmental impacts of inhalers. J. Clean. Prod. 2019, 237, 117733. [Google Scholar] [CrossRef]
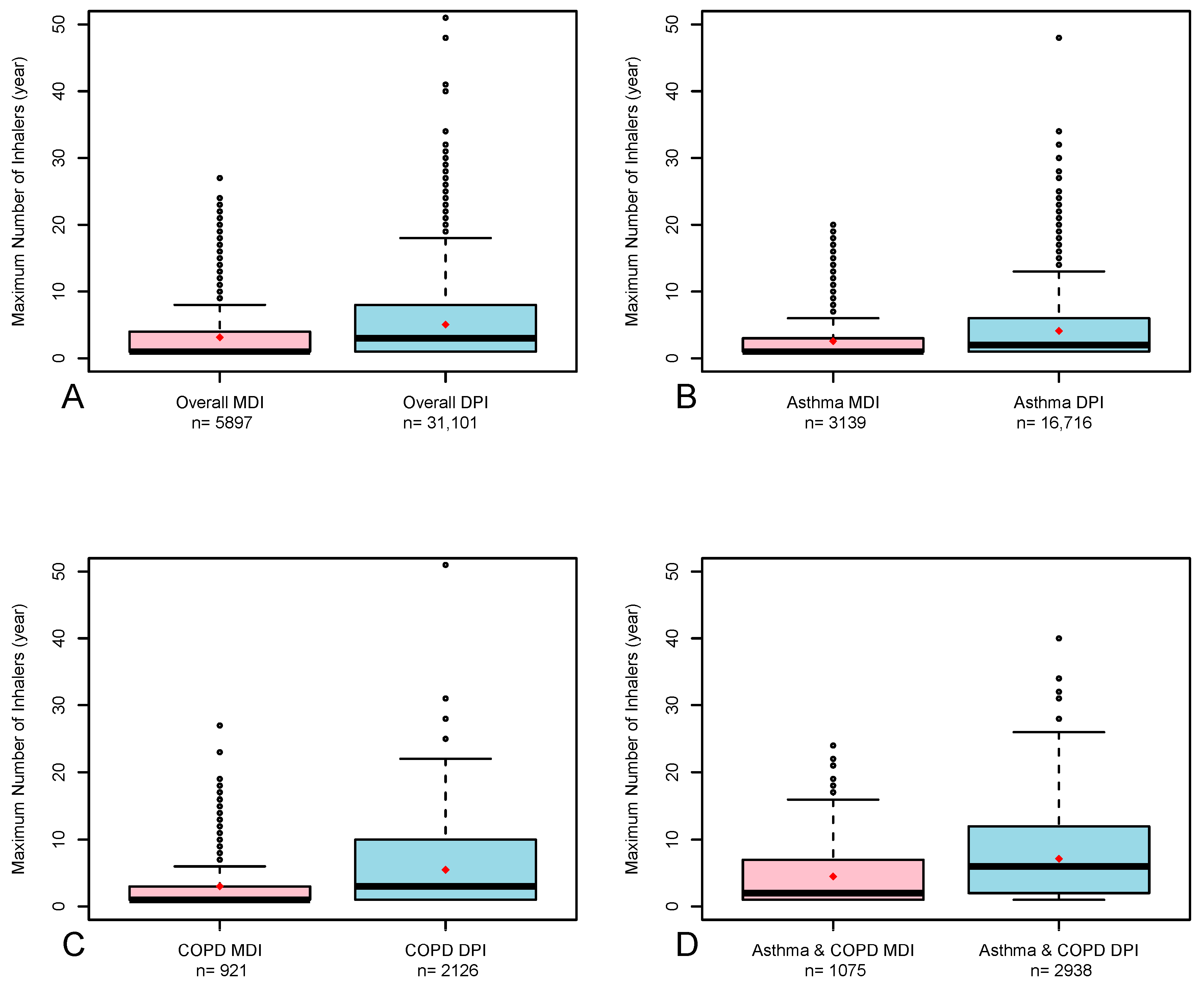
| Total | MDIs | DPIs | p | |
|---|---|---|---|---|
| No | 66,910 | 6469 | 60,441 | |
| Age | ||||
| Mean (SD) | 54.9 (19.6) | 54.3 (19.5) | 55.0 (19.6) | 0.005 |
| Gender (%) | ||||
| Male | 29,687 (44.4) | 2696 (41.7) | 26,991 (44.7) | <0.001 |
| BMI (%) | ||||
| <18.5 | 1706 (2.5) | 161 (2.5) | 1545 (2.6) | 0.403 |
| 18.5–<25 | 22,687 (33.9) | 2180 (33.7) | 20,507 (33.9) | |
| 25–<30 | 22,860 (34.2) | 2170 (33.5) | 20,690 (34.2) | |
| ≥30 | 19,657 (29.4) | 1958 (30.3) | 17,699 (29.3) | |
| Smoking status (%) | ||||
| never | 38,849 (58.1) | 3754 (58.0) | 35,095 (58.1) | 0.968 |
| past or current | 28,061 (41.9) | 2715 (42.0) | 25,346 (41.9) | |
| Socioeconomic status (%) | ||||
| Low | 5797 (8.7) | 433 (6.7) | 5364 (8.9) | <0.001 |
| Medium | 42,493 (63.5) | 4483 (69.3) | 38,010 (62.9) | |
| High | 18,620 (27.8) | 1553 (24.0) | 17,067 (28.2) | |
| Charlson comorbidity index (SD) | 5 (3.8) | 5 (3.8) | 5 (3.8) | 0.147 |
| Asthma | COPD | Asthma–COPD Overlap | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | pMDIs | DPIs | p | Total | pMDIs | DPIs | p | Total | pMDIs | DPIs | p | |
| No (%) | 35,431 | 3601 | 31,830 | 12,456 | 1408 | 11,048 | 19,023 | 1460 | 17,563 | |||
| Age | ||||||||||||
| Mean (SD) | 45.5 (18.4) | 45.8 (18.5) | 45.4 (18.4) | 0.311 | 67.4 (12.3) | 66.3 (12.5) | 67.5 (12.3) | 0.001 | 64.4 (16.0) | 63.7 (16.5) | 64.5 (16.0) | 0.074 |
| Gender (%) | ||||||||||||
| Male | 13,872 (39.2) | 1331 (37.0) | 12,541 (39.4) | 0.005 | 7631 (61.3) | 816 (58.0) | 6815 (61.7) | 0.007 | 8184 (43.0) | 549 (37.6) | 7635 (43.5) | <0.001 |
| BMI (%) | ||||||||||||
| <18.5 | 981 (2.8) | 96 (2.7) | 885 (2.8) | 0.623 | 361 (2.9) | 42 (3.0) | 319 (2.9) | 0.149 | 364 (1.9) | 23 (1.6) | 341 (1.9) | 0.714 |
| 18.5–<25 | 13,722 (38.7) | 1379 (38.3) | 12,343 (38.8) | 3792 (30.4) | 408 (29.0) | 3384 (30.6) | 5173 (27.2) | 393 (26.9) | 4780 (27.2) | |||
| 25–<30 | 11,591 (32.7) | 1165 (32.4) | 10,426 (32.8) | 4444 (35.7) | 485 (34.4) | 3959 (35.8) | 6825 (35.9) | 520 (35.6) | 6305 (35.9) | |||
| ≥30 | 9137 (25.8) | 961 (26.7) | 8176 (25.7) | 3859 (31.0) | 473 (33.6) | 3386 (30.6) | 6661 (35.0) | 524 (35.9) | 6137 (34.9) | |||
| Smoking status (%) | ||||||||||||
| never | 25,857 (73.0) | 2560 (71.1) | 23,297 (73.2) | 0.008 | 3531 (28.3) | 435 (30.9) | 3096 (28.0) | 0.026 | 9461 (49.7) | 759 (52.0) | 8702 (49.5) | 0.078 |
| past or current | 9574 (27.0) | 1041 (28.9) | 8533 (26.8) | 8925 (71.7) | 973 (69.1) | 7952 (72.0) | 9562 (50.3) | 701 (48.0) | 8861 (50.5) | |||
| Socioeconomic status (%) | ||||||||||||
| Low | 2697 (7.6) | 218 (6.1) | 2479 (7.8) | <0.001 | 1206 (9.7) | 96 (6.8) | 1110 (10.0) | <0.001 | 1894 (10.0) | 119 (8.2) | 1775 (10.1) | 0.009 |
| Medium | 21,011 (59.3) | 2357 (65.5) | 18,654 (58.6) | 8484 (68.1) | 1080 (76.7) | 7404 (67.0) | 12,998 (68.3) | 1046 (71.6) | 11,952 (68.1) | |||
| High | 11,723 (33.1) | 1026 (28.5) | 10,697 (33.6) | 2766 (22.2) | 232 (16.5) | 2534 (22.9) | 4131 (21.7) | 295 (20.2) | 3836 (21.8) | |||
| Charlson comorbidity index (SD) | 3.3 (2.9) | 3.4 (3) | 3.2 (2.9) | 0.003 | 7.5 (3.5) | 7.3 (3.6) | 7.6 (3.5) | 0.024 | 6.7 (3.7) | 6.6 (3.7) | 6.7 (3.7) | 0.2 |
| No Matching | Propensity Score Exact Matching | |||||
|---|---|---|---|---|---|---|
| n | Median [IQR] | p Value | n | Median [IQR] | p Value | |
| Entire population | ||||||
| 66,910 | 36,998 | |||||
| MDIs (%) | 6469 (9.7%) | 1 [1, 4] | <0.001 | 5897 (15.9) | 1 [1, 4] | <0.001 |
| DPIs (%) | 60,441 (90.3) | 3 [1, 9] | 31,101 (84.1) | 3 [1, 8] | ||
| Only asthma | ||||||
| 35,431 | 19,855 | |||||
| MDIs (%) | 3601 (10.2) | 1 [1, 3] | <0.001 | 3139 (15.8) | 1 [1, 3] | <0.001 |
| DPIs (%) | 31,830 (89.8) | 2 [1, 7] | 16,716 (84.2) | 2 [1, 6] | ||
| Only COPD | ||||||
| 12,456 | 3047 | |||||
| MDIs (%) | 1408 (11.3) | 1 [1, 3] | <0.001 | 921 (30.2) | 1 [1, 3] | <0.001 |
| DPIs (%) | 11,048 (88.7) | 3 [1, 10] | 2126 (69.8) | 3 [1, 10] | ||
| Asthma–COPD overlap | ||||||
| 19,023 | 4013 | |||||
| MDIs (%) | 1460 (7.7) | 2 [1, 7] | <0.001 | 1075 (26.8) | 2 [1, 7] | <0.001 |
| DPIs (%) | 17,563 (92.3) | 7 [2, 12] | 2938 (73.2) | 6 [2, 12] | ||
| No Matching | Propensity Score Exact Matching | |||||
|---|---|---|---|---|---|---|
| n | Median [IQR] | p Value | n | Median [IQR] | p Value | |
| Entire population | ||||||
| 50,667 | 35,484 | |||||
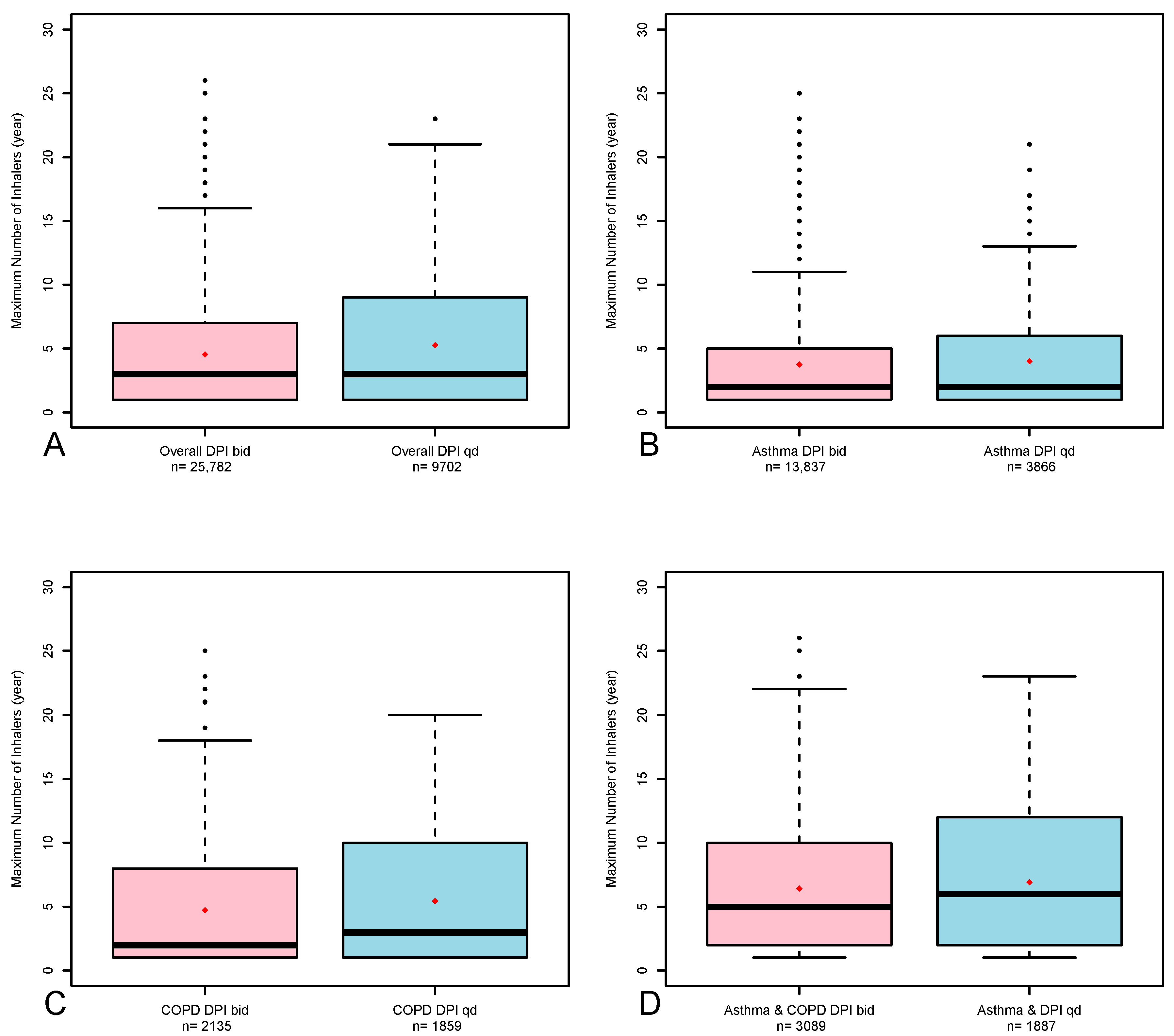
| Once-a-day (%) | 11,746 (23.2) | 3 [1, 10] | 9702 (27.3) | 3 [1, 9] | ||
| <0.001 | <0.001 | |||||
| Twice-a-day (%) | 38,921 (76.8) | 3 [1, 7] | 25,782 (72.7) | 3 [1, 7] | ||
| Only asthma | ||||||
| 27,704 | 17,703 | |||||
| Once-a-day (%) | 4751 (17.1) | 2 [1, 6] | 3866 (21.8) | 2 [1, 6] | ||
| <0.001 | 0.053 | |||||
| Twice-a-day (%) | 22,953 (82.9) | 2 [1, 6] | 13,837 (78.2) | 2 [1, 5] | ||
| Only COPD | ||||||
| 9438 | 3994 | |||||
| Once-a-day (%) | 3762 (39.9) | 3 [1, 10] | 1859 (46.8) | 3 [1, 10] | ||
| <0.001 | 0.001 | |||||
| Twice-a-day (%) | 5676 (60.1) | 2 [1, 8] | 2135 (53.2) | 2 [1, 8] | ||
| Asthma–COPD overlap | ||||||
| 13,525 | 4976 | |||||
| Once-a-day (%) | 3233 (23.9) | 6 [2, 12] | 1887 (38.7) | 6 [2, 12] | ||
| <0.001 | 0.01 | |||||
| Twice-a-day (%) | 10,292 (76.1) | 5 [2, 10] | 3089 (63.3) | 5 [2, 10] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shlomi, D.; Oberman, B.; Halevy, Y.; Kushnir, S.; Meir, H.; Reichenberg, Y. Pressurized Metered-Dose Inhaler Versus Dry Powder Inhaler Adherence Among Individuals with Asthma and COPD. Adv. Respir. Med. 2025, 93, 44. https://doi.org/10.3390/arm93050044
Shlomi D, Oberman B, Halevy Y, Kushnir S, Meir H, Reichenberg Y. Pressurized Metered-Dose Inhaler Versus Dry Powder Inhaler Adherence Among Individuals with Asthma and COPD. Advances in Respiratory Medicine. 2025; 93(5):44. https://doi.org/10.3390/arm93050044
Chicago/Turabian StyleShlomi, Dekel, Bernice Oberman, Yehonatan Halevy, Shiri Kushnir, Hadas Meir, and Yael Reichenberg. 2025. "Pressurized Metered-Dose Inhaler Versus Dry Powder Inhaler Adherence Among Individuals with Asthma and COPD" Advances in Respiratory Medicine 93, no. 5: 44. https://doi.org/10.3390/arm93050044
APA StyleShlomi, D., Oberman, B., Halevy, Y., Kushnir, S., Meir, H., & Reichenberg, Y. (2025). Pressurized Metered-Dose Inhaler Versus Dry Powder Inhaler Adherence Among Individuals with Asthma and COPD. Advances in Respiratory Medicine, 93(5), 44. https://doi.org/10.3390/arm93050044






