FeNO 350 mL/s: Unlocking the Small Airways to Achieve Clinical Remission in Severe Asthma—A Pilot Study
Abstract
Highlights
- Baseline FeNO measured at 350 mL/s (FeNO350) was a significant predictor of clinical remission in severe asthma after one year of biologic therapy.
- An optimal FeNO350 cutoff of ≥18 ppb showed high specificity (92.8%) and good predictive accuracy (AUC = 0.799) for identifying patients likely to achieve remission.
- FeNO350 may serve as a non-invasive, effort-independent biomarker for identifying patients with small airway inflammation who are more likely to respond to biologics.
- Integrating FeNO350 into routine assessment could help guide precision medicine strategies and optimize biologic therapy in severe asthma management.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Questionnaires
2.4. Lung Function
2.5. Measurement of Exhaled Nitric Oxide (FeNO) 50 and 350 mL/s
2.6. Impulse Oscillometry (IOS)
- R5–R20 (kPa·s·L−1): This represents the difference in resistance between 5 Hz and 20 Hz, serving as a surrogate marker for peripheral (small) airway resistance. Values exceeding 0.07 kPa·s·L−1 are indicative of small airways dysfunction (SAD) [16].
- X5 (kPa·s·L−1): Reactance at 5 Hz, reflecting the elastic properties of the distal lung; more negative values suggest reduced compliance and increased stiffness of the peripheral airways.
- Fres (Hz): Resonant frequency at which the reactance equals zero, representing the balance point between capacitive and inertial properties of the respiratory system.
- AX (kPa/L): The area under the reactance curve from 5 Hz to Fres, used as an index of the overall load on the peripheral airways and lung compliance [17].
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Inter-Group Comparisons at Baseline (T0)
3.3. Inter-Group Comparisons After One Year (T1)
3.4. Intra-Group Comparisons over Time
Subgroup Analysis: Benralizumab-Treated Patients
3.5. Predictive Value of FeNO350
3.6. Diagnostic Accuracy of FeNO350
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nunes, C.; Pereira, A.M.; Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pr. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Global Initiative for Asthma. 2023. Available online: www.ginasthma.org (accessed on 22 October 2023).
- Postma, D.S.; Brightling, C.; Baldi, S.; Berge, M.V.D.; Fabbri, L.M.; Gagnatelli, A.; Papi, A.; Van der Molen, T.; Rabe, K.F.; Siddiqui, S.; et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): Baseline data from a prospective cohort study. Lancet Respir. Med. 2019, 7, 402–416, Erratum in Lancet Respir. Med. 2019, 7, e28. [Google Scholar] [CrossRef] [PubMed]
- Buels, K.S.; Fryer, A.D. Muscarinic receptor antagonists: Effects on pulmonary function. Handb. Exp. Pharmacol. 2012, 208, 317–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cottini, M.; Licini, A.; Lombardi, C.; Bagnasco, D.; Comberiati, P.; Berti, A. Small airway dysfunction and poor asthma control: A dangerous liaison. Clin. Mol. Allergy. 2021, 19, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Canonica, G.W.; Blasi, F.; Carpagnano, G.E.; Guida, G.; Heffler, E.; Paggiaro, P.; Allegrini, C.; Antonelli, A.; Aruanno, A.; Bacci, E.; et al. Severe Asthma Network Italy Definition of Clinical Remission in Severe Asthma: A Delphi Consensus. J. Allergy Clin. Immunol. Pr. 2023, 11, 3629–3637. [Google Scholar] [CrossRef] [PubMed]
- McDowell, P.J.; McDowell, R.; Busby, J.; Eastwood, M.C.; Patel, P.H.; Jackson, D.J.; Mansur, A.; Patel, M.; Burhan, H.; Doe, S.; et al. Clinical remission in severe asthma with biologic therapy: An analysis from the UK Severe Asthma Registry. Eur. Respir. J. 2023, 62, 2300819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansen, S.; Søndergaard, M.B.; von Bülow, A.; Bjerrum, A.-S.; Schmid, J.; Rasmussen, L.M.; Johnsen, C.R.; Ingebrigtsen, T.; Håkansson, K.E.J.; Johansson, S.L.; et al. Clinical Response and Remission in Patients With Severe Asthma Treated With Biologic Therapies. Chest 2024, 165, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Portacci, A.; Nolasco, S.; Detoraki, A.; Vatrella, A.; Calabrese, C.; Pelaia, C.; Montagnolo, F.; Scioscia, G.; Valenti, G.; et al. Features of severe asthma response to anti-IL5/IL5r therapies: Identikit of clinical remission. Front. Immunol. 2024, 15, 1343362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, M.; Kay, S.; Pike, J.; Williams, A.; Rosenzweig, J.R.C.; Hillyer, E.V.; Price, D. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: Analysis of a multinational cross-sectional survey. Prim. Care Respir. J. 2009, 18, 41–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plaza, V.; Fernández-Rodríguez, C.; Melero, C.; Cosío, B.G.; Entrenas, L.M.; de Llano, L.P.; Gutiérrez-Pereyra, F.; Tarragona, E.; Palomino, R.; López-Viña, A.; et al. Validation of the ‘Test of the Adherence to Inhalers’ (TAI) for Asthma and COPD Patients. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 142–152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jia, C.E.; Zhang, H.P.; Lv, Y.; Liang, R.; Jiang, Y.Q.; Powell, H.; Fu, J.J.; Wang, L.; Gibson, P.G.; Wang, G. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2013, 131, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.-C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R.; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katsoulis, K.; Kipourou, M.; Quaranta, V.N. Predicting Bronchial Hyperresponsiveness in Patients with Asthma: The Role of Impulse Oscillometry. Arch. Bronconeumol. (Ed. Impr.) 2022, 58, 722–724. [Google Scholar] [CrossRef] [PubMed]
- King, G.G.; Bates, J.; Berger, K.I.; Calverley, P.; De Melo, P.L.; Dellacà, R.L.; Farre, R.; Hall, G.; Ioan, I.; Irvin, C.G.; et al. Technical standards for respiratory oscillometry. Eur. Respir. J. 2020, 55, 1900753. [Google Scholar] [CrossRef] [PubMed]
- Italian Medicines Agency (AIFA). Criteria for Prescribing Biologic Therapies in Severe Asthma. Available online: https://www.aifa.gov.it (accessed on 1 January 2025).
- Chan, R.; Lipworth, B.J. Impact of Biologic Therapy on the Small Airways Asthma Phenotype. Lung 2022, 200, 691–696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carr, T.F.; Altisheh, R.; Zitt, M. Small airways disease and severe asthma. World Allergy Organ. J. 2017, 10, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipworth, B.; Manoharan, A.; Anderson, W. Unlocking the quiet zone: The small airway asthma phenotype. Lancet Respir. Med. 2014, 2, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Usmani, O.S.; Singh, D.; Spinola, M.; Bizzi, A.; Barnes, P.J. The prevalence of small airways disease in adult asthma: A systematic literature review. Respir. Med. 2016, 116, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Nakwan, N.; Ruklerd, T.; Perkleang, T.; Taptawee, P. The levels and correlations of FeNO, blood eosinophils and lung function in well-controlled asthma. Adv. Respir. Med. 2022, 90, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Comhair, S.A.; Gaston, B.; Thunnissen, F.B.; Farver, C.; Thomassen, M.J.; Kavuru, M.; Hammel, J.; Abu-Soud, H.M.; Erzurum, S.C. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl. Acad. Sci. USA 2001, 98, 2622–2627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kostikas, K.; Brindicci, C.; Patalano, F. Blood Eosinophils as Biomarkers to Drive Treatment Choices in Asthma and COPD. Curr. Drug Targets 2018, 19, 1882–1896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petsky, H.L.; Cates, C.J.; Kew, K.M.; Chang, A.B. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): A systematic review and meta-analysis. Thorax 2018, 73, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Pianigiani, T.; Luzzi, S.; Dilroba, A.; Meocci, M.; Salvadori, E.; Alderighi, L.; Bergantini, L.; D’alessandro, M.; Sestini, P.; Bargagli, E.; et al. Evaluation of multiple-flows exhaled nitric oxide and its clinical significance in severe asthmatic patients treated with biologics: A prospective real-life study. J. Asthma 2024, 61, 1619–1628. [Google Scholar] [CrossRef]
- Abdo, M.; Watz, H.; Veith, V.; Kirsten, A.-M.; Biller, H.; Pedersen, F.; von Mutius, E.; Kopp, M.V.; Hansen, G.; Waschki, B.; et al. Small airway dysfunction as predictor and marker for clinical response to biological therapy in severe eosinophilic asthma: A longitudinal observational study. Respir. Res. 2020, 21, 278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, R.; Lipworth, B.J. Real-life effects of benralizumab on airway oscillometry in severe eosinophilic asthma. BMJ Open Respir. Res. 2023, 10, e001472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
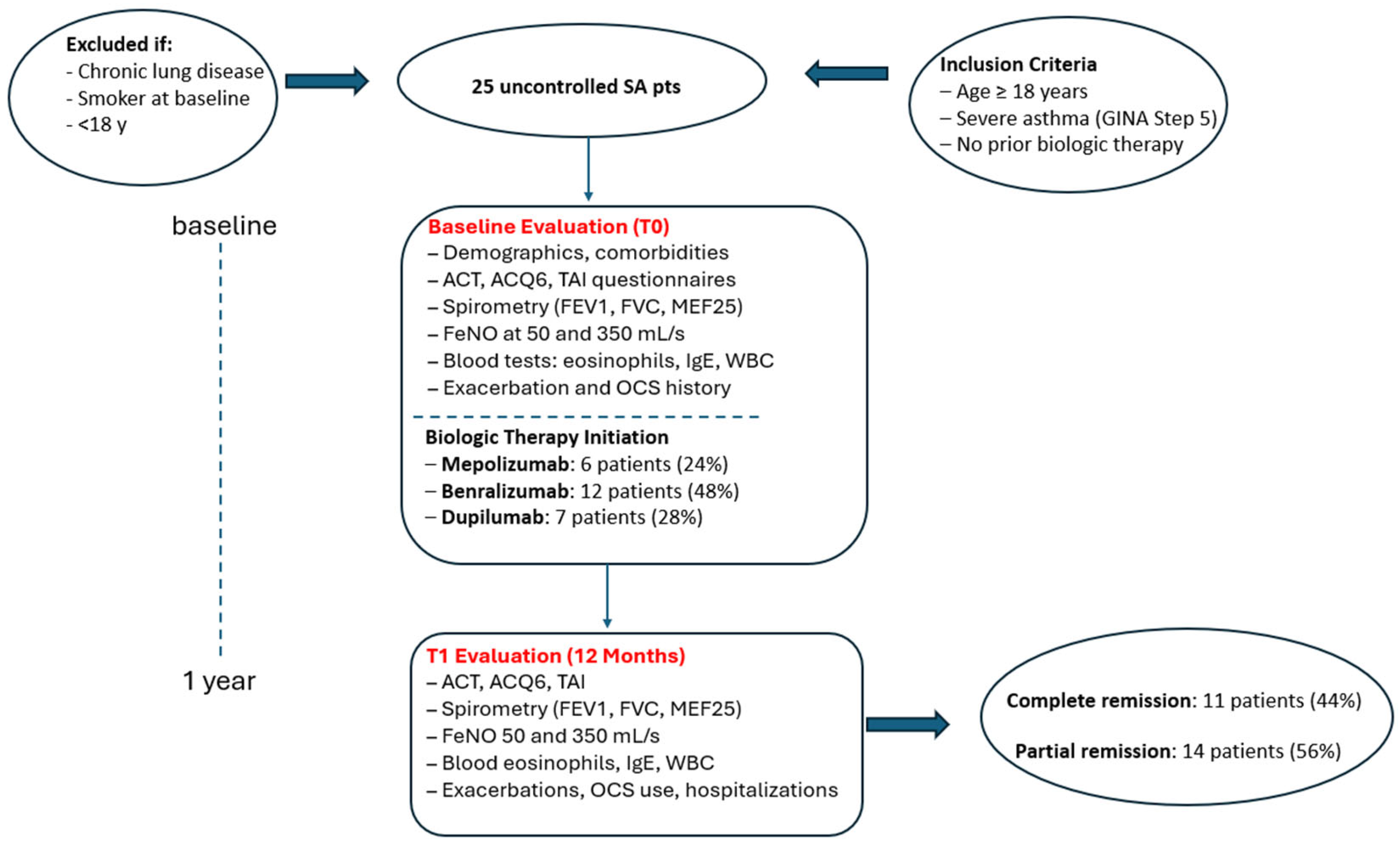
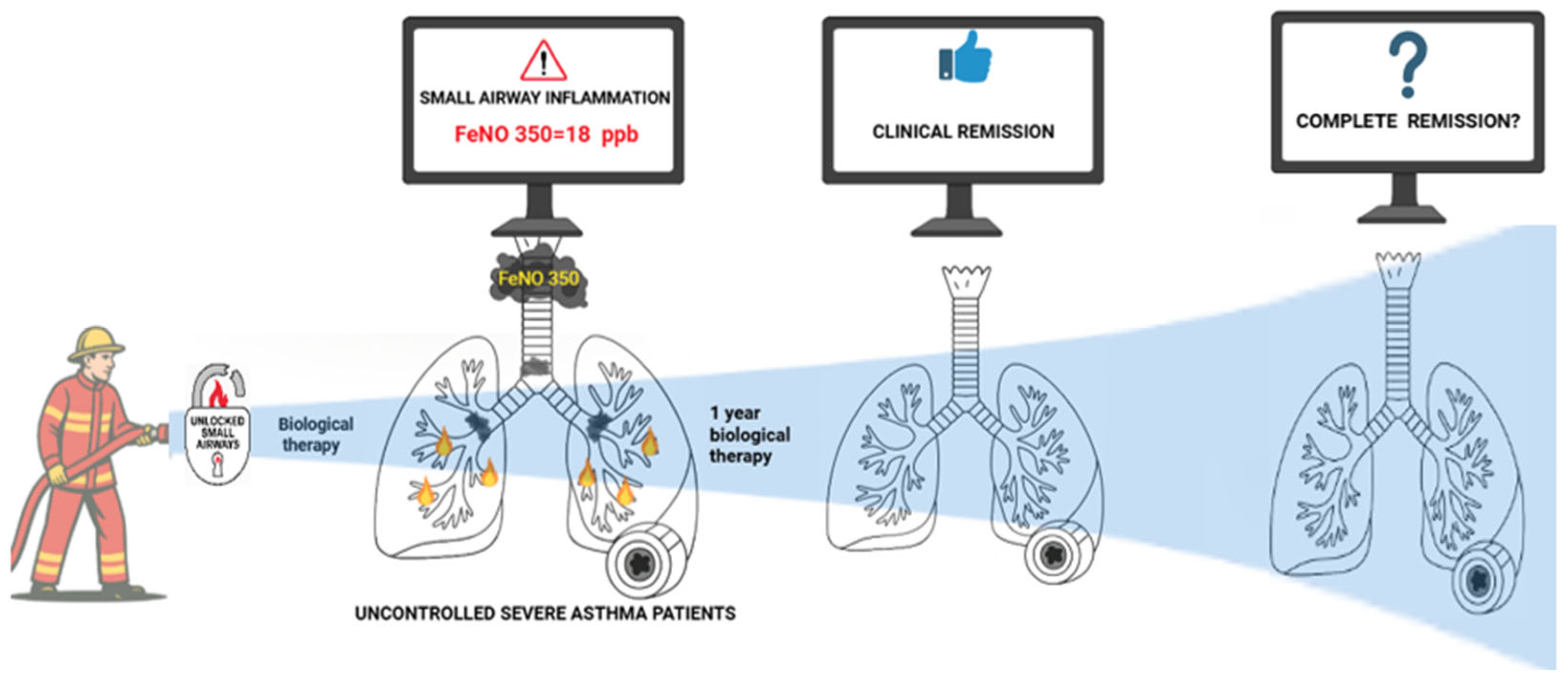
| Parameters | Population N = 25 | Uncontrolled Asthma N = 14 | Clinical Remission N = 11 | p-Value |
|---|---|---|---|---|
| Age y | 51 (41; 59) | 51.5 (43.5; 59) | 38.5 (21; 56) | 0.848 |
| Sex f | 11 (44) | 8 (57.1) | 3 (27.3) | 0.138 |
| BMI kg/m2 | 26 (24; 30) | 27.5 (24; 30) | 23.5 (21; 26) | 0.144 |
| History of smoke n (%) | 0.325 | |||
| Current | 0 (0) | 0 (0) | ||
| Past | 9 (36) | 5 (45.5) | ||
| No | 16 (64) | 6 (54.5) | ||
| Duration of asthma y median (IQ 25; 75) | 20 (3; 38) | 20(8.5; 39) | 11(1; 21) | 0.297 |
| Age at asthma diagnosis y median (IQ 25; 75) | 23 (15; 44) | 23 (12.5; 45) | 2.5 (1; 4) | 0.247 |
| EGPA n (%) | 3 (12) | 1 (7.1) | 2 (18.2) | 0.407 |
| Eosinophilic Pneumonia n (%) | 0 (0) | |||
| Bronchiectasis n (%) | 0 (0) | |||
| Hypereosinophilic Syndrome n (%) | 1 (4) | 0 (0) | 1 (9.1) | 0.440 |
| Chronic_Rhinosinusitis n (%) | 14 (56) | 6 (42.9) | 8 (72.7) | 0.138 |
| Nasal Poliposis n (%) | 14 (56) | 6 (42.9) | 8 (72.7) | 0.138 |
| Urticaria n (%) | 1 (4.0) | 1(7.1) | 0 (0) | 0.560 |
| Vocal Cord Dysfunction n (%) | 0 (0; 0) | |||
| GERD n (%) | 11 (44) | 7 (50) | 4(36.4) | 0.393 |
| OSAS n (%) | 3 (12) | 2 (14.3) | 1 (9.1) | 0.593 |
| Anxiety n (%) | 5 (20) | 4 (28.6) | 1 (9.1) | 0.245 |
| Population N = 25 | T0 Baseline | T1 After 1 Year N = 25 | |||||
|---|---|---|---|---|---|---|---|
| Uncontrolled Asthma Pt N = 14 | Clinical Remission Pt N = 11 | p-Value | Uncontrolled Asthma Pt N = 14 | Clinical Remission Pt N = 11 | p-Value | ||
| Biological Therapy n (%) | 0.140 | ||||||
| Mepolizumab | 6 (24) | 2 (14.3) | 4 (36.4) | ||||
| Bernalizumab | 12 (48) | 6 (42.9) | 6 (54.5) | ||||
| Dupilumab | 7 (28) | 6 (42.9) | 1.(9.1) | ||||
| Exacerbations (y) median (IQ 25; 75) | 2.5 (1; 5) | * 2.5 (1; 5.5) | ° 2.5 (1; 4) | 0.283 | 0 (0; 1) | 0 (0; 0) | 0.058 |
| Hospitalizations (y) median (IQ 25; 75) | 0 (0; 1) | 0 (0; 0.5) | 0.5 (0; 1) | 0.783 | 0 (0; 0) | 0 (0; 0) | 1 |
| Infectious exacerbations (y) median (IQ 25; 75) | 0 (0; 1) | 0 (0; 0) | 1 (1; 1) | 0.430 | 0 (0; 0) | 0 (0; 0) | 0.200 |
| Unscheduled visits (y) median (IQ 25; 75) | 1 (0; 2) | * 1 (0; 2) | 0.5 (0; 1) | 0.117 | 0 (0; 0) | 0 (0; 0) | 1 |
| OCS (y) median (IQ 25; 75) | 2.5 (1; 5) | * 2.5 (1; 5.5) | ° 2.5 (1; 4) | 0.236 | 0 (0; 0) | 0 (0; 0) | 0.109 |
| Average annual corticosteroid dosage (mg) median (IQ 25; 75) | 17.5 (0.07; 0.34) | 0.175 (0.07; 0.37) | 0.17 (0.07; 0.27) | ||||
| ACT median (IQ 25; 75) | 14 (10; 17) | * 12 (9.50; 16.50) | ° 19.50 (16; 23) | 0.100 | 23.50 (22; 25) | 25 (23; 25) | 0.295 |
| ACQ6 median (IQ 25; 75) | 2.20 (1.45; 3) | 2.21 (1.43) | ° 2.57 | 0.681 | 2.15 (1.12; 3.21) | 0.81 (0.73; 0.88) | 0.005 |
| TAI median (IQ 25; 75) | 54 (54; 54) | 54 (54; 54) | 54 (54; 54) | 1 | 54 (54; 54) | 54 (54; 54) | 1 |
| % FEV1 median (IQ 25; 75) | 65 (55; 86) | 65 (56; 82) | ° 70.5 (55; 86) | 0.742 | 71.50 (58; 97) | 91 (64.5; 98) | 0.642 |
| FEV1 (L) median (IQ 25; 75) | 1.98 (1.55; 2.41) | 1.88 (1.51; 2.23) | ° 2.41 (1.99; 2.84) | 0.396 | 2.06 (1.85; 2.58) | 3.10 (1.94; 3.57) | 0.112 |
| % FVC median (IQ 25; 75) | 84.50 (75; 97) | 84.50 (74.5; 97) | ° 86 (75; 97) | 0.742 | 92.50 (80; 104) | 101 (82; 103) | 0.681 |
| FVC (L) median (IQ 25; 75) | 2.93 (2.63; 4.30) | 2.81 (2.53; 3.20) | ° 3.64 (2.38; 4.58) | 0.155 | 2.99 (2.51; 3.76) | 3.88 (3.45; 4.73) | 0.080 |
| % FEV1/FVC median (IQ 25; 75) | 81.50 (71; 85) | 81.50 (71; 85) | 78 (71; 85) | 0.565 | 85 (70; 94) | 90 (68; 97) | 0.848 |
| % REV median (IQ 25; 75) | 1 (0; 3) | 1 (0; 3.5) | 1 (0; 2) | 0.595 | 2 (2; 3) | 3 (2; 3) | 0.860 |
| R5-20 kPa·L−1·s−1 IQ (25; 75) | 0.10 (0.09; 0.13) | 0.09 (0.07; 0.11) | 0.11 (0.10; 0.15) | 0.123 | 0.1 (0.07; 0.11) | 0.12 (0.08; 0.16) | 0.322 |
| Fres Hz IQ (25; 75) | 17 (15; 19) | 16 (14; 18) | 19 (16; 19) | 0.061 | 18 (15; 19) | 17 (15; 20) | 0.956 |
| AX kPa/L IQ (25; 75) | 1.10 (0.5; 1.3) | 1.30 (1.0; 1.4) | 0.60 (0.5; 1.1) | 0.297 | 1.20 (0.9; 1.4) | 0.90 (0.6; 1.1) | 0.334 |
| X5 kPa·L−1·s IQ (25; 75) | −0.12 (−0.20; −0.11) | −0.12 (−0.20; −0.11) | −0.12 (−0.16; −0.12) | 0.657 | −0.14 (−0.18; −0.11) | −0.21 (−0.85; −0.11) | 0.087 |
| % MEF25 median (IQ 25; 75) | 25 (15; 34) | 25 (15; 33.5) | 26 (16; 36) | 0.443 | 34.5 (18; 50) | 27 (16.5; 65.5) | 0.742 |
| FeNO50 (ppb) median (IQ 25; 75) | 20 (8; 27) | 23.50 (6.50; 30.50) | 26 (16; 36) | 0.476 | 17 (7; 39) | 23 (17.5; 26.5) | 0.956 |
| FeNO350 (ppb) median (IQ 25; 75) | 10 (5; 17) | 10 (4.5; 16.5) | ° 13.5 (8; 19) | 0.012 | 10 (4; 18) | 9 (6.5; 9.5) | 0.459 |
| WBC (cells/μL) median (IQ 25; 75) | 8170 (7250; 9300) | 8185 (716; 9510) | 7710 (7250; 8170) | 0.547 | 7105 (5800; 10,510) | 7690 (6095; 8125) | 0.956 |
| EOS (cells/μL) median (IQ 25; 75) | 389.3 (232; 700) | 284 (231; 638) | ° 600 (500; 700) | 0.198 | 185 (0; 470) | 0 (0; 40) | 0.084 |
| % EOS median (IQ 25; 75) | 4.36 (3.2; 7.9) | 4.10 (2.9; 7.33) | ° 7.73 (6.90; 8.75) | 0.080 | 2.61 (0; 6.61) | 0 (0; 0.6) | 0.170 |
| IgE (cells/μL) median (IQ 25; 75) | 248.50 (48; 485) | 230 (42.4; 476.5) | 600 (221; 980) | 0.273 | # | ||
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Parameters | OR | %CI 95 | p-value | OR | %CI | p-value |
| Age y | 0.993 | 0.929–1.062 | 0.841 | 0.932 | 0.843–1.031 | 0.932 |
| Sex f | 0.281 | 0.052–1.536 | 0.222 | 0.322 | 0.036–2.907 | 0.312 |
| BMI kg/m2 | 0.833 | 0.651–1.066 | 0.147 | |||
| History of smoking Current Former No | 0.480 | 0.091–2.523 | 0.386 | |||
| Years of asthma y | 0.96 | 0.916–1.018 | 0.204 | |||
| Age at asthma onset y | 1.035 | 0.979–1.094 | 0.220 | |||
| Chronic Rhinosinusitis | 3.282 | 0.574–18751 | 0.181 | |||
| Nasal Poliposis | 3.556 | 0.651–19412 | 0.143 | |||
| GERD | 0.571 | 0.114–2.872 | 0.497 | |||
| OSAS | 0.600 | 0.047–7.630 | 0.694 | |||
| Anxiety | 0.250 | 0.024–2.648 | 0.250 | |||
| Biological Therapy Omalizumab Mepolizumab Bernalizumab Dupilumab | 0.301 | 0.082–1.101 | 0.070 | |||
| Exacerbations (y) | 0.787 | 0.517–1.184 | 0.245 | |||
| Hospitalizations (y) | 0.600 | 0.128–3.392 | 0.619 | |||
| Infectious exacerbations (y) | 2.250 | 0.304–16.632 | 0.427 | |||
| Unscheduled visits (y) | 0.531 | 0.218–1.297 | 0.531 | |||
| OCS (y) | 0.749 | 0.483–1.160 | 0.195 | |||
| Average annual corticosteroid dosage (mg) | 0.014 | 0.000–8.634 | 0.192 | |||
| ACT | 1.144 | 0.970–1.347 | 0.110 | |||
| ACQ6 | 1.163 | 0.603–2.243 | 0.653 | |||
| % FEV1 | 0.995 | 0.962–1.030 | 0.784 | |||
| FEV1 (L) | 1.770 | 0.631–4.965 | 0.278 | |||
| % FVC | 0.988 | 0.941–1.037 | 0.619 | |||
| FVC (L) | 2.005 | 0.753–5.341 | 0.164 | |||
| % FEV1/FVC | 0.987 | 0.935–1.041 | 0.210 | |||
| % REV | 1.110 | 0.981–1.382 | 0.351 | |||
| % MEF25 | 1.004 | 0.97–1.033 | 0.759 | |||
| FeNO50 (ppb) | 1.018 | 0.986–1.051 | 0.264 | |||
| FeNO350 (ppb) | 1.115 | 1.008–1.234 | 0.035 * | 1.143 | 1.007–1.298 | 0.039 * |
| WBC (cells/μL) | 1.000 | 0.999–1.000 | 0.657 | |||
| EOS (cells/μL) | 1.001 | 0.999–1.004 | 0.228 | |||
| % EOS | 1.203 | 0.954–1516 | 0.119 | |||
| IgE (cells/μL) | 1.003 | 0.998–1.008 | 0.215 | |||
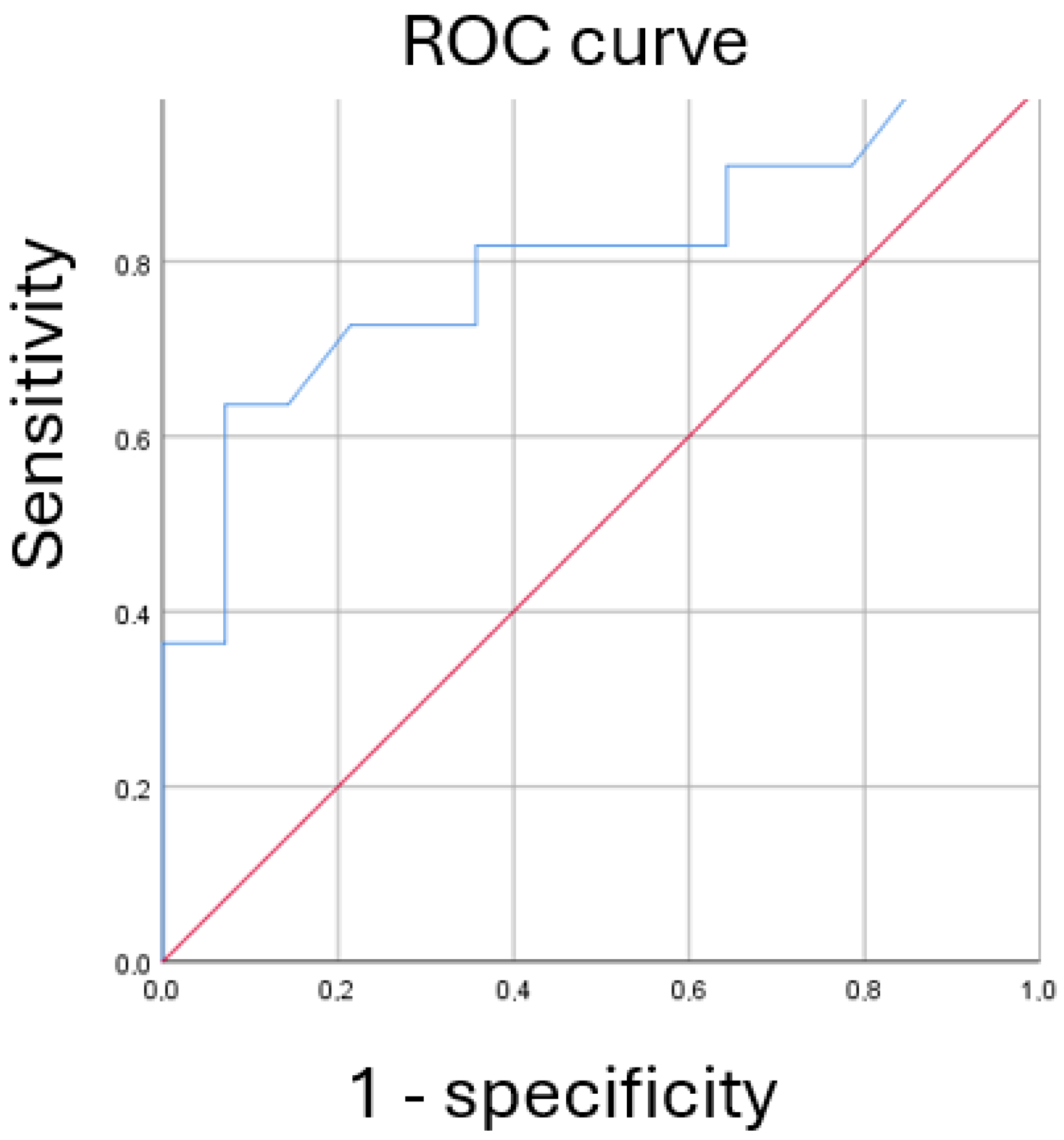
| Cutoff | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 18 | 63.6 (35.4; 84.8) | 92.8 (68.5; 98.7) | 87.5 (52.9; 97.7) | 76.4 (90.4; 52.7) |
| 10 | 81.8 (48.2–97.7) | 64.3 (35.1–87.2) | 64.3 (31.6–86.1) | 81.8 (48.2–97.7) |
| 14 | 72.7 (39.0–94.0) | 64.3 (35.1–87.2) | 61.5 (31.6–86.1) | 75.0 (42.8–94.5) |
| 20 | 54.5 (23.4–83.3) | 92.9 (66.1–99.8) | 85.7 (42.1–99.6) | 72.2 (46.5–90.3) |
| 24 | 36.4 (10.9–69.2) | 92.9 (66.1–99.8) | 80.0 (28.3–99.5) | 65.0 (40.8–84.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaranta, V.N.; Portacci, A.; Maselli, L.; Tornesello, M.; Granito, M.; Rociola, G.; Dragonieri, S.; Carpagnano, G.E. FeNO 350 mL/s: Unlocking the Small Airways to Achieve Clinical Remission in Severe Asthma—A Pilot Study. Adv. Respir. Med. 2025, 93, 37. https://doi.org/10.3390/arm93050037
Quaranta VN, Portacci A, Maselli L, Tornesello M, Granito M, Rociola G, Dragonieri S, Carpagnano GE. FeNO 350 mL/s: Unlocking the Small Airways to Achieve Clinical Remission in Severe Asthma—A Pilot Study. Advances in Respiratory Medicine. 2025; 93(5):37. https://doi.org/10.3390/arm93050037
Chicago/Turabian StyleQuaranta, Vitaliano Nicola, Andrea Portacci, Leonardo Maselli, Marta Tornesello, Maria Granito, Gennaro Rociola, Silvano Dragonieri, and Giovanna Elisiana Carpagnano. 2025. "FeNO 350 mL/s: Unlocking the Small Airways to Achieve Clinical Remission in Severe Asthma—A Pilot Study" Advances in Respiratory Medicine 93, no. 5: 37. https://doi.org/10.3390/arm93050037
APA StyleQuaranta, V. N., Portacci, A., Maselli, L., Tornesello, M., Granito, M., Rociola, G., Dragonieri, S., & Carpagnano, G. E. (2025). FeNO 350 mL/s: Unlocking the Small Airways to Achieve Clinical Remission in Severe Asthma—A Pilot Study. Advances in Respiratory Medicine, 93(5), 37. https://doi.org/10.3390/arm93050037








