Highlights
What are the main findings?
- Comparative analyses showed that inhaled aztreonam did not increase the risk of common adverse events (cough, dyspnea, fever, headache) compared with placebo or other inhaled antibiotics; however, serious grade 3/4 respiratory disorders were more frequent.
- A reduced risk of pulmonary function decline was observed in patients receiving inhaled aztreonam, with most adverse events being mild, manageable, and reversible.
What is the implication of the main finding?
- The overall tolerability and safety profiles support the use of inhaled aztreonam in children and adolescents with cystic fibrosis, provided that clinicians monitor for severe respiratory reactions and rare hepatotoxic effects.
- Future trials with larger sample sizes and longer follow-up should evaluate dosing strategies and investigate rare adverse events to refine risk–benefit assessments in pediatric populations.
Abstract
Respiratory infections and chronic lung disease are major contributors to morbidity in children. Aztreonam lysine for inhalation (AZLI) delivers high local antibiotic concentrations while limiting systemic exposure; however, its safety in younger patients remains uncertain. This systematic review and meta-analysis searched MEDLINE, CENTRAL, and Google Scholar for randomized and observational studies reporting adverse events in children and adolescents (≤18 years) receiving AZLI, with no date limit. Fourteen studies were included. Most studies were moderate-to-high quality. Comparative analysis showed no clinically relevant increase in common adverse events relative to placebo or other inhaled antibiotics. The pooled relative risk for severe respiratory disorders (grade 3/4) was 1.65 (95% CI 1.07–2.57), suggesting a higher incidence of serious respiratory events, while a protective effect against decline in pulmonary function was observed (RR 0.70, 95% CI 0.54–0.90). Adverse events were generally mild; serious adverse events and hospitalizations were infrequent and comparable between groups. Cumulative prevalence estimates indicated that respiratory irritation occurred in 10–25% of patients, whereas systemic effects were uncommon. Overall, AZLI appears to have an acceptable tolerability and safety profile in children and adolescents, though careful monitoring is warranted, especially for severe respiratory events.
1. Introduction
Respiratory infections remain a leading challenge in pediatric health worldwide. Acute lower respiratory tract infections, including bacterial and viral pneumonias, are the single largest infectious cause of death in children, killing about 740,180 children under five years of age in 2019, and accounting for 14% of all under-five deaths [1]. The burden of respiratory disease extends beyond acute infections. Chronic conditions such as bronchiectasis, primary ciliary dyskinesia, and cystic fibrosis (CF) predispose children to persistent lower-airway infections that are difficult to treat. These disorders often harbor multidrug-resistant bacteria—including Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, Burkholderia spp., and non-tuberculous mycobacteria—necessitating intensive antimicrobial strategies [2]. Among chronic disorders, CF is the most common inherited condition associated with recurring endobronchial infections. Registry data indicate that there are roughly 30,000 people with CF in the United States and 70,000 worldwide [3]. Pulmonary complications are the major cause of death in CF, accounting for nearly 60% of CF-related mortality [3]. P. aeruginosa colonization becomes increasingly common with age: in a multinational cohort, about half of children with CF acquired P. aeruginosa by 5.1 years of age, and 25% developed chronic colonization by 14.7 years [4]. Historical reports suggest that about 80% of individuals are infected by the age of 18 years [5]. Early infection is associated with a more rapid decline in lung function and worse survival [5]. Even with eradication strategies, persistent P. aeruginosa infection remains a significant cause of morbidity, highlighting the need for effective and safe inhaled therapies.
Systemic antipseudomonal antibiotics enhance outcomes but are constrained by toxicity and the requirement for intravenous access. Inhaled delivery gets a lot of the drug to the site of infection while minimizing how much of it gets into the rest of the body [6]. Aztreonam lysine for inhalation (AZLI) is a synthetic monobactam whose activity is mediated by inhibition of bacterial cell wall assembly, and works against gram-negative bacteria, such as P. aeruginosa. It binds with high affinity to penicillin-binding protein-3 (PBP3) in bacteria, blocking the transpeptidase step of peptidoglycan cross-linking. PBP3 inhibition triggers autolytic enzymes and membrane rupture, resulting in bactericidal effects [6].
The U.S. Food and Drug Administration (FDA) approved AZLI in 2010 to help CF patients with chronic P. aeruginosa infection breathe better [7]. After that, guideline committees included AZLI in standard care. According to the Cystic Fibrosis Foundation (CFF), AZLI should be given to those over 6 years old who have mild, moderate, or severe lung illness and a chronic P. aeruginosa infection to improve lung function and quality of life [8]. Subsequent trials showed that long-term alternate-month AZLI therapy maintained lung function and quality-of-life benefits without increasing resistance, and increasing the time to need antibiotics [2,9]. These data established AZLI as a key option for suppressing chronic P. aeruginosa infections in pediatric CF.
Inhaled antibiotics generally have favorable safety profiles compared with systemic therapy, yet respiratory irritation and systemic effects remain concerns. Across clinical trials, AZLI has been well tolerated, but the most frequently reported adverse reactions include cough (32–35%), headache (6–11%), nasal congestion (7–10%), rhinorrhea (~7%), and bronchospasm (6–10%) [2]. Occasional bronchoconstriction has prompted recommendations for a monitored test dose in patients with severe lung disease. Existing studies, however, have several limitations: many were conducted before widespread use of CF transmembrane conductance regulator (CFTR) modulators, enrolled relatively small numbers of children and adolescents, and prioritized efficacy over systematic adverse-event collection; evidence in very young children is sparse, and one randomized trial in patients colonized with Burkholderia cepacia found no improvement in lung function despite similar adverse-event rates [2]. Given that CFTR modulators have changed the course of cystic fibrosis, inhaled antibiotics such as AZLI warrant a fresh safety and relevance evaluation. Furthermore, post-marketing pharmacovigilance has highlighted unexpected safety signals. A recent disproportionality analysis of the U.S. Food and Drug Administration Adverse Event Reporting System, including 11,627 reports listing aztreonam as the primary suspect. It identified 127 preferred-term safety signals. Rare but serious events were reported, such as cholestatic liver injury, hypoprothrombinemia, hemoptysis, pulmonary hemorrhage, drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis. For AZLI specifically, the median time to onset of adverse events was approximately one year [10]. Such evidence would inform clinicians, caregivers, and guideline committees about the balance between benefits and harms, support shared decision-making, and identify knowledge gaps for future research.
The lack of a specific quantitative synthesis of AZLI safety in children and adolescents presents difficulties for clinicians and policymakers. Current meta-analyses generally aggregate data across extensive age ranges or prioritize efficacy outcomes, resulting in ambiguity on the incidence and severity of adverse events in younger patients. A systematic review and meta-analysis of adverse events linked to inhaled aztreonam can aggregate data from trials and observational studies to ascertain the prevalence of particular side effects and evaluate risks related to control therapy. Such evidence would elucidate the equilibrium between benefits and detriments, facilitate collaborative decision-making, and pinpoint knowledge deficiencies for subsequent research endeavors.
The primary objective of this review is to quantify the comparative risk of adverse events associated with inhaled aztreonam in children and adolescents. Secondary objectives include quantifying the pooled prevalence of adverse events associated with AZLI in the pediatric population. The primary research inquiries are as follows:
- Do the risks of specific adverse events differ between AZLI and placebo or other inhaled antibiotics?
- What is the pooled prevalence of adverse outcomes among children and adolescents receiving AZLI?
2. Materials and Methods
2.1. Register and Guidelines
This systematic review was designed in accordance with the PRISMA 2020 statement guidelines [11] and was prospectively registered in PROSPERO under registration number CRD420251084278. The study commenced on 2 July 2025, with completion anticipated for 2 August 2025.
2.2. Eligibility Criteria (PICOS)
Eligible studies evaluated children and adolescents (<18 years at treatment initiation) of both sexes, all pediatric conditions, country, language, or care setting (outpatient or inpatient). We included randomized trials and observational studies that investigated AZLI in any formulation, dose, duration, or device, whether administered alone or in combination with other treatments. All comparators were accepted (placebo, standard therapy, other inhaled antimicrobials, or comparisons between regimens). Preclinical studies (animal or in vitro models) were excluded.
2.3. Information Sources
Search was conducted in the MEDLINE database (via PubMed) and CENTRAL (Cochrane Central Register of Controlled Trials), with no language or date restrictions. For grey literature, we consulted Google Scholar and performed manual searches of article reference lists and citations (snowballing). We also examined clinical trial registries when available. All records were imported into Zotero for organization and deduplication. Title/abstract and full-text screening was conducted in Rayyan and in the Excel sheets [12]. We intentionally excluded EMBASE because of the overlap with the main databases and did not yield any unique eligible studies beyond those already identified.
2.4. Search Strategy
We employed a sensitive search strategy based on keywords, synonyms, and MeSH terms, covering three domains: drug (aztreonam and its synonyms), route of administration (inhalation/nebulization/aerosol), and pediatric population. The complete PubMed search string was as follows:
(“aztreonam” OR “Azthreonam” OR “Az-threonam” OR “Azactam” OR “SQ-26,776” OR “SQ 26,776” OR “SQ26,776” OR “Aztreon *” OR “azonam” OR “primbactam” OR “nebactam”) AND (“inhal*” OR “nebul*” OR “aerosol*” OR “atomaz*” OR “vapor*” OR “Nebulizers and Vaporizers”[Mesh] OR “Inhalation”[Mesh] OR “Administration, Inhalation”[Mesh]) AND ((infan* OR newborn* OR new-born * OR neonat* OR baby* OR babies OR toddler* OR minors OR boy OR boys OR boyhood OR girl * OR twin * OR kid OR kids OR child* OR stepchild OR step-child OR preschool OR schoolchild OR pediatrics[mh] OR pediatric* OR paediatric* OR peadiatric* OR school*[tiab] OR premature * OR preterm OR Child[Mesh] OR Infant[Mesh])).
The syntax was adapted for other databases (CENTRAL, Google Scholar).
2.5. Study Selection Process
Study selection was performed in two stages. During the screening of titles and abstracts, two reviewers independently and blindly assessed each record; disagreements were discussed and, when necessary, resolved with the involvement of a third reviewer. The same approach was applied to the full-text review, with standardized documentation of exclusion reasons in a PRISMA flow diagram: population outside the target age range, ineligible intervention, lack of safety and tolerability data, not an original article, not a clinical study, and unavailability of abstract and/or full text.
2.6. Data Extraction
Two reviewers independently extracted data using a standardized form that had been previously piloted. For each study, we collected information on identification (author, year of publication, study period), country, study design, setting (outpatient or inpatient), sample size, age (mean or median and range), diagnosis, and eligibility criteria. We detailed the intervention (dose, frequency—for example, BID/TID, inhalation device, treatment duration, total study period, and follow-up) and the comparator (type of control, if present). Discrepancies were resolved by consensus or, if needed, by a third reviewer.
2.7. Risk of Bias Assessment
Risk of bias was evaluated using the Cochrane RoB 2 tool for randomized clinical trials [13]. Two independent reviewers applied the tool and discussed the results; persistent disagreements were resolved by consensus. These judgments were integrated into the interpretation of results and the assessment of certainty of evidence using GRADE. “Traffic light/robvis” diagrams will be used to visually present the results.
2.8. Comparative Meta-Analysis
When a comparator group was present, we calculated the relative risk (RR) of adverse events using random-effects models by inverse variance. To handle zero-event data, a continuity correction of 0.5 was applied only to zero cells, and studies with double zeros were included [14]. Between-study heterogeneity was quantified using τ2 and the I2 statistic. The between-study variance (τ2) was estimated using restricted maximum likelihood (REML) [15], and confidence intervals were calculated using the Hartung–Knapp–Sidik–Jonkman (HKSJ) method [16]. From the pooled RR and 95% prediction interval (PI), we derived the corresponding risk in the aztreonam group (assumed risk). Tests for publication bias were conducted when there were ten or more studies.
2.9. Proportional Meta-Analysis
For studies with and without comparator groups, cumulative prevalence was calculated, and the proportion of participants experiencing at least one adverse event was synthesized using random-effects models with τ2 using REML on the Freeman–Tukey (double arcsine) scale with back-transformation and 95% prediction interval (PI). Confidence intervals for each study were obtained using the exact binomial (Clopper–Pearson) method; the pooled confidence interval was calculated using the HKSJ method [17].
2.10. Software
All analyses were conducted using R (RStudio 2025.05.01).
2.11. Certainty of Evidence (GRADE)
The certainty of evidence was evaluated for each outcome using the GRADE approach [18]. For comparative outcomes, the summary of Findings tables presents the RR and its 95% CI (HKSJ), the assumed risk (median of risks in the control group), the corresponding risk in the aztreonam group, the absolute risk increase, when RR > 1 (or indication of benefit when RR < 1). For proportional meta-analyses, we report the pooled prevalence with 95% CI. The GRADE rating starts at “high” for randomized trials and “low” for observational studies, including single-arm studies. The studies were downgraded for risk of bias, inconsistency, indirectness, imprecision, or publication bias; and upgrades when applicable (large effect, dose-response gradient, or residual confounding opposing the observed effect) [19].
3. Results
3.1. Selection and Inclusion of Records in the Systematic Review
The systematic search of Medline through PubMed, CENTRAL, and Google Scholar yielded 6545 entries, excluding duplicates, of which 93 articles were evaluated in full text. Figure A1 shows that 14 studies were included for qualitative analysis after the eligibility criteria were applied. These studies were published between 1987 and 2023, with more published in the last 15 years. This shows that there is more interest in the therapeutic effects of inhaled aztreonam in children [9,20,21,22,23,24,25,26,27,28,29,30,31,32].
As shown in Table 1, the conduct period for the included studies ranged from 2003 to 2021. Methodological robustness was demonstrated by the predominance of randomized clinical trials (64% of included studies), encompassing phase 2 and 3 trials, double-blind, placebo-controlled designs, as well as open-label and dose-escalation studies [9,21,26,27,28,29,30,31]. Observational studies accounted for 29% of the sample and included both open single-arm designs and studies with active comparators [20,22,24,32]. Additionally, a post hoc analysis of a multicenter trial was included, further broadening the methodological spectrum [31].

Table 1.
Characteristics and main findings of studies evaluating inhaled aztreonam safety and tolerability in children and adolescents.
Most studies were conducted in the United States, with six single-center studies in that country [9,20,21,27,28,29]. Four additional multicenter studies involved intercontinental collaborations between the USA, Canada, Australia, and New Zealand, while three studies were conducted jointly in Europe and the USA. One study did not report the geographic location of its conduct [30].
An analysis of the 14 included studies revealed significant diversity in the age profile of participants assessed for the safety of inhaled aztreonam. While most trials included both adults and children, three studies [22,30,31] focused exclusively on the pediatric population, underscoring the focus and relevance of the results for children and adolescents with cystic fibrosis (CF). The age range of patients treated with inhaled aztreonam was broad, from infants as young as 3 months to young adults and elderly individuals, especially in studies with mixed samples. Nonetheless, most participants were aged between 3 months and less than 18 years, with mean ages in pediatric studies primarily ranging from 6 to 15 years [21,24,27,29,30]. This age representation guarantees that the safety facts provided herein are directly relevant to pediatric and adolescent demographics. Concerning sex, the study samples exhibited sufficient female representation. Only two studies [25,29] did not specify the sex of participants. The clinical characteristics of the studied populations were also highly heterogeneous. All studies involved children and adolescents with a confirmed diagnosis of cystic fibrosis (CF). However, the clinical criteria differed: certain trials concentrated on patients with chronic Pseudomonas aeruginosa infection, while others encompassed children with recent or initial infection, in addition to cases with chronic Burkholderia infection. There was also a range of pulmonary function criteria, including cases with stable illness and moderate to severe lung disease. This helped evaluate the safety of inhaled aztreonam in varied clinical settings for children with CF.
Some studies utilized single-arm or cohort designs lacking a control group [19,21,24,29,31], whilst others integrated a placebo with regular medicines, illustrating the intricacies of clinical practice [20]. Most trials utilized placebo groups, typically consisting of lactose or saline solutions, as comparators [8,22,24,25,26,27]. Some studies also looked at aztreonam directly against other routinely used antibiotics, like inhaled tobramycin [23] and combinations of azlocillin and intravenous tobramycin [28]. Other research chooses to look at different doses or concentrations of aztreonam, which makes it possible to see how safe it is in a variety of situations.
Analysis of studies on inhaled aztreonam in children and adolescents with cystic fibrosis revealed that the predominant regimen was 75 mg three times daily, typically administered in 28-day cycles followed by rest periods. This design was used in most trials [20,21,22,23,24,25,27,29], which made it easy to compare the results. Some studies tried different doses and lengths of time; however, they did not change the fact that the 28-day cycle was the most common. This cycle was valuable for testing the safety and effectiveness of the intervention in varied clinical situations.
The assessed clinical indications were diverse, including the eradication or early prevention of Pseudomonas aeruginosa infection, maintenance and bacterial suppression, and the management of acute exacerbations. The 75 mg TID regimen for 28 days demonstrated efficacy in eliminating the germs and reducing respiratory symptoms during investigations focused on eradication. In prolonged therapeutic contexts, inhaled aztreonam enhanced quality of life, lung function, and diminished exacerbations, while exhibiting a safety profile akin to that of a placebo. Investigations into acute exacerbations and pharmacokinetics indicated that the medication was well-tolerated, even when administered concurrently with other pharmaceuticals.
The studies included used inhaled administration, mostly with electronic equipment like the eFlow®/Altera® nebulizer (PARI Pharma GmbH Gräfelfing, Germany), which may be used by people of all ages and in diverse clinical settings [8,20,21,22,24,25,27,29,31]. Protocols sometimes suggested the use of bronchodilators before giving aztreonam, and self-administration under supervision was common. The majority of investigations indicated the utilization of various concomitant medications, encompassing inhaled antibiotics (such as tobramycin, colistin), azithromycin, mucolytics, bronchodilators, hypertonic saline solutions, corticosteroids, pancreatic enzymes, and vitamins [8,20,24,25,26,28].
There was a lot of variety in how long the trials on inhaled aztreonam lasted, with follow-up periods ranging from 13 days to five years. Furthermore, the diverse time points utilized for outcome collecting and analysis demonstrate rigorous scientific practices, encompassing evaluations throughout various therapy phases and after many cycles. Studies included both early monitoring of adverse events—through assessments completed hours after administration—and long-term follow-up, with regular visits lasting up to five years, enhancing the thoroughness of safety and efficacy analyses across clinical settings.
Outcomes analyzed were comprehensive, including microbiological eradication, changes in lung function (FEV1), respiratory symptoms, and exacerbation rates, as well as tolerability, laboratory safety, and bacterial resistance. This multiplicity of outcomes reflects a balanced concern for both efficacy and safety.
As shown in Table A1, the risk of bias analysis, conducted using the RoB 2 tool, revealed that only four studies were classified as having low risk of bias, demonstrating methodological robustness primarily through adequate randomization and transparent outcome reporting [4,20,22,25]. These studies serve as references for the most reliable interpretation of inhaled aztreonam safety data in children and adolescents. On the other hand, the majority of included studies—nine of the fourteen analyzed—were classified as high risk of bias [8,19,21,23,24,25,27,31]. The main limitations identified related to lack of blinding, substantial participant losses during follow-up, absence of intention-to-treat analysis, and insufficient or selective reporting of evaluated outcomes. Notably, in domains relating to intervention adherence, handling of missing data, and selection of reported outcomes, several studies were classified as high risk or “some concerns,” further compromising the reliability of the findings [12,21,24,28].
3.2. Comparative Meta-Analysis of Adverse Events
The comparative meta-analysis reveals that the administration of inhaled aztreonam in children and adolescents does not correlate with a clinically significant rise in the majority of prevalent adverse events when compared to controls, as illustrated in Table 2. In high-frequency respiratory outcomes, productive sputum exhibited a relative risk (RR) of 0.83 (95% CI 0.67–1.02; I2 2.3%), sputum congestion RR 0.84 (0.54–1.31; I2 0%), rales/crackles RR 0.84 (0.60–1.20; I2 0%), and dyspnea RR 0.76 (0.51–1.13; I2 0%). The narrow confidence intervals and limited heterogeneity offer moderate evidence of the absence of increased harm.

Table 2.
Summary of comparative risks for adverse effects associated with inhaled aztreonam treatments in children and adolescents: results from comparative meta-analysis.
A major exception was noted for severe (grade 3/4) respiratory problems, where aztreonam markedly elevated the risk (RR 1.65; 1.07–2.57; I2 0%); however, the certainty is low due to methodological constraints and imprecision. From a functional standpoint, the medication was linked to a reduced probability of deterioration in pulmonary function (PFT), with a relative risk of 0.70 (0.54–0.90; I2 0%), indicating a strong and potentially clinically protective outcome.
Among systemic effects, fatigue (RR 0.71; 0.38–1.31; I2 0%), pyrexia (RR 1.11; 0.82–1.50; I2 0%), and headache (RR 1.03; 0.70–1.52; I2 0%) exhibited no statistically significant increases. Regarding gastrointestinal signs, abdominal pain/tenderness exhibited a relative risk (RR) of 0.62 (0.15–2.63; I2 63%), vomiting had an RR of 0.97 (0.48–1.96; single study), and diarrhea presented an RR of 0.36 (0.10–1.26; single study); dysgeusia demonstrated an RR of 4.65 (0.27–81.62; single study). The evidence for these outcomes is highly questionable, as it originates from a restricted number of studies or small samples, with confidence intervals covering significant effects in both directions.
Regarding laboratory abnormalities, namely hepatotoxicity, the relative risk (RR) was 4.00 (1.08–14.75), while for general laboratory abnormalities, the RR was 2.00 (0.85–4.73). Both findings were from a singular experiment with 22 patients, demonstrating no substantial heterogeneity; hence, these data necessitate careful interpretation.
Data on serious adverse events (SAEs) (RR 1.14; 0.68–1.90; I2 32.3%), hospitalizations (RR 0.88; 0.54–1.44; I2 18.4%), and mortality (RR 1.27; 0.52–3.10; I2 0%) were infrequent and exhibited no significant differences between groups; however, the certainty is classified as low to very low due to the infrequency of outcomes and considerable imprecision. All forest plots are presented in Figure A2.
Owing to the limited number of papers used in this meta-analysis (n < 10), no formal assessment of publication bias was performed, utilizing funnel plots or statistical tests for asymmetry, as advised by the Cochrane Handbook [14]. Searches of grey literature and trial registries were conducted to mitigate the likelihood of publication bias, and the potential constraints associated with this bias were qualitatively analyzed.
3.3. Proportional Meta-Analysis of Adverse Events
For the cumulative prevalence meta-analyses of adverse events, as shown in Table A2, among respiratory outcomes, mild events such as productive sputum showed a pooled prevalence of 0.25 (95% CI 0.08–0.47; I2 98.2%, Q 455.9, p < 0.0001), while sputum congestion presented 0.19 (0.03–0.43; I2 97.0%). Among potentially severe events, bronchospasm/wheezing occurred in 0.10 of participants (0.02–0.21; I2 93.3%), and grade 3/4 respiratory disorders reached 0.15 (0.06–0.27; I2 94.7%). The high heterogeneity (I2 > 90% for all these outcomes) suggests the influence of clinical differences (e.g., age, baseline lung function) or methodological differences between studies.
Among systemic manifestations, fever/pyrexia was relatively common, with a prevalence of 10.9 (2.4–23.7; I2 96.1%, Q 206.5, p < 0.0001). Fatigue and headache showed similar estimates—0.13 (0.01–0.34; I2 96.6%) and 0.13 (0.05–0.24; I2 92.3%), respectively—again with substantial variability. Gastrointestinal events such as abdominal pain (4%, 1–11; I2 75%) and vomiting (5%, 3–8; I2 90.8%) occurred in up to one in twenty patients, but with wide confidence intervals and marked heterogeneity, reflecting considerable uncertainty in the estimates.
Regarding severe complications, serious adverse events (SAEs) had a prevalence of 0.11 (0.03–0.23; I2 96.1%, Q 285.7, p < 0.0001). Hospitalizations occurred with greater frequency, recorded at 15.3 (4.9–29.7; I2 97.3%), although mortality remained infrequent at 0.02 (0.00–0.36) with I2 0%. Although fatalities are infrequent, the substantial variety in serious adverse events and hospitalizations suggests potential methodological and clinical discrepancies, necessitating additional analyses (e.g., meta-regression) to ascertain causes of heterogeneity.
Laboratory abnormalities (1.7, 0–9.4; I2 75.2%) and audiological abnormalities (1.7, 0–9.4; I2 75.2%) exhibited borderline heterogeneity, indicating that a limited number of severe observations may distort the estimations. Extremely rare occurrences, such as lung transplantation (0.00, 0.00–0.001; I2 0%) and allergic responses (0.00, 0.00–0.50; I2 0%), remained nearly negligible; nonetheless, the data are highly questionable due to the restricted number of studies and patients.
4. Discussion
4.1. Overview of the Evidence Base
This review indicates that the most common adverse events (AEs) did not increase in a clinically relevant way with the use of inhaled aztreonam in children and adolescents. One important exception was observed for grade 3/4 respiratory disorders, for which risk increased with AZLI. On the other hand, the drug demonstrated a signal of protection against lung function decline: the significant reduction in the occurrence of pulmonary function deterioration (PFT) suggests a potential clinical benefit. Rare outcomes such as serious adverse events, hospitalizations, and mortality showed no statistical difference; however, the certainty of evidence is low due to the limited number of events. Laboratory abnormalities, especially hepatotoxicity, were evaluated in only one trial and suggested an increased risk, but the small sample size limits firm conclusions. Cumulative prevalence estimates revealed that mild respiratory events are common in the context of inhaled aztreonam. Severe events remained infrequent. Uncertainty remains high because confidence intervals are wide and inter-study heterogeneity is substantial.
4.2. Clinical Interpretation and Significance
From a practical standpoint, the data suggest that the safety profile of inhaled aztreonam is acceptable for most children and adolescents with CF when compared to placebo or reference therapies. The absence of a significant increase in events such as productive sputum, congestion, dyspnea, fatigue, and fever indicates that the local effects of the drug are predictable and generally manageable. The reduction in the risk of lung function decline suggests a potential clinical benefit, especially in patients with stable or declining pulmonary function. The observed increase in severe respiratory disorders, although based on few events, should be considered in the risk–benefit assessment: these conditions include exacerbations or bronchospasm that may require medical intervention. In clinical practice, treatment should be individualized, with respiratory monitoring particularly during the first weeks of use and in patients with advanced lung disease.
The high heterogeneity observed in many outcomes likely reflects clinical and methodological differences between studies. Age (infants vs. adolescents), severity of baseline pulmonary function, and infection status (initial vs. chronic infection by P. aeruginosa or Burkholderia) are factors that may modify the risk of AEs. The presence of comorbidities or colonization by multiple pathogens, as well as concomitant use of bronchodilators, mucolytics, azithromycin, and saline solutions, may influence both the incidence and the perception of adverse events. Dosing regimens also varied (75 mg TID for 14, 28, or 56 days) and may impact the accumulation of irritative effects. Follow-up duration and outcome definitions (for example, “dyspnea” versus “chest discomfort”) differed, contributing to the dispersion of results.
The present findings are largely consistent with eradication and long-term use studies from the past decade. The ALPINE2 study, a phase 3b trial comparing 14- versus 28-day regimens in 149 children with newly isolated P. aeruginosa, found similar eradication rates between groups (55.9% vs. 63.4%); treatment-emergent adverse events were similar, and no new safety signals were detected [31]. An earlier open-label study with 105 children confirmed that inhaled aztreonam was effective and well tolerated; 89.1% of patients were free of Pa at the end of treatment, and 58% remained negative at all subsequent assessments [22]. These results complement our meta-analysis by reinforcing that the tolerability of aztreonam is comparable between 14- and 28-day cycles. In adults, 2025 pharmacovigilance data based on the FAERS database identified new potential signals, such as cholestatic liver injury, hypoprothrombinemia, and hemoptysis, with a median onset of one year after starting AZLI [10]. Such events were rare and reported mainly in older adults, but highlight the need for liver monitoring, even in pediatric patients undergoing repeated or prolonged treatments.
Inhaled aztreonam achieves concentrations far above the MIC of P. aeruginosa directly within the airway lumen, providing minimal systemic exposure. This local administration explains the low frequency of systemic events (fatigue, fever, headache) and the absence of renal or hematological toxicity. On the other hand, deposition of the antibiotic in the airways can cause local irritation and induce bronchospasm or transient increases in sputum production. The observed increase in grade 3/4 respiratory disorders may reflect individual hypersensitivity, bronchial irritation, or interaction with thick secretions in patients with advanced lung disease. Laboratory abnormalities (hepatotoxicity) may be related to prolonged systemic absorption during repeated cycles, as suggested by pharmacovigilance signals in adults [10].
This review stands out for its comprehensive search strategy, which included multiple databases and grey literature, and for the independence of reviewers, reducing the risk of selection bias. The predominance of high-quality RCTs with similar dosing regimens (75 mg TID for 28 days) enabled the aggregation of comparable data. The inclusion of studies up to 2023, as well as consideration of long-term follow-up data, enhances the currency of the safety estimates.
4.3. Limitations
Several limitations must be acknowledged. Many estimates were derived from small samples and rare events, increasing imprecision and resulting in very high heterogeneity. There was variation in outcome definitions and in methods of event ascertainment (spontaneous reporting vs. standardized clinical evaluation), which hinders comparability. Most studies had a short duration (one 28-day cycle); data on prolonged and repeated use are scarce. Subgroup analysis (age, lung function, and initial vs. chronic infection) was not possible due to the lack of individual-level data, and selective publication of positive or safety-favorable RCTs cannot be excluded.
4.4. Certainty of Evidence (GRADE)
Applying the GRADE approach, the overall certainty for common respiratory events (productive sputum, congestion, dyspnea, fever) ranged from moderate to low, as RCTs provided precise estimates and the risk of bias was small; however, heterogeneity and imprecision reduced reliability. For rare or laboratory events (hepatotoxicity, hematological and renal abnormalities), certainty was very low, as estimates were based on single studies with few children. The main reasons for downgrading were risk of bias (unclear allocation concealment in some RCTs), imprecision (wide confidence intervals), inconsistency, and possible publication bias.
4.5. Implications for Clinical Practice and Future Research
The findings indicate that inhaled aztreonam can be administered relatively safely and is well-tolerated by children and adolescents with cystic fibrosis to eliminate or inhibit Pseudomonas aeruginosa, particularly when utilizing a regimen of 75 mg three times a day for 28 days. Monitoring should encompass an initial evaluation of respiratory function and vigilant observation for indications of bronchospasm or wheezing, especially in patients with severe pulmonary illness. Families and caregivers must be advised about the potential for moderate symptoms (sputum, weariness) and the infrequency of severe occurrences. For patients enduring numerous cycles or possessing risk factors for liver illness, laboratory evaluation of liver function and coagulation is advised, given the few indicators of cholestasis and hypoprothrombinemia observed in adult pharmacovigilance studies [10]. The choice to utilize should weigh the advantages of bacterial eradication or suppression and quality of life against the potential for severe respiratory exacerbations.
Subsequent investigations ought to incorporate randomized controlled trials (RCTs) including higher sample sizes and extended follow-up periods, proficient in identifying infrequent occurrences (hepatotoxicity, hematotoxicity, severe bronchospasm). The examination of various dosing schedules (e.g., 14 vs. 28 days) must persist to enhance adherence without jeopardizing efficacy or safety, as investigated in ALPINE2 [31]. Individual patient data meta-analyses and meta-regressions could investigate the observed heterogeneity by evaluating modifiers such as age, baseline lung function, infection type, and concurrent medications. Post-marketing safety studies and those utilizing extensive databases are crucial for detecting long-term uncommon signals, as indicated by pharmacovigilance analysis.
5. Conclusions
Inhaled aztreonam shows an acceptable safety and tolerability profile in children and adolescents with cystic fibrosis—adverse events are predominantly mild–moderate, reversible, and comparable to placebo, with no signal for excess mortality or hospitalization and a suggestion of preserved lung function. Nevertheless, the higher rate of grade 3/4 respiratory events and occasional laboratory abnormalities warrants close monitoring—especially with prolonged use—and supports the need for larger, longer, standardized studies to refine subgroup-specific risk. Meanwhile, clinicians should individualize therapy and monitor patients closely.
Author Contributions
All authors contributed equally to every stage of the work, including study conception and design, data acquisition, analysis and interpretation, manuscript drafting and critical revision. All authors approved the final version and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AE | Adverse Event(s) |
| ALPINE2 | Aztreonam Lysine for Pseudomonas Infection Eradication 2 (trial name) |
| Altera® | Altera Nebulizer System |
| AZLI | Aztreonam Lysine for Inhalation |
| BID | Bis in Die |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| CF | Cystic Fibrosis |
| CFF | Cystic Fibrosis Foundation |
| CI | Confidence Interval |
| Clopper–Pearson | Clopper–Pearson Exact Method |
| CRD | Centre for Reviews and Dissemination |
| DRESS | Drug Reaction with Eosinophilia and Systemic Symptoms |
| eFlow® | eFlow Nebulizer System |
| FAERS | FDA Adverse Event Reporting System |
| FDA | Food and Drug Administration |
| FEV1 | Forced Expiratory Volume in 1 Second |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| HKSJ | Hartung–Knapp–Sidik–Jonkman |
| I2 | I-squared statistic |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| MeSH | Medical Subject Headings |
| MIC | Minimum Inhibitory Concentration |
| NICE | National Institute for Health and Care Excellence |
| NLM | National Library of Medicine |
| P. aeruginosa | Pseudomonas aeruginosa |
| Pa | Pseudomonas aeruginosa |
| PFT | Pulmonary Function Test(s) |
| PICOS | Population, Intervention, Comparator, Outcomes, Study design |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| R | R Programming Language |
| RCT | Randomized Controlled Trial |
| REML | Restricted Maximum Likelihood |
| RoB 2 | Risk of Bias 2 |
| RR | Relative Risk |
| RStudio | RStudio IDE |
| SAE | Serious Adverse Event(s) |
| TID | Ter in Die |
| U.S. | United States |
| τ2 | Tau-squared |
Appendix A
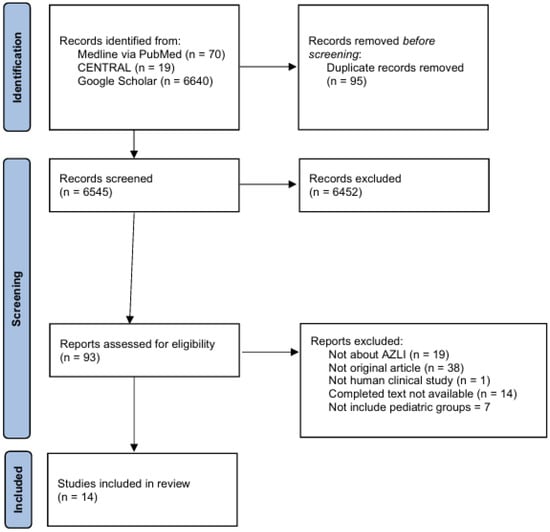
Figure A1.
PRISMA flow diagram of study identification, screening, and inclusion for the systematic review of inhaled aztreonam tolerability and safety in pediatric populations. Note: AZLI: Aztreonam lysine for inhalation; CENTRAL: Cochrane Central Register of Controlled Trials; n: Number of records or studies. This PRISMA flow diagram details the multi-stage process of identifying, screening, and including studies for this systematic review, following the PRISMA 2020 guidelines. “Reports excluded” refers to studies excluded after full-text assessment, with detailed reasons listed. “Duplicate records removed” denotes deduplication across databases. Screening was conducted on titles and abstracts, followed by full-text assessment for eligibility. Only studies including pediatric populations were retained.

Table A1.
Risk of bias for studies of inhaled aztreonam in children and adolescents.
Table A1.
Risk of bias for studies of inhaled aztreonam in children and adolescents.
| Study | Domain 1 | Domain 2.1 | Domain 2.2 | Domain 3 | Domain 4 | Domain 5 | Overall Judgment (RoB2) |
|---|---|---|---|---|---|---|---|
| Gilchrist (2023) [31] | L | L | L | S | L | S | S |
| Keating (2021) [20] | S | S | H | H | S | S | H |
| Flume (2016) [21] | L | L | L | L | L | S | S |
| Tiddens (2015) [22] | H | S | H | H | S | S | H |
| Tullis (2014) [23] | L | L | L | L | L | S | S |
| Assael (2012) [24] | L | S | H | S | S | S | H |
| Wainwright (2011) [25] | L | L | L | L | H | S | H |
| Oermann (2010) [32] | S | L | H | S | S | S | H |
| Retsch-Bogart (2009) [26] | L | L | H | L | L | S | H |
| McCoy (2008) [9] | L | L | H | L | L | S | H |
| Retsch-Bogart (2008) [27] | L | L | L | L | S | S | S |
| Gibson (2006) [28] | L | L | L | L | H | S | H |
| Pryka (1987) [29] | S | S | H | H | H | S | H |
| Tiddens (2015) [30] | S | S | H | L | H | S | H |
Abbreviations and Notes: H: High risk of bias; L: Low risk of bias; S: Some concerns; RoB2: Risk Of Bias 2 tool. Domain 1: Randomization process; Domain 2.1: Deviations from intended interventions (effect of assignment); Domain 2.2: Deviations from intended interventions (effect of adherence); Domain 3: Missing outcome data; Domain 4: Measurement of the outcome; Domain 5: Selection of the reported result. All judgments are based on the RoB2 criteria. “Overall Judgment (RoB2)” reflects the highest risk rating across all domains for each study. For detailed criteria, see RoB2 guidance. Colors denote risk level: green (L) for low risk, yellow (S) for some concerns, and red (H) for high risk.

Table A2.
Cumulative pooled prevalence of adverse events associated with inhaled aztreonam in children and adolescents: results from a proportional meta-analysis.
Table A2.
Cumulative pooled prevalence of adverse events associated with inhaled aztreonam in children and adolescents: results from a proportional meta-analysis.
| Adverse Events | Pooled Prevalence % (95% CI) | No of Participants (Studies) | Heterogeneity (I2, Q, p) | Quality of the Evidence (GRADE) |
|---|---|---|---|---|
| Hemoptysis | 0.15 [0.01–0.43] | 625 (4) | I2 = 95.4%, p < 0.0001, Q = 64.77 | Very low 1,2,3,6 |
| Productive sputum | 0.25 [0.08–0.47] | 960 (9) | I2 = 98.2%, p < 0.0001, Q = 455.92 | Very low 1,2,3,6,7 |
| Sputum increased congestion | 0.19 [0.03–0.43] | 732 (6) | I2 = 97.0%, p < 0.0001, Q = 166.46 | Very low 1,2,6 |
| Fatigue | 0.13 [0.01–0.34] | 701 (5) | I2 = 96.6%, p < 0.0001, Q = 118.97 | Low 1,2,5 |
| Dyspnea | 0.14 [0.03–0.32] | 773 (6) | I2 = 95.4%, p < 0.0001, Q = 109.80 | Low 1,2,6 |
| Acute Respiratory Infections | 0.10 [0.00–0.45] | 319 (5) | I2 = 95.7%, p < 0.0001, Q = 93.90 | Low 1,2,6 |
| Influenza | 0.01 [0.01–0.02] | 150 (2) | I2 = 0.0%, p = 0.98, Q = 0.00 | Low 1,2,3,4 |
| Alterations in Oxyhemoglobin Saturation | 0.0 [0.0–2.3] | 74 (1) | –* | Very low 1,2,3,5 |
| Crackles or rales | 0.14 [0.02–0.33] | 558 (4) | I2 = 88.8%, p < 0.0001, Q = 26.80 | Low 1,2,3 |
| Chest pain or discomfort | 0.11 [0.04–0.21] | 632 (5) | I2 = 86.4%, p < 0.0001, Q = 29.34 | Low 1,2,6 |
| Bronchospasm or wheezing | 0.10 [0.02–0.21] | 815 (7) | I2 = 93.3%, p < 0.0001, Q = 89.03 | Low 1,2,3,5 |
| Grade 3/4 respiratory disorders | 0.15 [0.06–0.27] | 992 (7) | I2 = 94.7%, p < 0.0001, Q = 113.00 | Low 1,2,5 |
| Decreased pulmonary function (PFT) | 0.06 [0.02–0.11] | 959 (9) | I2 = 87.3%, p < 0.0001, Q = 63.10 | Low 1,2,6 |
| Lung transplant | 0.00 (0.00–0.0010) | 291 (3) | I2 = 0.0%, p = 0.97, Q = 0.05 | Very low 1,2,4,5,6 |
| Fever or pyrexia | 10.9 [2.4–23.7] | 965 (9) | I2 = 96.1%, p < 0.0001, Q = 206.50 | Low 1,2,4,5,6 |
| Rhinorrhea, rhinitis, congestion, or sinusitis | 0.13 [0.08–0.19] | 1022 (9) | I2 = 87.2%, p < 0.0001, Q = 62.46 | Low 1,2,6 |
| Pharyngolaryngeal irritation | 0.16 [0.05–0.31] | 843 (7) | I2 = 95.9%, p < 0.0001, Q = 144.70 | Low 1,2,3,6 |
| Abdominal pain or tenderness | 4 [1–11] | 495 (5) | I2 = 75.0%, p = 0.003, Q = 16.02 | Low 1,2,3,6 |
| Vomiting | 5 [3–8] | 241 (2) | I2 = 90.8%, p = 0.001, Q = 10.88 | Very low 1,2,3,4,6 |
| Dysgeusia | 0.07 [0.02–0.15] | 74 (1) | –* | Very low 1,2,5,6 |
| Decreased appetite | 0.17 [0.00–0.75] | 545 (3) | I2 = 97.9%, p < 0.0001, Q = 96.30 | Low 1,2,6 |
| Allergic reactions | 0.00 [0.00–0.50] | 167 (3) | I2 = 0.0%, p = 0.84, Q = 0.35 | Low 1,2,4,5 |
| Headache | 0.13 [0.05–0.24] | 843 (7) | I2 = 92.3%, p < 0.0001, Q = 78.43 | Low 1,2,6 |
| Audiological abnormalities | 1.7 [0.0–9.4] | 354 (4) | I2 = 75.2%, p = 0.007, Q = 12.12 | Low 1,2,6 |
| Hepatic abnormalities | 0.23 [0.00–1.00] | 146 (2) | I2 = 97.4%, p < 0.0001, Q = 38.94 | Very low 1,2,4,5,6 |
| Hematological abnormalities | 0.00 [0.00–0.15] | 11 (1) | –* | Very low 1,2,4,5,6 |
| Renal abnormalities | 0.00 [0.00–0.14] | 146 (2) | I2 = 0.0%, p = 0.50, Q = 0.45 | Very low 1,2,4,5,6 |
| Laboratory abnormalities | 1.7 [0.0–9.4] | 354 (4) | I2 = 75.2%, p = 0.007, Q = 12.12 | Low 1,2,6 |
| Serious adverse events (SAE) | 0.11 [0.03–0.23] | 1124 (12) | I2 = 96.1%, p < 0.0001, Q = 285.70 | Low 1,2,3,6 |
| Hospitalizations | 15.3 [4.9–29.7] | 1.517 (11) | I2 = 97.3%, p < 0.0001, Q = 376.70 | Low 1,2,3,6 |
| Deaths | 0.02 [0.00–0.36] | 1124 (12) | I2 = 0.0%, p = 0.72, Q = 7.95 | Low 1,2,3,4 |
| Withdrawal due to adverse events | 0.03 [0.01–0.05] | 1.634 (13) | I2 = 80.9%, p < 0.0001, Q = 62.95 | Low 1,2,6 |
| Missed dose (“Dose Missed”) | 0.01 [0.00–0.05] | 105 (1) | –* | Very low 1,2,5,6 |
| Diarrhea | 0.05 [0.02–0.10] | 150 (2) | I2 = 0.0%, p = 0.46, Q = 0.54 | Low 1,2,5 |
Abbreviations and Notes: CI: Confidence interval; GRADE: Grading of Recommendations, Assessment, Development and Evaluation; I2: Percentage of statistical heterogeneity; n: Number; PFT: Pulmonary function test; Q: Cochran’s heterogeneity statistic; RR: Relative risk; SAE: Serious adverse event. All prevalence estimates are pooled proportions calculated using a random-effects model and are presented as percentages with corresponding 95% confidence intervals (CIs). Heterogeneity is reported as I2 (percentage of total variation due to heterogeneity), Cochran’s Q, and p-value. GRADE quality assessments were downgraded for the following reasons: 1 = risk of bias; 2 = imprecision; 3 = applicability concerns; 4 = incomplete data; 5 = lack of numerical values; 6 = wide confidence interval. “–*” indicates the outcome was reported in only one study. Outcomes with a pooled prevalence of less than 1% are considered rare events. Deaths, serious adverse events (SAEs), and withdrawals are presented as proportions of the total cohort. Full statistical methods are detailed in the Methods section.
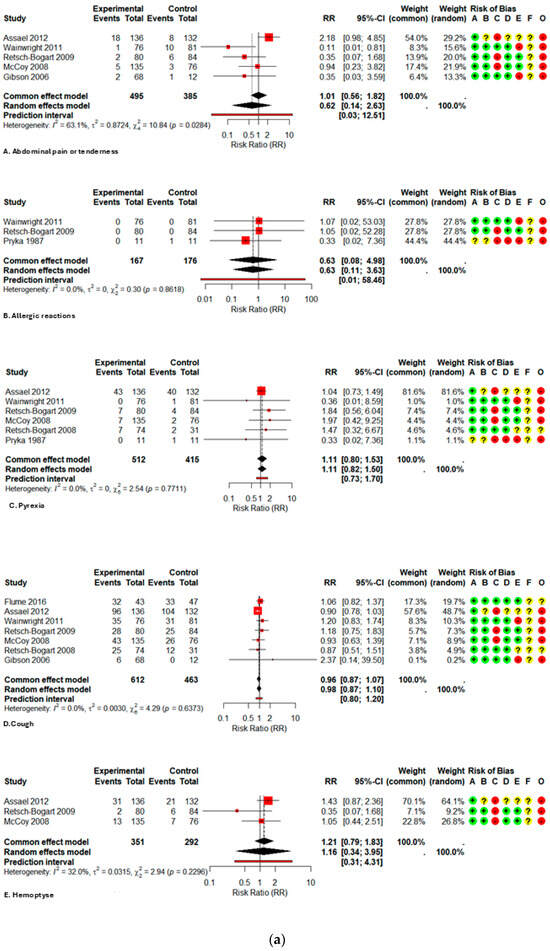
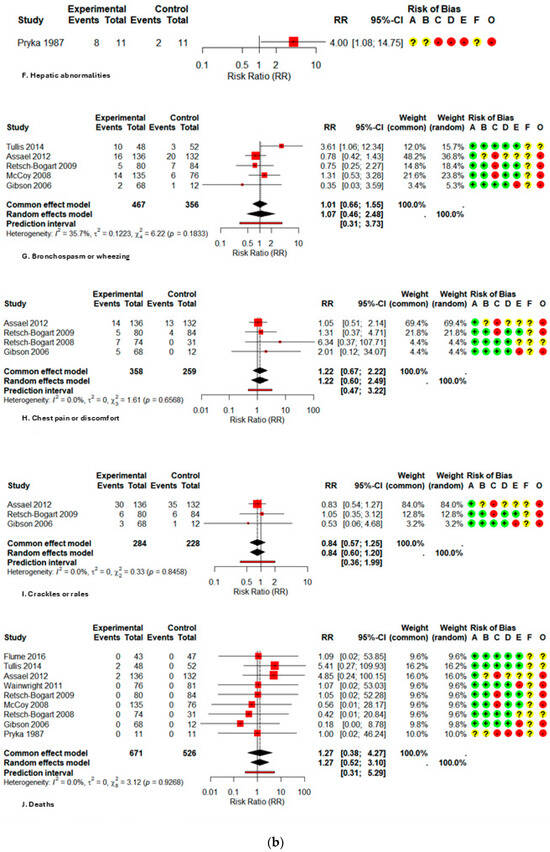
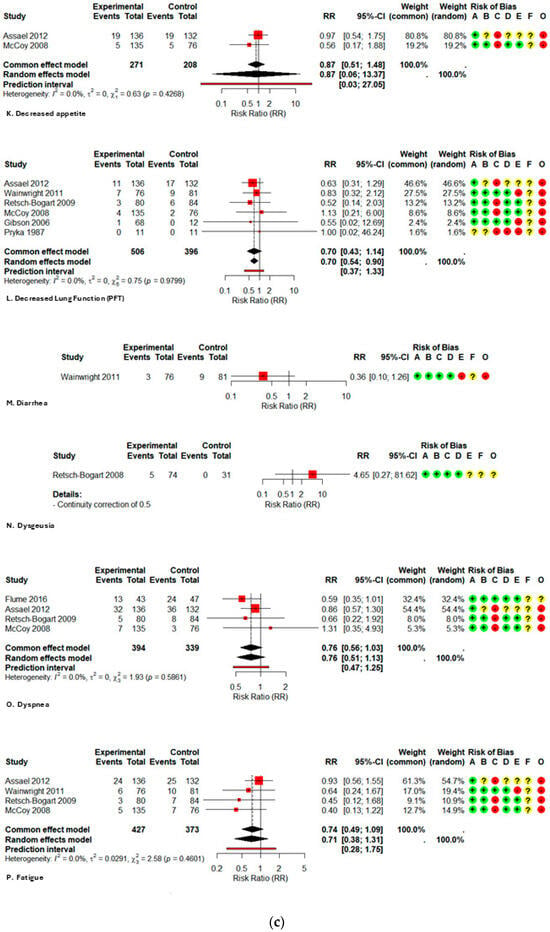
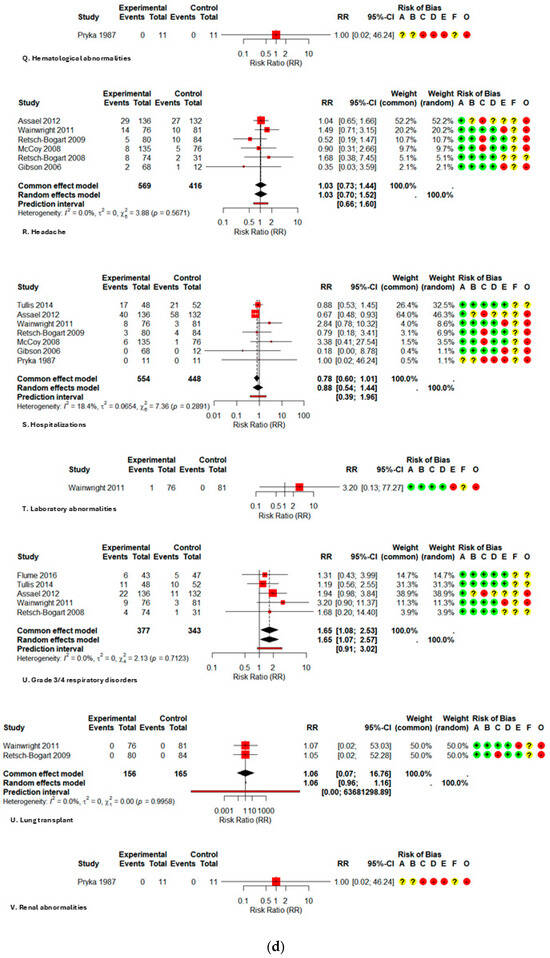
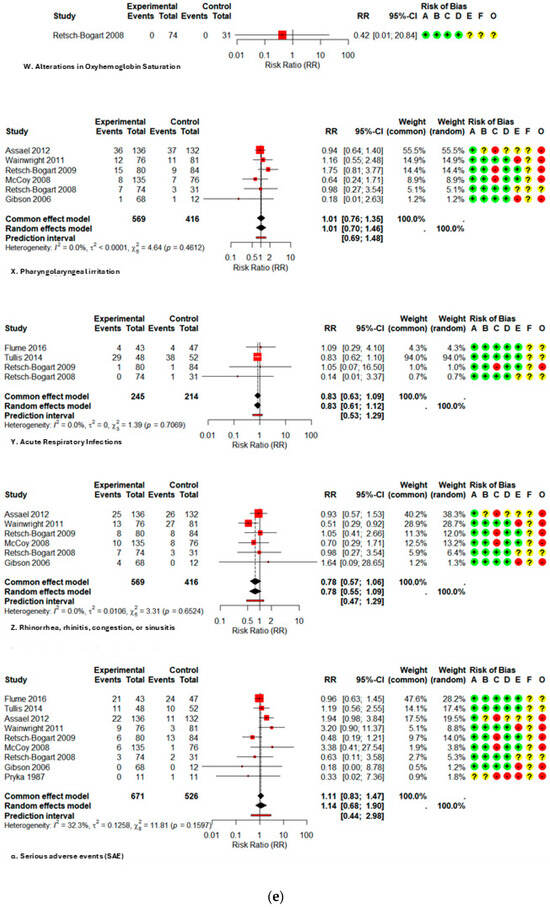
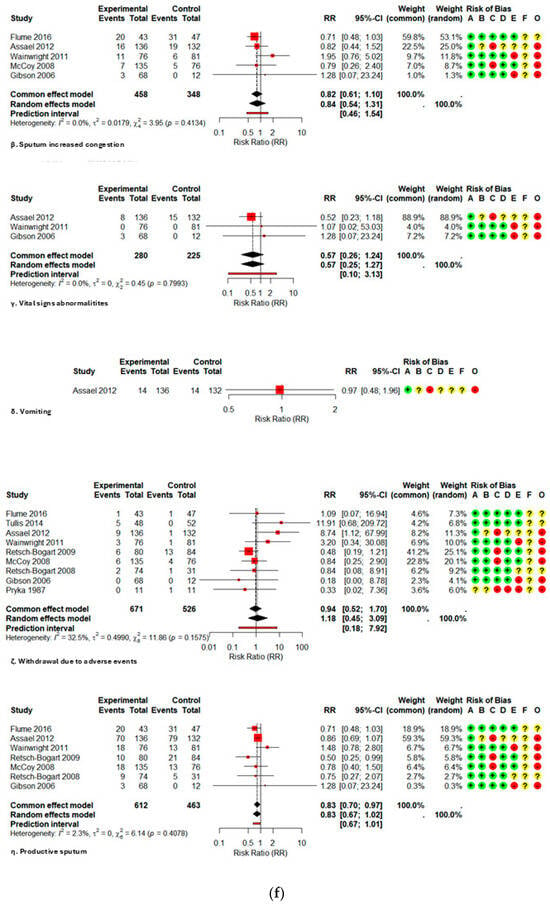
Figure A2.
Forest plot of risk ratios for each adverse event with AZLI in children and adolescents: (a) abdominal pain or tenderness, allergic reactions, pyrexia, cough, hemoptysis; (b) hepatic abnormalities, bronchospasm or wheezing, chest pain or discomfort, crackles or rales, deaths; (c) decreased appetite, decreased lung function, diarrhea, dysgeusia, dyspnea, fatigue; (d) hematological abnormalities, headache, hospitalizations, laboratory abnormalities, grade ¾ respiratory disorders, lung transplant, renal abnormalities; (e) alterations in oxyhemoglobin saturation, pharyngolaryngeal irritation, acute respiratory infections, rhinorrhea, rhinitis, congestion, sinusitis, serious adverse events; (f) sputum increased congestion, vital signs abnormalities, vomiting, withdrawal due to adverse events, productive sputum. Abbreviations and Notes: AZLI: Aztreonam lysine for inhalation; RR: Risk ratio; CI: Confidence interval; I2: Percentage of total variation across studies due to heterogeneity; τ2: Between-study variance in random-effects meta-analysis; Q: Cochran’s heterogeneity statistic; p: p-value; A–F: Risk of bias domains (A: randomization, B: intervention deviations, C: missing data, D: outcome measurement, E: reporting, F: additional); O: Overall risk of bias. Risk ratios >1 suggest higher risk in the aztreonam group; <1 suggest lower risk. Weights reflect the contribution of each study to pooled analysis. All analyses are based on inverse variance methods. A continuity correction of 0.5 was applied to studies with zero events in both groups to allow meta-analysis [9,21,23,24,25,26,27,28,29].
References
- World Health Organization: WHO. Pneumonia in Children. Available online: http://www.who.int/news-room/fact-sheets/detail/pneumonia#:~:text=Pneumonia%20is%20the%20single%20largest,affects%20children%20and%20families%20everywhere (accessed on 7 July 2025).
- Maselli, D.; Keyt, H.; Restrepo, M. Inhaled Antibiotic Therapy in Chronic Respiratory Diseases. Int. J. Mol. Sci. 2017, 18, 1062. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Collaco, J.M. Cystic Fibrosis. Pediatr. Rev. 2021, 42, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Mésinèle, J.; Ruffin, M.; Kemgang, A.; Guillot, L.; Boëlle, P.-Y.; Corvol, H. Risk factors for Pseudomonas aeruginosa airway infection and lung function decline in children with cystic fibrosis. J. Cyst. Fibros. 2022, 21, 45–51. [Google Scholar] [CrossRef]
- Crull, M.R.; Ramos, K.J.; Caldwell, E.; Mayer-Hamblett, N.; Aitken, M.L.; Goss, C.H. Change in Pseudomonas aeruginosa prevalence in cystic fibrosis adults over time. BMC Pulm. Med. 2016, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Georgopapadakou, N.H.; Smith, S.A.; Sykes, R.B. Mode of action of azthreonam. Antimicrob. Agents Chemother. 1982, 21, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Cayston FDA Approval History, Drugs.com. Available online: https://www.drugs.com/history/cayston.html#:~:text=FDA%20Approved%3A%20Yes%20,for%3A%20Pneumonia%20with%20Cystic%20Fibrosis (accessed on 7 July 2025).
- Elson, E.C.; Mermis, J.; Polineni, D.; Oermann, C.M. Aztreonam Lysine Inhalation Solution in Cystic Fibrosis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2019, 13, 1179548419842822. [Google Scholar] [CrossRef]
- McCoy, K.S.; Quittner, A.L.; Oermann, C.M.; Gibson, R.L.; Retsch-Bogart, G.Z.; Montgomery, A.B. Inhaled Aztreonam Lysine for Chronic Airway Pseudomonas aeruginosa in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 921–928. [Google Scholar] [CrossRef]
- Cai, L.; Zhou, H.; Wu, N.; Hong, L.; Lin, Z. Safety concerns of aztreonam: A real-world disproportionality analysis based on FDA Adverse Event Reporting System. Expert. Opin. Drug Saf. 2025, 24, 325–344. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Flemyng, E.; Dwan, K.; Moore, T.H.; Page, M.J.; Higgins, J.P. Risk of Bias 2 in Cochrane Reviews: A phased approach for the introduction of new methodology. Cochrane Database Syst. Rev. 2020, 2020, ED000148. [Google Scholar] [CrossRef]
- Friedrich, J.O.; Adhikari, N.K.; Beyene, J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med. Res. Methodol. 2007, 7, 5. [Google Scholar] [CrossRef]
- Kelley, G.A. Statistical models for meta-analysis: A brief tutorial. World J. Methodol. 2012, 2, 27. [Google Scholar] [CrossRef]
- Röver, C.; Knapp, G.; Friede, T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med. Res. Methodol. 2015, 15, 99. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Pedro Lima, J.; Chu, X.; Guyatt, G.H.; Tangamornsuksan, W. Certainty of evidence, why? J. Bras. Pneumol. 2023, 49, e20230167. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Guyatt, G.H. Assessing the certainty of the evidence in systematic reviews: Importance, process, and use. Am. J. Epidemiol. 2025, 194, 1681–1686. [Google Scholar] [CrossRef]
- Keating, C.L.; Zuckerman, J.B.; Singh, P.K.; McKevitt, M.; Gurtovaya, O.; Bresnik, M.; Marshall, B.C.; Saiman, L. Pseudomonas aeruginosa Susceptibility Patterns and Associated Clinical Outcomes in People with Cystic Fibrosis following Approval of Aztreonam Lysine for Inhalation. Antimicrob. Agents Chemother. 2021, 65, e02327-20. [Google Scholar] [CrossRef]
- Flume, P.A.; Clancy, J.P.; Retsch-Bogart, G.Z.; Tullis, D.E.; Bresnik, M.; Derchak, P.A.; Lewis, S.A.; Ramsey, B.W. Continuous alternating inhaled antibiotics for chronic pseudomonal infection in cystic fibrosis. J. Cyst. Fibros. 2016, 15, 809–815. [Google Scholar] [CrossRef]
- Tiddens, H.A.W.M.; De Boeck, K.; Clancy, J.P.; Fayon, M.; Arets, H.G.M.; Bresnik, M.; Derchak, A.; Lewis, S.A.; Oermann, C.M. Open label study of inhaled aztreonam for Pseudomonas eradication in children with cystic fibrosis: The ALPINE study. J. Cyst. Fibros. 2015, 14, 111–119. [Google Scholar] [CrossRef]
- Tullis, D.E.; Burns, J.L.; Retsch-Bogart, G.Z.; Bresnik, M.; Henig, N.R.; Lewis, S.A.; LiPuma, J.J. Inhaled aztreonam for chronic Burkholderia infection in cystic fibrosis: A placebo-controlled trial. J. Cyst. Fibros. 2014, 13, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Assael, B.M.; Pressler, T.; Bilton, D.; Fayon, M.; Fischer, R.; Chiron, R.; LaRosa, M.; Knoop, C.; McElvaney, N.; Lewis, S.A.; et al. Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: A comparative efficacy trial. J. Cyst. Fibros. 2013, 12, 130–140. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Quittner, A.L.; Geller, D.E.; Nakamura, C.; Wooldridge, J.L.; Gibson, R.L.; Lewis, S.; Montgomery, A.B. Aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis, mild lung impairment, and P. aeruginosa. J. Cyst. Fibros. 2011, 10, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Retsch-Bogart, G.Z.; Quittner, A.L.; Gibson, R.L.; Oermann, C.M.; McCoy, K.S.; Montgomery, A.B.; Cooper, P.J. Efficacy and Safety of Inhaled Aztreonam Lysine for Airway Pseudomonas in Cystic Fibrosis. Chest 2009, 135, 1223–1232. [Google Scholar] [CrossRef]
- Retsch-Bogart, G.Z.; Burns, J.L.; Otto, K.L.; Liou, T.G.; McCoy, K.; Oermann, C.; Gibson, R.L. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr. Pulmonol. 2008, 43, 47–58. [Google Scholar] [CrossRef]
- Gibson, R.L.; Retsch-Bogart, G.Z.; Oermann, C.; Milla, C.; Pilewski, J.; Daines, C.; Ahrens, R.; Leon, K.; Cohen, M.; McNamara, S.; et al. Microbiology, safety, and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatr. Pulmonol. 2006, 41, 656–665. [Google Scholar] [CrossRef]
- Pryka, R.D. An Evaluation of the Safety and Efficacy of Aztreonam Versus Azlocillin Plus Tobramycin in the Treatment of Pulmonary Exacerbations in Cystic Fibrosis Patients. Ph.D. Thesis, University of Utah, Salt Lake City, UT, USA, 1987. Available online: https://collections.lib.utah.edu/ark:/87278/s6jt059v (accessed on 10 July 2025).
- Tiddens, H.A.W.M.; De Boeck, K.; Clancy, J.P.; Fayon, M.; Arets, H.G.M.; Bresnik, M.; Derchak, P.A.; Lewis, S.A.; Oermann, C.M. 110 Aztreonam for inhalation solution (AZLI) for eradication of new onset Pseudomonas aeruginosa (PA) infection in children with cystic fibrosis (CF): Evaluation of treatment failures. J. Cyst. Fibros. 2015, 14, S85. [Google Scholar] [CrossRef]
- Gilchrist, F.J.; Bui, S.; Gartner, S.; McColley, S.A.; Tiddens, H.; Ruiz, G.; Stehling, F.; Alani, M.; Gurtovaya, O.; Bresnik, M.; et al. ALPINE2: Efficacy and safety of 14-day vs. 28-day inhaled aztreonam for Pa eradication in children with cystic fibrosis. J. Cyst. Fibros. 2024, 23, 80–86. [Google Scholar] [CrossRef]
- Oermann, C.M.; Retsch-Bogart, G.Z.; Quittner, A.L.; Gibson, R.L.; McCoy, K.S.; Montgomery, A.B.; Cooper, P.J. An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis. Pediatr. Pulmonol. 2010, 45, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).