Clinical Utility of ctDNA Analysis in Lung Cancer—A Review
Abstract
Highlights
- ctDNA can be detected using both NGS techniques and various forms of PCR.
- ctDNA reflects changes in the tumor’s genetic makeup.
- Liquid biopsy with ctDNA is clinically approved in genetic profiling in NSCLC.
- ctDNA has potential as a biomarker for cancer relapse prediction and treatment efficacy.
Abstract
1. Introduction
2. Liquid Biopsy
3. Methods of ctDNA Detection
4. Lung Cancer Screening and Its Early Detection
5. The Utilization of ctDNA in Routine Clinical Practice
6. Applications of ctDNA in Lung Cancer
6.1. Treatment with Curative Intention
6.2. Treatment Monitoring
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung Cancer: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Lung Cancer—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10662965/?fbclid=IwAR03Mwo3JHzgLo7yvn6qHWlYdrTiGIxCrP_rXuLIH1n6BqRdAAl-jQZ22zw#R1 (accessed on 31 January 2024).
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung Cancer in Never Smokers—A Different Disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Lou, Y.; Dholaria, B.; Soyano, A.; Hodge, D.; Cochuyt, J.; Manochakian, R.; Ko, S.J.; Thomas, M.; Johnson, M.M.; Patel, N.M.; et al. Survival Trends among Non-Small-Cell Lung Cancer Patients over a Decade: Impact of Initial Therapy at Academic Centers. Cancer Med. 2018, 7, 4932–4942. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Aucamp, J.; Bronkhorst, A.J.; Badenhorst, C.P.S.; Pretorius, P.J. A Historical and Evolutionary Perspective on the Biological Significance of Circulating DNA and Extracellular Vesicles. Cell. Mol. Life Sci. 2016, 73, 4355–4381. [Google Scholar] [CrossRef]
- Fleischhacker, M.; Schmidt, B. Circulating Nucleic Acids (CNAs) and Cancer—A Survey. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2007, 1775, 181–232. [Google Scholar] [CrossRef]
- Lichtenstein, A.V.; Melkonyan, H.S.; David Tomei, L.; Umansky, S.R. Circulating Nucleic Acids and Apoptosis. Ann. N. Y. Acad. Sci. 2001, 945, 239–249. [Google Scholar] [CrossRef]
- Gahan, P.B.; Stroun, M. The Virtosome-a Novel Cytosolic Informative Entity and Intercellular Messenger. Cell Biochem. Funct. 2010, 28, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vigil, I.G.; Moreno-Martínez, A.K.; Wang, J.Y.; Roehrl, M.H.A.; Barrera-Saldaña, H.A. The Dawn of the Liquid Biopsy in the Fight against Cancer. Oncotarget 2018, 9, 2912. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. CtDNA as a Cancer Biomarker: A Broad Overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef]
- Mouliere, F.; Robert, B.; Peyrotte, E.; Del Rio, M.; Ychou, M.; Molina, F.; Gongora, C.; Thierry, A.R. High Fragmentation Characterizes Tumour-Derived Circulating DNA. PLoS ONE 2011, 6, e23418. [Google Scholar] [CrossRef]
- Mouliere, F.; El Messaoudi, S.; Pang, D.; Dritschilo, A.; Thierry, A.R. Multi-marker Analysis of Circulating Cell-free DNA toward Personalized Medicine for Colorectal Cancer. Mol. Oncol. 2014, 8, 927. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA Fragments in the Blood Plasma of Cancer Patients: Quantitations and Evidence for Their Origin from Apoptotic and Necrotic Cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and Death of Circulating Cell-Free DNA. Cancer Biol. Ther. 2019, 20, 1057. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Stoehlmacher, J.; Pantel, K.; Goekkurt, E. Detection and Monitoring of Cell-Free DNA in Blood of Patients with Colorectal Cancer. Ann. N. Y. Acad. Sci. 2008, 1137, 190–196. [Google Scholar] [CrossRef]
- Lanman, R.B.; Mortimer, S.A.; Zill, O.A.; Sebisanovic, D.; Lopez, R.; Blau, S.; Collisson, E.A.; Divers, S.G.; Hoon, D.S.B.; Scott Kopetz, E.; et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PLoS ONE 2015, 10, e0140712. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, M.; Dawson, S.J.; Tsui, D.W.Y.; Gale, D.; Forshew, T.; Piskorz, A.M.; Parkinson, C.; Chin, S.F.; Kingsbury, Z.; Wong, A.S.C.; et al. Non-Invasive Analysis of Acquired Resistance to Cancer Therapy by Sequencing of Plasma DNA. Nature 2013, 497, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Elazezy, M.; Joosse, S.A. Techniques of Using Circulating Tumor DNA as a Liquid Biopsy Component in Cancer Management. Comput. Struct. Biotechnol. J. 2018, 16, 370. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.M.; Kothari, P.D.; Mouliere, F.; Mair, R.; Somnay, S.; Benayed, R.; Zehir, A.; Weigelt, B.; Dawson, S.J.; Arcila, M.E.; et al. The Value of Cell-Free DNA for Molecular Pathology. J. Pathol. 2018, 244, 616. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid Biopsy: Monitoring Cancer-Genetics in the Blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883. [Google Scholar] [CrossRef]
- Santarpia, M.; Liguori, A.; D’Aveni, A.; Karachaliou, N.; Gonzalez-Cao, M.; Daffinà, M.G.; Lazzari, C.; Altavilla, G.; Rosell, R. Liquid Biopsy for Lung Cancer Early Detection. J. Thorac. Dis. 2018, 10, S882. [Google Scholar] [CrossRef]
- Santarpia, M.; Karachaliou, N.; González-Cao, M.; Altavilla, G.; Giovannetti, E.; Rosell, R. Feasibility of Cell-Free Circulating Tumor DNA Testing for Lung Cancer. Biomark. Med. 2016, 10, 417–430. [Google Scholar] [CrossRef]
- Adashek, J.J.; Janku, F.; Kurzrock, R. Signed in Blood: Circulating Tumor DNA in Cancer Diagnosis, Treatment and Screening. Cancers 2021, 13, 3600. [Google Scholar] [CrossRef]
- Lin, C.; Liu, X.; Zheng, B.; Ke, R.; Tzeng, C.M. Liquid Biopsy, CtDNA Diagnosis through NGS. Life 2021, 11, 890. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Sorber, L.; Zwaenepoel, K.; Jacobs, J.; De Winne, K.; Goethals, S.; Reclusa, P.; Van Casteren, K.; Augustus, E.; Lardon, F.; Roeyen, G.; et al. Circulating Cell-Free DNA and RNA Analysis as Liquid Biopsy: Optimal Centrifugation Protocol. Cancers 2019, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Sorber, L.; Zwaenepoel, K.; De Winne, K.; Van Casteren, K.; Augustus, E.; Jacobs, J.; Zhang, X.H.; Galdermans, D.; De Droogh, E.; Lefebure, A.; et al. A Multicenter Study to Assess EGFR Mutational Status in Plasma: Focus on an Optimized Workflow for Liquid Biopsy in a Clinical Setting. Cancers 2018, 10, 290. [Google Scholar] [CrossRef]
- van Ginkel, J.H.; van den Broek, D.A.; van Kuik, J.; Linders, D.; de Weger, R.; Willems, S.M.; Huibers, M.M.H. Preanalytical Blood Sample Workup for Cell-free DNA Analysis Using Droplet Digital PCR for Future Molecular Cancer Diagnostics. Cancer Med. 2017, 6, 2297. [Google Scholar] [CrossRef] [PubMed]
- Van Dessel, L.F.; Beije, N.; Helmijr, J.C.A.; Vitale, S.R.; Kraan, J.; Look, M.P.; De Wit, R.; Sleijfer, S.; Jansen, M.P.H.M.; Martens, J.W.M.; et al. Application of Circulating Tumor DNA in Prospective Clinical Oncology Trials—Standardization of Preanalytical Conditions. Mol. Oncol. 2017, 11, 295. [Google Scholar] [CrossRef]
- Warton, K.; Graham, L.J.; Yuwono, N.; Samimi, G. Comparison of 4 Commercial Kits for the Extraction of Circulating DNA from Plasma. Cancer Genet. 2018, 228–229, 143–150. [Google Scholar] [CrossRef]
- Martignano, F. Cell-Free DNA: An Overview of Sample Types and Isolation Procedures. Methods Mol. Biol. 2019, 1909, 13–27. [Google Scholar] [CrossRef]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef]
- Chen, S.; Liu, M.; Zhou, Y. Bioinformatics Analysis for Cell-Free Tumor DNA Sequencing Data. Methods Mol. Biol. 2018, 1754, 67–95. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Liu, Z.; Liang, G.; Gu, W.; Ge, Q. Technical Progress in Circulating Tumor DNA Analysis Using next Generation Sequencing. Mol. Cell. Probes 2020, 49, 101480. [Google Scholar] [CrossRef]
- Kastrisiou, M.; Zarkavelis, G.; Pentheroudakis, G.; Magklara, A. Clinical Application of Next-Generation Sequencing as A Liquid Biopsy Technique in Advanced Colorectal Cancer: A Trick or A Treat? Cancers 2019, 11, 1573. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Bi, J.; Bao, L. Genetic Profiling of Cancer with Circulating Tumor DNA Analysis. J. Genet. Genomics 2018, 45, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Leary, R.J.; Sausen, M.; Kinde, I.; Papadopoulos, N.; Carpten, J.D.; Craig, D.; O’Shaughnessy, J.; Kinzler, K.W.; Parmigiani, G.; Vogelstein, B.; et al. Detection of Chromosomal Alterations in the Circulation of Cancer Patients with Whole-Genome Sequencing. Sci. Transl. Med. 2012, 4, 162ra154. [Google Scholar] [CrossRef] [PubMed]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.Y.; Kaper, F.; Dawson, S.J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Sci. Transl. Med. 2012, 4, 136ra68. [Google Scholar] [CrossRef]
- Leary, R.J.; Kinde, I.; Diehl, F.; Schmidt, K.; Clouser, C.; Duncan, C.; Antipova, A.; Lee, C.; McKernan, K.; De La Vega, F.M.; et al. Development of Personalized Tumor Biomarkers Using Massively Parallel Sequencing. Sci. Transl. Med. 2010, 2, 20ra14. [Google Scholar] [CrossRef]
- Boscolo Bielo, L.; Trapani, D.; Repetto, M.; Crimini, E.; Valenza, C.; Belli, C.; Criscitiello, C.; Marra, A.; Subbiah, V.; Curigliano, G. Variant Allele Frequency: A Decision-Making Tool in Precision Oncology? Trends Cancer 2023, 9, 1058–1068. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic CtDNA Analysis Depicts Early-Stage Lung Cancer Evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef]
- Warton, K.; Mahon, K.L.; Samimi, G. Methylated Circulating Tumor DNA in Blood: Power in Cancer Prognosis and Response. Endocr. Relat. Cancer 2016, 23, R157. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. A Decade of Exploring the Cancer Epigenome—Biological and Translational Implications. Nat. Rev. Cancer 2011, 11, 726. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Z.; Meng, Y.; Liu, S.; Zhan, H. Blood-Based DNA Methylation Signatures in Cancer: A Systematic Review. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166583. [Google Scholar] [CrossRef]
- Luo, H.; Wei, W.; Ye, Z.; Zheng, J.; Xu, R. hua Liquid Biopsy of Methylation Biomarkers in Cell-Free DNA. Trends Mol. Med. 2021, 27, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Allen Chan, K.C.; Jiang, P.; Chan, C.W.M.; Sun, K.; Wong, J.; Hui, E.P.; Chan, S.L.; Chan, W.C.; Hui, D.S.C.; Ng, S.S.M.; et al. Noninvasive Detection of Cancer-Associated Genome-Wide Hypomethylation and Copy Number Aberrations by Plasma DNA Bisulfite Sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 18761–18768. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Jelovac, D.; Barnathan, E.; Blair, B.; Slater, S.; Powers, P.; Zorzi, J.; Jeter, S.C.; Oliver, G.R.; Fetting, J.; et al. Detection of Tumor PIK3CA Status in Metastatic Breast Cancer Using Peripheral Blood. Clin. Cancer Res. 2012, 18, 3462–3469. [Google Scholar] [CrossRef] [PubMed]
- Rothé, F.; Laes, J.F.; Lambrechts, D.; Smeets, D.; Vincent, D.; Maetens, M.; Fumagalli, D.; Michiels, S.; Drisis, S.; Moerman, C.; et al. Plasma Circulating Tumor DNA as an Alternative to Metastatic Biopsies for Mutational Analysis in Breast Cancer. Ann. Oncol. 2014, 25, 1959–1965. [Google Scholar] [CrossRef]
- Chan, K.C.A.; Jiang, P.; Zheng, Y.W.L.; Liao, G.J.W.; Sun, H.; Wong, J.; Siu, S.S.N.; Chan, W.C.; Chan, S.L.; Chan, A.T.C.; et al. Cancer Genome Scanning in Plasma: Detection of Tumor-Associated Copy Number Aberrations, Single-Nucleotide Variants, and Tumoral Heterogeneity by Massively Parallel Sequencing. Clin. Chem. 2013, 59, 211–224. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Weigelt, B.; Cortes, J.; Won, H.H.; Ng, C.K.Y.; Nuciforo, P.; Bidard, F.C.; Aura, C.; Saura, C.; Peg, V.; et al. Capturing Intra-Tumor Genetic Heterogeneity by de Novo Profiling of Circulating Cell-Free Tumor DNA: A-of-Principle. Ann. Oncol. 2014, 25, 1729. [Google Scholar] [CrossRef]
- Thierry, A.R.; Mouliere, F.; El Messaoudi, S.; Mollevi, C.; Lopez-Crapez, E.; Rolet, F.; Gillet, B.; Gongora, C.; Dechelotte, P.; Robert, B.; et al. Clinical Validation of the Detection of KRAS and BRAF Mutations from Circulating Tumor DNA. Nat. Med. 2014, 20, 430–435. [Google Scholar] [CrossRef]
- Zhao, Y.R.; Xie, X.; De Koning, H.J.; Mali, W.P.; Vliegenthart, R.; Oudkerk, M. NELSON Lung Cancer Screening Study. Cancer Imaging 2011, 11, S79. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Van Meerbeeck, J.P.; O’dowd, E.; Ward, B.; Van Schil, P.; Snoeckx, A. Lung Cancer Screening: New Perspective and Challenges in Europe. Cancers 2022, 14, 2343. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Huang, W.; Gao, Y.; Xu, W.; Tao, J.; Yang, M.; Li, L.; Ping, W.; Shen, H.; et al. Non-Invasive Diagnosis of Early-Stage Lung Cancer Using High-Throughput Targeted DNA Methylation Sequencing of Circulating Tumor DNA (CtDNA). Theranostics 2019, 9, 2056–2070. [Google Scholar] [CrossRef] [PubMed]
- Bittla, P.; Kaur, S.; Sojitra, V.; Zahra, A.; Hutchinson, J.; Folawemi, O.; Khan, S. Exploring Circulating Tumor DNA (CtDNA) and Its Role in Early Detection of Cancer: A Systematic Review. Cureus 2023, 15, e45784. [Google Scholar] [CrossRef]
- Panet, F.; Papakonstantinou, A.; Borrell, M.; Vivancos, J.; Vivancos, A.; Oliveira, M. Use of CtDNA in Early Breast Cancer: Analytical Validity and Clinical Potential. NPJ Breast Cancer 2024, 10, 50. [Google Scholar] [CrossRef]
- Chen, D.; Guo, J.; Huang, H.; Tian, L.; Xie, Y.; Wu, Q. Prognostic Value of Circulating Tumor DNA in Operable Non-Small Cell Lung Cancer: A Systematic Review and Reconstructed Individual Patient-Data Based Meta-Analysis. BMC Med. 2023, 21, 467. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L. The Prognostic Value of Circulating Tumor DNA in Ovarian Cancer: A Meta-Analysis. Technol. Cancer Res. Treat. 2021, 20, 1–12. [Google Scholar] [CrossRef]
- Taliento, C.; Morciano, G.; Nero, C.; Froyman, W.; Vizzielli, G.; Pavone, M.; Salvioli, S.; Tormen, M.; Fiorica, F.; Scutiero, G.; et al. Circulating Tumor DNA as a Biomarker for Predicting Progression-Free Survival and Overall Survival in Patients with Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis. Int. J. Gynecol. Cancer 2024, 34, 906–918. [Google Scholar] [CrossRef]
- Chidharla, A.; Rapoport, E.; Agarwal, K.; Madala, S.; Linares, B.; Sun, W.; Chakrabarti, S.; Kasi, A. Circulating Tumor DNA as a Minimal Residual Disease Assessment and Recurrence Risk in Patients Undergoing Curative-Intent Resection with or without Adjuvant Chemotherapy in Colorectal Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 10230. [Google Scholar] [CrossRef]
- Gandini, S.; Zanna, I.; De Angelis, S.P.; Cocorocchio, E.; Queirolo, P.; Lee, J.H.; Carlino, M.S.; Mazzarella, L.; Achutti Duso, B.; Palli, D.; et al. Circulating Tumour DNA and Melanoma Survival: A Systematic Literature Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2021, 157, 103187. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16, 1647–1662. [Google Scholar] [CrossRef]
- Yang, J.C.H.; Ahn, M.J.; Kim, D.W.; Ramalingam, S.S.; Sequist, L.V.; Su, W.C.; Kim, S.W.; Kim, J.H.; Planchard, D.; Felip, E.; et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J. Clin. Oncol. 2017, 35, 1288–1296. [Google Scholar] [CrossRef]
- Goss, G.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Lee, J.S.; Chang, G.C.; Crino, L.; Satouchi, M.; Chu, Q.; Hida, T.; et al. Osimertinib for Pretreated EGFR Thr790Met-Positive Advanced Non-Small-Cell Lung Cancer (AURA2): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2016, 17, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Swalduz, A.; Schiffler, C.; Curcio, H.; Ambasager, B.; Le Moel, G.; Debieuvre, D.; Dot, J.-M.; Duruisseaux, M.; Fournel, P.; Odier, L.; et al. LIBELULE: A Randomized Phase III Study to Evaluate the Clinical Relevance of Early Liquid Biopsy in Patients with Suspicious Metastatic Lung Cancer. J. Thorac. Oncol. 2024, 20, 437–450. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Zulato, E.; Tosello, V.; Nardo, G.; Bonanno, L.; Del Bianco, P.; Indraccolo, S. Implementation of Next Generation Sequencing-Based Liquid Biopsy for Clinical Molecular Diagnostics in Non-Small Cell Lung Cancer (NSCLC) Patients. Diagnostics 2021, 11, 1468. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO Recommendations on the Use of Circulating Tumour DNA Assays for Patients with Cancer: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Watanabe, K.; Tsuboi, M.; Sakamaki, K.; Nishii, T.; Yamamoto, T.; Nagashima, T.; Ando, K.; Ishikawa, Y.; Woo, T.; Adachi, H.; et al. Postoperative Follow-up Strategy Based on Recurrence Dynamics for Non-Small-Cell Lung Cancer. Eur. J. Cardio-Thorac. Surg. 2016, 49, 1624–1631. [Google Scholar] [CrossRef]
- Demicheli, R.; Fornili, M.; Ambrogi, F.; Higgins, K.; Boyd, J.A.; Biganzoli, E.; Kelsey, C.R. Recurrence Dynamics for Non-Small-Cell Lung Cancer: Effect of Surgery on the Development of Metastases. J. Thorac. Oncol. 2012, 7, 723–730. [Google Scholar] [CrossRef]
- Xia, L.; Mei, J.; Kang, R.; Deng, S.; Chen, Y.; Yang, Y.; Feng, G.; Deng, Y.; Gan, F.; Lin, Y.; et al. Perioperative CtDNA-Based Molecular Residual Disease Detection for Non-Small Cell Lung Cancer: A Prospective Multicenter Cohort Study (LUNGCA-1). Clin. Cancer Res. 2022, 28, 3308–3317. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394. [Google Scholar] [CrossRef]
- Zhong, R.; Gao, R.; Fu, W.; Li, C.; Huo, Z.; Gao, Y.; Lu, Y.; Li, F.; Ge, F.; Tu, H.; et al. Accuracy of Minimal Residual Disease Detection by Circulating Tumor DNA Profiling in Lung Cancer: A Meta-Analysis. BMC Med. 2023, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V.; et al. Adjuvant Vinorelbine plus Cisplatin versus Observation in Patients with Completely Resected Stage IB-IIIA Non-Small-Cell Lung Cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A Randomised Controlled Trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.P.; Vansteenkiste, J.; International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-Based Adjuvant Chemotherapy in Patients with Completely Resected Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Cortés, Á.A.; Urquizu, L.C.; Cubero, J.H. Adjuvant Chemotherapy in Non-Small Cell Lung Cancer: State-of-the-Art. Transl. Lung Cancer Res. 2015, 4, 191. [Google Scholar] [CrossRef]
- Qiu, B.; Guo, W.; Zhang, F.; Lv, F.; Ji, Y.; Peng, Y.; Chen, X.; Bao, H.; Xu, Y.; Shao, Y.; et al. Dynamic Recurrence Risk and Adjuvant Chemotherapy Benefit Prediction by CtDNA in Resected NSCLC. Nat. Commun. 2021, 12, 6770. [Google Scholar] [CrossRef]
- Cascone, T.; Awad, M.M.; Spicer, J.D.; He, J.; Lu, S.; Sepesi, B.; Tanaka, F.; Taube, J.M.; Cornelissen, R.; Havel, L.; et al. LBA1 CheckMate 77T: Phase III Study Comparing Neoadjuvant Nivolumab (NIVO) plus Chemotherapy (Chemo) vs. Neoadjuvant Placebo plus Chemo Followed by Surgery and Adjuvant NIVO or Placebo for Previously Untreated, Resectable Stage II–IIIb NSCLC. Ann. Oncol. 2023, 34, S1295. [Google Scholar] [CrossRef]
- Reck, M.; Gale, D.; Zhu, Z.; Harpole, D.; Taube, J.; Mitsudomi, T.; Van Luong, D.; Hochmair, M.J.; Lee, K.Y.; Horio, Y.; et al. LBA49 Associations of CtDNA Clearance (CL) during Neoadjuvant Tx with Pathological Response and Event-Free Survival (EFS) in Pts with Resectable NSCLC (R-NSCLC): Expanded Analyses from AEGEAN. Ann. Oncol. 2024, 35, S1239. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. IRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol. 2017, 18, e143. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, F.; Qiao, M.; Li, X.; Zhao, C.; Cheng, L.; Chen, X.; Zhou, C. The Role of Circulating Tumor DNA in Advanced Non-Small Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 671874. [Google Scholar] [CrossRef]
- Anagnostou, V.; Forde, P.M.; White, J.R.; Niknafs, N.; Hruban, C.; Naidoo, J.; Marrone, K.; Ashok Sivakumar, I.K.; Bruhm, D.C.; Rosner, S.; et al. Dynamics of Tumor and Immune Responses during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Res. 2019, 79, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Jones, G.; Beeler, J.F.; Plagnol, V.; Morris, C.; Mourlanette, J.; Delaunay, M.; Keller, L.; Rouquette, I.; Favre, G.; et al. Targeted Sequencing of Plasma Cell-Free DNA to Predict Response to PD1 Inhibitors in Advanced Non-Small Cell Lung Cancer. Lung Cancer 2019, 137, 1–6. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Narayan, A.; Kole, A.J.; Decker, R.H.; Teysir, J.; Carriero, N.J.; Lee, A.; Nemati, R.; Nath, S.K.; Mane, S.M.; et al. Early Assessment of Lung Cancer Immunotherapy Response via Circulating Tumor DNA. Clin. Cancer Res. 2018, 24, 1872–1880. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; van der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842. [Google Scholar] [CrossRef]
- Thompson, J.C.; Carpenter, E.L.; Silva, B.A.; Rosenstein, J.; Chien, A.L.; Quinn, K.; Espenschied, C.R.; Mak, A.; Kiedrowski, L.A.; Lefterova, M.; et al. Serial Monitoring of Circulating Tumor DNA by Next-Generation Gene Sequencing as a Biomarker of Response and Survival in Patients With Advanced NSCLC Receiving Pembrolizumab-Based Therapy. JCO Precis. Oncol. 2021, 5, 510–524. [Google Scholar] [CrossRef]
- Song, Y.; Hu, C.; Xie, Z.; Wu, L.; Zhu, Z.; Rao, C.; Liu, L.; Chen, Y.; Liang, N.; Chen, J.; et al. Circulating Tumor DNA Clearance Predicts Prognosis across Treatment Regimen in a Large Real-World Longitudinally Monitored Advanced Non-Small Cell Lung Cancer Cohort. Transl. Lung Cancer Res. 2020, 9, 269. [Google Scholar] [CrossRef]
- Almodovar, K.; Iams, W.T.; Meador, C.B.; Zhao, Z.; York, S.; Horn, L.; Yan, Y.; Hernandez, J.; Chen, H.; Shyr, Y.; et al. Longitudinal Cell-Free DNA Analysis in Patients with Small Cell Lung Cancer Reveals Dynamic Insights into Treatment Efficacy and Disease Relapse. J. Thorac. Oncol. 2017, 13, 112. [Google Scholar] [CrossRef]
- Anagnostou, V.; Ho, C.; Nicholas, G.; Juergens, R.A.; Sacher, A.; Fung, A.S.; Wheatley-Price, P.; Laurie, S.A.; Levy, B.; Brahmer, J.R.; et al. CtDNA Response after Pembrolizumab in Non-Small Cell Lung Cancer: Phase 2 Adaptive Trial Results. Nat. Med. 2023, 29, 2559. [Google Scholar] [CrossRef]
- Phan, T.T.; Tran, V.T.; Tran, B.T.; Ho, T.T.; Pho, S.P.; Le, A.T.; Le, V.T.; Nguyen, H.T.; Nguyen, S.T. EGFR-Plasma Mutations in Prognosis for Non-Small Cell Lung Cancer Treated with EGFR TKIs: A Meta-Analysis. Cancer Rep. 2022, 5, e1544. [Google Scholar] [CrossRef]
- Garrido, P.; Paz-Ares, L.; Majem, M.; Morán, T.; Trigo, J.M.; Bosch-Barrera, J.; Garcίa-Campelo, R.; González-Larriba, J.L.; Sánchez-Torres, J.M.; Isla, D.; et al. LungBEAM: A Prospective Multicenter Study to Monitor Stage IV NSCLC Patients with EGFR Mutations Using BEAMing Technology. Cancer Med. 2021, 10, 5878–5888. [Google Scholar] [CrossRef]
- Sorensen, B.S.; Wu, L.; Wei, W.; Tsai, J.; Weber, B.; Nexo, E.; Meldgaard, P. Monitoring of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-Sensitizing and Resistance Mutations in the Plasma DNA of Patients With Advanced Non–Small Cell Lung Cancer During Treatment With Erlotinib. Cancer 2014, 120, 3896. [Google Scholar] [CrossRef] [PubMed]
- Boysen Fynboe Ebert, E.; McCulloch, T.; Holmskov Hansen, K.; Linnet, H.; Sorensen, B.; Meldgaard, P. Clearing of Circulating Tumour DNA Predicts Clinical Response to Osimertinib in EGFR Mutated Lung Cancer Patients. Lung Cancer 2020, 143, 67–72. [Google Scholar] [CrossRef]
- Zhou, R.; Tong, F.; Zhang, Y.; Zhang, R.; Bin, Y.; Zhang, S.; Yang, N.; Dong, X. Genomic Alterations Associated with Pseudoprogression and Hyperprogressive Disease during Anti-PD1 Treatment for Advanced Non-Small-Cell Lung Cancer. Front. Oncol. 2023, 13, 1231094. [Google Scholar] [CrossRef]
- Guibert, N.; Mazieres, J.; Delaunay, M.; Casanova, A.; Farella, M.; Keller, L.; Favre, G.; Pradines, A. Monitoring of KRAS-Mutated CtDNA to Discriminate Pseudo-Progression from True Progression during Anti-PD-1 Treatment of Lung Adenocarcinoma. Oncotarget 2017, 8, 38056. [Google Scholar] [CrossRef]
- Pulla, M.P.; Awad, M.; Cascone, T.; Spicer, J.D.; He, J.; Lu, S.; Alexandru, A.; Watanabe, Y.; Cornelissen, R.; Koch, L.D.O.; et al. LBA50 Perioperative Nivolumab (NIVO) v Placebo (PBO) in Patients (Pts) with Resectable NSCLC: Clinical Update from the Phase III CheckMate 77T Study. Ann. Oncol. 2024, 35, S1239–S1240. [Google Scholar] [CrossRef]
- Page, R.D.; Drusbosky, L.M.; Dada, H.; Raymond, V.M.; Daniel, D.B.; Divers, S.G.; Reckamp, K.L.; Villalona-Calero, M.A.; Dix, D.; Odegaard, J.I.; et al. Clinical Outcomes for Plasma-Based Comprehensive Genomic Profiling Versus Standard-of-Care Tissue Testing in Advanced Non–Small Cell Lung Cancer. Clin. Lung Cancer 2022, 23, 72–81. [Google Scholar] [CrossRef]
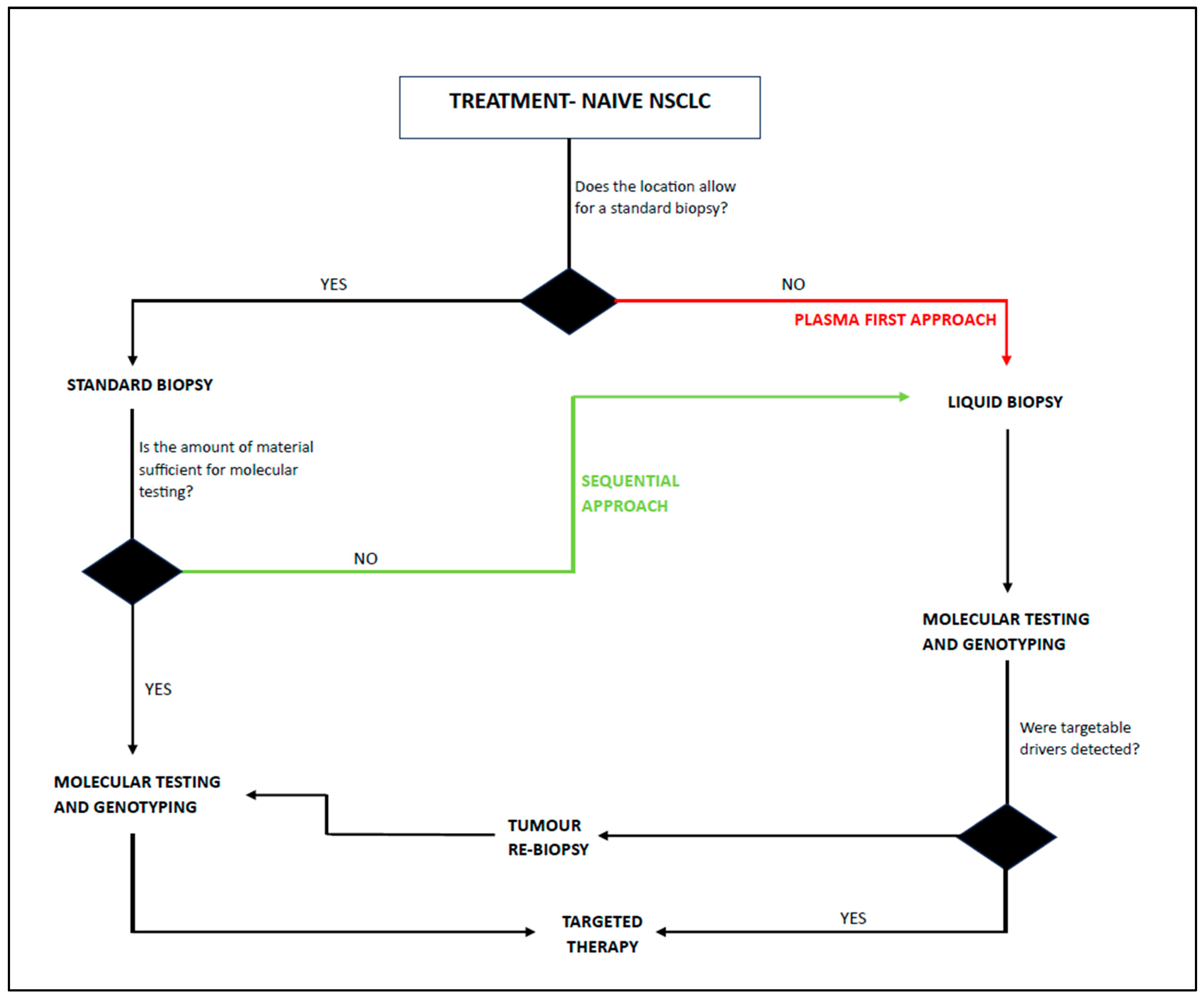
- Bote-De Cabo, H.; Siringo, M.; Conde, E.; Hernández, S.; López-Ríos, F.; Castelo-Loureiro, A.; García-Lorenzo, E.; Baena, J.; Herrera, M.; Belén Enguita, A.; et al. Clinical Utility of Combined Tissue and Plasma Next-Generation Sequencing in Patients With Advanced, Treatment-Naïve NSCLC. JTO Clin. Res. Rep. 2025, 6, 100778. [Google Scholar] [CrossRef]
- Xie, J.; Yao, W.; Chen, L.; Zhu, W.; Liu, Q.; Geng, G.; Fang, J.; Zhao, Y.; Xiao, L.; Huang, Z.; et al. Plasma CtDNA Increases Tissue NGS-Based Detection of Therapeutically Targetable Mutations in Lung Cancers. BMC Cancer 2023, 23, 294. [Google Scholar] [CrossRef]
- Palmero, R.; Taus, A.; Viteri, S.; Majem, M.; Carcereny, E.; Garde-Noguera, J.; Felip, E.; Nadal, E.; Malfettone, A.; Sampayo, M.; et al. Biomarker Discovery and Outcomes for Comprehensive Cell-Free Circulating Tumor DNA Versus Standard-of-Care Tissue Testing in Advanced Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2021, 5, 93–102. [Google Scholar] [CrossRef]
- Leighl, N.B.; Page, R.D.; Raymond, V.M.; Daniel, D.B.; Divers, S.G.; Reckamp, K.L.; Villalona-Calero, M.A.; Dix, D.; Odegaard, J.I.; Lanman, R.B.; et al. Clinical Utility of Comprehensive Cell-Free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non-Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 4691–4700. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, W.; Xiong, L.; Pan, F.; Niu, Y.; Chu, T.; Wang, H.; Zhao, Y.; Jiang, L. Use of Capture-Based next-Generation Sequencing to Detect ALK Fusion in Plasma Cell-Free DNA of Patients with Non-Small-Cell Lung Cancer. Oncotarget 2016, 8, 2771. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Rooney, M.; Nagy, R.J.; Lin, J.J.; Chin, E.; Ferris, L.A.; Ackil, J.; Lennerz, J.K.; Lanman, R.B.; Gainor, J.F.; et al. Molecular Analysis of Plasma from Patients with ROS1-Positive Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 816. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating Mutant DNA to Assess Tumor Dynamics. Nat. Med. 2007, 14, 985. [Google Scholar] [CrossRef]
- Lam, V.K.; Zhang, J.; Wu, C.C.; Tran, H.T.; Li, L.; Diao, L.; Wang, J.; Rinsurongkawong, W.; Raymond, V.M.; Lanman, R.B.; et al. Genotype-Specific Differences in Circulating Tumor DNA Levels in Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 601–609. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef]
- Franzi, S.; Seresini, G.; Borella, P.; Raviele, P.R.; Bonitta, G.; Croci, G.A.; Bareggi, C.; Tosi, D.; Nosotti, M.; Tabano, S. Liquid Biopsy in Non-Small Cell Lung Cancer: A Meta-Analysis of State-of-the-Art and Future Perspectives. Front. Genet. 2023, 14, 1254839. [Google Scholar] [CrossRef]

| Clinical Application of ctDNA | First Author | Year | Number of Subjects | Results | p | Limitations | Strenghts |
|---|---|---|---|---|---|---|---|
| MRD detection | Xia [79] | 2022 | 330 | The presence of postoperative MRD (ctDNA positivity at postoperative period) was a strong predictor for disease relapse. | <0.001 | Mixed I-III stage cohort with most patients in stage I. | Big cohort |
| Chen [65] | 2023 | 1686 | Positive ctDNA status at the baseline, postoperative, or longitudinal timepoints determine higher risk of recurrence. Persistent ctDNA-negativity had the lowest recurrence rate. | <0.001 | Lack of standardization in ctDNA assays and definition of ctDNA positivity. | Meta-analysis of 28 studies | |
| Zhong [82] | 2023 | 1251 | ctDNA MRD detection can predict relapse with high specifity but suboptimal sensitivity, whether at specific time point or during interval surveillance. ctDNA surveillance significantly increases sensitivity with a slight decrease in specifity. | <0.05 | Heterogenous group with SCLC and NSCLC patients. | Meta-analysis of 16 studies | |
| Pulla [106] | 2024 | 461 | ctDNA clearance prior to surgery is associated with pathological complete response benefit. | - | A need for confirmation in interventional studies. | Results confirmed with histopathological results | |
| Perioperative treatment | Reck [89] | 2024 | 283 | ctDNA clearance prior to surgery identifies patients with improved event-free survival (EFS) with higher concordance than pCR status. | - | A need for confirmation in interventional studies. | Revolutionary data |
| Wang [91] | 2021 | 1017 | Reduction in ctDNA levels during treatment is associated with longer OS and PFS in patients with advanced NSCLC receiving ICIs. | <0.001 | Studies based on different drugs, high proportion of patients with KRAS and TP53 mutations. | Meta-analysis of 10 studies | |
| Treatment monitoring | Song [97] | 2020 | 248 | ctDNA clearance during the course of treatment is related to longer PFS and OS regardless of treatment type. | <0.001 | Differences in timepoints of ctDNA evalution between studies. | Multicenter study comparing different treatment regimens |
| Phan [100] | 2022 | 4106 | Detection of EGFR plasma mutations before treatment and after EGFR TKI initiation is negative prognostic factor for PFS and OS. | <0.001 | Study considers the prognostic role of EGFR-plasma mutation as a single gene. | Meta-analysis of 35 studies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makar, K.; Wróbel, A.; Antczak, A.; Tworek, D. Clinical Utility of ctDNA Analysis in Lung Cancer—A Review. Adv. Respir. Med. 2025, 93, 17. https://doi.org/10.3390/arm93030017
Makar K, Wróbel A, Antczak A, Tworek D. Clinical Utility of ctDNA Analysis in Lung Cancer—A Review. Advances in Respiratory Medicine. 2025; 93(3):17. https://doi.org/10.3390/arm93030017
Chicago/Turabian StyleMakar, Kamil, Agata Wróbel, Adam Antczak, and Damian Tworek. 2025. "Clinical Utility of ctDNA Analysis in Lung Cancer—A Review" Advances in Respiratory Medicine 93, no. 3: 17. https://doi.org/10.3390/arm93030017
APA StyleMakar, K., Wróbel, A., Antczak, A., & Tworek, D. (2025). Clinical Utility of ctDNA Analysis in Lung Cancer—A Review. Advances in Respiratory Medicine, 93(3), 17. https://doi.org/10.3390/arm93030017







