Open and Closed Triple Inhaler Therapy in Patients with Uncontrolled Asthma
Abstract
Highlights
- The addition of a LAMA, for asthma symptoms and exacerbation control, in patients with persistent asthma, uncontrolled with medium or high dose ICS + LABA, is an effective treatment option;
- Multiple inhaler devices could be one of the reasons for suboptimal adherence because it is challenging for the patients to establish and sustain the correct technique for each inhaler.
- LAMAs should be used as an add-on treatment for the control of symptoms and exacerbations in patients with asthma that remains persistently uncontrolled despite treatment with ICS + LABA;
- The use of a singletriple inhaler simplifies the treatment in contrast to an open triple inhaler, and this fact could strengthen adherence.
Abstract
1. Introduction
2. Discussion
2.1. Asthma Control
2.2. Asthma Exacerbation Rate
2.3. Lung Function
2.4. Asthma-Related Biomarkers
2.5. Safety
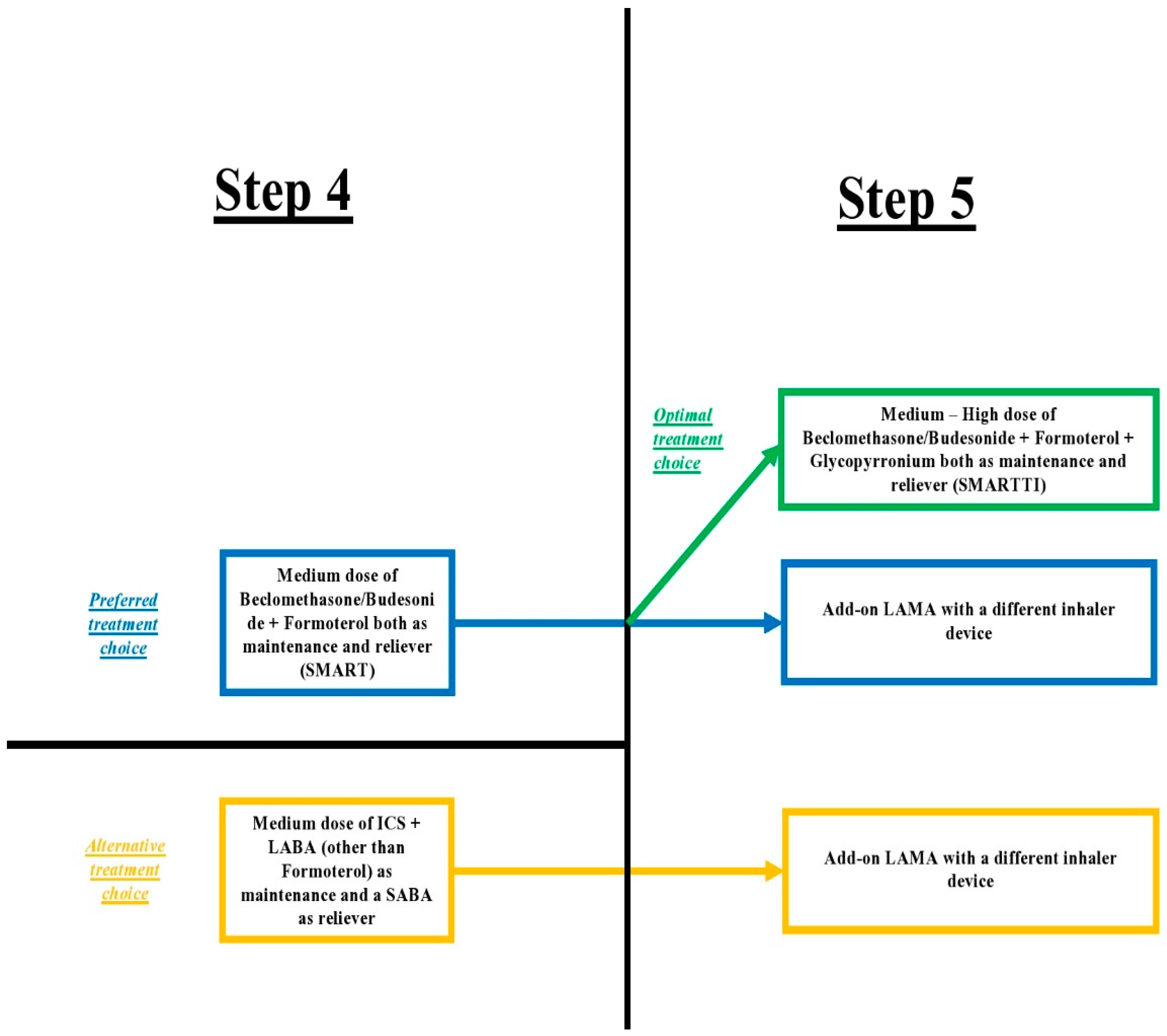
2.6. Single Maintenance and Reliever Therapy (SMART) vs. Single Triple Inhaler Therapy
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Byrne, P.M.; Pedersen, S.; Lamm, C.J.; Tan, W.C.; Busse, W.W.; START Investigators Group. Severe exacerbations and decline in lung function in asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Selroos, O.; Pietinalho, A.; Löfroos, A.B.; Riska, H. Effect of early vs. late intervention with inhaled corticosteroids in asthma. Chest 1995, 108, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: www.ginasthma.org (accessed on 10 May 2023).
- Marceau, C.; Lemière, C.; Berbiche, D.; Perreault, S.; Blais, L. Persistence, adherence, and effectiveness of combination therapy among adult patients with asthma. J. Allergy Clin. Immunol. 2006, 118, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Hekking, P.W.; Wener, R.R.; Amelink, M.; Zwinderman, A.H.; Bouvy, M.L.; Bel, E.H. The prevalence of severe refractory asthma. J. Allergy Clin. Immunol. 2015, 135, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef]
- Chung, K.F. Personalised medicine in asthma: Time for action. Eur. Respir. Rev. 2017, 26, 170064. [Google Scholar] [CrossRef]
- Chung, L.P.; Paton, J.Y. Two sides of the same coin?-Treatment of chronic asthma in children and adults. Front Pediatr. 2019, 7, 62. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Gross, N.J. Inhaled glycopyrrolate for the treatment of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1873–1888. [Google Scholar] [CrossRef]
- Puggioni, F.; Brussino, L.; Canonica, G.W.; Blasi, F.; Paggiaro, P.; Caminati, M.; Latorre, M.; Heffler, E.; Senna, G.; Severe Asthma Network in Italy (SANI) group. Frequency of tiotropium bromide use and clinical features of patients with severe asthma in a real-life setting: Data from the severe asthma network in Italy (sani) registry. J. Asthma Allergy 2020, 13, 599–604. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Bolaki, M.; Karagiannis, K.; Trachalaki, A.; Ierodiakonou, D.; Stamatopoulou, V.; Chatzinikolaou, C.; Mastrodimou, S.; Stamataki, E.; Pitsidianakis, G.; et al. Real-life Cretan asthma registry focused on severe asthma: On behalf of ‘The Cretan registry of the use of Biologics in Severe Asthma’. Exp. Ther. Med. 2021, 22, 1239. [Google Scholar] [CrossRef]
- Kirkland, S.W.; Vandenberghe, C.; Voaklander, B.; Nikel, T.; Campbell, S.; Rowe, B.H. Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma. Cochrane Database Syst. Rev. 2017, 1, CD001284. [Google Scholar] [CrossRef]
- Craig, S.S.; Dalziel, S.R.; Powell, C.V.; Graudins, A.; Babl, F.E.; Lunny, C. Interventions for escalation of therapy for acute exacerbations of asthma in children: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 8, CD012977. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Sanfilippo, F.; Cuttone, G.; Dezio, V.; Falcone, M.; Brancati, S.; Crimi, C.; Astuto, M. Use of ketamine in patients with refractory severe asthma exacerbations: Systematic review of prospective studies. Eur. J. Clin. Pharmacol. 2022, 78, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Guglani, V.; Jat, K.R. Ketamine versus aminophylline for acute asthma in children: A randomized, controlled trial. Ann. Thorac. Med. 2016, 11, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Nedel, W.; Costa, R.; Mendez, G.; Marin, L.; Vargas, T.; Marques, L. Negative results for ketamine use in severe acute bronchospasm: A randomised controlled trial. Anaesthesiol. Intensive Ther. 2020, 52, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Page, C.; O’Shaughnessy, B.; Barnes, P. Pathogenesis of COPD and Asthma. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2017; Volume 237, pp. 1–21. [Google Scholar] [CrossRef]
- Gosens, R.; Grainge, C. Bronchoconstriction and airway biology: Potential impact and therapeutic opportunities. Chest 2015, 147, 798–803. [Google Scholar] [CrossRef]
- Gosens, R.; Gross, N. The mode of action of anticholinergics in asthma. Eur. Respir. J. 2018, 52, 1701247. [Google Scholar] [CrossRef]
- Rodrigo, G.J.; Castro-Rodriguez, J.A. Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta-analysis. Thorax 2005, 60, 740–746. [Google Scholar] [CrossRef]
- Cazzola, M.; Braido, F.; Calzetta, L.; Matera, M.G.; Piraino, A.; Rogliani, P.; Scichilone, N. The 5T approach in asthma: Triple Therapy Targeting Treatable Traits. Respir. Med. 2022, 200, 106915. [Google Scholar] [CrossRef]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M. The St George’s Respiratory Questionnaire. Respir. Med. 1991, 85, 25–31. [Google Scholar] [CrossRef]
- Juniper, E.F.; O’Byrne, P.M.; Guyatt, G.H.; Ferrie, P.J.; King, D.R. Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 1999, 14, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Guyatt, G.H.; Epstein, R.S.; Ferrie, P.J.; Jaeschke, R.; Hiller, T.K. Evaluation of impairment of health related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax 1992, 47, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M.; Littlejohns, P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am. Rev. Respir. Dis. 1992, 145, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Bousquet, J.; Abetz, L.; Bateman, E.D. Identifying “well-controlled” and “not well-controlled” asthma using the Asthma Control Questionnaire. Respir. Med. 2006, 100, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Svensson, K.; Mörk, A.C.; Ståhl, E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir. Med. 2005, 99, 553–558. [Google Scholar] [CrossRef]
- Juniper, E.F.; Guyatt, G.H.; Willan, A.; Griffith, L.E. Determining a minimal important change in a disease-specific quality of life questionnaire. J. Clin. Epidemiol. 1994, 47, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Virchow, J.C.; Kuna, P.; Paggiaro, P.; Papi, A.; Singh, D.; Corre, S.; Zuccaro, F.; Vele, A.; Kots, M.; Georges, G.; et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): Two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 2019, 394, 1737–1749. [Google Scholar] [CrossRef]
- Gessner, C.; Kornmann, O.; Maspero, J.; van Zyl-Smit, R.; Krüll, M.; Salina, A.; Gupta, P.; Bostel, S.; Fucile, S.; Conde, L.G.; et al. Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: A randomised, Phase IIIb, non-inferiority study (ARGON). Respir. Med. 2020, 170, 106021. [Google Scholar] [CrossRef]
- Lee, L.A.; Bailes, Z.; Barnes, N.; Boulet, L.P.; Edwards, D.; Fowler, A.; Hanania, N.A.; Kerstjens, H.A.M.; Kerwin, E.; Nathan, R.; et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): A double-blind, randomised, phase 3A trial. Lancet. Respir. Med. 2021, 9, 69–84. [Google Scholar] [CrossRef]
- Kerstjens, H.A.M.; Maspero, J.; Chapman, K.R.; van Zyl-Smit, R.N.; Hosoe, M.; Tanase, A.M.; Lavecchia, C.; Pethe, A.; Shu, X.; D’Andrea, P.; et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): A randomised, double-blind, controlled phase 3 study. Lancet Respir. Med. 2020, 8, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Ishiura, Y.; Fujimura, M.; Ohkura, N.; Hara, J.; Kasahara, K.; Ishii, N.; Tamaki, T.; Shimizu, T.; Nomura, S. Effect of triple therapy in patients with asthma-COPD overlap. Int. J. Clin. Pharmacol. Ther. 2019, 57, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, S.; Kim, J.H.; Kim, S.H.; Lee, T.; Yoon, S.Y.; Kim, M.H.; Moon, J.Y.; Yang, M.S.; Jung, J.W.; et al. A Randomized, Noninferiority Trial Comparing ICS + LABA with ICS + LABA + LAMA in Asthma-COPD Overlap (ACO) Treatment: The ACO Treatment with Optimal Medications (ATOMIC) Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 1304–1311.e2. [Google Scholar] [CrossRef]
- Braido, F.; Tiotiu, A.; Guidos-Fogelbach, G.; Baiardini, I.; Cosini, F.; Correia de Sousa, J.; Bikov, A.; Novakova, S.; Labor, M.; Kaidashev, I.; et al. Manifesto on inhaled triple therapy in asthma: An Interasma (Global Asthma Association-GAA) document. J. Asthma. 2022, 59, 2402–2412. [Google Scholar] [CrossRef]
- George, M.; Bender, B. New insights to improve treatment adherence in asthma and COPD. Patient Prefer. Adherence 2019, 13, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.A.; Pedersen, S.; Busse, W.W.; Tan, W.C.; Chen, Y.Z.; Ohlsson, S.V.; Ullman, A.; Lamm, C.J.; O’Byrne, P.M.; START Investigators Group. Early intervention with budesonide in mild persistent asthma: A randomised, double-blind trial. Lancet 2003, 361, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.; Ware, S.; Marks, G.; Salome, C.; Jenkins, C.; Woolcock, A. Differences between asthma exacerbations and poor asthma control. Lancet 1999, 353, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Ko, H.K.; Lu, M.S.; Chou, C.L.; Su, K.C.; Hsu, C.C.; Chou, K.T.; Chen, T.J.; Perng, D.W.; Chou, Y.C. Independent risk factors for death in patients admitted for asthma exacerbation in Taiwan. NPJ Prim. Care Respir. Med. 2020, 30, 7. [Google Scholar] [CrossRef]
- Roberts, G.; Patel, N.; Levi-Schaffer, F.; Habibi, P.; Lack, G. Food allergy as a risk factor for life-threatening asthma in childhood: A case-controlled study. J. Allergy Clin. Immunol. 2003, 112, 168–174. [Google Scholar] [CrossRef]
- Alvarez, G.G.; Schulzer, M.; Jung, D.; FitzGerald, J.M. A systematic review of risk factors associated with near-fatal and fatal asthma. Can. Respir. J. 2005, 12, 265–270. [Google Scholar] [CrossRef]
- Suissa, S.; Blais, L.; Ernst, P. Patterns of increasing β-agonist use and the risk of fatal or near-fatal asthma. Eur. Respir. J. 1994, 7, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Averell, C.M.; Laliberté, F.; Duh, M.S.; Wu, J.W.; Germain, G.; Faison, S. Characterizing real-world use of tiotropium in Asthma in the USA. J. Asthma Allergy 2019, 12, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Fairburn-Beech, J.; Sato, K.; Kaise, T. Clinical characteristics, treatment patterns, disease burden, and persistence/adherence in patients with asthma initiating inhaled triple therapy: Real-world evidence from Japan. Curr. Med. Res. Opin. 2020, 36, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Singh, D.; Virchow, J.C.; Canonica, G.W.; Vele, A.; Georges, G. Normalisation of airflow limitation in asthma: Post-hoc analyses of TRIMARAN and TRIGGER. Clin. Transl. Allergy 2022, 12, e12145. [Google Scholar] [CrossRef]
- Singh, D.; Virchow, J.C.; Canonica, G.W.; Vele, A.; Kots, M.; Georges, G.; Papi, A. Determinants of response to inhaled extrafine triple therapy in asthma: Analyses of TRIMARAN and TRIGGER. Respir. Res. 2020, 21, 285. [Google Scholar] [CrossRef]
- Kerstjens, H.A.; Engel, M.; Dahl, R.; Paggiaro, P.; Beck, E.; Vandewalker, M.; Sigmund, R.; Seibold, W.; Moroni-Zentgraf, P.; Bateman, E.D. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl. J. Med. 2012, 367, 1198–1207. [Google Scholar] [CrossRef]
- Chipps, B.; Mosnaim, G.; Mathur, S.K.; Shaikh, A.; Khoury, S.; Gopalan, G.; Palli, S.R.; Lamerato, L.; Casciano, J.; Dotiwala, Z.; et al. Add-on tiotropium versus step-up inhaled corticosteroid plus long-acting beta-2-agonist in real-world patients with asthma. Allergy Asthma Proc. 2020, 41, 248–255. [Google Scholar] [CrossRef]
- Peters, S.P.; Kunselman, S.J.; Icitovic, N.; Moore, W.C.; Pascual, R.; Ameredes, B.T.; Boushey, H.A.; Calhoun, W.J.; Castro, M.; Cherniack, R.M.; et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl. J. Med. 2010, 363, 1715–1726. [Google Scholar] [CrossRef]
- Johansson, G.; Andreasson, E.B.; Larsson, P.E.; Vogelmeier, C.F. Cost effectiveness of budesonide/formoterol for maintenance and reliever therapy versus salmeterol/fluticasone plus salbutamol in the treatment of asthma. Pharmacoeconomics 2006, 24, 695–708. [Google Scholar] [CrossRef]
- Lundborg, M.; Wille, S.; Bjermer, L.; Tilling, B.; Lundgren, M.; Telg, G.; Ekström, T.; Selroos, O. Maintenance plus reliever budesonide/formoterol compared with a higher maintenance dose of budesonide/formoterol plus formoterol as reliever in asthma: An efficacy and cost-effectiveness study. Curr. Med. Res. Opin. 2006, 22, 809–821. [Google Scholar] [CrossRef]
| Reference | Study Type | Participants | Main Findings | Main Limitations |
|---|---|---|---|---|
| Puggioni 2020 [10] | Retrospective observational study. | 698 patients from Severe Asthma Network in Italy (SANI). | Severe asthmatics under treatment with long-acting muscarinic antagonists (LAMAs) experience significantly worse asthma control and worse disease-related quality of life before LAMA initiation. Asthmatic patients, who are already on LAMA treatment, have a significantly higher annual exacerbation rate. In LAMAusers, the fraction of exhaled nitric oxide (FeNO) and serum total immunoglobin E (IgE) levels were comparable with those of non-LAMAusers. | These patients tended to be ex-smokers and to have later-onset asthma in comparison to patients who did not receive LAMAs. They were also more often diagnosed with concomitant bronchiectasis and used to receive oral corticosteroid (OCS) treatment courses and long-term treatment with monoclonal antibodies more frequently compared to non-LAMAusers. |
| Virchow 2019 [30] | Two randomized controlled trials (TRIMARAN & TRIGGER). | 1155 asthma patients in TRIMARAN & 1437 in TRIGGER. | In the TRIGGER study, participants on close triple therapy experienced considerable improvement in their asthma control questionnaire (ACQ) score and in the proportion of days free of asthma symptoms at 52 weeks, but not in rescue medication use. In the TRIMARAN study, no significant differences between the study groups in any of the aforementioned parameters were observed. In both studies, a significant reduction in the rate of moderate and severe exacerbations was observed. In both studies, a significant improvement in both pre-dose and peak forced expiratory volume in 1 s (FEV1), as well as in morning peak expiratory flow (PEF), was observed. In both studies, adverse events, as well as serious adverse events, were comparable among the study groups. | No differences were found in the TRIGGER study, as far as asthma control, between open and closed triple inhaler therapy arms. No significant differences between the open and closed treatment combinations in asthma exacerbation rate control were found in either study. No significant differences between the open and the closed triple inhaler therapy study arms in lung function were found in either study. |
| Gessner 2020 [31] | Randomized, non-inferiority study (ARGON). | 1426 patients with asthma. | Singletriple inhaler therapy was non-inferior to open triple inhaler use as far as the asthma quality of life questionnaire (AQLQ) improvement at 24 weeks is concerned. The single triple inhaler therapy proved superior in the ACQ and St. George respiratory questionnaire (SGRQ) improvement. Closed triple inhaler therapy with a high ICS dose was superior to the open triple inhaler therapy regarding the improvement of trough FEV1 and of morning and evening PEF. Adverse events were comparable among study groups. | Partially blinded design. Relatively short duration of the trial for evaluation of exacerbations (24 weeks). No significant differences between the studied groups in asthma exacerbation rate control were observed. While current smokers were included, they accounted for a minor subgroup (2.2%);thus, no firm conclusions can be drawn regarding the effect of current smoking and its potential influence on the study outcomes. |
| Lee 2021 [32] | Randomized controlled trial (CAPTAIN). | 2439 patients with asthma. | Τhe minimal clinically important improvement in the ACQ was significantly greater regarding closed triple inhaler therapy. The addition of LAMA increased the FEV1, between 82 and 110 mL in the different study arms, with different drug doses—and these differences were statistically significant across all study arms and were supported by the analysis of FEV1 at 3 h post-dose. | There was no clinically important improvement in SGRQ between study arms. In the patient groups with higher baseline FeNO and blood eosinophil count, clinic trough FEV1 and annual moderate and/or severe exacerbation rate were not affected by LAMA initiation but by inhaled corticosteroids (ICS) dosage. |
| Kerstjens 2020 [33] | Randomized controlled trial (IRIDIUM). | 3092 patients with asthma. | ACQ questionnaire improvement was significantly greater in the closed triple inhaler therapy group compared to the group that received only ICS + long-acting β2-agonists (LABA) therapy. The addition of a LAMA reduced the overall risk for an exacerbation along with the risk for moderate and/or severe exacerbation. The addition of LAMA to ICS + LABA increased the FEV1 between 65 and 119 mL. Adverse events were comparable among study groups. | The reduction in the overall risk for an exacerbation and the risk for moderate and/or severe exacerbation was more evident in high ICS dosages. The study was not powered to provide conclusive answers for some comparisons and endpoints. This is a carefully controlled study and, therefore, not necessarily reflective of a real-world setting. |
| Ishiura 2019 [34] | Randomized, open-label crossover pilot study. | 17 asthma chronic obstructive pulmonary disease (COPD) overlap (ACO) patients. | No statistically significant difference in asthma control, measured with ACT, when a LAMA was added to the treatment with ICS + LABA. Significant improvement in multiple pulmonary function tests (PFTs) and impulse oscillometry (IOS) parameters after LAMA treatment initiation. The beneficial effect of LAMA in patients with ACO seems to be independent of FeNO level, total serumIgE, or eosinophil blood count. | The results by themselves cannot express the etiology of the clinically beneficial effect. A specific, formal definition of ACO has yet to be determined. The number of patients enrolled in this study was insufficient to detect benefits with respect to healthcare outcomes. This study was also relatively short for the assessment of patient-reported outcomes. |
| Park 2021 [35] | Multicenter, 48-week, randomized, non-inferiority trial (ATOMIC). | 303 patients with ACO. | No statistically significant difference in asthma control, measured with ACT, when a LAMA was added to the treatment with ICS + LABA. There is no clear evidence whether ICS + LABA + LAMA treatment reduces the time to first exacerbation. Significant improvement in multiple pulmonary function tests (PFTs) and impulse oscillometry (IOS) parameters after LAMA treatment initiation. | The study uses a non-inferiority study design. The incidence of adverse events was much less than expected. The definition of ACO is still controversial. This study was conducted as an open-label study, where subject bias can affect the result. |
| Braido 2022 [36] | Review. | Previously published studies. | It seems plausible to suggest the addition of a LAMA, for asthma symptom control, in patients with persistent asthma, uncontrolled with medium or high dose ICS + LABA treatment, either in a separate or the same inhaler device, which is more cost-effective. The addition of a LAMA to the standard treatment with ICS + LABA probably reduces the risk of moderate and/or severe asthma exacerbations and should be used in cases wheredual treatment is inadequate in the prevention of asthma exacerbations. The addition of LAMA to the standard ICS + LABA treatment regimen undoubtedly significantly improves lung function in patients with inadequately controlled asthma. Some asthma traits that might be predictive ofbetter response to treatment with LAMA could be a previous smoking history and fixed airway obstruction. Existing evidence suggests that the safety profile of adding LAMA to the standard treatment with ICS + LABA is excellent. | Available data related to the impact of triple inhaled therapy on asthma control and quality of life are conflicting. Currently, there are no reliable biomarkers that could identify patients with severe refractory asthma eligible for LAMA treatment initiation. |
| Averell 2019 [44] | Retrospective observational study. | 1821 patients treated with tiotropium. | Asthma exacerbations were more frequent in patients receiving various control medications, including LAMAs. | It was not possible to confirm that tiotropium was prescribed specifically for the treatment of asthma. Treatment patterns identified in this study may change over time. |
| Suzuki 2020 [45] | Retrospective, observational cohort study. | 1546 patients with asthma and 199 patients with ACO. | The exacerbation rate was significantly lower in the year following the triple treatment initiation compared to patients receiving ICS + LABA alone. | The exclusion of patients who withdrew from the health insurance database may have resulted in the exclusion of patients who died due to severe asthma. Approximate severity was estimated using ICS daily dose, meaning that severity could only be approximated for patients on a fixed daily ICS dose and not for patients on a variable ICS dose. |
| Papi 2022 [46] | Post-hoc analyses of TRIMARAN and TRIGGER | 1155 asthma patients in TRIMARAN and1437 in TRIGGER. | Significant reduction in the rate of moderate and severe exacerbations with seasonal variation, as they were greater in winter compared to the other seasons (20.3% and between 8.6% and 12.0%, respectively). The addition of a LAMA to the dual therapy of ICS + LABA in patients with asthma that remained uncontrolled led to a clearly beneficial effect on lung function. | These results came from post-hoc analyses. The conclusions were drawn on mean data rather than individual patient analyses and didnot provide a comprehensive mechanistic explanation for the results that we observed. |
| Singh 2020 [47] | Post-hoc analyses of TRIMARAN and TRIGGER. | 1155 asthma patients in TRIMARAN and1437 in TRIGGER. | Patients with higher reversibility in post-bronchodilator FEV1 (>400 mL) experienced greater exacerbation reduction benefits. In the TRIMARAN study, the sub-group of patients with low eosinophil blood count (<300 cells/μL) experienced greater benefits in lung function. | No differences were observed between patients with different blood eosinophil levels in exacerbation rate reduction. In the TRIGGER study, eosinophil blood count was not associated with the lung function benefit. |
| Kerstjens 2012 [48] | Two replicate, randomized, controlled trials. | 912 patients with asthma. | The addition of a LAMA to ICS + LABA increased the time to the first severe exacerbation by 56 days and significantly reduced the risk for a severe exacerbation by 21%. Both pre-dose (trough) and peak FEV1 were significantly increased after LAMA initiation. No deaths were reported. | Inconsistency in the results between the two trials. A larger placebo response was seen in trial 1 than in trial 2. The trials were supported by two pharmaceutical companies. |
| Cazzola 2022 [21] | Review. | Previously published studies. | The addition of a LAMA to the standard treatment with ICS + LABA probably reduces the risk of moderate and/or severe asthma exacerbations and should be used in cases wheredual treatment is inadequate in the prevention of asthma exacerbations. The addition of LAMA to the standard ICS + LABA treatment regimen undoubtedly significantly improves lung function in patients with inadequately controlled asthma. | The role of several recognizable disease traits in treatment decisions is still under investigation. The study was supported by a pharmaceutical company. |
| Chipps 2020 [49] | Retrospective cohort study. | 7857 patients with asthma. | The addition of LAMA to ICS + LABA decreased the risk of exacerbations by 35% versus ICS dose escalation. | Lack of information about events and rate of events that did not result in a paid claim. Actual SABA use, inhaler technique, medication adherence, and influence of comorbidities on uncontrolled symptoms. |
| Peters 2010 [50] | Three-way, double-blind, triple-dummy crossover trial. | 210 patients with asthma. | The addition of LAMA improved lung function and daily symptom control. | Evaluated only a small number of patients, with no treatment lasting longer than 14 weeks. Could not examine either the rate of asthma exacerbations or long-term safety issues. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotoulas, S.-C.; Tsiouprou, I.; Domvri, K.; Ntontsi, P.; Pataka, A.; Porpodis, K. Open and Closed Triple Inhaler Therapy in Patients with Uncontrolled Asthma. Adv. Respir. Med. 2023, 91, 288-300. https://doi.org/10.3390/arm91040023
Kotoulas S-C, Tsiouprou I, Domvri K, Ntontsi P, Pataka A, Porpodis K. Open and Closed Triple Inhaler Therapy in Patients with Uncontrolled Asthma. Advances in Respiratory Medicine. 2023; 91(4):288-300. https://doi.org/10.3390/arm91040023
Chicago/Turabian StyleKotoulas, Serafeim-Chrysovalantis, Ioanna Tsiouprou, Kalliopi Domvri, Polyxeni Ntontsi, Athanasia Pataka, and Konstantinos Porpodis. 2023. "Open and Closed Triple Inhaler Therapy in Patients with Uncontrolled Asthma" Advances in Respiratory Medicine 91, no. 4: 288-300. https://doi.org/10.3390/arm91040023
APA StyleKotoulas, S.-C., Tsiouprou, I., Domvri, K., Ntontsi, P., Pataka, A., & Porpodis, K. (2023). Open and Closed Triple Inhaler Therapy in Patients with Uncontrolled Asthma. Advances in Respiratory Medicine, 91(4), 288-300. https://doi.org/10.3390/arm91040023










