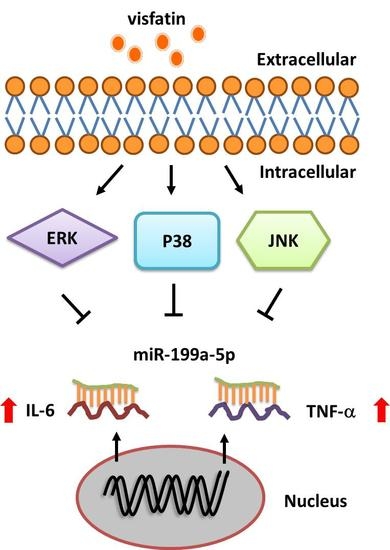
Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways
Abstract
:1. Introduction
2. Results
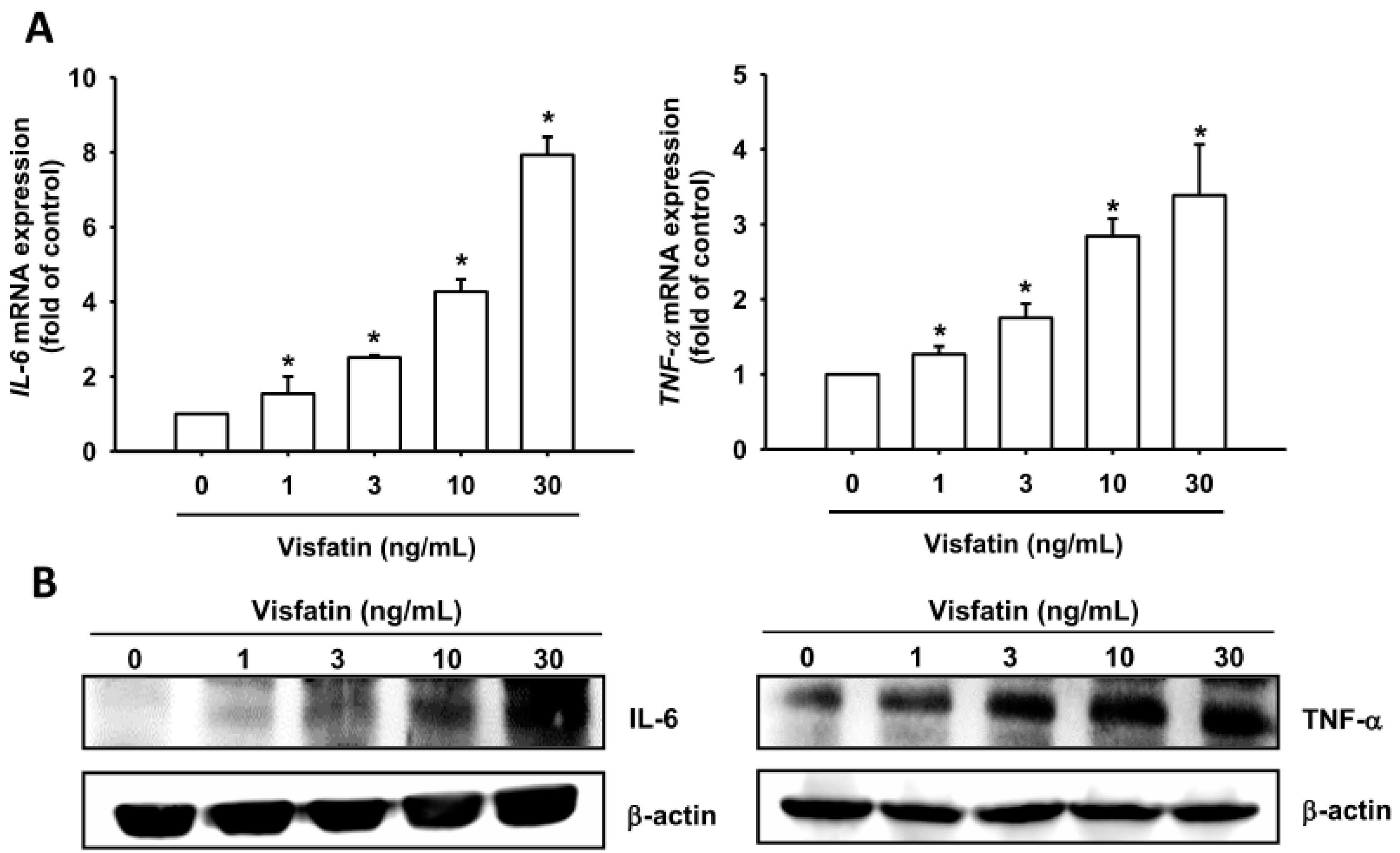
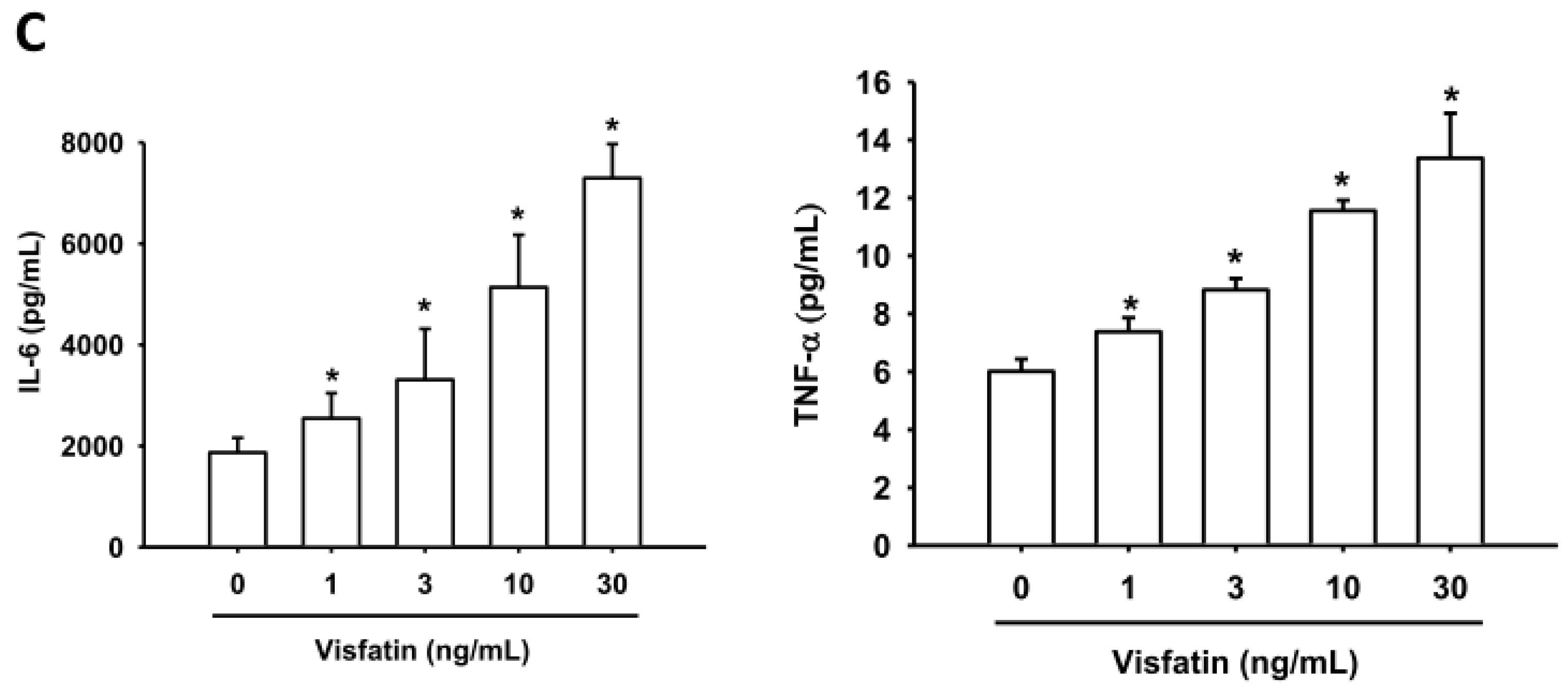
2.1. Visfatin Promotes IL-6 and TNF-α Expression in Human Osteoarthritis Synovial Fibroblasts (OASFs)
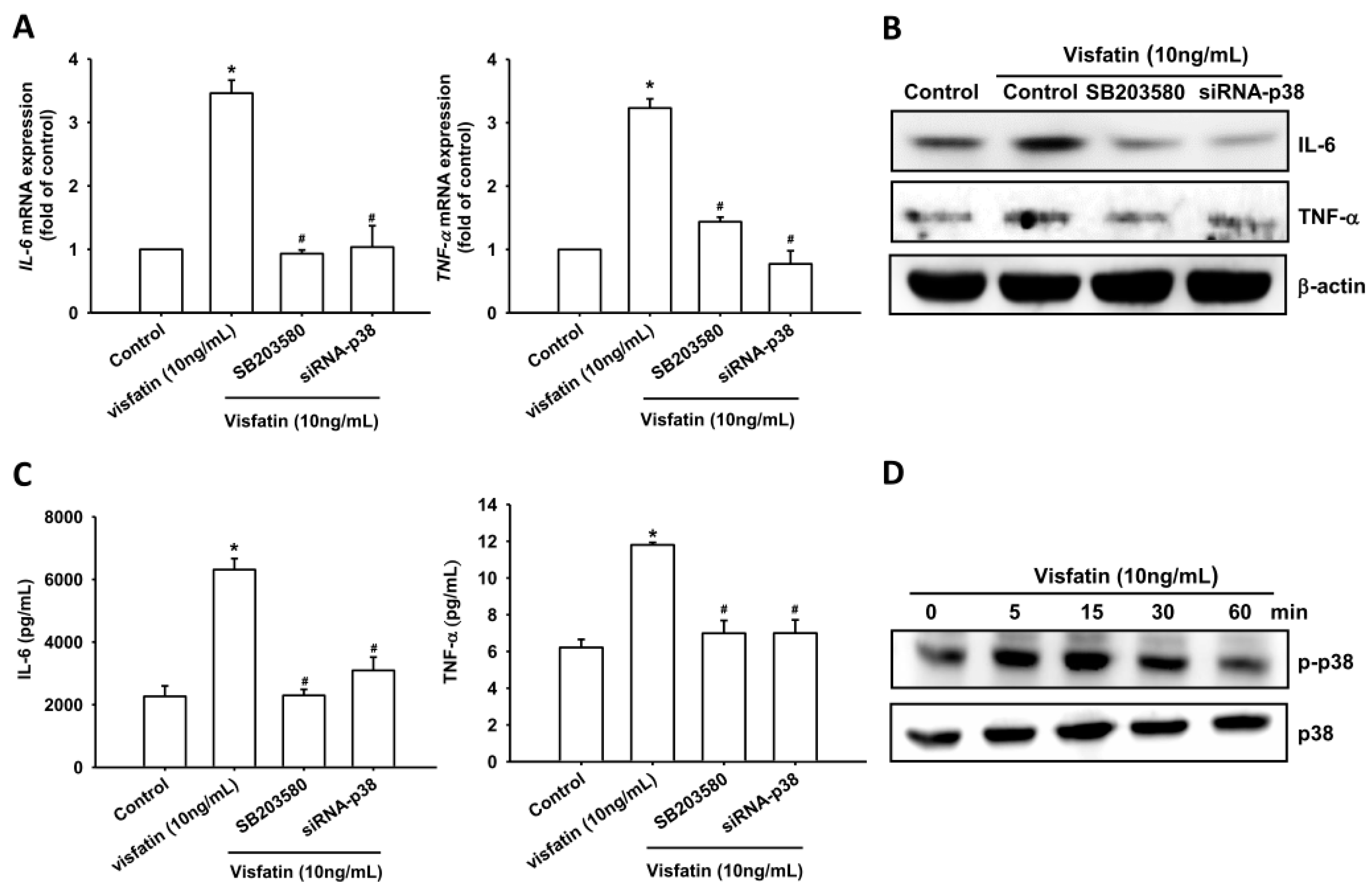
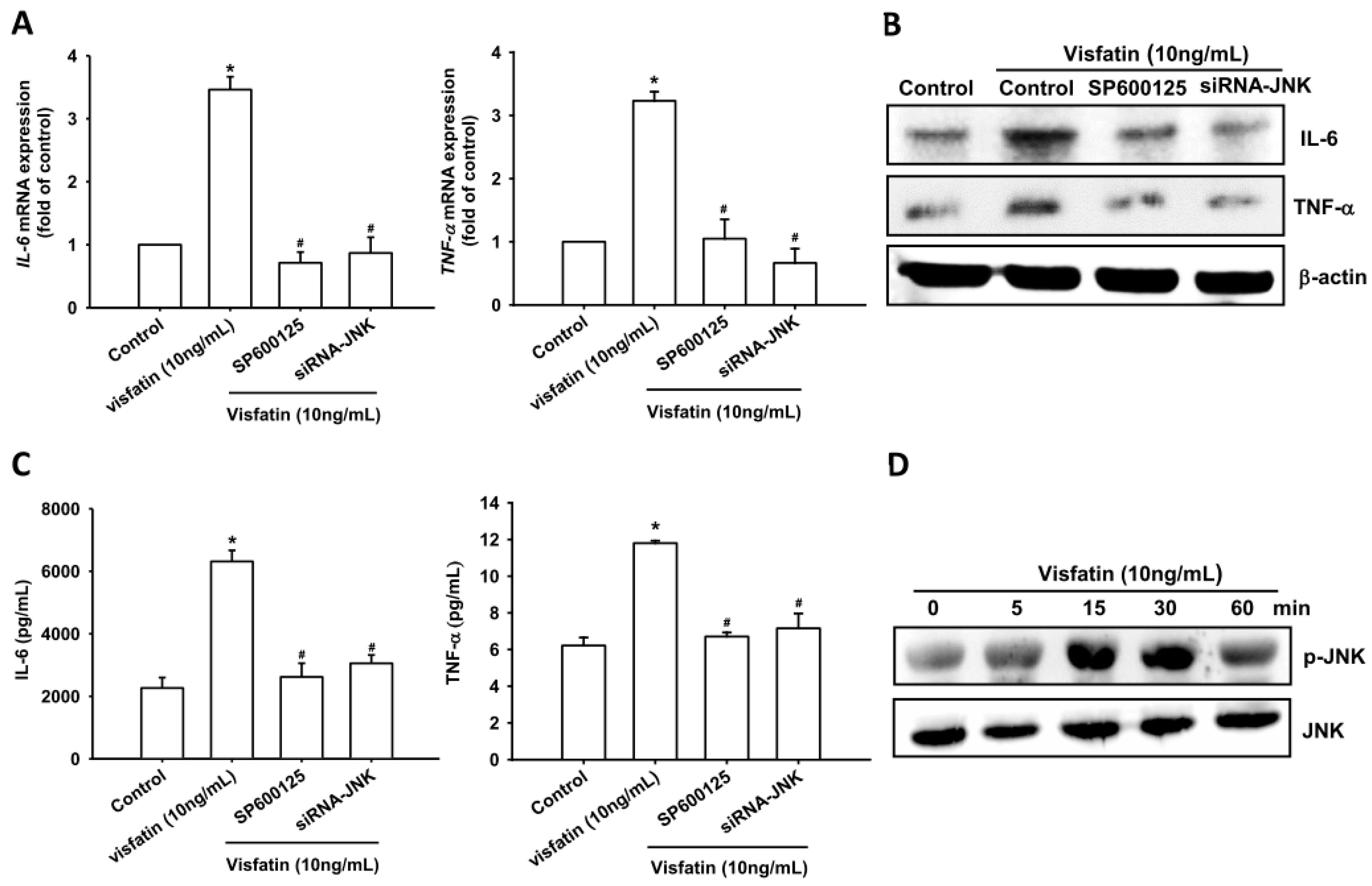
2.2. Visfatin Increases IL-6 and TNF-α Expression via the MAPK Signaling Pathway
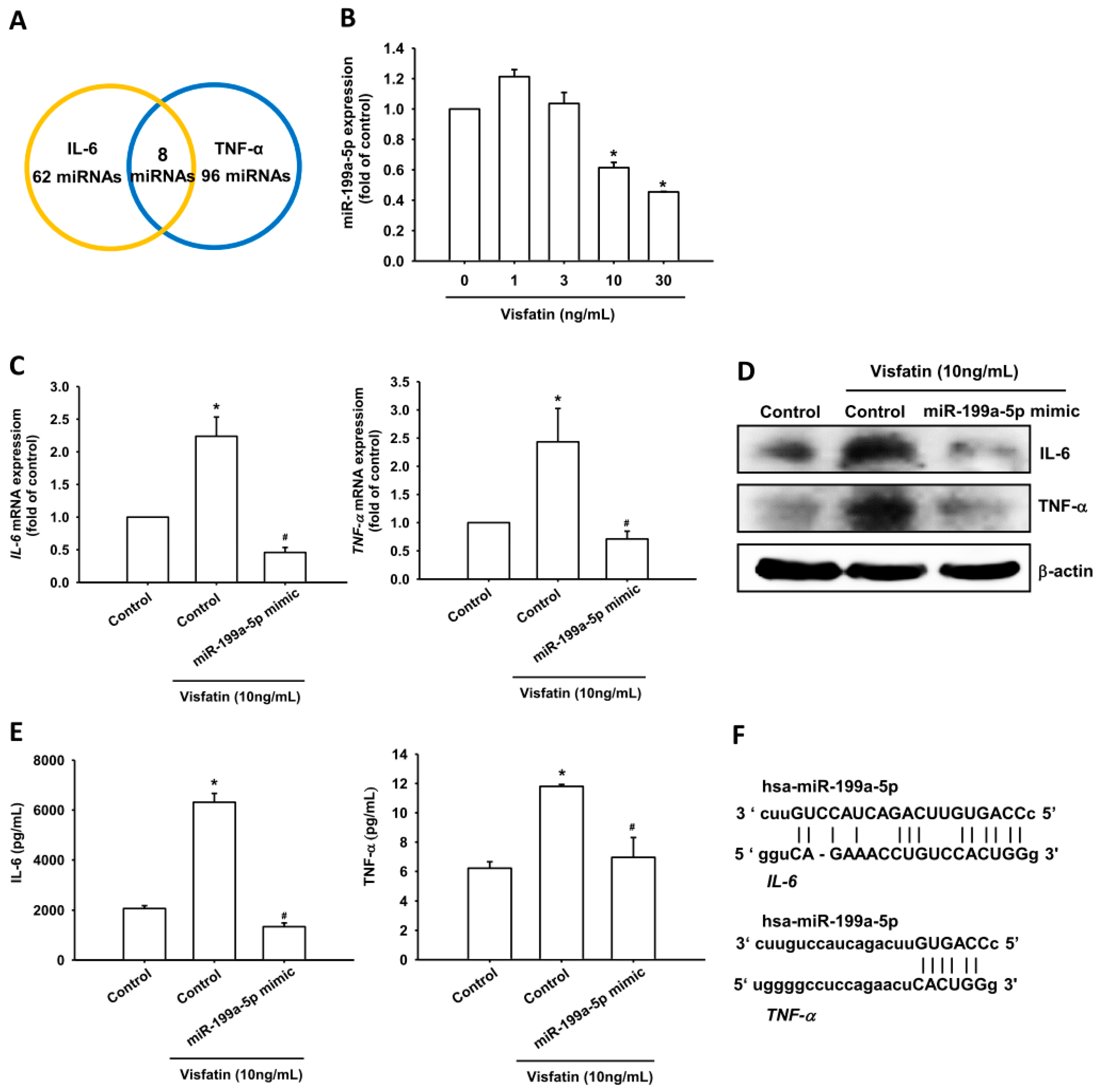
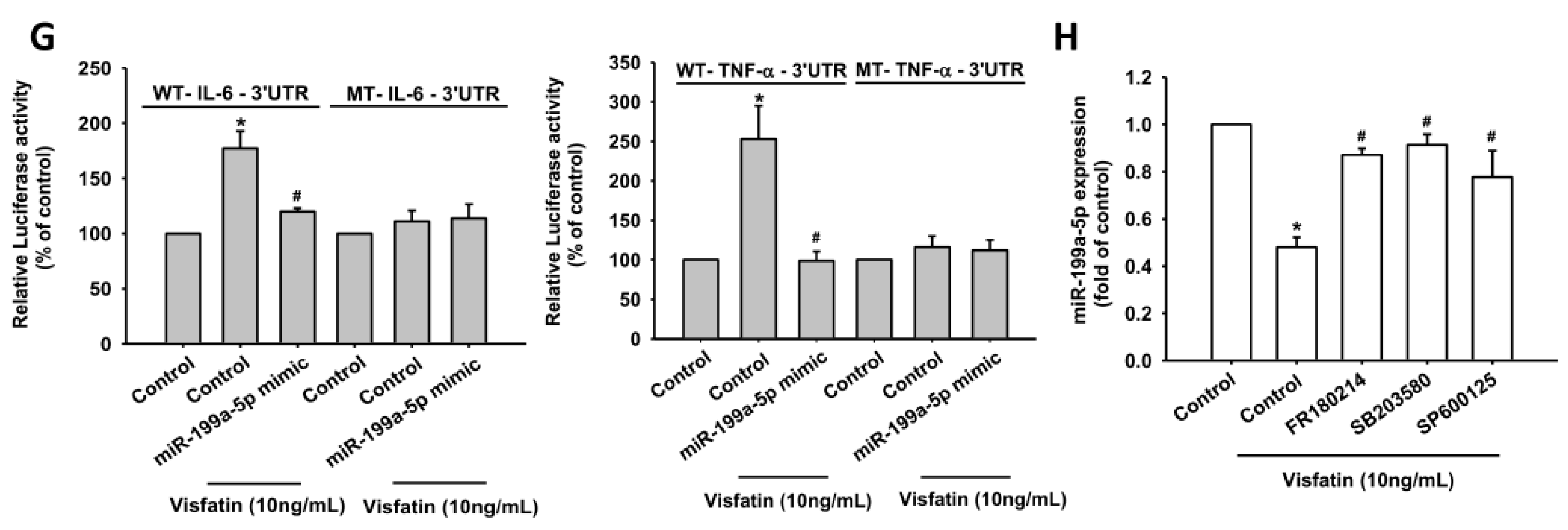
2.3. Visfatin Increases IL-6 and TNF-α Production in OASFs by Inhibiting miR-199a-5p Expression
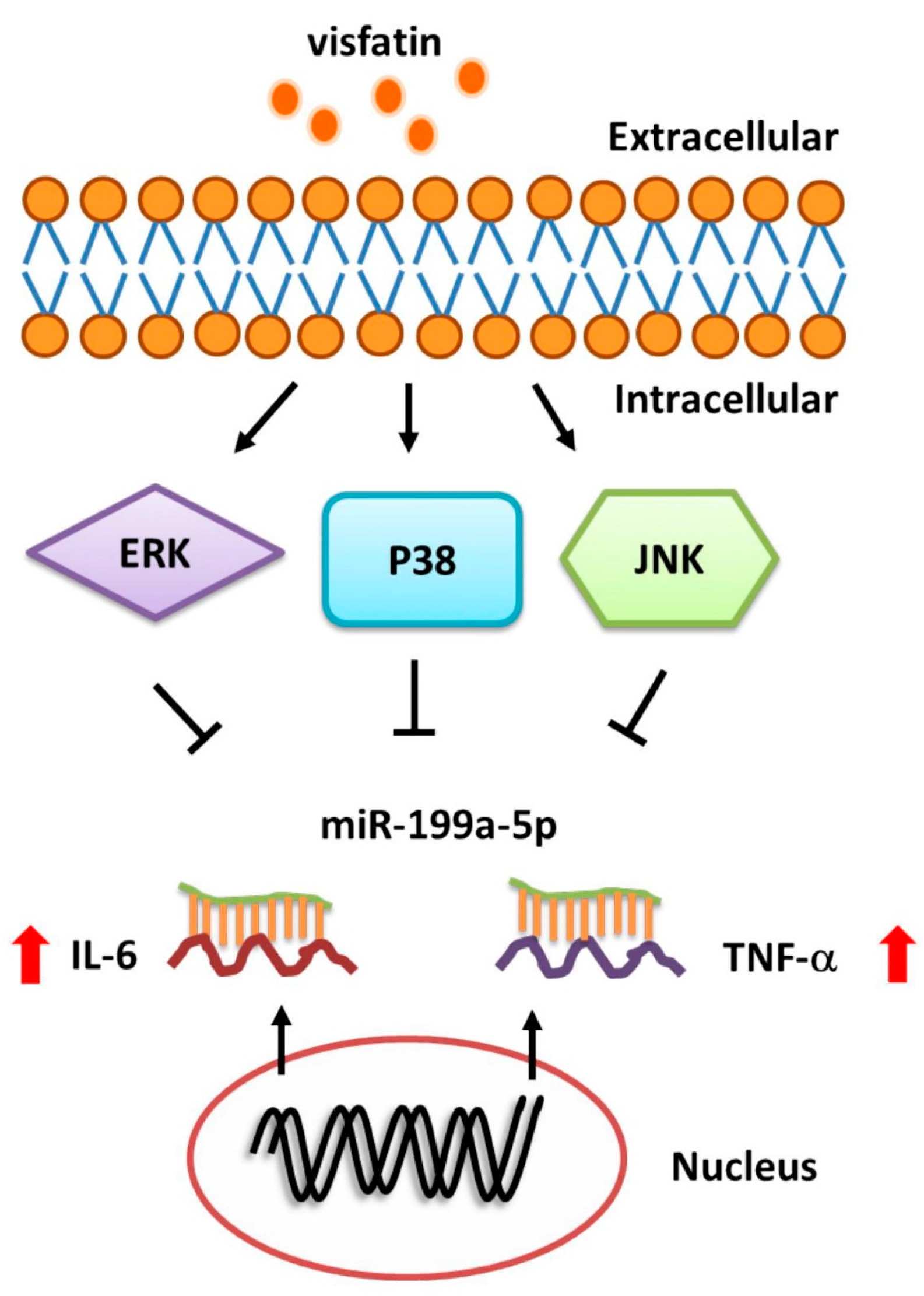
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Measurement of IL-6 and TNF-α
4.4. Real-Time Quantitative PCR of mRNA and miRNA
4.5. Western Blot Analysis
4.6. Plasmid Construction and Luciferase Assay
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bresnihan, B. Pathogenesis of joint damage in rheumatoid arthritis. J. Rheumatol. 1999, 26, 717–719. [Google Scholar] [PubMed]
- Grossman, J.M.; Brahn, E. Rheumatoid arthritis: Current clinical and research directions. J. Women Health 1997, 6, 627–638. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the american rheumatism association. Arthritis Rheumatol. 1986, 29, 1039–1049. [Google Scholar]
- Bai, T.; Chen, C.C.; Lau, L.F. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J. Immunol. 2010, 184, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Barksby, H.E.; Hui, W.; Wappler, I.; Peters, H.H.; Milner, J.M.; Richards, C.D.; Cawston, T.E.; Rowan, A.D. Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: Implications for cartilage destruction and repair. Arthritis Rheumatol. 2006, 54, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 2004, S27–S36. [Google Scholar] [CrossRef]
- Tang, C.H.; Hsu, C.J.; Fong, Y.C. The CCL5/CCR5 axis promotes interleukin-6 production in human synovial fibroblasts. Arthritis Rheumatol. 2010, 62, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The chingford study. Arthritis Rheumatol. 2009, 60, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Honsawek, S.; Deepaisarnsakul, B.; Tanavalee, A.; Yuktanandana, P.; Bumrungpanichthaworn, P.; Malila, S.; Saetan, N. Association of the IL-6 −174G/C gene polymorphism with knee osteoarthritis in a thai population. Genet. Mol. Res. 2011, 10, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J.; Cerami, A. Tumor necrosis factor, other cytokines and disease. Annu. Rev. Cell Biol. 1993, 9, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.J.; Wang, S.H.; Chen, Y.H.; Chang, S.S.; Hwang, T.L.; Leu, Y.L.; Tseng, Y.C.; Li, C.Y.; Chen, Y.L. Viscolin reduces vcam-1 expression in TNF-α-treated endothelial cells via the JNK/NF-κB and ROS pathway. Free Radic. Biol. Med. 2011, 51, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Di Giovine, F.S.; Nuki, G.; Duff, G.W. Tumour necrosis factor in synovial exudates. Ann. Rheumatol. Dis. 1988, 47, 768–772. [Google Scholar] [CrossRef]
- Saxne, T.; Palladino, M.A., Jr.; Heinegard, D.; Talal, N.; Wollheim, F.A. Detection of tumor necrosis factor α but not tumor necrosis factor β in rheumatoid arthritis synovial fluid and serum. Arthritis Rheumatol. 1988, 31, 1041–1045. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Chen, Y.; Wang, W. The current progress in understanding the molecular functions and mechanisms of visfatin in osteoarthritis. J. Bone Miner. Metab. 2016, 34, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Giannitti, C.; Cheleschi, S.; Simpatico, A.; Pascarelli, N.A.; Galeazzi, M. Circulating levels of adiponectin, resistin, and visfatin after mud-bath therapy in patients with bilateral knee osteoarthritis. Int. J. Biometeorol. 2015, 59, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.M.; Chen, C.P.; Huang, K.C.; Shieh, D.C.; Cheng, H.C.; Tzeng, C.Y.; Chen, K.H.; Chiu, Y.C.; Tang, C.H. Adiponectin increases MMP-3 expression in human chondrocytes through adipor1 signaling pathway. J. Cell. Biochem. 2011, 112, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Lee, W.L.; Hsu, C.J.; Lu, T.T.; Wang, L.H.; Xu, G.H.; Tang, C.H. Adiponectin induces oncostatin M expression in osteoblasts through the PI3K/AKT signaling pathway. Int. J. Mol. Sci. 2016, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Fuh, L.J.; Huang, C.C.; Hsu, C.J.; Su, C.M.; Liu, S.C.; Lin, Y.M.; Tang, C.H. Enhancement of CCL2 expression and monocyte migration by CCN1 in osteoblasts through inhibiting miR-518a-5p: Implication of rheumatoid arthritis therapy. Sci. Rep. 2017, 7, 421. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.C.; Yeh, M.H.; Chen, Y.J.; Liu, J.F.; Tang, C.H.; Huang, W.C. Lapatinib increases motility of triple-negative breast cancer cells by decreasing miRNA-7 and inducing RAF-1/MAPK-dependent interleukin-6. Oncotarget 2015, 6, 37965–37978. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jing, Z.; Cheng, G. MicroRNAs: New regulators of Toll-like receptor signalling pathways. BioMed Res. Int. 2014, 2014, 945169. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, Y.; Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H317–H325. [Google Scholar] [CrossRef] [PubMed]
- Santini, P.; Politi, L.; Vedova, P.D.; Scandurra, R.; Scotto d’Abusco, A. The inflammatory circuitry of miR-149 as a pathological mechanism in osteoarthritis. Rheumatol. Int. 2014, 34, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Budd, E.; Waddell, S.; de Andres, M.C.; Oreffo, R.O.C. The potential of microRNAs for stem cell-based therapy for degenerative skeletal diseases. Curr. Mol. Biol. Rep. 2017, 3, 263–275. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, A.; Im, G.I. Micrornas in orthopaedic research: Disease associations, potential therapeutic applications, and perspectives. J. Orthop. Res. Soc. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Hou, S.M.; Tsai, C.H.; Huang, C.Y.; Yang, W.H.; Tang, C.H. Thrombin induces heme oxygenase-1 expression in human synovial fibroblasts through protease-activated receptor signaling pathways. Arthritis Res. Ther. 2012, 14, R91. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Lago, R.; Gomez, R.; Lago, F.; Dieguez, C.; Gomez-Reino, J.J.; Gualillo, O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann. Rheumatol. Dis. 2006, 65, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, H.J.; Chen, H.S.; Cheng, S.Y.; Hsu, H.C.; Tang, C.H. Thrombin promotes matrix metalloproteinase-13 expression through the PKCδ/c-Src/EGFR/PI3K/Akt/AP-1 signaling pathway in human chondrocytes. Mediat. Inflamm. 2013, 2013, 326041. [Google Scholar] [CrossRef] [PubMed]
- Latimer, H.R.; Veal, E.A. Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction. Mol. Cells 2016, 39, 40–45. [Google Scholar] [PubMed]
- Bayoumi, A.S.; Sayed, A.; Broskova, Z.; Teoh, J.P.; Wilson, J.; Su, H.; Tang, Y.L.; Kim, I.M. Crosstalk between long noncoding RNAs and microRNAs in health and disease. Int. J. Mol. Sci. 2016, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. Micrornas: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.J.; Yang, W.H.; Liu, S.C.; Tsai, C.H.; Hsu, H.C.; Tang, C.H. Transforming growth factor β1 enhances heme oxygenase 1 expression in human synovial fibroblasts by inhibiting microRNA 519b synthesis. PLoS ONE 2017, 12, e0176052. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Chiu, C.P.; Tsai, C.H.; Hung, C.Y.; Li, T.M.; Wu, Y.C.; Tang, C.H. Soya-cerebroside, an extract of cordyceps militaris, suppresses monocyte migration and prevents cartilage degradation in inflammatory animal models. Sci. Rep. 2017, 7, 43205. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.E.; Tsai, C.H.; Chang, Z.L.; Su, C.M.; Wang, S.W.; Hwang, W.L.; Tang, C.H. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem. Pharmacol. 2013, 85, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Li, Y.; Huang, B.F.; Zhao, Y.M.; Yuan, H.; Guo, D.; Su, C.M.; Hu, G.N.; Wang, Q.; Long, T.; et al. EGFR conjunct FSCN1 as a novel therapeutic strategy in triple-negative breast cancer. Sci. Rep. 2017, 7, 15654. [Google Scholar] [CrossRef] [PubMed]
- Lerner, I.; Baraz, L.; Pikarsky, E.; Meirovitz, A.; Edovitsky, E.; Peretz, T.; Vlodavsky, I.; Elkin, M. Function of heparanase in prostate tumorigenesis: Potential for therapy. Clin. Cancer Res. 2008, 14, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chen, S.Y.; Tsai, H.C.; Hsu, H.C.; Tang, C.H. Thrombin induces epidermal growth factor receptor transactivation and CCL2 expression in human osteoblasts. Arthritis Rheumatol. 2012, 64, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Lin, C.; Lu, C.C.; Kuo, S.C.; Tsao, J.W.; Juan, Y.N.; Chiu, H.Y.; Lee, F.Y.; Yang, J.S.; Tsai, F.J. YC-1 induces G0/G1 phase arrest and mitochondria-dependent apoptosis in cisplatin-resistant human oral cancer CAR cells. Biomedicine 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-H.; Tsai, C.-H.; Huang, Y.-L.; Fong, Y.-C.; Tang, C.-H. Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 190. https://doi.org/10.3390/ijms19010190
Wu M-H, Tsai C-H, Huang Y-L, Fong Y-C, Tang C-H. Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. International Journal of Molecular Sciences. 2018; 19(1):190. https://doi.org/10.3390/ijms19010190
Chicago/Turabian StyleWu, Min-Huan, Chun-Hao Tsai, Yuan-Li Huang, Yi-Chin Fong, and Chih-Hsin Tang. 2018. "Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways" International Journal of Molecular Sciences 19, no. 1: 190. https://doi.org/10.3390/ijms19010190